Introduction
Spinal cord injury (SCI) is a devastating condition,
which involves the sudden loss of sensory, motor and autonomic
functions distal to the areas of trauma. No effective therapy
exists for treating the neurological deficits of major SCI. Stem
cell-based therapies have introduced novel possibilities for the
repair and restoration of neuronal functions following SCI
(1–5). Transplantation of bone marrow
mesenchymal stem cells (BMSCs), which represent an accessible
autologous stem cell source, has reached the stage of clinical
investigation and may be a promising treatment for SCI (6). According to previous reports, the
mechanism underlying BMSC-induced recovery is primarily associated
with the secretion of numerous cytokines and growth factors,
including neurotrophic factors (NTFs), brain-derived neurotrophic
factor (BDNF), glial cell line-derived neurotrophic factor (GDNF)
and nerve growth factor (NGF); the angiogenic factor, vascular
endothelial growth factor (VEGF) and the inflammation-associated
cytokines, transforming growth factor (TGF)-β1 and interleukin
(IL)-10 (7–9). These factors likely promote endogenous
repair mechanisms by stimulating neurite outgrowth and
angiogenesis, and by promoting immunomodulatory effects (7–9).
However, BMSC isolation is invasive, the abundance of stem cells in
the bone marrow is very low, and the proliferative capacity and
differentiation abilities of stem cells decrease with age (10). Due to these shortcomings, clinical
applications with BMSCs are limited. Thus, there is a requirement
to identify and evaluate other autologous sources of adult stem
cells.
In a previous study, we demonstrated that dermal
papilla cells (DPCs) expressed mesenchymal stem cell markers,
including cluster of differentiation (CD)44, CD90 and CD105, and
exhibited multipotent stem cell characteristics, similar to BMSCs
(11). Notably, DPCs secreted higher
amounts of BDNF and GDNF, and promoted rat pheochromocytoma cell
(PC12 cell) neural differentiation and axonal outgrowth more
effectively compared with BMSCs in vitro. On this basis,
DPCs may exhibit similar characteristics to BMSCs, including the
secretion of NTFs, angiogenic factors and inflammation-associated
cytokines. Thus, DPCs may potentially be utilized in autologous
clinical stem cell applications for treating SCI.
In the present study, the expression of associated
cytokines in DPCs and BMSCs was examined, which were isolated from
the same donor rat. Thereafter, these cells were transplanted into
a completely transected lesion site of the spinal cord to further
investigate their therapeutic efficacies in repairing SCI in
vivo.
Materials and methods
Animals
A total of 3 adult male green fluorescence protein
(GFP)-transgenic SD rats (6 weeks old; ~200 g) were purchased from
Xing Xingming Biomedical Technology (Shanghai) Co., Ltd. (Shanghai,
China) and 27 adult female SD rats (7 weeks old; ~250 g) were
purchased from Beijing Hua Fu Kang Biotechnology Co., Ltd.
(Beijing, China). All the rats were kept under identical housing
conditions (temperature, 18–26°C; humidity, 40–70%) with a 12 h
light-dark cycle, and given access to water and food ad
libitum. All experimental procedures were approved by the
Ethics Committee of Jilin University (Changchun, China) and
conformed to their regulatory standards.
Isolation and cultivation of DPCs and
BMSCs from GFP-transgenic rats
DPCs were isolated from the vibrissa of
GFP-transgenic rats according to previously described protocols
(11). Subsequently, the papillae
were cultured in proliferation medium consisting of Dulbecco's
modified Eagle's medium (DMEM)/Nutrient Mixture F-12 (1:1) medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% (v/v) fetal bovine serum (FBS; HyClone; GE
Healthcare Lie Sciences, Logan, UT, USA) and 10 ng/ml basic
fibroblast growth factor (bFGF; PeproTech EC, Ltd., London, UK).
Half of the medium was changed every 3 days. The DPCs that migrated
out of the explants were digested with 0.25% trypsin (Invitrogen;
Thermo Fisher Scientific, Inc.) in 0.02% ethylenediaminetetraacetic
acid (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), after which
they were expanded in new culture dishes at a seeding density of
1×104 cells/cm2. The cells were passaged when
~80% confluence was attained. Finally, the DPCs at passage 6 were
prepared for transplantation. As a control, BMSCs were isolated
from the same donor rat and cultured using a previously described
method (11).
Semi-quantitative reverse
transcription polymerase chain reaction (RT-PCR)
Total RNA from DPCs and BMSCs was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols. cDNA was synthesized
from 500 ng total RNA using the Takara RNA PCR kit (AMV) version
3.0 (Takara Biotechnology Co., Ltd. Dalian, China), only the
reagents for the reverse transcription protocol were used. The
reaction condition was as follows: 45°C for 30 min, 99°C for 5 min
and 5°C for 5 min. PCR was performed with 1 µl cDNA in a 20 µl
reaction volume using 2X Taq MasterMix (Beijing ComWin Biotech Co.,
Ltd., Beijing, China), according to the manufacturer's protocols.
The reaction condition was as follows: Initial denaturation at 94°C
for 2 min, then 30 cycles of degeneration at 94°C for for 30 sec,
annealing at 50–58°C for 30 sec and extension at 72°C for 50 sec,
and final extension at 72°C for 10 min. PCR products were
separeated by 1.5% agarose gel electrophoresis with ethidium
bromide and visualized using an ultraviolet transilluminator.
Sequence information for the primers used is presented in Table I.
 | Table I.Primers and annealing temperatures
used for semi-quantitative polymerase chain reaction. |
Table I.
Primers and annealing temperatures
used for semi-quantitative polymerase chain reaction.
| Gene | Primer sequence (5′
to 3′) | Annealing
temperature |
|---|
| HGF |
Forward-CCTTCGAGCTATCGCGGTAAAGAC | 56°C |
|
|
Reverse-TCAAGAGTGTAGCACCATGGCCTC |
|
| NGF |
Forward-GGACGCAGCTTTCTATCCTG | 56°C |
|
|
Reverse-GTCCGTGGCTGTGGTCTTAT |
|
| GDNF |
Forward-GACTCCAATATGCCCGAAGA | 56°C |
|
|
Reverse-ATGGTAAACCAGGCTGTCGT |
|
| BDNF |
Forward-TGGCTGACACTTTTGAGCAC | 56°C |
|
|
Reverse-GCAGCCTTCCTTCGTGTAAC |
|
| CNTF |
Forward-TGGCTAGCAAGGAAGATTCG | 56°C |
|
|
Reverse-ACCTTCAAGCCCCATAGCTT |
|
| VEGFA |
Forward-ACCAAAGAAAGATAGAACAAAG | 50°C |
|
|
Reverse-GGTGAGAGGTCTAGTTCCCGA |
|
| FLK-1 |
Forward-GCCAATGAAGGGGAACTGAAGAC | 50°C |
|
|
Reverse-TCTGACTGCTGGTGATGCTGTC |
|
| FGF2 |
Forward-ATCACTTCGCTTCCCGCACT | 55°C |
|
|
Reverse-AGTATGGCCTTCTGTCCAGG |
|
| TGF-β1 |
Forward-CTCTGCAGGCGCAGCTCTG | 55°C |
|
|
Reverse-GGACTCTCCACCTGCAAGAC |
|
| IL-10 |
Forward-CAATAACTGCACCCACTTCC | 55°C |
|
|
Reverse-ATTCTTCACCTGCTCCACTGC |
|
| TNF-α |
Forward-CCCAGACCCTCACACTCAGAT | 55°C |
|
|
Reverse-TTGTCCCTTGAAGAGAACCTG |
|
| IL-6 |
Forward-CCGGAGAGGAGACTTCACAG | 55°C |
|
|
Reverse-GAGCATTGGAAGTTGGGGTA |
|
| IL-1β |
Forward-CAGGAAGGCAGTGTCACTCA | 53°C |
|
|
Reverse-GAAGACAAACCGCTTTTCCA |
|
| GAPDH |
Forward-ATGGGAAGCTGGTCATCAAC | 58°C |
|
|
Reverse-GGATGCAGGGATGATGTTCT |
|
RT-quantitative PCR (RT-qPCR)
Total RNA from DPCs and BMSCs was extracted using
TRIzol reagent, according to the manufacturer's protocols, then
reverse transcribed into cDNA using the Takara RNA PCR kit (AMV)
Version 3.0 (Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocols. Only the reagents for the reverse
transcription protocol were used. To determine the expression
levels of NTFs (BDNF, GDNF, CNTF, HGF and NGF), angiogenic factor
VEGF(A) and inflammation-associated cytokines [TGF-β1, tumor
necrosis factor (TNF)-α, IL-6 and IL-1β] in DPCs. qPCR was
performed using TransStart® Top Green qPCR SuperMix
(Beijing TransGen Biotech, Co., Ltd., Beijing, China). GAPDH was
used as an internal reference to normalize mRNA expression levels.
qPCR was performed in a PCR System 7300 (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The thermocyling conditions were as
follows: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15
sec and 60°C for 1 min, 95°C for 15 sec, 60°C for 1 min, and 95°C
for 15 sec. The expression levels of mRNAs were measured using the
cycle-threshold values and then the results were converted to
fold-changes (12). Sequence
information for the primers used is presented in Table II.
 | Table II.Primers used for quantitative
polymerase chain reaction. |
Table II.
