Introduction
The occurrence of pressure ulcer is a serious
complication in health care centres (1). Reduced mobility, sensory impairment and
poor nutritional status are the significant factors that increase
the risk of pressure ulcer (2).
Nutrition plays a key role in pressure ulcer healing and evidence
has shown that nutritional support reduces the incidence of
pressure ulcer in at-risk patients by 25% (3). Malnutrition is considered an important
risk factor for the development of pressure ulcer (4). It has been proven that poor dietary
intake is associated with the development of pressure ulcer as well
as delayed healing time (5).
Nutritional supplements are conducive to shortening the pressure
ulcer healing time. In addition to regular food intake, providing
an oral nutritional supplement is a logical way to promote tissue
repair (6).
Prospective studies have shown that compared with
patients whose nutritional intake was adequate, patients who had an
adequate intake of protein had faster healing rates of pressure
ulcers (7,8). Protein is essential for tissue cell
repair and it is necessary for the repair of epithelial tissue
(9). High protein diets may improve
the healing of pressure ulcer. A number of studies have
demonstrated that high protein intake is beneficial for pressure
ulcer healing (8,10,11). In
addition, many animal pressure ulcer models have been developed to
test the effect of protein in this field (12,13). The
aforementioned studies showed that high protein intake was helpful
to pressure ulcer healing, but which protein level is most
beneficial in the recovery from pressure ulcer remain to be
determined. In this regard, the relevant research is rare.
In the present study, rat models of stage II
pressure ulcer were established in order to investigate the effect
of different level protein intake on healing of pressure ulcer.
Materials and methods
Animals
A total of 100 healthy male rats with a weight of
280±20 g were purchased from Experimental Animal Center of Hebei
Medical University (Hebei, China), and the animal certificate
number was 1607209. The use of animals was in accordance with the
specification for laboratory animal use of People's Hospital of
Hebei Province. The study was approved by the Ethics Committee of
the Hebei General Hospital (Hebei, China).
Feed
Feeds with different protein intake were prepared
(Table I). The protein levels of the
feed were referred to Medical Laboratory Animal Standards
promulgated by the Ministry of Health of the People's Republic of
China in 1992. The standards stipulated that the protein level of
the feed was 18–25%.
 | Table I.Feed with different protein levels
(g/100 g). |
Table I.
Feed with different protein levels
(g/100 g).
|
| Protein level |
|---|
|
|
|
|---|
| Ingredients | 10% | 15% | 20% | 25% |
|---|
| Corn flour (g) | 50 | 30 | 30 | 22 |
| Flour (g) | 50 | 56 | 40 | 32 |
| Soy flour (g) | 3 | 4 | 8 | 15 |
| Soybean meal (g) | 4 | 4 | 10 | 8 |
| Bone meal (g) | 3 | 3 | 6 | 10 |
| Fish meal (g) | 6 | 3 | 6 | 10 |
| Salt (g) | 0.05 | 0.05 | 0.05 | 0.05 |
| Cod liver oil
(g) | 0.012 | 0.012 | 0.012 | 0.012 |
| Mineral (g) | 0.16 | 0.16 | 0.16 | 0.16 |
| Multidimensional
nutrients (g) | 0.12 | 0.12 | 0.12 | 0.12 |
| Energy (kJ) | 358.55 | 364.75 | 369.88 | 375.90 |
| Nitrogen content
(%) | 1.6 | 2.4 | 3.2 | 4.0 |
Grouping
The rats were housed at a constant room temperature
(20–25°C) and humidity (40–60%) with standard feed (20% protein
level) for one week. After being numbered according to body weight,
the rats were randomly divided into 4 groups (groups A, B, C and D)
using the random number table. The number of rats in each group was
25.
Construction of rats with stage II
pressure ulcer models
All the rats were subjected to the same surgical
procedure. Before surgery, they were fasted and water deprived for
8 h. Each rat was subjected to depilatory treatment with a skin
area of 4×4 cm after they were anesthetized by intraperitoneal
injection of 10% chloral hydrate (0.3 ml/100 g body weight). The
depilatory area was at the buttock on the left side of the back
center line. After disinfection of the skin with 5%
povidone-iodine, an incision with a length of 2 cm was performed.
