Introduction
The liver is a vital organ that serves a role in the
metabolism of endogenous and exogenous substances where
physiological imbalances cause cellular oxidative stress and the
formation of toxic free radicals (1). Increased accumulation of intracellular
reactive oxygen and nitrogen species together with decreased
antioxidant defense results in hepatotoxicity that may progress to
liver dysfunction, carcinoma and failure (1). Therefore, developing preventive
therapeutic strategies against hepatic oxidative stress and
toxicity remains an important issue. Plants contain a number of
bioactive secondary metabolites, including flavonoids, polyphenols,
alkaloids, saponins and terpenoids, which possess radical
scavenging and hepatoprotective activities (2–6).
The genus Atriplex (subfamily
Chenopodiaceae), commonly known as lagoon or sprawling saltbush are
widely distributed in arid and semi-arid regions, including the
Middle East (7). Globally, ~400
species of Atriplex herbs and shrubs have been recognized
(7,8). Of these, the protein-rich shoots of
A. halimus are an important fodder for sheep, goats and
camels (9). In addition, the
protein-rich leaves of A. lampa have been proposed as a
potential dietary supplement for animals and humans (10). In traditional medicine, A.
halimus decoction has been used to treat syphilis (11) and its leaves have been used to treat
heart disease, diabetes and rheumatism in the Arabian Peninsula
(12). In addition, methanol and
hexane extracts of the aerial parts of A. halimus have been
demonstrated to have antimicrobial activity (13). A previous study, in which
phytochemical analysis was performed on the aerial parts of A.
halimus, revealed the presence of myricetin, quercetin,
isorhamnetin glycosides, phenolic acids and esters (14). Recently, triterpenoids isolated from
A. laciniata demonstrated antibacterial, antioxidant and
antiurease activities (15),
including anticholinesterase effects against Alzheimer's and other
neurological disorders (16). The
fungicidal effects of A. semibaccata, A. portulacoides and
A. infalata have been previously reported (17) and the molluscicidal and larvicidal
activities of A. inflata have also been identified (18). Furthermore, Gođevac et al
(19) revealed that flavonoid
glycosides isolated from the aerial parts of A. littoralis
exhibited protection against in vitro biochemical and
cytogenetic damage to human lymphocytes (19).
In Saudi Arabia, of the 10 reported species of
saltbush, A. coriacea, A. dimorphostegia, A. farinosa, A glauca,
A. halimus, A. leucoclada and A. tatarica are native,
whereas A. canescens, A. semibaccata and A. suberecta
were introduced and naturalized (20). A. suberecta I. Verd is a herb
with thin and narrow leaves, separate male and female flowers and
capsulated fruits (20,21). Compared with other species, there
have been few phytochemical and bioactivity studies on A.
suberecta. To the best of our knowledge, the only previous
study into A. suberecta leaf protein concentrate, suggested
its nutritional value was due to its high lysine content (21). The aim of the present study was to
investigate the in vitro and in vivo antioxidative
and hepatoprotective potential of A. suberecta
ethanol-extract (ASEE), including standardization and validation by
chromatography.
Materials and methods
Collection of plant material and
extract preparation
The clean and healthy aerial shoots of Atriplex
suberecta I. Verd were collected from Jazan (Saudi Arabia) and
authenticated (voucher specimen no. 16386) by a plant taxonomist at
the College of Pharmacy, King Saud University (Riyadh, Saudi
Arabia). Briefly, the air-dried leaf powder (300 g) was soaked in
70% ethanol (Merck KGaA, Darmstadt, Germany) for 2 days at room
temperature and filtered (Whatman® Filter paper, grade
1; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The extraction
process was repeated twice with the same solvent, followed by
evaporation using a rotary evaporator (BÜCHI Labortechnik AG,
Flawil, Switzerland) under reduced pressure at 40°C. The obtained
semi-solid ASEE (31.5 g) was stored at −20°C prior to use.
Human hepatoblastoma cell cultures and
drugs
The human hepatoblastoma cell line, HepG2 (22) was maintained in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
supplemented with 10% heat-inactivated bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 1X penicillin-streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) and 1X sodium pyruvate (GE Healthcare Life
Sciences, Logan, UT, USA) at 37°C in a humidified chamber
containing 5% CO2. Silymarin, 2,7-dichlorofluorescein
(DCFH), ascorbic acid (all Sigma-Aldrich; Merck KGaA) and gallic
acid (Fluka; Honeywell International Inc., Morris Plains, NJ, USA)
were also purchased.
Free-radical scavenging activity of
ASEE
The free-radical scavenging ability of ASEE against
1,1-diphenyl-2-picrylhydrazyl (DPPH) was evaluated quantitatively
as described previously (23) with
minor modifications. In brief, 100 µl of different concentrations
(31.25, 62.5, 125 and 250 µg/ml) of the ASEE was mixed with 40 µl
DPPH (0.2 mM in methanol) in a 96-well microplate. The control was
prepared using the solvent (methanol) only in addition to the same
amount of DPPH reagent to remove any inherent solvent effect.
Ascorbic acid was used as the standard. Following 30 min incubation
at 25°C the decrease in absorbance (Abs) was measured at λ=517 nm
using a microplate reader (ELx800; BioTek Instruments, Inc.,
Winooski, VT, USA). The experiment was performed in triplicate and
the radical scavenging activity was calculated from the following
equation: Percentage radical scavenging activity = [1 -
(Abssample/Abscontrol)] × 100.
Lipid peroxidation assay
The lipid peroxidation activity of ASEE was
evaluated using the β-carotene bleaching method as previously
described (24) with minor
modifications. Briefly, 0.25 mg β-carotene was dissolved in 0.5 ml
chloroform and added to flasks containing 12.5 µg linoleic acid and
100 mg Tween-40. The chloroform was evaporated at 43°C using a
Savant™ Universal SpeedVac™ Vacuum system
concentrator (Thermo Fisher Scientific, Inc.). The resultant
mixture was immediately diluted to 25 ml with distilled water and
agitated vigorously for 2–3 min to form an emulsion. A 200 µl
aliquot of the emulsion was added to a 96-well plate containing 50
µl ASEE or 500 µg/ml gallic acid (standard). A control containing
solvent (emulsion) was also prepared. The test was performed in
triplicate and the plate was incubated at 50°C for 2 h. The Abs was
read at λ=470 nm at 30 min intervals using a microplate
spectrophotometer. The antioxidant activity was estimated using two
different methods; initially the kinetic curve was obtained by
plotting Abs of each sample against time and then the antioxidant
activity was expressed as percentage inhibition of lipid
peroxidation using the following equation: Percentage inhibition =
[(As120 - Ac120)/(Ac0 - Ac120)] × 100, where As120 and Ac120 are
the Abs of the sample and control at 120 min, respectively, and Ac0
is the Abs of the control at 0 min.
In vitro hepatoprotection assay of
ASEE
HepG2 cells were seeded in a 96-well flat-bottom
plate (0.5×105 cells/well) and grown for 24 h as
described above. Liver cytotoxicity was induced by DCFH
(IC50 100 µg/ml) treatment as previously described
(25). ASEE was initially dissolved
in DMSO (200 mg/ml) and further diluted in RPMI-1640 medium to
prepare four doses (25, 50, 100 and 200 µg/ml) and an untreated
control containing DMSO only (volume equivalent to 200 µg/ml ASEE).