Primers used for quantitative
polymerase chain reaction.
| Gene | Primer sequence (5′
to 3′) |
|---|
| NGF |
Forward-ACCTCTTCGGACACTCTGGA |
|
|
Reverse-TCCAACCCACACACTGACAC |
| BDNF |
Forward-TGGGTTACACGAAGGAAGGC |
|
|
Reverse-ATCCTTATGAACCGCCAGCC |
| CNTF |
Forward-GGCAAGCACTGATCGTTGGA |
|
|
Reverse-TGGAAGGTACGGTAAGCCTG |
| HGF |
Forward-ACAGCTTTTTGCCTTCGAGC |
|
|
Reverse-GCAAGAATTTGTGCCGGTGT |
| GDNF |
Forward-TGACTTGGGTTTGGGCTACG |
|
|
Reverse-GGTAAACCAGGCTGTCGTCT |
| IL-1β |
Forward-TCCATGGTGGATTATGCTCA |
|
|
Reverse-TTCTGTTCCTGCTCGAGGTT |
| IL-10 |
Forward-ACTGCTAGTTTGCCTGCTCTT |
|
|
Reverse-ATGTGGGTCTGGCTGACTGG |
| IL-6 |
Forward-CTGCCCTTCAGGAACAGCTATG |
|
|
Reverse-GGCAGTGGCTGTCAACAACAT |
| TNF-α |
Forward-GAGATGTGGAACTGGCAGAGGA |
|
|
Reverse-TCAGTAGACAGAAGAGCGTGGTG |
| TGF-β1 |
Forward-CTCCCGTGGCTTCTAGTGC |
|
|
Reverse-GCCTTAGTTTGGACAGGATCTG |
| VEGFA |
Forward-CCTGGCTTTACTGCTGTACCT |
|
|
Reverse-GCTGGTAGACGTCCATGAACT |
| GAPDH-1 |
Forward-GAAGGTCGGTGTGAACGGAT |
|
|
Reverse-ACCAGCTTCCCATTCTCAGC |
| GAPDH-2 |
Forward-ATGGGAAGCTGGTCATCAAC |
|
|
Reverse-GGATGCAGGGATGATGTTCT |
Spinal cord transection and acute
injection of cells
The female SD rats were randomly assigned to the
DPC-transplant group (n=10), the BMSC-transplant group (n=9) and a
control group (n=8). Subsequently, the rats were deeply
anesthetized by intraperitoneal injection with 10% chloral hydrate
(400 mg/ml; Sigma-Aldrich; Merck KGaA). The SCI models were
generated according to Medalha's method (13). Briefly, the hair over the thoracic
area was shaved, the skin was disinfected with iodine and T8
vertebra were completely transected. A ~1.5-mm-long segment of
spinal cord was cut using iridectomy scissors and the debris were
removed by micro-aspiration. Once hemostasis was achieved, the gap
was filled with 7 µl DPCs or BMSCs (106 cells total) in
rat-tail collagen I (2 mg/ml; Tianjin Weikai Bioeng, Ltd., Tianjin,
China) using a 10-µl pipettor. As a control, 7 µl collagen I
without cells was microinjected into the lesion cavity. Following
exposure at room temperature for 45 min, the lesion site was
covered with the fat pad, and the skin was sewn back together.
Bladders were manually flushed twice daily until the animals were
euthanized (on days 14 and 21 post-transplantation) and penicillin
(4×104 units/rat; North China Pharmaceutical Co., Ltd.,
Shijiazhuang, China) was injected intraperitoneally daily for 4
days postoperatively as prophylaxis against urinary tract
infection. In addition, animals were immunosuppressed daily by
subcutaneous administration of cyclosporine A (1 mg/100 g; Novartis
Pharma Stein AG, Stein, Switzerland) beginning 1 day prior to
grafting and continuing until the termination of the
experiment.
Gel properties
To examine whether the graft mixture may gelatinize
effectively and influence the transplantation process, as well as
the gel concentration on cell survival, an extra 7 µl graft mixture
remaining post-transplantation was added to a 35-mm culture dish
and incubated for 45 min in a 37°C/5% CO2 incubator.
Subsequently, 1 ml DMEM/F-12 culture medium containing 10% FBS was
added, and the cells were cultured further.
Small animal in vivo-imaging
detection
To examine lesion filling and the survival of
DPC/BMSC grafts, the rats (n=3 at each time point per group) were
anesthetized by intraperitoneal injection with 10% chloral hydrate
(400 mg/ml; Sigma-Aldrich; Merck KGaA), then perfused with PBS
followed by 4% paraformaldehyde (Beijing Dingguo Changsheng
Biotechnology, Co., Ltd, Beijing, China), on days 14 and 21
post-transplantation, respectively. Subsequently, the spinal cord
(2 cm around the epicenter) was surgically dissected, fixed in 4%
paraformaldehyde at 4°C for 48 h, and transferred to 30% sucrose
(Sigma-Aldrich; Merck KGaA) at 4°C for 72 h. Finally, the segments
of the spinal cords were placed into a new culture dish and the
fluorescence intensities around the lesion/graft sites were
detected with a Small Animal Imaging system (Xenogen Corporation,
Alameda, CA, USA) at 488 nm excitation.
Assessment of lesion areas
Following fixation and dehydration, the spinal cord
segments were embedded with Optimal Cutting Temperature compound
(Leica Microsystems GmbH, Wetzlar, Germany), and serial sections of
20-µm thickness were prepared. Every sixth section per rat sample
was hydrated in PBS and hematoxylin and eosin staining of the
sections was performed. Briefly, the sections were stained with the
hematoxylin solution for 5 min, rinsed in running tap water, then
differentiated with 1% hydrochloric acid ethanol for several
seconds, followed by rinsing in running tap water. The sections
were stained with weak ammonia for 1–2 sec, rinsed in tap water and
stained with eosin for 15 min. The sections were dehydrated with
ascending concentration gradient of ethyl alcohol (in 80% ethyl
alcohol for 1–2 sec, 95% ethyl alcohol for 1–2 sec, 100% ethyl
alcohol I for 5 min and 100% ethyl alcohol II for 10 min). The
sections were cleared with xylol I for 10 min and xylol II for 10
min (Beijing Dingguo Changsheng Biotechnology, Co., Ltd., Beijing,
China). All protocols were performed at room temperature. Finally,
the sections were mounted with neutral resin and observed under a
light microscope at ×40 magnification. Finally, the images were
captured and merged with Adobe Photoshop CS5 software (version
12.0; Adobe Systems, Inc., San Jose, CA, USA) and cellSens
Dimension software (version 1.0; Olympus Corporation, Tokyo, Japan)
was used to assess the lesion areas.
Immunocytofluorescence histochemical
staining
To assess axonal outgrowth, angiogenesis and
inflammation, immunocytofluorescence histochemical staining was
conducted in the present study. Briefly, every sixth section per
rat sample was hydrated in PBS for 25 min at room temperature;
then, samples were permeabilized with 0.1% Triton X-100
(Sigma-Aldrich; Merck KGaA) for 45 min at room temperature. Normal
goat serum (10%; Zhongshan Jinqiao Biotechnology Co., Ltd.,
Beijing, China) was used to block non-specific binding for 1 h at
room temperature. Every sixth section per rat sample was then
incubated overnight with primary antibodies at 4°C (detailed
information regarding the primary antibodies is presented in
Table III), followed by incubation
with Alexa Fluor® 488-conjugated or 555-conjugated
secondary antibodies (1:400; cat. nos. 4412 and 4413; Cell
Signaling Technology, Inc., Danvers, MA, USA) for 1 h at room
temperature. Unbound antibody was removed by thoroughly washing
with PBS. For the negative control, the primary antibodies were
omitted. Finally, the sections were mounted using Anti-Fade
mounting medium with DAPI (Vector Laboratories, Inc., Burlingame,
CA, USA). All images were obtained using an IX71 fluorescence
microscope (Olympus Corporation). The duration of exposure and the
parameter settings were identical in ≥3 independent experiments
conducted with samples from different groups.
 | Table III.Primary antibody information. |
Table III.