Fascia and tissue were separated by tweezers and a sterile steel
disk (diameter 15 mm, thickness 0.3 mm, weight 0.6 g, autoclave
sterilization) was implanted under the muscle. The incision was
stitched with polyglycolic acid suture and 5% povidone-iodine was
used to disinfect the suture to prevent infection. Three days after
surgery, a magnetic disk (diameter 15 mm, thickness 2.5 mm, weight
2.6 g, with magnetic flux up to 1,500 gauss) was placed outside the
skin in the area where the steel disk was placed (14). The pressure between the steel disk
and magnet disk could cause pressure on the muscles and skin, which
potentially led to local tissue ischemia. Two hours after
pressurization, the magnets were removed for 0.5 h for ischemia
reperfusion. This pressurization process was cycled 5 times a day
and the stage II pressure ulcer was formed after 2–3 days of cyclic
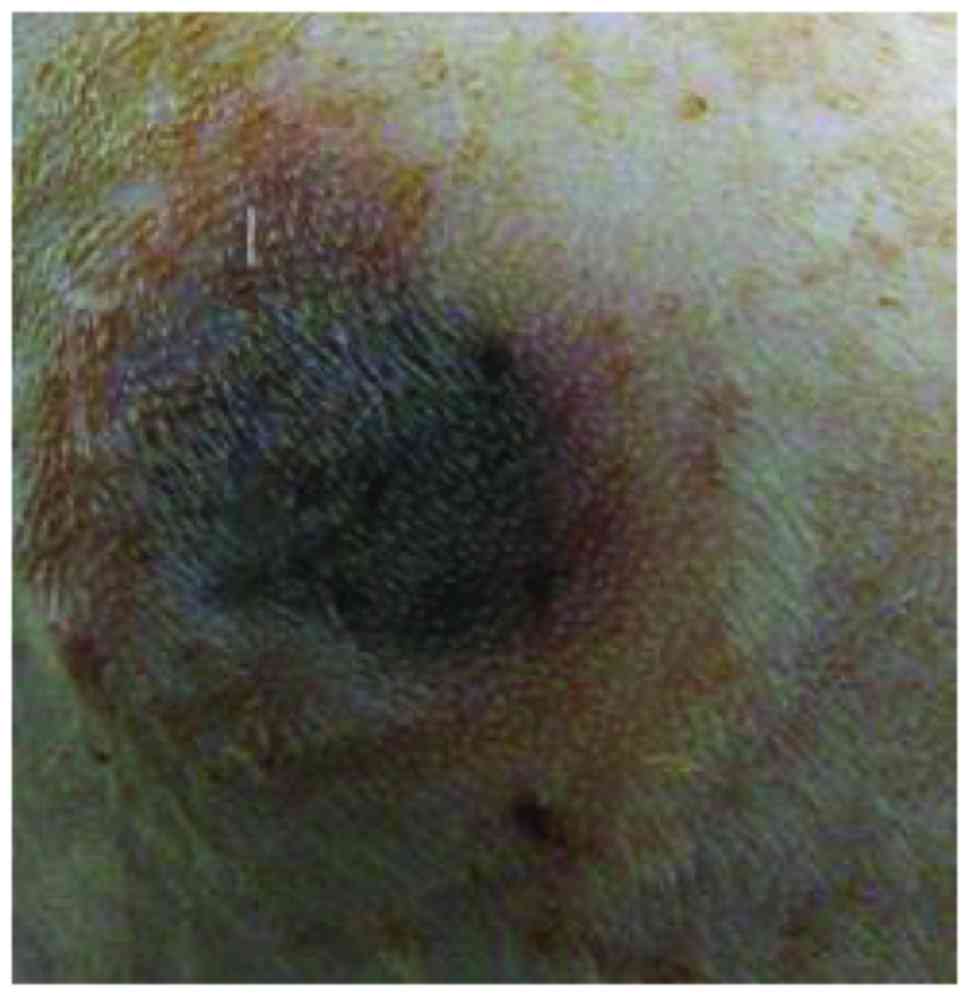
pressure operation. The criteria of successful stage II pressure
ulcer models were: The color of the pressure ulcer was dark red,
the skin around the pressure ulcer was redish, bleeding and exudate
did not occur, obvious pain was showed by the rats.
Intervention method
Rats were fed with standard feed before and during
the construction of stage II pressure ulcer models. The pressure
ulcer area and body weight of the rats were measured and recorded
immediately after the pressure ulcer was formed. After stage II
pressure ulcer models were constructed, the rats in each group were
fed with different protein levels of feed. The protein levels of
the feed in groups A, B, C and D were 10, 15, 20 and 25%,
respectively. The amount of feed was 20 g/rat/day and water was
adequate.