As determined previously (data not shown), the final concentration
of DMSO used never exceeded >0.1% and therefore was tolerated by
the cultured cells. The culture monolayers were replaced with
culture medium containing DCFH (100 µg/ml) and a dose of ASEE,
including the untreated as well as the DCFH only-treated controls.
The treated cells were incubated for 48 h at 37°C in a
CO2 incubator, followed by an MTT assay (TACS MTT Cell
Proliferation Assay kit, Trevigen, Gaithersburg, MD, USA) according
to the manufacturer's instructions. Briefly, MTT reagent (10
µl/well) was added to the cells and incubated for 3 h. The lysis
buffer (100 µl/well) was gently added and further incubated for
~1.5 h. The Abs was recorded at λ=570 nm by a microplate reader,
and data was analyzed using non-linear regression (Excel software
2010; Microsoft Corporation, Redmond, WA, USA) to determine the
percentage cell survival as follows: Percentage cell survival =
[(Abssample -
Absblank)/(Abscontrol - Absblank)]
× 100.
Anti-apoptotic signaling assay of
ASEE
To determine the anti-apoptotic effect of ASEE,
caspase-3 and −7 activation was measured using an
Apo-ONE® homogenous caspase-3/-7 assay kit (Promega
Corporation, Madison, WI, USA) following the manufacturer's
protocol. Briefly, HepG2 toxicity was induced with DCFH (100 µg/ml)
and treated with ASEE (25, 50, 100 and 200 µg/m) for 48 h as
described above. Caspase-3/-7 reagent (100 µl/per well) was added
and mixed by gently rocking the culture plate. Treated cultures
were incubated for 5–6 h in the dark at room temperature and the
Abs was measured at λ=570 nm. Non-linear regression analysis was
performed to determine the percentage cell proliferation and
caspase activity as follows: Percentage cell proliferation =
[(Abssample -
Absblank)/(Abscontrol - Absblank)]
× 100.
Animals and acute toxicity test
A total of 30 male Wistar rats (weight, 200–220 g;
age, 8–9 weeks) received from the Experimental Animal Care Center,
King Saud University (Riyadh, Saudi Arabia) were kept in
polycarbonate cages in a sterile room under a controlled 12 h
dark/light cycle at 25±2°C with 50–60% humidity. The animals were
provided standard rodent chow diet (Grain Silos & Flour Mills
Org., Riyadh, Saudi Arabia) and water ad libitum. The
animals were divided into five test groups (n=6/group/cage) that
were each fed different doses of ASEE (50, 100, 250 and 500
mg/kg.bw), including a control group that was fed normal saline
instead of ASEE. All ASEE treated rats, including the control group
were observed continuously and uninterruptedly for 1 h and then at
30 min intervals for 4 h for any gross behavioral change and
general motor activities, including writhing, convulsion, response
to tail pinching, gnawing, pupil size and feeding behavior, and
additionally monitored for up to 72 h for any mortality. No
behavioral change was observed in the treated or control rats. The
present study was approved by the Ethics Committee of the
Experimental Animal Care Society (King Saud University, Riyadh,
Saudi Arabia) and adhered to its guidelines.
Experimental design and treatment
Upon acclimatization to the laboratory conditions
for 1 week, the rats (n=30) were randomized and assorted into five
groups (GI-GV) with 6 rats in each group. The GI group was fed
orally with normal saline (1 ml) and served as the untreated
control, the GII group received carbon tetrachloride
(CCl4) in liquid paraffin (1:1) only, 1.25 m/kg
intraperitoneally (IP). The GIII, GIV and GV groups also received
CCl4 and the GIII and GIV were also treated with ASEE
100 and 200 mg/kg, respectively whereas GV was treated with
silymarin (10 mg/kg) used as a comparison to the current
experimental and clinical standard (23,26). All
treatment was administered for 3 weeks
Rat sacrifice, blood collection and
liver tissue preparation
Following 3 weeks of treatment, rats (weight,
200–220 g) were anesthetized with pentobarbital sodium (50 mg/kg,
intraperitoneally; Sigma-Aldrich; Merck KGaA) and sacrificed by
cervical dislocation. Death was confirmed by monitoring the
heartbeat, absence of withdrawal to paw pinch and non-response of
pupils to light. While under anesthesia, rats blood was collected
with a 23G needle via cardiac puncture and sera were separated at
1,000 × g for 10 min at 4°C, and stored at −20°C until biochemical
analysis. The livers were quickly removed and fixed in 10% neutral
buffered formalin (NBF) for 48 h at room temperature. The fixed
specimens were processed overnight for dehydration, clearing and
paraffin impregnation using an automatic tissue processor (Sakura
Finetek Europe B.V., The Netherlands) and cut into 4-µm-thick
sections using a rotary microtome (RM2245; Leica Microsystems GmbH,
Wetzlar, Germany).
Estimation of serum marker enzymes,
bilirubin, lipid profile and total protein
Serum glutamate oxaloacetate (SGOT) (Reflotron GOT,
cat. no. 10745120; Roche Diagnostics GmbH, Mannheim, Germany),
serum pyruvate transaminase (SGPT) (Reflotron GPT, cat. no.
10745138; Roche Diagnostics GmbH), alkaline phosphatase (ALP)
(Reflotron ALP, cat. no. 11622773; Roche Diagnostics GmbH),
γ-glutamyl transferase (GGT) (Reflotron GGT, cat. no. 10745081;
Roche Diagnostics GmbH), bilirubin (Reflotron BIL, cat. no.
10905321; Roche Diagnostics GmbH), total cholesterol (TC)
(Reflotron Cholesterol, cat. no. 10745065; Roche Diagnostics GmbH),
triglycerides (TG) (Reflotron TG, cat. no. 10745049; Roche
Diagnostics GmbH) and high-density lipoproteins (HDL) (Reflotron
HDL Cholesterol, cat. no. 11208756; Roche Diagnostics GmbH) were
estimated using test-specific commercial kits and a Reflotron Plus
Analyzer (Woodley Equipment Co., Ltd., Horwich, Lancashire, UK).
Very low-density lipoproteins (VLDL) and low-density lipoproteins
(LDL) were calculated using the two following standard formulas:
TG/5 and [Cholesterol-(VLDL+HDL)], respectively. The serum total
protein (TP) was estimated using a kit (Crescent Diagnostics,
Jeddah, Saudi Arabia) and the following equation: TP =
(Abssample/Absstandard) × concentration of
standard.
Determination of tissue
malondialdehyde (MDA) and non-protein sulfhydryl (NP-SH)
For tissue MDA the method reported by Utley et
al (27) was followed. Briefly,
the liver tissues were homogenized in 0.15 M KCl at 40°C
(Potter-Elvehjem type C homogenizer) to give a 10% w/v homogenate.
The Abs of the solution was then read at λ=532 nm and the MDA
content (nmol/g wet tissue) was calculated by reference to a
standard curve of MDA solution. Hepatic NP-SH was measured
according to the method of Sedlak and Lindsay (28). The tissues were homogenized in
ice-cold 0.02 mM EDTA and the Abs (λ=412 nm) was measured following
the addition of 5,5′dithio-bis(2-nitrobenzoic acid) against the
control.