Primary antibody information.
| Antibody | Dilution | Supplier | Catalogue
number |
|---|
| GFP | 1:1,000 | Abcam (Cambridge,
UK) | ab6556 |
| NF-200 | 1:200 | EMD Millipore
(Billerica, MA, USA) | Mab5262 |
| GFAP | 1:400 | Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany) | G3893 |
| CD31 | 1:20 | Abcam | ab28364 |
| CD11b | 1:50 | Bio-Rad
Laboratories, Inc., Hercules, CA, USA | MCA275GA |
Behavioral testing
Hindlimb motor functions of the DPC-transplant,
BMSC-transplant and control group were evaluated weekly by two
independent, blinded observers, based on the Basso Beattie
Bresnahan (BBB) motor score (14).
The number of the rats that received the behavioral test at each
time point is presented in Table
IV.
 | Table IV.Number of rats that underwent a
behavioral test at each time point. |
Table IV.
Number of rats that underwent a
behavioral test at each time point.
|
| Day |
|---|
|
|
|
|---|
| Group | 0 | 7 | 14 | 21 |
|---|
| Dermal papilla
cell-transplant | 10 | 6 | 6 | 3 |
| Bone marrow
mesenchymal stem cell-transplant | 9 | 8 | 6 | 3 |
| Control | 8 | 7 | 7 | 4 |
Statistical analysis
Statistical analysis was performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard error of the mean from ≥3
independent experiments. Multiple-group comparisons were conducted
using one-way analysis of variance followed by Least Significant
Difference post hoc comparisons (equal variances assumed) or Dunn's
post hoc test (unequal variances) for multiple comparisons. Two
groups were compared using a Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Isolation and cultivation of DPCs and
BMSCs from GFP-transgenic rats
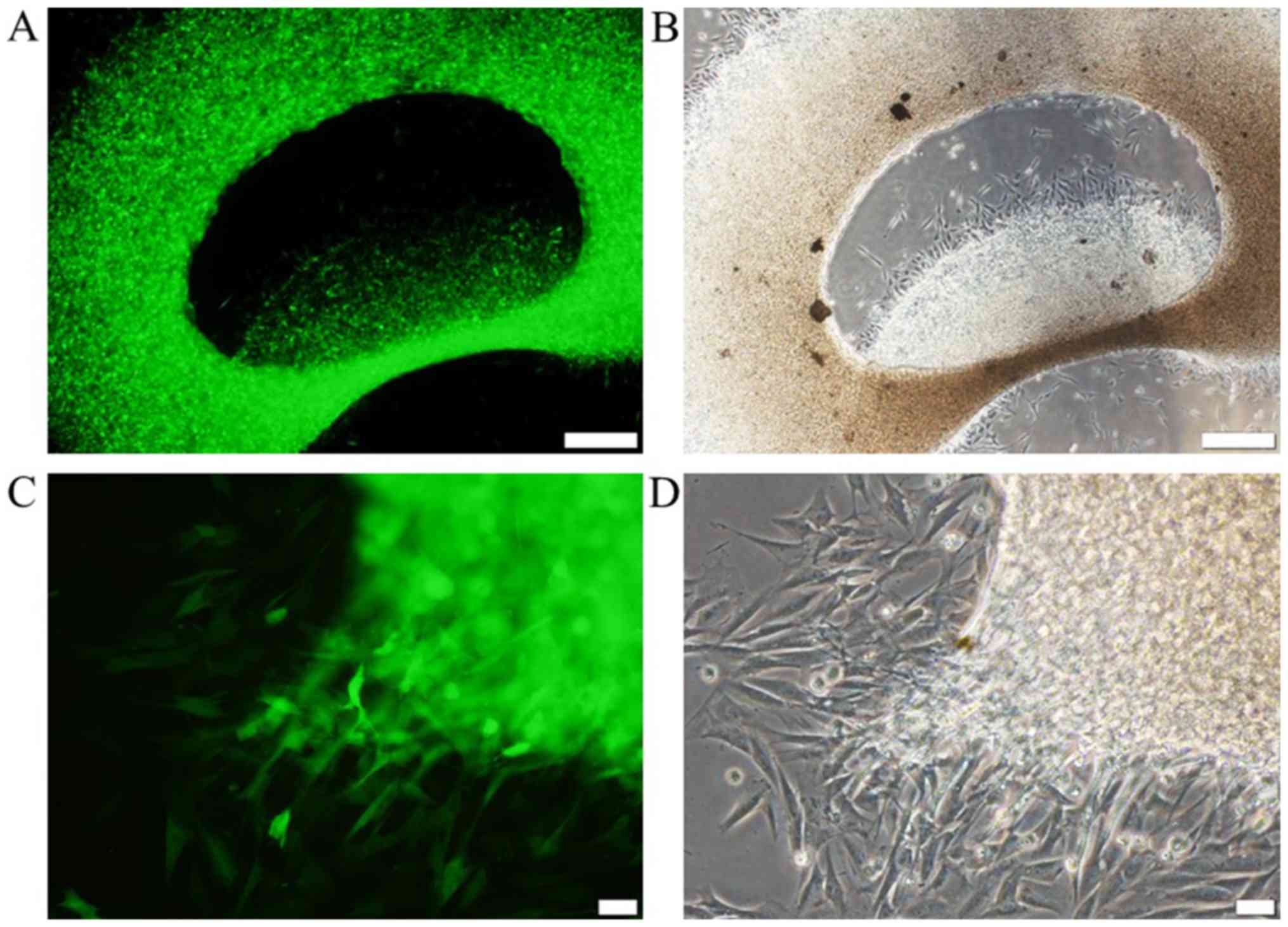
After 6 days of cultivation, typical spindle-shaped,
fibrocyte-like cells were cultured from the explants, which were
all positive for GFP (Fig. 1A and
B). The cells demonstrated stable growth following 6 passages;
cells revealed fibrocyte-associated morphology and expressed GFP
(Fig. 1C and D). As a control, BMSCs
were isolated and cultured from the same donor rat. Following
culture for 10 days, the clusters consisted of numerous cells that
were all positive for GFP (Fig. 1E and
F). These controls revealed stable GFP expression after 6
passages (Fig. 1G and H).
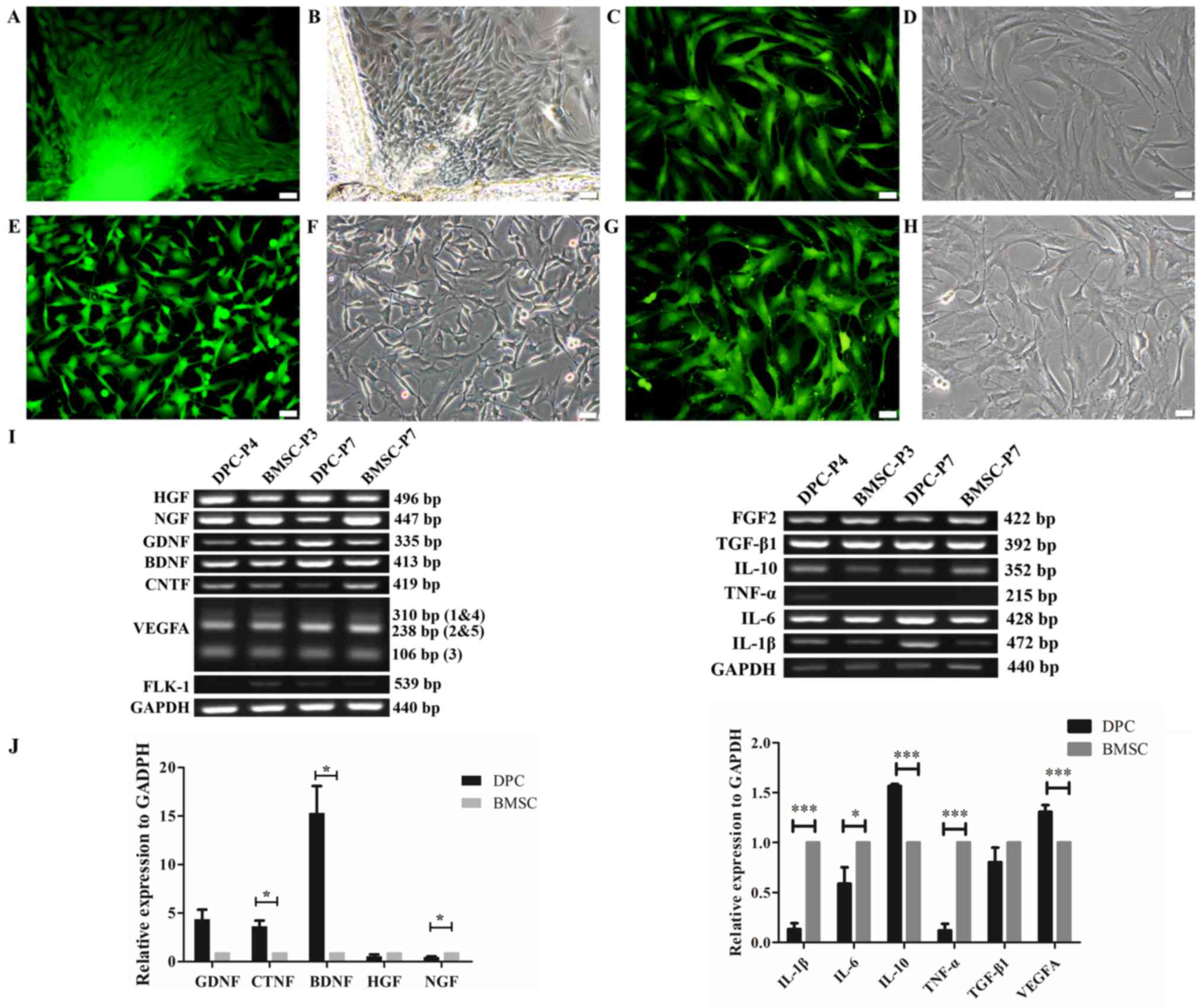 | Figure 1.Isolation and cultivation of DPCs and
BMSCs from GFP-transgenic rats, and expression of spinal cord
injury repair-associated cytokines. (A-G) Isolation and cultivation
of DPCs and BMSCs from GFP-transgenic rats. (A) Direct
GFP-fluorescence and (B) phase-contrast images demonstrated the
morphological characteristics of primary DPCs. (C and D) DPCs at
passage 6. (E) Direct GFP-fluorescence and (F) phase-contrast
images revealed the morphological characteristics of BMSCs. (G and
H) BMSCs at passage 6. (I) Semi-quantitative RT-PCR analysis was
performed to confirm the expression of NTFs, angiogenic factors and
inflammation-associated cytokines in DPCs. (J) RT-qPCR analysis