Monitoring of healing time, pressure
ulcer area and rat body weight
Healing time, pressure ulcer area, and rat body
weight were monitored at different times. Evaluation criteria for
pressure ulcer healing were as follows: The redness of the skin
around the pressure ulcer had subsided, the scab of the pressure
ulcer was shed, the new skin color was consistented with the
surrounding normal skin color.
Monitoring of ALB and Hb levels
Before surgery, after surgery, and 3, 7 and 14 days
after rat models were constructed, 3 rats in each group were
randomly selected to obtain abdominal aortic blood samples. Albumin
(ALB) and hemoglobin (Hb) levels were measured by spectrophotometry
after the blood samples were diluted with Drabkin's solution and
Bromocresol green, respectively.
Hematoxylin and eosin (H&E)
staining
After the pressure ulcer models were established,
pressure ulcer tissue samples were obtained. Pressure ulcer tissue
samples of each group were obtained after 14 days feeding with
different feeds. Conventional H&E staining was performed to
observe the histological structure.
Statistical analysis
Data were analyzed by SPSS 19.0 software (SPSS,
Inc., Chicago, IL, USA) and expressed as mean ± SD. Multiple
comparisons were performed by analysis of variance (ANOVA) followed
by post hoc test (LSD test). P<0.05 was considered to indicate a
statistically sgnificant difference.
Results
Effects of protein on healing
time
In each group, 22 rats were modeled successfully
(Fig. 1) and the pressure ulcer
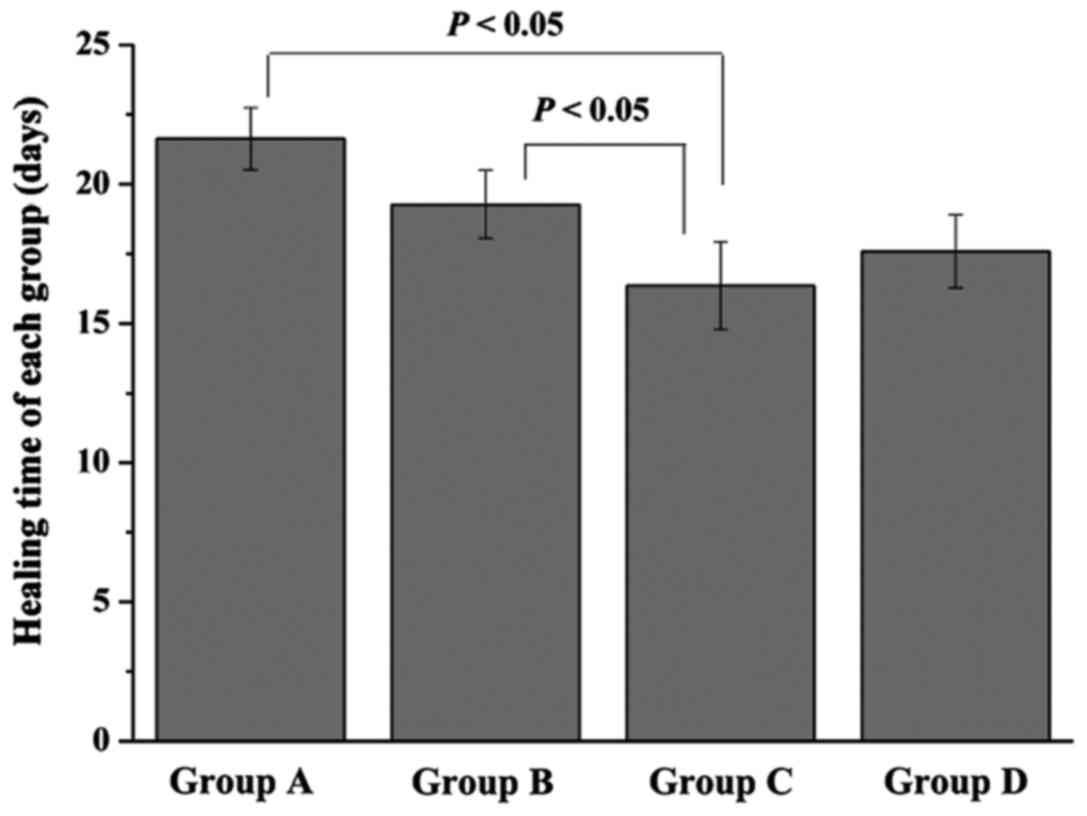
healing time of each group was measured (Fig. 2). Rats in group A and B needed
21.63±1.12 and 19.27±1.22 day, respectively, for their ulcers to
heal. However, the healing time of group C was only 16.36±1.57
days, which was significantly lower than that of group A and B
(P<0.05). Furthermore, when compared with group C, a little
longer healing time was found in group D (17.58±1.31 days), but
without significant difference.