Microscopy and histopathological
evaluation
Morphological investigation of the cultured HepG2
cells was performed under a microscope to investigate any changes
in the cells cultured with different concentrations of ASEE and
DCFH at 24 and 48 h post-treatment. The sections of liver tissues
fixed in NBF (for 48 h at room temperature) were stained with
hematoxylin and eosin for 2–3 min at room temperature as previously
described (29). Tissue sections
were histopathologically examined under a light microscope
(OMX1200C; Nikon Corporation, Tokyo, Japan) and images (at
magnifications ×200 and ×400) were captured using a mounted digital
camera.
Qualitative phytochemical screening of
ASEE
Phytochemical screening tests for major secondary
metabolites, including alkaloids, flavonoids, tannins and saponins
were performed using standard procedures as described previously
(30–32). Briefly, for alkaloids 0.5 mg ASEE was
dissolved in 2% hydrochloric acid (Sigma-Aldrich, Merck KGaA) and
filtered. Fresh Mayer's reagent (0.68 g mercuric chloride and 2.5 g
potassium iodide; Sigma-Aldrich; Merck KGaA) prepared in distilled
water (50 ml, final volume) was added to the 3 ml ASEE solution in
a test tube. The formation of a yellow precipitate confirmed the
presence of alkaloids. For flavonoids, 5 ml ASEE solution was
treated with several drops of 20% sodium hydroxide (Sigma-Aldrich;
Merck KGaA) in a test tube. The appearance of an intense yellow
color that turned colorless following the addition of diluted
hydrochloric acid was indicative of flavonoids. For tannins, 0.25
mg ASEE was dissolved in 10 ml water in a test tube and several
drops of 5% ferric chloride (Sigma-Aldrich; Merck KGaA) were added.
The development of a brown-green or blue-black color indicated the
presence of tannins. For saponins, 0.5 mg ASEE was dissolved in 10
ml water in a test tube and agitated vigorously to form a thick
persistent froth, which represented a positive result for
saponin.
Standardization of ASEE by the
validated high-performance thin-layer chromatography (HPTLC)
method
The reverse phase (RP)-HPTLC method was used to
standardize the 70% ethanol extract of A. subrecta as
described previously (33). The
chromatography was performed on a 10×10 cm precoated silica gel
F254 RP-HPTLC plate using rutin as the standard reference. Several
mobile phases were tried to obtain a good resolution and separation
of the different compounds present in the ASEE. Based on
observations, acetonitrile and water were selected in the ratio of
4:6 as a suitable mobile phase to perform the standardization of
ASEE. The standard and the samples were applied on the HPTLC plate
by an Automatic TLC Sampler-4 (CAMAG Chemie-Erzeugnisse &
Adsorptionstechnik AG, Muttenz, Switzerland). The plate was
developed under controlled condition in an Automated Developing
Chamber-2 and scanned by TLC Scanner-3 (λ=363 nm) (both CAMAG
Chemie-Erzeugnisse & Adsorptionstechnik AG).
Statistical analysis
Data are presented as the mean ± standard error of
three (in vitro) and six (in vivo) determinants.
Total variation present in a set of data was estimated by one-way
analysis of variance followed by Dunnett's post hoc test. Excel
2010 (Microsoft, Tulsa, OK, USA) was used to analyze the data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Antioxidant activity of ASEE
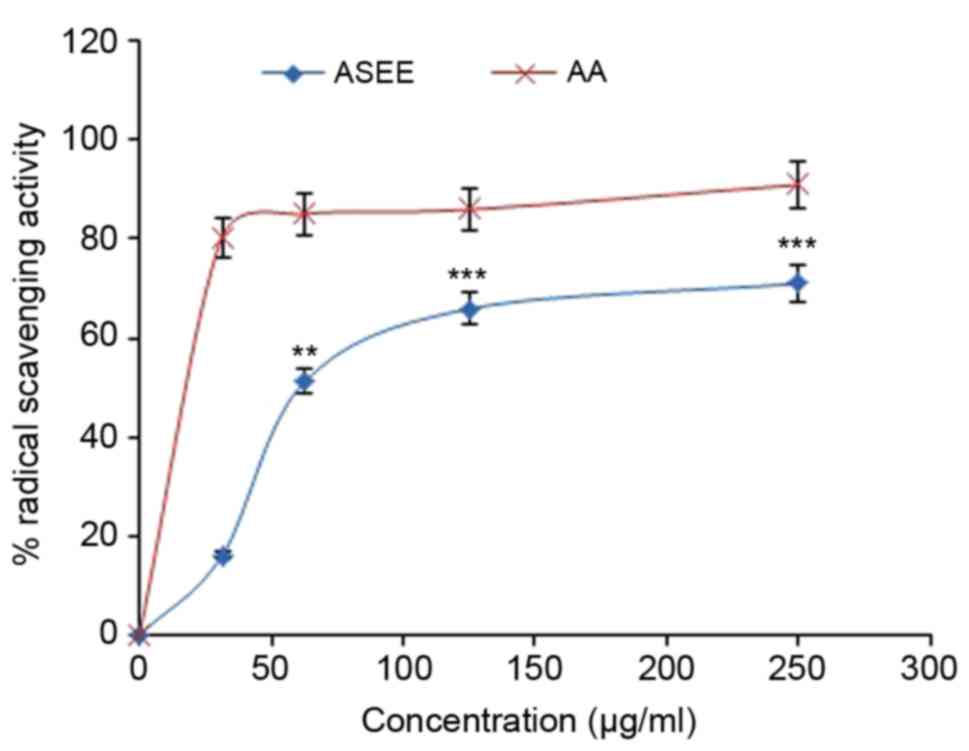
The DPPH radical scavenging activity of ASEE was
observed to be dose-dependent and 15.9, 51.1, 65.9 and 71.5% at
concentrations of 31.25, 62.5, 125 and 250 µg/ml, respectively,
compared with ascorbic acid (Fig.