further revealed relative expression levels of NTFs, angiogenic
factors and inflammation-associated cytokines in DPCs. *P<0.05,
***P<0.001, n=3. Scale bars=20 µm. BDNF, brain-derived
neurotrophic factor; BMSCs, bone marrow mesenchymal stem cells;
CNTF, ciliary neurotrophic factor; DPCs, dermal papilla cells;
FGF2, fibroblast growth factor 2; FLK1, vascular endothelial growth
factor receptor 2; GDNF, glial cell line-derived neurotrophic
factor; GFP, green fluorescent protein; HGF, hepatocyte growth
factor; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10,
interleukin-10; NGF, nerve growth factor; NTFs, neurotrophic
factors; P, passage number; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; TGF-β1,
transforming growth factor-β1; TNF-α, tumor necrosis factor-α;
VEGFA, vascular endothelial growth factor A. |
Expression of NTFs, angiogenic factors
and inflammation-associated cytokines in DPCs
Semi-quantitative RT-PCR analysis revealed that the
NTFs, including hepatocyte growth factor (HGF), NGF, GDNF, BDNF and
ciliary neurotrophic factor (CNTF) (Fig.
1I); the angiogenic factors, VEGFA (all 3 transcriptional
variants) and FGF2 (Fig. 1I); and
the inflammation-associated cytokines TGF-β1, IL-10, IL-6 and IL-1β
(Fig. 1I) were expressed in DPCs at
passage 4 (DPC-P4) and passage 7 (DPC-P7), as well as in BMSCs at
passage 3 (BMSC-P3) and passage 7 (BMSC-P7). The expression level
of the angiogenic factors vascular endothelial growth factor
receptor 2 (FLK1) was low in DPCs at passage 4 (DPC-P4) and BMSCs
at passage 7 (BMSC-P7). In addition, the expression level of the
inflammation-associated cytokine TNF-α was low in DPCs at passage
4.
Additionally, RT-qPCR analysis revealed that,
compared with BMSCs, DPCs exhibited significantly higher expression
levels of NTFs, BDNF and CNTF, angiogenic factor VEGFA and
anti-inflammatory cytokine IL-10. Similar expression levels of
NTFs, GDNF and HGF, and anti-inflammatory TGF-β1 were observed in
DPCs and BMSCs. Significantly lower expression levels of NGF and
pro-inflammatory cytokines TNF-α, IL-6 and IL-1β were observed in
DPCs compared with BMSCs (Fig.
1J).
Spinal cord transection and acute
injection of cells
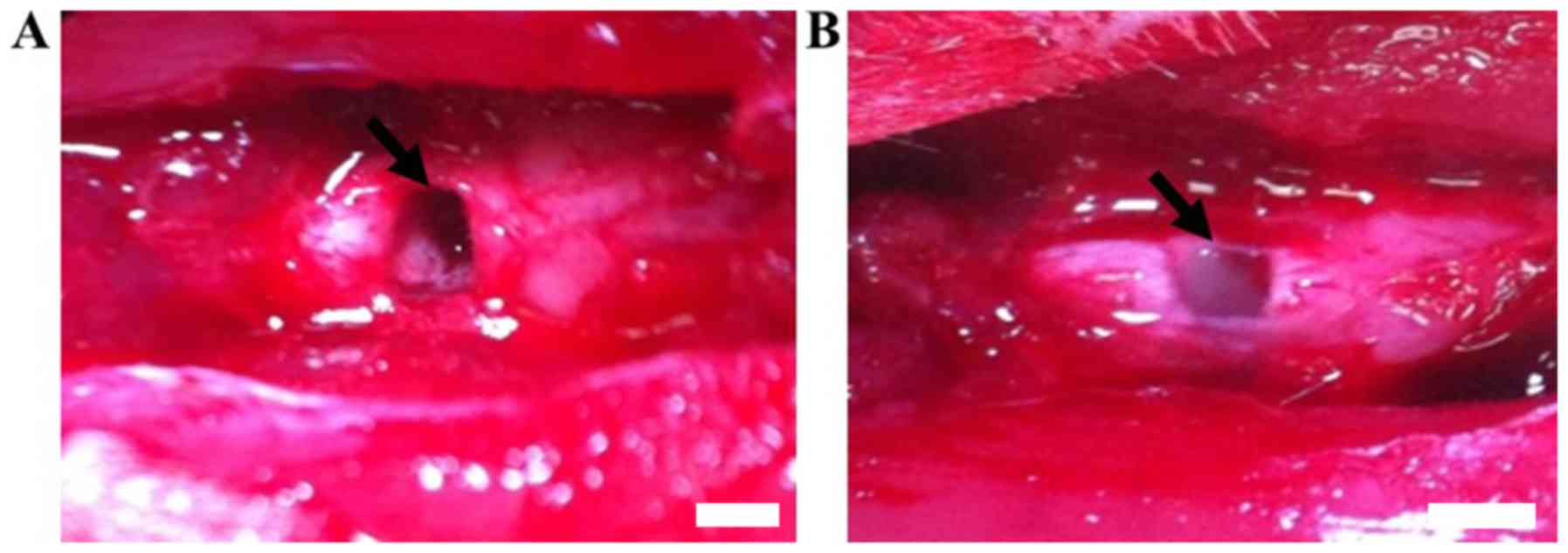
T8 vertebra was completely transected and a ~1.5 mm
lesion cavity was created using a combination of iridectomy
scissors and microaspiration (Fig.
2A), after which the gap was filled with 7 µl DPCs or BMSCs in
rat-tail collagen I. Following exposure at room temperature for 45
min, the appearance of the graft mixture became white, translucent
and gel-like (Fig. 2B).
Gel-associated properties
Following suspension of the GFP-positive cells in
collagen I and culturing in an incubator at 37°C for 45 min, the
graft mixture gelatinized (data not shown). Subsequently, DMEM/F-12
culture medium supplemented with 10% FBS was added, and the cells
were cultured further. Following overnight growth, most cells in
the graft mixture survived and numerous fibrocyte-like cells had
begun to migrate from the gel (Fig.
3).