Effects of protein on pressure ulcer
area
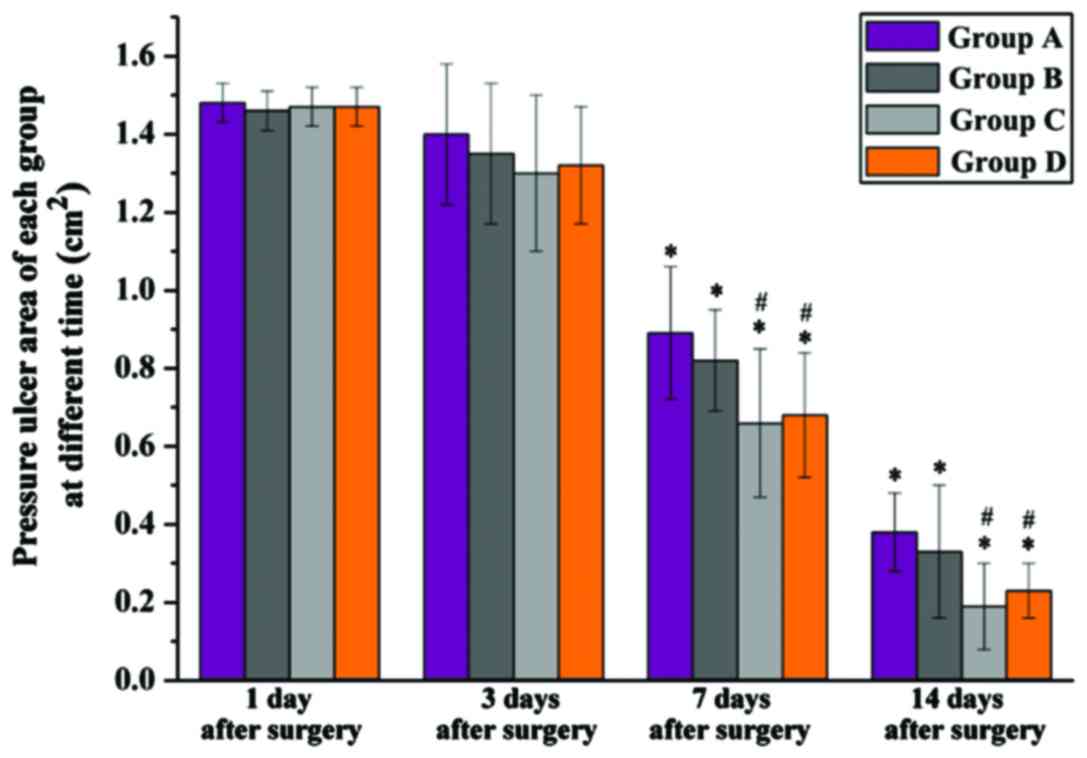
The pressure ulcer area was measured over time. No
significant difference was found in the pressure ulcer area among
groups after the models were constructed. Seven days after surgery
the pressure ulcer area of each group was significantly reduced
when compared with that of 1 day after surgery (P<0.05). The
same situation applied to 14 days after surgery. Of note was that,
at these times, the pressure ulcer area of group C (0.19±0.11
cm2) and group D (0.23±0.07 cm2) was
significantly lower than that of group A (0.38±0.10 cm2)
and group B (0.33±0.17 cm2) (P<0.05). Group D had a
larger pressure ulcer area compared to group C, but without
significant difference (Fig. 3).