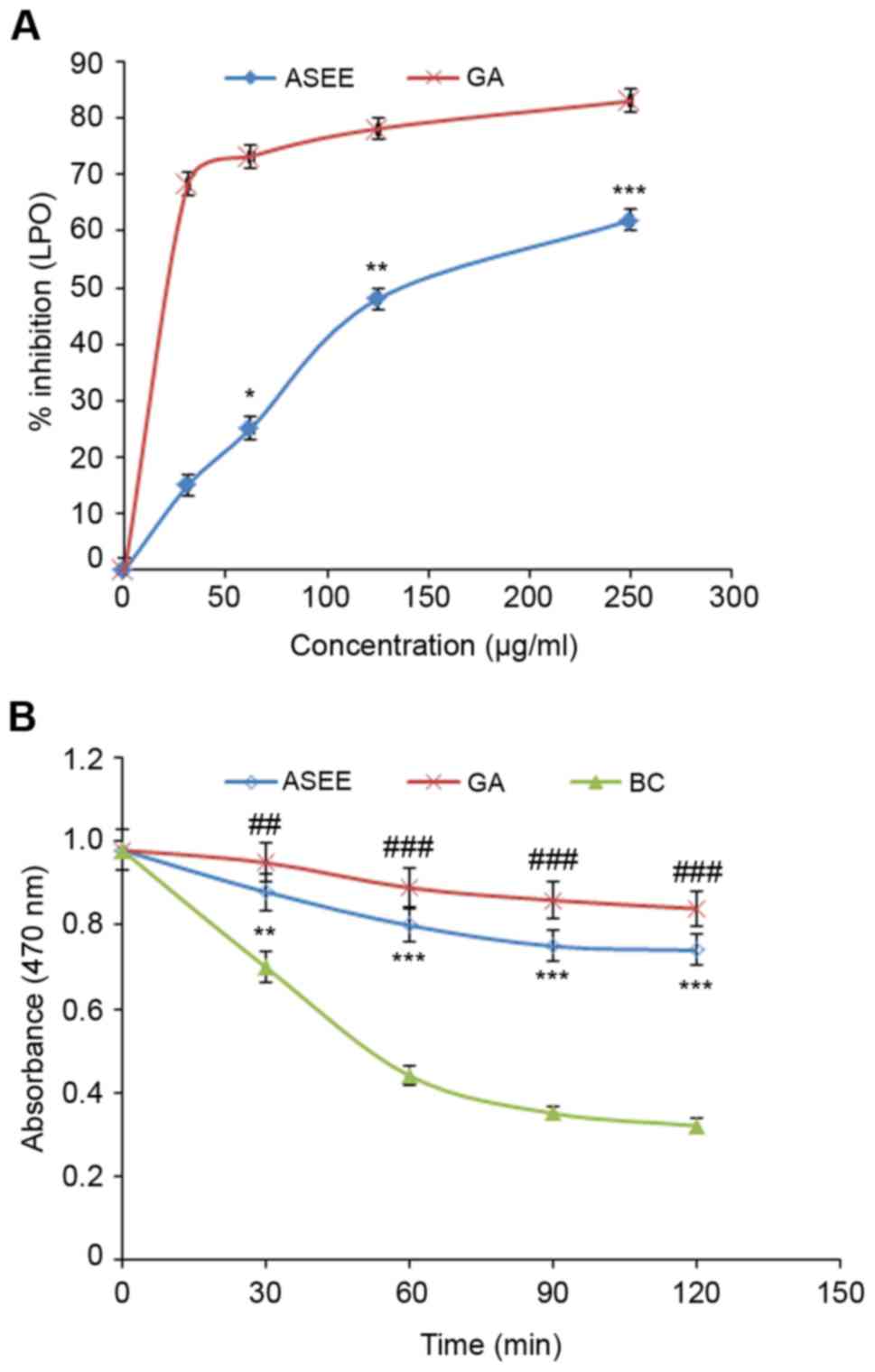
1). Similarly, in the β-carotene-linoleic acid assay ASEE
significantly inhibited lipid peroxidation in a dose-dependent
manner (P<0.01, P<0.001; Fig.
2) confirming its antioxidative potential.
In vitro cytoprotective and
anti-apoptotic effect of ASEE on HepG2 cells
Visual observation under a microscope revealed the
marked cytotoxic effect of DCFH on the HepG2 cells, which was
indicated by apoptosis or altered morphology compared with the
untreated cells (data not shown). However, treatment with ASEE
resulted in morphological recovery against DCFH toxicity at 24 and
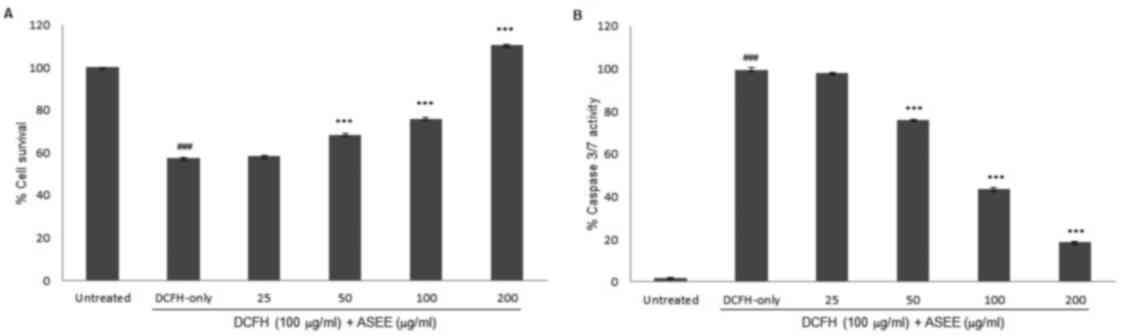
48 h (data not shown). An MTT assay revealed attenuation of the
HepG2 cells toxicity by ASEE in a dose-dependent manner (Fig. 3A). Treatment with 50, 100 and 200
µg/ml ASEE significantly restored the cells proliferation to 68, 76
and 110%, respectively compared with the untreated cells
(P<0.001; Fig. 3A). Furthermore,
in the anti-apoptotic signaling assay, ASEE at doses of 50, 100 and
200 µg/ml significantly downregulated caspase-3/-7 activity to 76,
43 and 18%, respectively compared with the DCFH-only group
(P<0.001; Fig. 3B).
Normalization of liver biochemical
markers by ASEE
The acute toxicity test revealed the toleration of
ASEE (500 mg/kg.bw) and healthy survival of the animals (data not
shown). Furthermore, the therapeutic potential of ASEE (100 and 200
mg/kg.bw) was examined against CCl4-induced in
vivo hepatotoxicity. In CCl4-only treated rats,
serum SGOT, SGPT, GGT ALP and bilirubin levels were significantly
elevated compared with the control group (P<0.001; Table I), which demonstrated significant
hepatotoxicity. By contrast, the administration of ASEE (200 mg/kg)
significantly normalized these parameters in line with silymarin,
compared with the control group (P<0.01 and P<0.001; Table I). The SGOT, SGPT, GGT ALP and
bilirubin levels in the ASEE (200 mg/kg) +CCl4 groups
were significantly reduced compared with the CCL4 only
group (P<0.05, P<0.01 and P<0.001). In addition, in the
CCl4-injured rats with altered serum lipid profiles,
ASEE (200 mg/kg) treatment significantly reduced the cholesterol,
TG and VLDL levels, and improved the HDL level, which was
comparable to that of the silymarin treated group (P<0.01 and
P<0.001; Table II). Furthermore,
compared with the increase in MDA level, a decrease in NP-SH tissue
and decrease in TP concentrations in the CCL4 only group
was indicated. ASEE (200 mg/kg) significantly normalized these
parameters in CCl4-injured rats (P<0.01 and
P<0.001; Table III).
 | Table I.Effect of ASEE on
CCl4-induced hepatotoxicity-associated parameters in
rats. |
Table I.