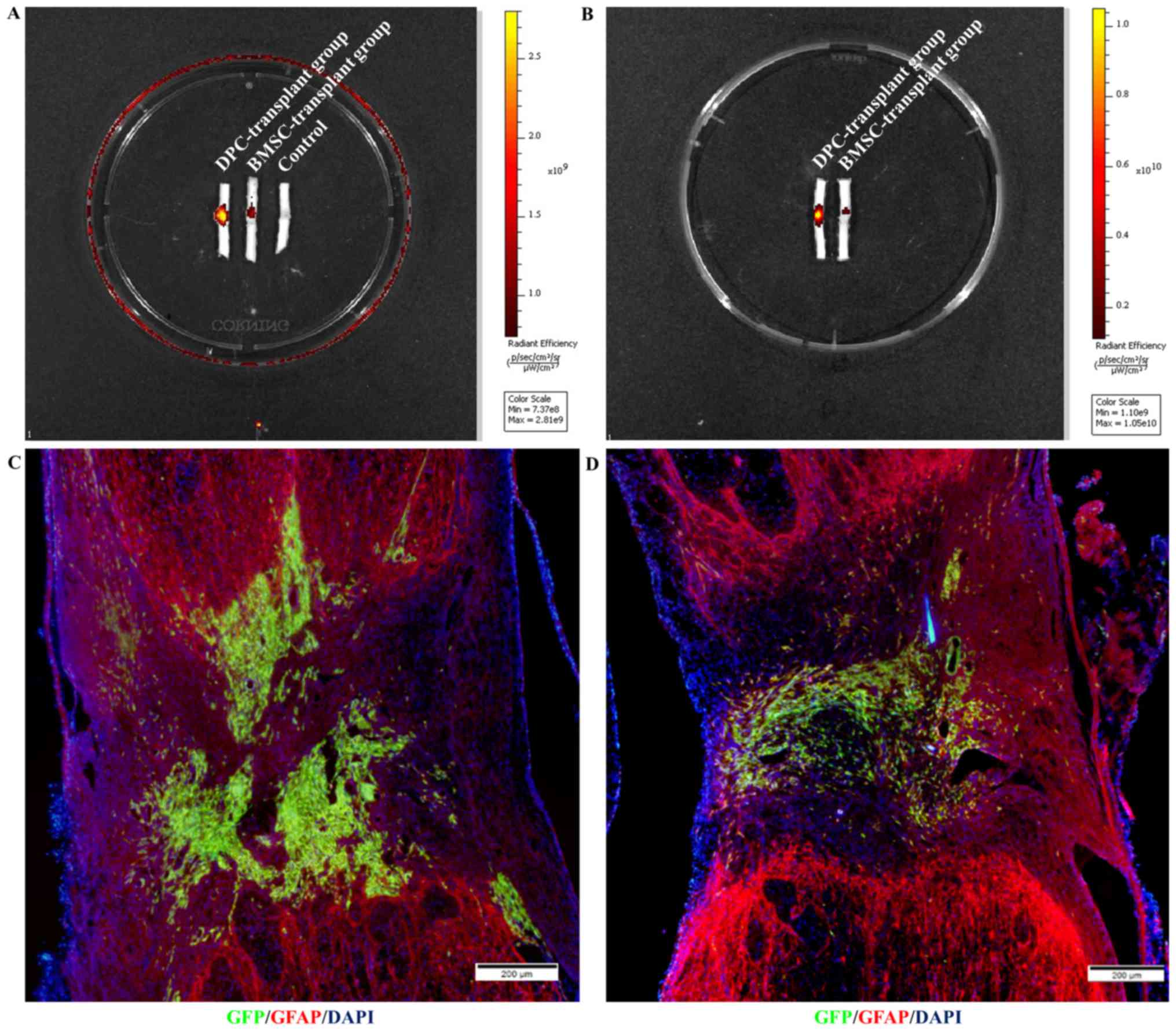
Survival and filling with DPCs/BMSCs
grafts
On day 14 (Fig. 4A)
and 21 (Fig. 4B)
post-transplantation, the immunofluorescence intensity surrounding
the lesion/graft site transplanted with DPCs was stronger compared
with the BMSC-transplant group and control group. There was no
control group on day 21 as no cell transplanted in the control
group on 14 day post transplantation. Additionally, a
low-magnification overview of GFP (graft) and glial fibrillary
acidic protein (host) immunolabeling on day 21 post-transplantation
further revealed that DPCs (Fig. 4C)
exhibited greater survival compared with BMSCs (Fig. 4D).
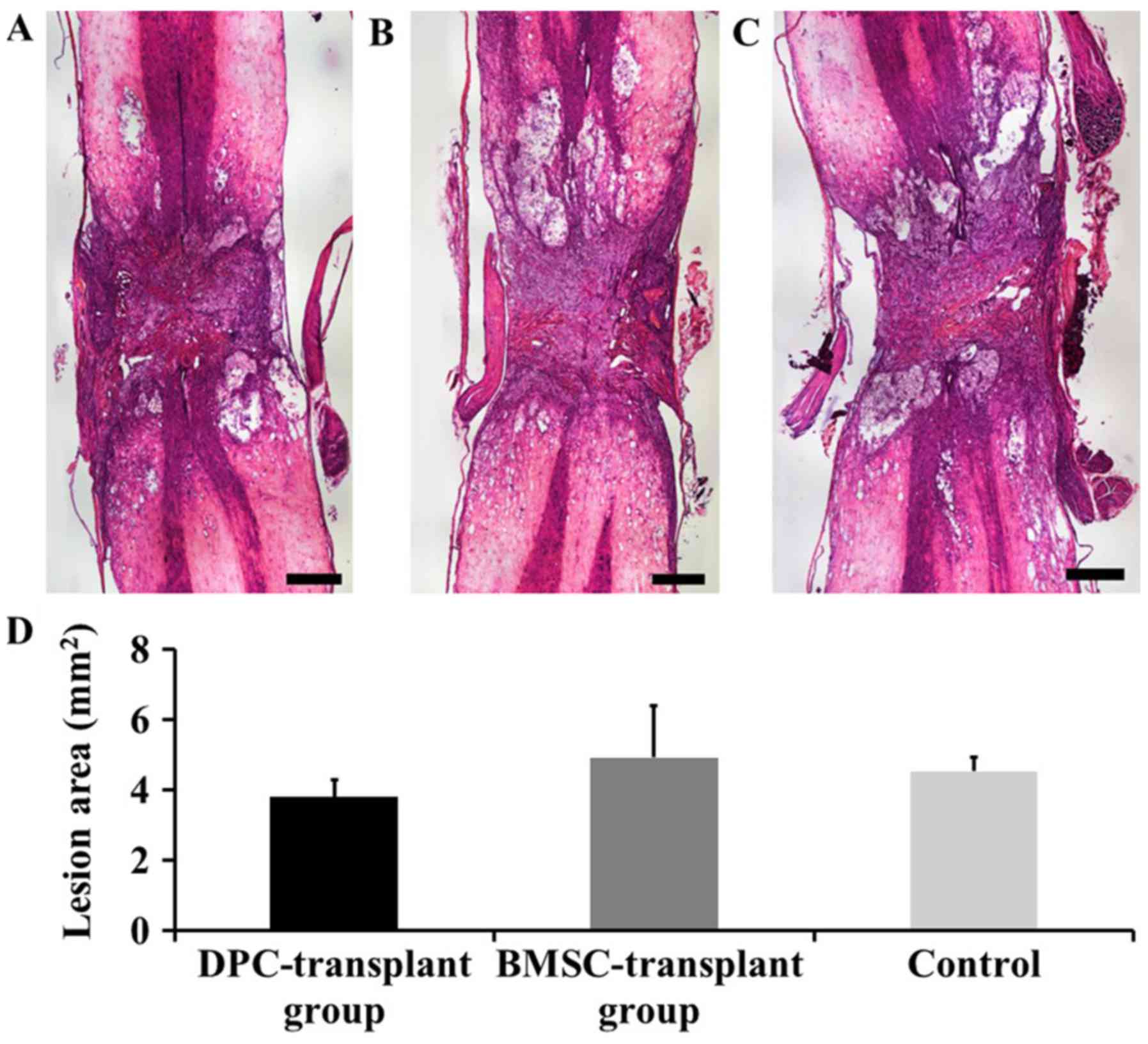
Assessment of lesion areas
On day 21 post-transplantation, the lesion areas of
spinal cords (n=3 in each group) transplanted with DPCs (Fig. 5A), BMSCs (Fig. 5B), or collagen alone (Fig. 5C) were 3.79±0.47, 4.90±1.47 and
4.52±0.40 mm2, respectively; thus, no significant
difference was observed (P>0.05; Fig.
5D).