Effects of protein on body weight
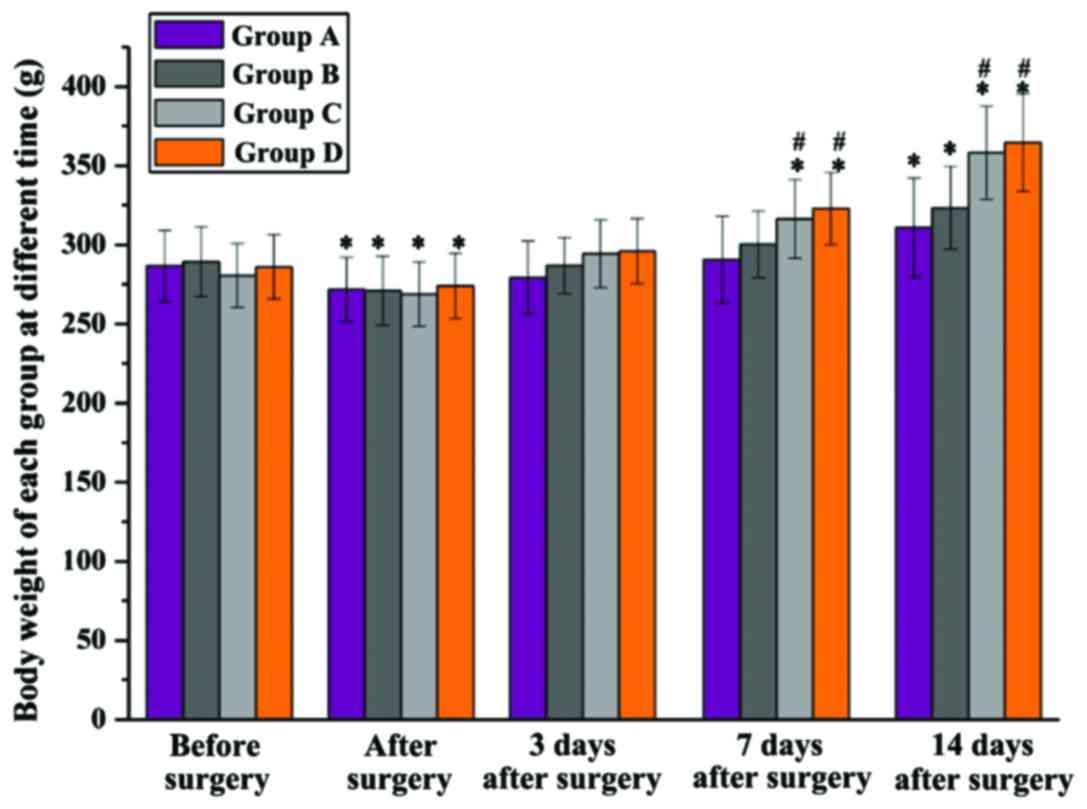
The rat body weights of each group were recorded at
different times (Fig. 4). Before
surgery, there was no significant difference in body weight among
the 4 groups, and it was significantly decreased in all groups
after the models were constructed, but again without significant
difference among groups. Three days after surgery, all the rat body
weights were back to normal, and 7 days after surgery, a
significantly higher body weight in groups C and D was found when
compared with group A and B at the same time (P<0.05). Fourteen
days after surgery, compared to after surgery, the body weight of
group A and B was significantly increased, but it was significantly
lower than that of groups C and D. Rats in group D had a higher
body weight compared to group C, but with no significant
difference.
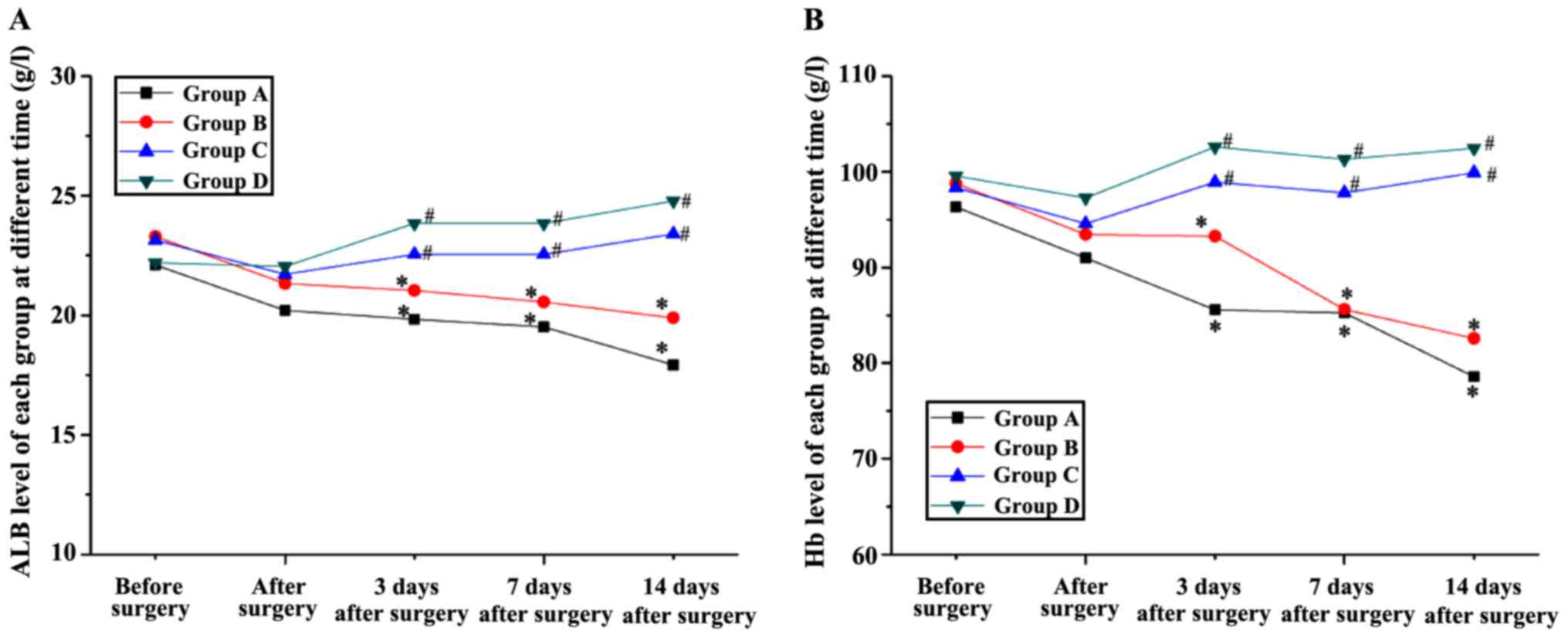
Effects of protein on ALB and Hb