Effect of ASEE on
CCl4-induced hepatotoxicity-associated parameters in
rats.
| Treatment
group | Dose (mg/kg) | SGOT (U/l) | SGPT (U/l) | ALP (U/l) | GGT (U/l) | Bilirubin
(mg/dl) |
|---|
| Control |
0 | 107.45±5.31 | 28.83±2.2 | 321.66±13.88 |
4.06±0.32 | 0.54±0.01 |
|
CCl4 |
0 |
294.83±8.33b |
230.83±9.62b |
515.16±13.70b |
12.85±0.98b |
2.16±0.08b |
| ASEE +
CCl4 | 100 |
309.33±5.93c |
209±7.47c |
509.66±12.48c |
13±0.53c |
2.08±0.05c |
| ASEE +
CCl4 | 200 |
286±6.29c |
193±6.52d |
467±11.00c |
10.66±0.38c |
1.91±0.06a |
| Silymarin +
CCl4 |
10 |
136.66±6.00e |
85.66±4.31e |
396.33±7.62e |
5.58±0.28e |
1.06±0.06e |
 | Table II.Effect of ASEE on
CCl4-induced lipid profile change in rats. |
Table II.
Effect of ASEE on
CCl4-induced lipid profile change in rats.
| Treatment
group | Dose mg/kg | Cholesterol
(mg/dl) | Triglycerides
(mg/dl) | HDL (mg/dl) | LDL (mg/dl) | VLDL (mg/dl) |
|---|
| Control |
0 | 109.83±3.94 |
59.01±2.74 | 55.18±2.4 |
42.84±3.22 |
11.8±0.54 |
|
CCl4 |
0 |
206±4.53a |
151.16±4.61a |
26.25±1.79a |
149.51±4.28a |
30.23±0.92a |
| ASEE +
CCl4 | 100 |
192.33±7.03b |
133.83±5.61c |
26.91±1.3b |
138.65±8.21b |
26.76±1.12c |
| ASEE +
CCl4 | 200 |
169.83±5.28d |
120±6.67d |
26.85±1.14b |
118.98±5.94c |
24±1.33d |
| Silymarin +
CCl4 |
10 |
147.66±4.88d |
110.16±5.26d |
40.41±2.97c |
85.21±5.98d |
22.02±1.05d |
 | Table III.Effect of ASEE on biochemical
parameters of liver tissues in CCl4-treated rats. |
Table III.
Effect of ASEE on biochemical
parameters of liver tissues in CCl4-treated rats.
| Treatment
group | Dose (mg/kg) | MDA (nmol/g) | NP-SH (nmol/g) | TP (g/l) |
|---|
| Control |
0 | 0.50±0.02 | 7.39±0.53 | 113.76±2.81 |
|
CCl4 |
0 |
4.82±0.29b |
3.86±0.44b |
49.11±1.82a |
| ASEE +
CCl4 | 100 |
4.63±0.21c |
5.38±0.47d |
46.30±2.59 |
| ASEE +
CCl4 | 200 |
3.54±0.24d |
5.70±0.44d |
65.06±2.58e |
| Silymarin +
CCl4 |
10 |
1.37±0.16e |
6.52±0.31e |
91.81±4.08e |
Histopathological improvement by
ASEE
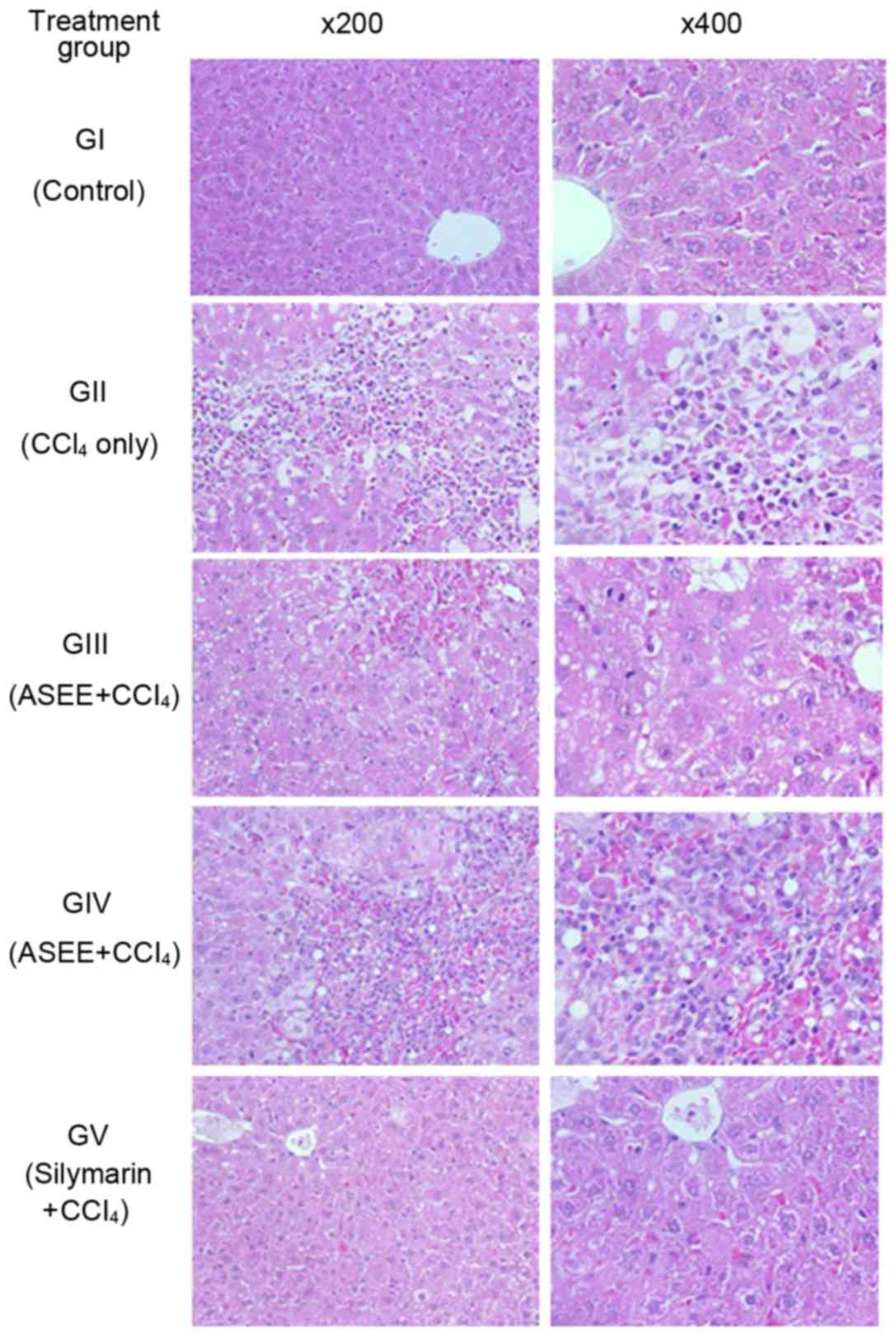
The rat liver histopathological analysis revealed
CCl4-induced necrotic and fatty degenerative changes
(panel GII; Fig. 4) as compared to
the control group (panel GI; Fig.
4). In the ASEE group (100 mg/kg.bw/day), congested central
vein with mild necrosis and fatty changes were observed (panel
GIII; Fig. 4). In addition, the
higher dose of ASEE (200 mg/kg.bw/day) normalized the hepatocyte
lesion and resulted in a full recovery (panel GIV; Fig. 4), comparable to that observed in the
silymarin group (panel GV; Fig. 4).
The histopathological data therefore confirmed the in vivo
hepatoprotective efficacy of ASEE.