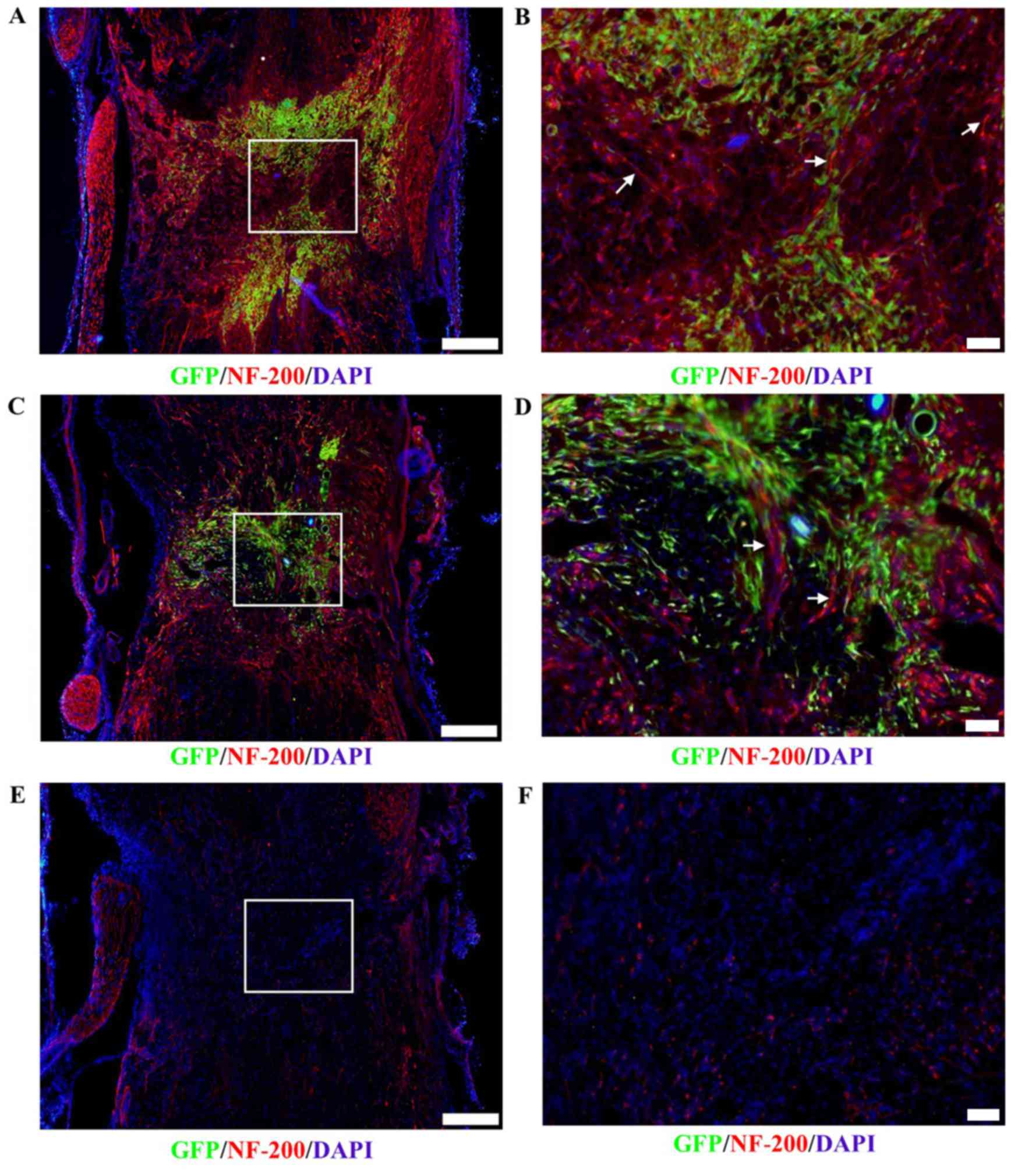
Axonal outgrowth
Immunofluorescence staining revealed that axonal
growth (arrow) into the lesion sites of the spinal cord
transplanted with DPCs (Fig. 6A and
B) was greater than that of the BMSC-transplant group (Fig. 6C and D) and control group (Fig. 6E and F) on day 21
post-transplantation. These results indicated that axonal growth in
the host may be more notably promoted by transplanted DPCs, and it
was associated with positive NF-200 expression and a lack of GFP
expression.
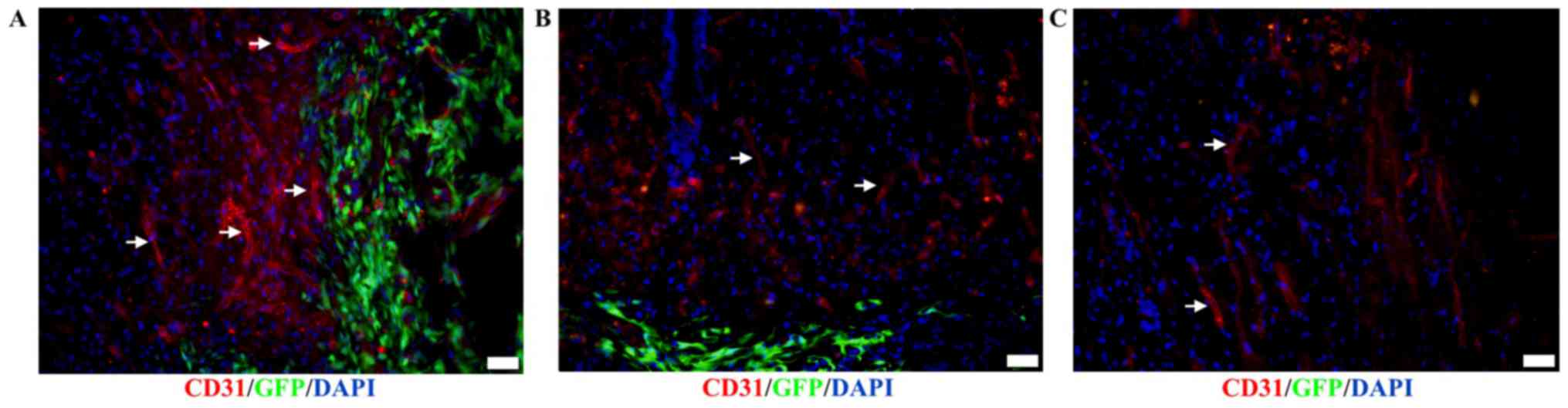
Vascular regeneration
Immunofluorescence staining also revealed that
vascular regeneration around the lesion sites of spinal cords
transplanted with DPCs (Fig. 7A) was
greater than that observed in the BMSC-transplant group (Fig. 7B) and the control group (Fig. 7C) on day 21 post-transplantation.
These results indicated that DPC transplantation notably promoted
revascularization in the host, which was positive for the vascular
endothelial cell specific marker CD31 and negative for GFP.
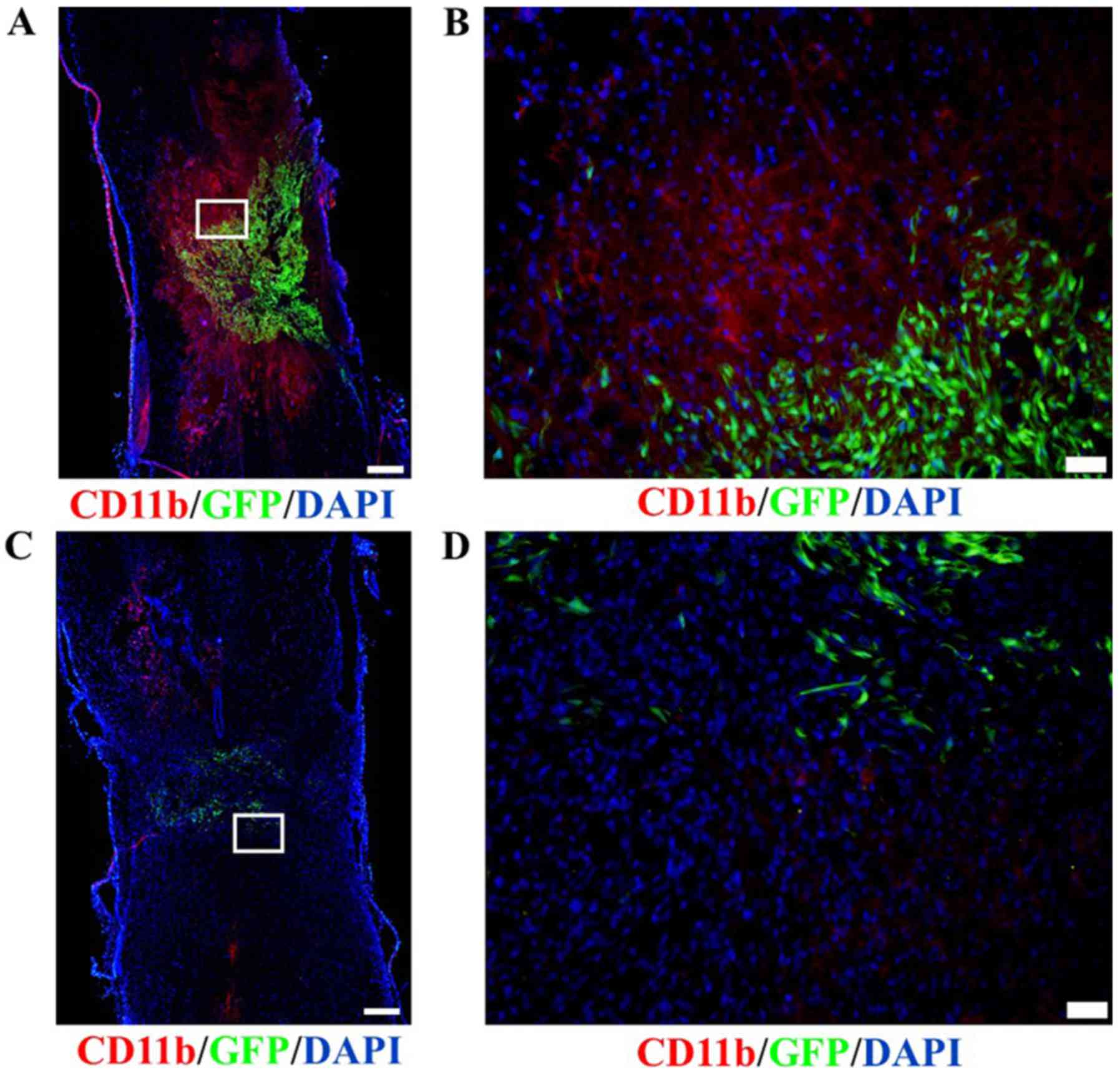
Macrophage infiltration
Immunofluorescence staining revealed that the number
of macrophages infiltrating around the lesion sites of spinal cords
transplanted with DPCs was greater than that observed in the
BMSC-transplant group (Fig. 8), as
determined by CD11b staining.