levels
Changes of ALB and Hb levels over time are shown in
Fig. 5, respectively. No significant
difference was found in ALB or Hb levels among groups before
surgery. From 3 days after surgery, a significant decrease ALB and
Hb levels was found in groups A and B when compared with that of
before surgery. Moreover, these two levels of group C and D were
significantly lower than that of group A and B. No significant
difference was found in the ALB or Hb levels between group C and D,
however, both of these levels of group C were closer to normal
level and the ALB level of group D at 14 days after surgery was
obviously a little higher than that before surgery.
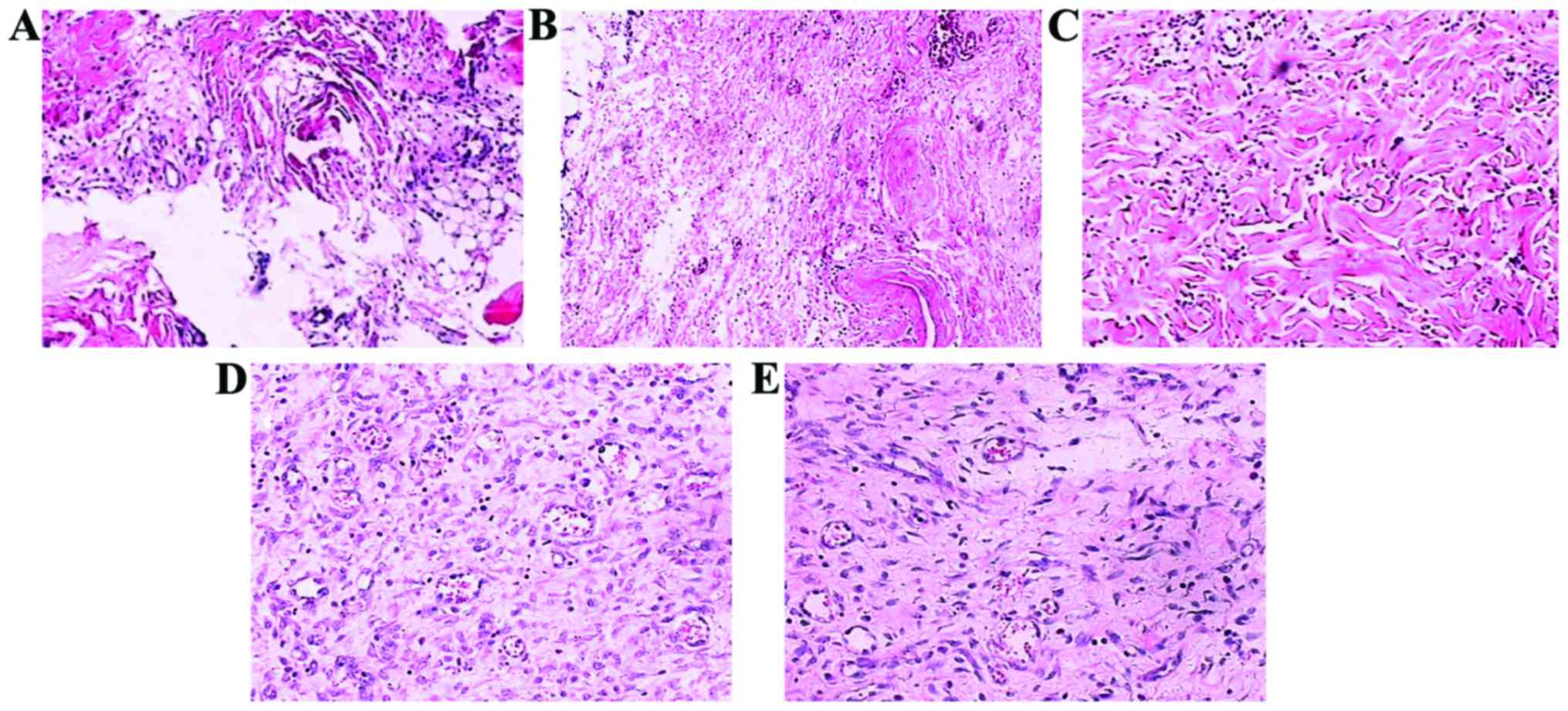
Histological structure changes
After the pressure ulcer models were formed, the
structural integrity of the pressure ulcer tissue was very poor and
tissue fracture phenomenon was observed. In addition, broken cell
structure occurred (Fig. 6A).