Phytochemical screening of ASEE
The qualitative phytochemical screening revealed the
presence of flavonoids, alkaloids, tannins and saponins in ASEE
(data not shown).
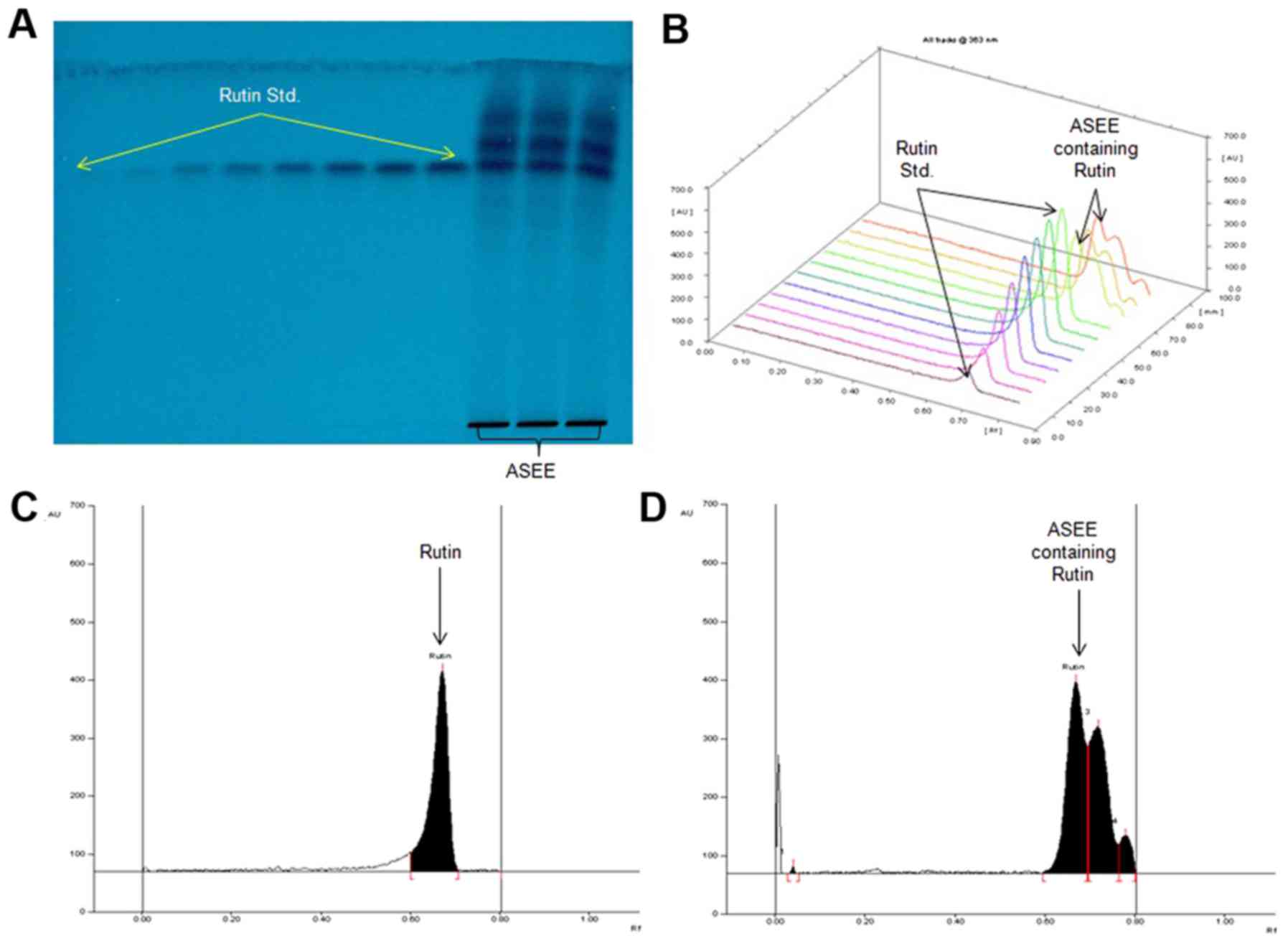
Chromatographic quantification of
rutin in ASEE
ASEE was further standardized by a validated
RP-HPTLC method using rutin as an antioxidant biomarker. Of the
various solvent combinations tested, acetonitrile and water (4:6;
v/v) was indicated as the optimal mobile phase for the estimation
of rutin in ASEE (Fig. 5A and B). A
sharp and compact spot of rutin was identified at
Rf=0.67 (Fig. 5C), with
clear separation along with different phytoconstituents of ASEE
(Fig. 5D) at the optimized mobile
phase volume (20 ml) and saturation time (20 min). The estimated
content of rutin in ASEE was 1.94 µg/mg (dry weight).
Discussion
Cellular oxidative stress is a process where
reactive oxygen and nitrogen species, common toxic products of
redox reactions, are increased (1).
Oxidative stress is closely associated with the occurrence and
development of various conditions, including cirrhosis and
carcinoma, which are chronic liver diseases (34). The healthy body has a set of hepatic
antioxidant enzymes to prevent and neutralize free-radical induced
cellular damage (35). However,
exposure to a hepatotoxic agent may cause the generation of free
radicals to exceed the protective effects of the antioxidant
enzymes (34,35). The effectiveness of hepatoprotective
agents is therefore dependent on their ability to attenuate the
harmful free radicals and to maintain normal liver functions
(3,4,34). In
the present study, the in vitro and in vivo
antioxidative and hepatoprotective potential of ASEE was
investigated.
DPPH is a molecule containing a stable free radical,
which upon receiving an electron from antioxidant agents undergoes
reduction in the intensity of its purple solution, and hence in
absorbance (23). As it is
recommended to conduct more than one in vitro assay
(36), in the present study the
antioxidant activity of ASEE was also confirmed by β-carotene
bleaching. In the β-carotene bleaching method linoleic acid
generated free radicals attack unsaturated β-carotene to undergo
oxidation and subsequently cause it to lose its orange color.
During in vitro DPPH free radical scavenging and
β-carotene-linoleic acid bleaching assays, ASEE demonstrated
antioxidant activity that appeared to be close to the levels of
ascorbic and galic acids. Notably, flavonol glycosides from the
aerial parts of A. halimus have been revealed to have a
clear DPPH radical scavenging ability (37). In addition, septanosides isolated
from A. portulacoides have recently been highlighted for
their in vitro antioxidant activity using DPPH,
ABTS+, Fe3+ and catalase assays (38).
DCFH is typically used to estimate in vitro
oxidative stress generated by free-radicals through the oxidation
of DCFH into the fluorescent DCF (39). In addition, it is also used as a
potent cytotoxic agent against an array of human cell lines
(25). In the in vitro
hepatoblastoma cell culture model used in the present study, ASEE
promoted HepG2 cell proliferation and recovery against
DCFH-toxicity in a dose-dependent manner. Apoptotic cell death
caused by reactive oxygen or nitrogen molecules is a well known
phenomenon (34,35). In the present study the
apoptotic-signaling assay revealed a dose-dependent inhibition of
caspase-3/-7 activation by ASEE against DCFH-induced HepG2 cell
death. In conclusion, ASEE exhibited a promising antioxidative and
cytoprotective salutation against chemical-toxicity.