Behavioral testing
On days 0, 7, 14 and 21 post-transplantation, the
BBB scores of the DPC-transplant, BMSC-transplant and control
groups were all 0, indicating that no functional improvement
occurred (data not shown).
Discussion
The combined data from the present and a previous
study reveal that, similar to BMSCs, DPCs express and secrete
various cytokines and growth factors, which promote the
reconstruction of a supportive microenvironment for neural
regeneration in SCI lesion sites following transplantation
(11). However, the therapeutic
functions of DPCs were more favorable than BMSCs, which further
indicated that DPCs may be a valuable alternative source of stem
cells for autologous cell therapy to treat SCI.
Stem cells, which are self-renewing and highly
multipotent, are potentially useful for repairing neuronal
functions following SCI. According to previous reports, BMSC
transplantation is an effective method for treating SCI (15,16), but
BMSCs must be isolated by aspiration, which is traumatic and
painful (17). In addition, the
percentage of BMSCs in the bone marrow decreases with age, as do
their differentiation abilities (18). Conversely, DPCs can be isolated by
directly removing hairs, which is a non-traumatic procedure, and
hair follicles are renewable organs. Furthermore, hair undergoes
repeated cycles of growth (anagen phase), regression (catagen
phase) and rest (telogen phase) throughout the lives of mammals
(19,20). In addition, we previously
demonstrated that DPCs, with partial properties of neural crest
stem cells, secrete greater amounts of BDNF and GDNF compared with
BMSCs (11). Thus, the abilities of
DPCs to promote SCI repair in vivo were compared in the
present study.
At present, numerous types of SCI models have been
established, including the impact-injury model, the crush-injury
model, and the complete transection and aspiration-injury model
(13,21,22).
Among these, the complete transection and aspiration-injury model
does not require expensive and complicated equipment, and may be
easily generated via relatively simple surgical process. Therefore,
it has been widely used to study axonal regeneration and outgrowth
at injury sites. For these reasons, this SCI model was selected for
analysis in the present study.
Biomaterial scaffolds provide a bridge to connect
lost tissues, an adhesion site for implanted or host cells, thus
are widely used in the research of SCI repair via stem cell
transplantation (23,24). Fibronectin, as a carrier of NTFs, has
been reported to provide a suitable axon-growth environment within
rat SCI sites (25); however,
fibronectin is expensive and the solidity of the gel formed by
mixing fibrinogen with thrombin is hard to control. Furthermore,
the distribution of cells mixed with fibronectin is nonuniform. By
contrast, collagen I possesses the properties of good
biocompatibility, biodegradability and low immunogenicity, and has
been widely used in tissue-regeneration research (26). In addition, particular concentrations
of collagen I remain at a liquid state at 4°C and form a solid
scaffold of particular physical strength at 37°C, which integrates
well with peripheral tissue, exhibits good plasticity
post-transplantation (27). This
results in uniform distribution of the cells suspended with
collagen for transplantation, provides mechanical support for
transplanted cells and provides a highly porous network structure
that allows for cell expansion, neurite extension, and transport of
nutrients, all of which are essential for cell survival (27). Thus, collagen I served as a scaffold
in the present study. Additionally, collagen I, as a promising
three-dimensional cell culture scaffold, maintains the survival and
migration ability of NPSCs under optimal conditions (0.5–0.75
mg/ml) (27). In the present study,
it was also observed that the dispersion and nonuniform
distribution of the cells transplanted was effectively avoided by
using 2 mg/ml collagen I as scaffold.
Immediately following a traumatic event, the
microenvironment of the spinal cord undergoes acute inflammation,
which is unfavorable for the survival of transplants. This factor
is almost always a barrier for cell-based therapy in the acute
phase, but in the present study, the DPCs survived well in the
lesion site of spinal cord, and the amount was greater than that of
BMSCs. In addition, the number of macrophages infiltrated in the
DPC-transplanted group was greater compared with the
BMSC-transplanted group. Based on the expression of
inflammation-associated cytokines (IL-10 and TGF-β1), which have
important roles in regulating the polarization of pro-inflammatory
M1-type macrophages into anti-inflammatory M2-type macrophages
(28,29), DPCs may have modified the
inflammatory environment by regulating the conversion of M1 to M2
macrophages. The regulatory effects of DPCs on polarization of
macrophages from M1 to M2 phenotype may be investigated in the
future.
Liu et al (30) reported that nestin-positive dermal
and bulge area cells may differentiate into neuronal and glial
cells after transplantation to injured spinal cords. Conversely,
DPC differentiation into neurons or astrocytes was not observed in
the present study; the cell cultivation conditions used may have
influenced the cellular differentiation ability. Generally, BMSCs
are expanded via culturing in conventional monolayers. To confirm
the therapeutic effect of DPCs on promoting SCI repair under the
same culture condition used with BMSCs, DPCs were also expanded in
monolayers, instead of in serum-free medium supplemented with
epidermal growth factor and bFGF. Culturing DPCs in monolayers may
have altered the differentiation abilities of nestin-positive NCSCs
to differentiate into DPCs, thereby inhibiting their capacity for
differentiation into neuronal and glial cells in SCI lesion sites.
Furthermore, the results of the present study revealed that,
similar to BMSCs, DPCs may also stimulate neural tissue
regeneration, primarily via the secretion of numerous cytokines and
growth factors. Although DPCs differentiated into neurons, the rate
of differentiation may not have been high enough to serve a crucial
function during the repair of SCI.
The SCI environment tends to promote the astrocytic
differentiation of neural stem cells (NSCs). Lu et al
(25) reported that NSCs embedded
into fibrin matrices containing BNDF and GDNF, which differentiated
into numerous cellular phenotypes following transplantation into
severe SCI sites and subsequently stimulated axonal outgrowth
effectively. In a previous study by our group, DPCs actively
secrete BDNF and GDNF compared with BMSCs, and notably induce the
neuronal differentiation of PC12 cells and promote the outgrowth of
neuritis (11). Thus, following
transplantation, DPCs may secrete BDNF and GDNF to form a more
beneficial microenvironment for the repair of SCI lesions, which
eventually promote axon sprouting.
In the present study, it was also observed that
angiogenesis in the DPC-transplanted group was markedly pronounced.
Based on the higher expression levels of angiogenic factor VEGFA in
DPCs, the promotion of angiogenesis in the DPC-transplanted group
may be due to the secretion of the VEGFA.
Additionally, histological repair was also observed,
including enhanced lesion filling; more axons formed within the
lesion sites with DPC grafts; however, the axons did not completely
develop into the lesion site so as to form axonal connections with
the caudal end during the short observation period. Thus, the
recovery of hindlimb motor function with BBB testing was not
observed in either the DPC-transplant group or the BMSC-transplant
group. In the future, the in vivo experimental period may be
extended to 3–6 months to further examine cell fates, the formation
of neural networks across sites of complete spinal transection and
the recovery of motor functions.