Fourteen days after feeding with the different feeds, the damage
degree of all rat pressure ulcer tissues was significantly
improved. However, the structural integrity of the pressure ulcer
tissue of rats in group A and B remained very poor, and the cell
structure still displayed a certain degree of damage (Fig. 6B and C). At the same time, the
pressure ulcer tissue of rats in group C and D had significantly
better structural integrity, and the integrity of the cell
structure had significantly improved, and the tissue fracture
phenomenon had disappeared (Fig. 6D and
E).
Discussion
In the present study, the effect of protein level on
pressure ulcer healing was studied. The results showed that 14 days
after the models were constructed, rats with 20% protein content
feed had the shortest healing time and the smallest pressure ulcer
area. Furthermore, body weight, ALB and Hb levels were much closer
to the normal level. H&E staining result also suggested that
the pressure ulcer healing degree of rats with 20% protein content
feed was much better than the others. These results indicated that
adequate protein intake has a positive effect on pressure ulcer
healing, while excessive or insufficient protein intake is not
conducive to healing.
Deep tissue injury is considered as the mechanism
for pressure ulcer formation (15,16).
Protein is the raw material for tissue cell repair and it is
necessary for the repair of epithelial tissue. Adequate protein
intake is essential to accelerate pressure ulcer healing (17). In addition, more high-quality
proteins are required during the ulcer healing process. Previous
findings have shown that ulcer healing time would be prolonged if
the protein is insufficient and a serious lack of protein would
cause ulcer not to heal (18). Our
study further demonstrated that protein is beneficial to pressure
ulcer healing, albeit not in excess. Gautam et al (19) reported that high protein consumption
diets may induce disorders and increase the burden on metabolic
organs, causing a negative impact on body functions. Excessive
protein intake also could lead to overweight, which would increase
the burden on the body organs and further delay the healing of
pressure ulcers. Our results are consistent with the study by
Gautam et al (19). The
healing degree of rats with the most protein intake (25%) was lower
than that of rats with 20% protein intake, although the difference
was not significant.
Protein deficiency has been recognized as an
independent risk factor for predicting the development of pressure
ulcers (20–22). Hb and ALB levels are two common
monitoring indicators (23).
Reduction in tissue repair function and decreased immunity may
occur when the ALB level is reduced, which eventually leads to an
increased risk of pressure ulcer and delay the healing time. In
addition, if the Hb level was reduced, decreased oxygen carrying
capacity would occur (24),
resulting in tissue stress tolerance decrease and healing of
pressure ulcer delay (25). The
results in this study has shown that the more protein intake in
rats, the faster the Hb and ALB levels increased, indicating that
the protein intake helps to increase the Hb and ALB levels and then
they further promote the healing of the pressure ulcer. Protein is
an essential component in the process of tissue repair, which has
the ability to improve the body's resistance and to maintain the
water balance between plasma and tissue (26). Long-term protein deficiency is likely
to cause hypoproteinemia. As a result, water in plasma may
penetrate into tissue and cause tissue edema, and this response
eventually leads to weakened tissue repair ability (27). In addition, hypoproteinemia can lead
to decreased anti-infective capacity of injured tissue (28). Of course, excessive protein intake
can also have a negative impact because hyperglycemia can increase
the burden on organs and then delay in tissue repair ability occurs
(29,30). In this study, in addition to healing
time and pressure ulcer area of the two indicators, H&E
staining result also proved the above view point. We also found
that ALB level changes were more sensitive than Hb level ones and
it could respond more timely to the nutritional status of rats with
pressure ulcer. We consider that ALB level changes can be the first
step to predict the risk of pressure ulcer, but further
investigations should determine the reason for this phenomenon.