To further confirm the in vitro effects, the
in vivo therapeutic potential of ASEE was examined in
CCl4-injured livers of Wistar rats. CCl4 is a
common hepatotoxin used in the experimental study of liver diseases
that induces free-radical generation in liver tissues (23,26).
Clinically, CCl4-induced acute hepatotoxicity manifests
as jaundice and elevated levels of liver enzymes, followed by
hepatic necrosis (40). In a
previous study, A. lentiformis ethanol and n-butanol
extracts were reported to have antioxidant activities, including
normalization of liver functions by a significant increase in serum
alanine transferase levels (41). In
the present study, the significant elevation of serum SGOT, SGPT,
GGT, ALP, bilirubin and TP was observed in CCl4-treated
rats, which indicates damage to the hepatic tissues. Treatment with
ASEE demonstrated its therapeutic ability to normalize the serum
biomarkers via attenuation of CCl4 toxicity at a
comparable level to treatment with silymarin. In addition to this,
ASEE also normalized the serum cholesterol, TC, LDL and HDL levels
in the CCl4 treated rats.
MDA is used as a marker for lipid peroxidation of
the cell membrane, which may cause cell damage (42). The level of MDA was reduced in ASEE
treated rats suggesting its cytoprotective and curative activities
against CCl4. In addition, the liver NP-SH level in
CCl4-treated animals was significantly diminished
compared with the control group, suggesting oxidative
hepatocellular damage. The administration of ASEE or silymarin
replenished NP-SH in the CCl4-treated animals
demonstrating its protective activity.
The histopathological changes observed in the liver
tissues revealed that the administration of ASEE caused the
recovery of hepatic damage. This was revealed by the presence of
normal hepatic cords and the absence of necrosis and lesser fatty
infiltration in CCl4-treated rats. These results
indicate the in vivo hepatoprotective effects of ASEE by
abating the chemical-induced oxidative and apoptotic pathways.
Furthermore, the antioxidative and hepatoprotective
activities of ASEE may be attributed to the presence of antioxidant
flavonoids, alkaloids, polyphenols and saponins as confirmed by the
qualitative phytochemical screening. The hepatoprotective activity
of flavonoids is due to their ability to scavenge and reduce
cellular free radicals. Rutin, a natural bioflavonoid that is
distributed in a range of medicinal plants, is known for its
pharmacological properties, including strong its antioxidant and
anti-lipid peroxidative activities (43,44).
Previously, the in vivo hepatoprotective efficacy of rutin
in CCl4-treated BALB/cN mice has been reported (45). The identification of rutin in A.
subrecta by the validated HTPLC method is in agreement with
previous findings and endorses its therapeutic attribution to the
prevention and treatment of liver diseases. In conclusion, to the
best of our knowledge this is the first investigation into the
hepatoprotective effects of A. subrecta and it has revealed
its promising antioxidative and hepatoprotective potential against
chemical-induced in vitro and in vivo liver injury.
These results were supported by the phytochemical analysis and
identification of rutin, a well-known antioxidant flavonoid in the
plant. Therefore, A. subrecta may be a valuable source of
natural antioxidant or health protective agent to manage oxidative
stress-associated diseases. However, further investigation into its
phytochemical properties and active principles, including an
assessment of any other therapeutic contributions is required.
Acknowledgements
The authors would like to thank Mr. Malik Saud from
the College of Pharmacy, King Saud University (Riyadh, Saudi
Arabia) for his assistance with animal care.
Funding
The study was supported by the Deanship of
Scientific Research, King Saud University, Riyadh, Saudi Arabia
(grant no. RG-1435-053).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MKP conceptualized and designed the study,
participated in the in vitro and in vivo experiments,
analyzed the data and wrote the manuscript. AHA performed the in
vitro experiments, analyzed data and contributed to writing the
paper. MSAD and AJAR collected and extracted the plant material and
participated in phytochemical analysis. PA performed the HPTLC
study and analyzed data. KEI performed the liver histopathology.
MSA and SR participated in the in vivo study and data
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Experimental Animal Care Society (King Saud
University, Riyadh, Saudi Arabia) and adhered to its
guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Williams R: Global challenges in liver
disease. Hepatology. 44:521–526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aniya Y, Koyama T, Miyagi C, Miyahira M,
Inomata C, Kinoshita S and Ichiba T: Free radical scavenging and
hepatoprotective actions of the medicinal herb, Crassocephalum
crepidioides from the Okinawa Islands. Biol Pharm Bull.
28:19–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farghali H, Canová NK and Zakhari S:
Hepatoprotective properties of extensively studied medicinal plant
active constituents: Possible common mechanisms. Pharm Biol.
53:781–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katikova Olu, Kostin IaV and Tishkin VS:
Hepatoprotective effect of plant preparations. Eksp Klin Farmakol.
65:41–43. 2002.(In Russian). PubMed/NCBI
|
|
5
|
Ahmad N, Fazal H, Abbasi BH, Anwar S and
Basir A: DPPH free radical scavenging activity and phenotypic
difference in hepatoprotective plant (Silybum marianum L.).
Toxicol Ind Health. 29:460–467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olalye MT and Rocha JB: Commonly used
tropical medicinal plants exhibit distinct in vitro antioxidant
activities against hepatotoxins in rat liver. Exp Toxicol Pathol.
58:433–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le Houerou HN: The role of saltbushes
(Atriplex spp.) in arid land rehabilitation in the
Mediterranean Basin: A review. Agroforest Sys. 18:107–148. 1992.
View Article : Google Scholar
|
|
8
|
Khalil JK, Sawaya WN and Hyder SZ:
Nutrient composition of Αtriplex leaves grown in
Saudi-Arabia. J Range Manage. 39:104–107. 1986. View Article : Google Scholar
|
|
9
|
Walker DJ, Lutts S, Sánchez-García M and
Correal E: Atriplex halimus L.: Its biology and uses. J Arid
Environ. 100:111–121. 2001.
|
|
10
|
Fernández SS, Padilla AP and Mucciarelli
S: Protein extraction from Atriplex lampa leaves: Potential
use as forage for animals used for human diets. Plant Foods Hum
Nutr. 54:251–259. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rolleston JD: I. The folk-lore of venereal
disease. Br J Vener Dis. 18:1–13. 1942.PubMed/NCBI
|
|
12
|
Said O, Fulder S, Khalil K, Azaizeh H,
Kassis E and Said B: Maintaining a physiological blood glucose
level with ‘Glucolevel’, a combination of four anti-diabetes plants
used in the traditional arab herbal medicine. Evid Based Complement
Alternat Med. 5:421–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abdel Rahman SM, Abd-Ellatif SA, Deraz SF
and Khalil AA: Antibacterial activity of some wild medicinal plants
collected from western Mediterranean coast, Egypt: Natural
alternatives for infectious disease treatment. Afr J Biotechnol.
10:10733–10743. 2011. View Article : Google Scholar
|
|
14
|
Clauser M, Dall'Acqua S, Loi MC and
Innocenti G: Phytochemical investigation on Atriplex halimus
L. from Sardinia. Nat Prod Res. 27:1940–1944. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ali B, Tabassum R, Riaz N, Yaqoob A,
Khatoon T, Tareen RB, Jabbar A, Nasim FU and Saleem M: Bioactive
triterpenoids from Atriplex lasiantha. J Asian Nat Prod Res.