In summary, the data of the present study
demonstrated that DPCs promote greater tissue repair following
complete SCI compared with BMSCs, including the promotion of
neurite outgrowth and greater revascularization following the
secretion of neurotrophic factors, angiogenic factors and
inflammation-associated cytokines. The findings of the present
study suggest that these cells may be a promising resource for
autologous cell therapy in the treatment of SCIs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81572139), the
National Natural Science Foundation of China (grant no. 81571199),
the Young Teachers' Innovation Projects from Jilin University
(grant no. 450060521193), the Bethune Project from Jilin University
(grant no. 2015430) and the Outstanding Young Teacher's Training
Program from Jilin University (grant no. 419080500576).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and XM isolated and cultured dermal papilla cells
and bone marrow mesenchymal stem cells, performed reverse
transcription-polymerase chain reaction, assayed the gel
properties, assessed the lesion areas, performed
immunocytofluorescence histochemical staining, analyzed data and
wrote the manuscript. SL and JinX performed acute injections of the
cells. ZZ and DS performed behavioral testing. JiaX and XH
performed reverse transcription-quantitative polymerase chain
reaction. GC designed the study, established the rat models of
spinal injury and performed small animal in vivo imaging. YL
designed the study and provided research funding.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jilin University (Changchun, China) and conformed to
their regulatory standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BDNF
|
brain-derived neurotrophic factor
|
|
bFGF
|
basic fibroblast growth factor
|
|
BMSCs
|
bone marrow mesenchymal stem cells
|
|
CNTF
|
ciliary neurotrophic factor
|
|
DPCs
|
dermal papilla cells
|
|
FBS
|
fetal bovine serum
|
|
FGF2
|
fibroblast growth factor 2
|
|
FLK-1
|
vascular endothelial growth factor
receptor 2
|
|
GDNF
|
glial cell line-derived neurotrophic
factor
|
|
GFP
|
green fluorescent protein
|
|
HGF
|
hepatocyte growth factor
|
|
IL
|
interleukin
|
|
NCSCs
|
neural crest stem cells
|
|
NGF
|
nerve growth factor
|
|
NSCs
|
neural stem cells
|
|
NTFs
|
neurotrophic factors
|
|
SCI
|
spinal cord injury
|
|
TGF-β1
|
transforming growth factor-β1
|
|
TNF-α
|
tumor necrosis factor-α
|
|
VEGFA
|
vascular endothelial growth factor
A
|
References
|
1
|
Nakamura M and Okano H: Cell
transplantation therapies for spinal cord injury focusing on
induced pluripotent stem cells. Cell Res. 23:70–80. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang L, Lu X, Zhu R, Qian T, Tao Y, Li K,
Zheng J, Zhao P, Li S, Wang X and Li L: Adipose-derived stem cells
expressing the neurogenin-2 promote functional recovery after
spinal cord injury in rat. Cell Mol Neurobiol. 36:657–667. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mothe AJ and Tator CH: Advances in stem
cell therapy for spinal cord injury. J Clin Invest. 122:3824–3834.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yokota K, Kobayakawa K, Kubota K, Miyawaki
A, Okano H, Ohkawa Y, Iwamoto Y and Okada S: Engrafted neural
stem/progenitor cells promote functional recovery through synapse
reorganization with spared host neurons after spinal cord injury.
Stem Cell Reports. 5:264–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang YX, Sun JJ, Zhang M, Hou XH, Hong J,
Zhou YJ and Zhang ZY: Propofol injection combined with bone marrow
mesenchymal stem cell transplantation better improves
electrophysiological function in the hindlimb of rats with spinal
cord injury than monotherapy. Neural Regen Res. 10:636–643. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sabapathy V, Tharion G and Kumar S: Cell
therapy augments functional recovery subsequent to spinal cord
injury under experimental conditions. Stem Cells Int.
2015:1321722015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neirinckx V, Cantinieaux D, Coste C,
Rogister B, Franzen R and Wislet-Gendebien S: Concise review:
Spinal cord injuries: How could adult mesenchymal and neural crest
stem cells take up the challenge? Stem Cells. 32:829–843. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ritfeld GJ, Patel A, Chou A, Novosat TL,
Castillo DG, Roos RA and Oudega M: The role of brain-derived
neurotrophic factor in bone marrow stromal cell-mediated spinal
cord repair. Cell Transplant. 24:2209–2220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cantinieaux D, Quertainmont R, Blacher S,
Rossi L, Wanet T, Noël A, Brook G, Schoenen J and Franzen R:
Conditioned medium from bone marrow-derived mesenchymal stem cells
improves recovery after spinal cord injury in rats: An original
strategy to avoid cell transplantation. PLoS One. 8:e695152013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Zou X, Zhao J, Wu X, E L, Feng L,
Wang D, Zhang G, Xing H and Liu H: Site-specific characteristics of
bone marrow mesenchymal stromal cells modify the effect of aging on
the skeleton. Rejuvenation Res. 2016 Mar 15;(Epub ahead of print).
View Article : Google Scholar
|
|
11
|
Li M, Liu JY, Wang S, Xu H, Cui L, Lv S,
Xu J, Liu S, Chi G and Li Y: Multipotent neural crest stem
cell-like cells from rat vibrissa dermal papilla induce neuronal
differentiation of PC12 cells. Biomed Res Int. 2014:1862392014.
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Medalha CC, Jin Y, Yamagami T, Haas C and
Fischer I: Transplanting neural progenitors into a complete
transection model of spinal cord injury. J Neurosci Res.
92:607–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Molina AE, Cristante AF, de Barros TE Sr,
Molina MS and Molina TP: A computerized system for the application
of Basso, Beattie and Bresnahan scale in Wistar rats. Acta Ortop
Bras. 23:179–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakano N, Nakai Y, Seo TB, Homma T, Yamada
Y, Ohta M, Suzuki Y, Nakatani T, Fukushima M, Hayashibe M and Ide
C: Effects of bone marrow stromal cell transplantation through CSF
on the subacute and chronic spinal cord injury in rats. PLoS One.
8:e734942013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dasari VR, Veeravalli KK and Dinh DH:
Mesenchymal stem cells in the treatment of spinal cord injuries: A
review. World J Stem Cells. 6:120–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klar AS, Zimoch J and Biedermann T: Skin
tissue engineering: Application of adipose-derived stem cells.
Biomed Res Int. 2017:97470102017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li C, Wei G, Gu Q, Wen G, Qi B, Xu L and
Tao S: Donor age and cell passage affect osteogenic ability of rat
bone marrow mesenchymal stem cells. Cell Biochem Biophys.
72:543–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoffman RM: The hair follicle as a gene
therapy target. Nat Biotechnol. 18:20–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Driskell RR, Clavel C, Rendl M and Watt
FM: Hair follicle dermal papilla cells at a glance. J Cell Sci.
124:1179–1182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tyor WR, Avgeropoulos N, Ohlandt G and
Hogan EL: Treatment of spinal cord impact injury in the rat with
transforming growth factor-beta. J Neurol Sci. 200:33–41. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walker MJ, Walker CL, Zhang YP, Shields
LB, Shields CB and Xu XM: A novel vertebral stabilization method
for producing contusive spinal cord injury. J Vis Exp.
5:e501492015.
|
|
23
|
Kim M, Park SR and Choi BH: Biomaterial
scaffolds used for the regeneration of spinal cord injury (SCI).
Histol Histopathol. 29:1395–1408. 2014.PubMed/NCBI
|
|
24
|
Assunção-Silva RC, Gomes ED, Sousa N,
Silva NA and Salgado AJ: Hydrogels and cell based therapies in
spinal cord injury regeneration. Stem Cells Int. 2015:9480402015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu P, Wang Y, Graham L, McHale K, Gao M,
Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, et al:
Long-distance growth and connectivity of neural stem cells after
severe spinal cord injury. Cell. 150:1264–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nectow AR, Marra KG and Kaplan DL:
Biomaterials for the development of peripheral nerve guidance
conduits. Tissue Eng Part B Rev. 18:40–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watanabe K, Nakamura M, Okano H and Toyama
Y: Establishment of three-dimensional culture of neural
stem/progenitor cells in collagen Type-1 Gel. Restor Neurol
Neurosci. 25:109–117. 2007.PubMed/NCBI
|
|
28
|
Boehler RM, Kuo R, Shin S, Goodman AG,
Pilecki MA, Gower RM, Leonard JN and Shea LD: Lentivirus delivery
of IL-10 to promote and sustain macrophage polarization towards an
anti-inflammatory phenotype. Biotechnol Bioeng. 111:1210–1221.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang F, Wang H, Wang X, Jiang G, Liu H,
Zhang G, Wang H, Fang R, Bu X, Cai S and Du J: TGF-β induces
M2-like macrophage polarization via SNAIL-mediated suppression of a
pro-inflammatory phenotype. Oncotarget. 7:52294–52306.
2016.PubMed/NCBI
|
|
30
|
Liu F, Uchugonova A, Kimura H, Zhang C,
Zhao M, Zhang L, Koenig K, Duong J, Aki R, Saito N, et al: The
bulge area is the major hair follicle source of nestin-expressing
pluripotent stem cells which can repair the spinal cord compared to
the dermal papilla. Cell Cycle. 10:830–839. 2011. View Article : Google Scholar : PubMed/NCBI
|