17:843–850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamal Z, Ullah F, Ayaz M, Sadiq A, Ahmad
S, Zeb A, Hussain A and Imran M: Anticholinesterase and antioxidant
investigations of crude extracts, subsequent fractions, saponins
and flavonoids of Atriplex laciniata L.: Potential
effectiveness in Alzheimer's and other neurological disorders. Biol
Res. 48:212015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boughalleb N, Trabelsi L and
Harzallah-Skhiri F: Antifungal activity from polar and non-polar
extracts of some Chenopodiaceae wild species growing in Tunisia.
Nat Prod Res. 23:988–997. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamed N, Njeh F, Damak M, Ayadi A,
Mezghani-Jarraya R and Hammami H: Molluscicidal and larvicidal
activities of Atriplex inflata aerial parts against the
mollusk Galba truncatula, intermediate host of Fasciola
hepatica. Rev Inst Med Trop Sao Paulo. 57:473–479. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gođevac D, Stanković J, Novaković M,
Anđelković B, Dajić-Stevanović Z, Petrović M and Stanković M:
Phenolic compounds from Atriplex littoralis and their
radiation-mitigating activity. J Nat Prod. 78:2198–2204. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Al-Turki TA, Omer S and Ghafoor A: A
synopsis of the genus Atriplex L. (Chenopodiaceae) in Saudi
Arabia. Feddes Repertorium. 111:261–293. 2000. View Article : Google Scholar
|
|
21
|
Cid JA, Petenatti E, Arellano M, Muzaber J
and de Mucciarelli SL: Biological value of protein from leaves of
Atriplex suberecta. Arch Latinoam Nutr. 41:421–427. 1991.(In
Spanish). PubMed/NCBI
|
|
22
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: HepG2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
23
|
Lee YJ, Kim DB, Lee J, Cho JH, Kim B, Choi
HS, Lee BY and Lee OH: Antioxidant activity and anti-adipogenic
effects of wild herbs mainly cultivated in Korea. Molecules.
18:12937–12950. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miller HE: A simplified method for the
evaluation of antioxidants. J Am Oil Chem Soc. 48:911971.
View Article : Google Scholar
|
|
25
|
Arbab AH, Parvez MK, Al-Dosari MS,
Al-Rehaily AJ, Al-Sohaibani M, Zaroug EE, AlSaid MS and Rafatullah
S: Hepatoprotective and antiviral efficacy of Acacia
mellifera leaves fractions against hepatitis B virus. Biomed
Res Int. 2015:9291312015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parveen R, Baboota S, Ali J, Ahuja A,
Vasudev SS and Ahmad S: Effects of silymarin nanoemulsion against
carbon tetrachloride-induced hepatic damage. Arch Pharm Res.
34:767–774. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Utley HG, Bernheim F and Hochstein P:
Effect of sulfhydryl reagents on peroxidation in microsomes. Arch
Biochem Biophys. 18:29–32. 1967. View Article : Google Scholar
|
|
28
|
Sedlak J and Lindsay RH: Estimation of
total, protein-bound, and nonprotein sulfhydryl groups in tissue
with Ellman's reagent. Anal Biochem. 25:192–205. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Culling CF: Handbook of Histopathological
and Histochemical Techniques. 3rd. Butterworth-Heinemann, Oxford,
UK: 1974
|
|
30
|
Satyajit D, Sarkar ZL and Gray AI: Natural
Products Isolation. 2nd edition. Humana Press; Totowa, NJ; 2006
|
|
31
|
Kar A: Pharmacognosy and
Pharmacobiotechnology. (2nd). New Age International. (New Delhi,
India). 2007.
|
|
32
|
Tiwari P, Mandeep BK, Kaur KG and Kaur H:
Phytochemical screening and extraction: A review. Interl Pharma
Sci. 1:98–1062. 2011.
|
|
33
|
Alam P, Parvez MK, Arbab AH and Al-Dosari
MS: Quantitative analysis of rutin, quercetin, naringenin and
gallic acid by validated RP- and NP-HPTLC methods for the quality
control of anti-HBV active extract of Guiera senegalensis.
Phrm Biol. 55:1317–1323. 2017. View Article : Google Scholar
|
|
34
|
Wang Z, Li Z, Ye Y, Xie L and Li W:
Oxidative stress and liver cancer: Etiology and therapeutic
targets. Oxid Med Cell Long. 2016:78915742016.
|
|
35
|
Rahman K: Studies on free-radicals,
antioxidants, and co-factors. Clin Interven Aging. 2:219–236.
2007.
|
|
36
|
Kedare SB and Singh RP: Genesis and
development of DPPH method of antioxidant assay. J Food Sci
Technol. 48:412–422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kabbash A and Shoeib N: Chemical and
biological investigation of some secondary metabolites in
Atriplex halimus growing in Egypt. Nat Prod Commun.
7:1465–1468. 2012.PubMed/NCBI
|
|
38
|
Ben Nejma A, Nguir A, Ben Jannet H, Hamza
MA, Daïch A, Othman M and Lawson AM: New septanoside and
20-hydroxyecdysone septanoside derivative from Atriplex
portulacoides roots with preliminary biological activities.
Bioorg Med Chem Lett. 25:1665–1670. 2015. View Article : Google Scholar
|
|
39
|
Rota C, Chignell CF and Mason RP: Evidence
for free radical formation during the oxidation of
2′-7′-dichlorofluorescin to the fluorescent dye
2′-7′-dichlorofluorescein by horseradish peroxidase: Possible
implications for oxidative stress measurements. Free Radic Biol
Med. 27:873–881. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tirkey N, Pilkhwal S, Kuhad A and Chopra
K: Hesperidin, a citrus bioflavonoid, decreases the oxidative
stress produced by carbon tetrachloride in rat liver and kidney.
BMC Pharmacol. 5:22005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Awaad AS, Maitland DJ, Donia AR, Alqasoumi
SI and Soliman GA: Novel flavonoids with antioxidant activity from
a Chenopodiaceous plant. Pharm Biol. 50:99–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Suhail M, Suhail S, Gupta BK and Bharat V:
Malondialdehyde and antioxidant enzymes in maternal and cord blood,
and their correlation in normotensive and preeclamptic women. J
Clin Med Res. 1:150–157. 2009.PubMed/NCBI
|
|
43
|
Janbaz KH, Saeed SA and Gilani AH:
Protective effect of rutin on paracetamol- and CCl4-induced
hepatotoxicity in rodents. Fitoterapia. 73:557–563. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hosseinzadeh H and Nassiri-Asl M: Review
of the protective effects of rutin on the metabolic function as an
important dietary flavonoid. J Endocrinol Invest. 37:783–788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Domitrović R, Jakovac H, Vasiljev Marchesi
V, Vladimir-Knežević S, Cvijanović O, Tadić Z, Romić Z and Rahelić
D: Differential hepatoprotective mechanisms of rutin and quercetin
in CCl(4)-intoxicated BALB/cN mice. Acta Pharmacol Sin.
33:1260–1270. 2012. View Article : Google Scholar : PubMed/NCBI
|