In conclusion, the present study suggests that
adequate protein intake is conducive to pressure ulcer healing.
Protein plays a key role in tissue repair processes, and
insufficient or too much protein intake exerts an adverse effect on
the pressure ulcer healing. This study has important guiding
significance for the clinical treatment of patients with pressure
ulcers.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZQ, WZ and YW contributed to the conception and
design of the study and drafted the study. YZ analyzed and
interpreted the hematoxylin and eosin (H&E) staining. YT, SS
and XL interpreted the data of the study and revised the manuscipt.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Hebei General Hospital (Hebei, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Defloor T, Vanderwee K, Bouzegta N, Van
Durme T and Gobert M: Assessing the adequacy of pressure ulcer
prevention in Belgian geriatric hospital wards. J Clin Nurs.
19:412010.
|
|
2
|
Chapman BR, Mills KJ, Pearce LM and Crowe
TC: Use of an arginine-enriched oral nutrition supplement in the
healing of pressure ulcers in patients with spinal cord injuries:
An observational study. Nutr Diet. 68:208–213. 2011. View Article : Google Scholar
|
|
3
|
Stratton RJ, Ek AC, Engfer M, Moore Z,
Rigby P, Wolfe R and Elia M: Enteral nutritional support in
prevention and treatment of pressure ulcers: A systematic review
and meta-analysis. Ageing Res Rev. 4:422–450. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Houwing RH, Rozendaal M, Wouters-Wesseling
W, Beulens JW, Buskens E and Haalboom JR: A randomised,
double-blind assessment of the effect of nutritional
supplementation on the prevention of pressure ulcers in
hip-fracture patients. Clin Nutr. 22:401–405. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guenter P, Malyszek R, Bliss DZ, Steffe T,
O'Hara D, LaVan F and Monteiro D: Survey of nutritional status in
newly hospitalized patients with stage III or stage IV pressure
ulcers. Adv Skin Wound Care. 13:164–168. 2000.PubMed/NCBI
|
|
6
|
Heyman H, Van De Looverbosch DE, Meijer EP
and Schols JM: Benefits of an oral nutritional supplement on
pressure ulcer healing in long-term care residents. J Wound Care.
17(476–478): 4802008.
|
|
7
|
Benati G, Delvecchio S, Cilla D and Pedone
V: Impact on pressure ulcer healing of an arginine-enriched
nutritional solution in patients with severe cognitive impairment.
Arch Gerontol Geriatr Suppl. 7:43–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Breslow RA, Hallfrisch J, Guy DG, Crawley
B and Goldberg AP: The importance of dietary protein in healing
pressure ulcers. J Am Geriatr Soc. 41:357–362. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu S, Thompson K and Leask A: Leask, CCN2
expression by fibroblasts is not required for cutaneous tissue
repair. Wound Repair Regen. 22:119–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wijewardena A, Lajevardi SS, Vandervord E,
Vandervord J, Lang TC, Fulcher G and Jackson CJ: Activated protein
C to heal pressure ulcers. Int Wound J. 13:986–991. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fogh K, Nielsen CB and Dam W: Effect of
amelogenin ECM protein on the healing of chronic leg ulcers with
atrophie blanche. J Wound Care. 21:612–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nguyen PK, Smith AL and Reynolds KJ: A
literature review of different pressure ulcer models from 1942–2005
and the development of an ideal animal model. Australas Phys Eng
Sci Med. 31:223–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salcido R, Popescu A and Ahn C: Animal
models in pressure ulcer research. J Spinal Cord Med. 30:107–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wassermann E, van Griensven M, Gstaltner
K, Oehlinger W, Schrei K and Redl H: A chronic pressure ulcer model
in the nude mouse. Wound Repair Regen. 17:480–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ankrom MA, Bennett RG, Sprigle S, Langemo
D, Black JM, Berlowitz DR and Lyder CH: National Pressure Ulcer
Advisory Panel: Pressure-related deep tissue injury under intact
skin and the current pressure ulcer staging systems. Adv Skin Wound
Care. 18:35–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Black J, Baharestani MM, Cuddigan J,
Dorner B, Edsberg L, Langemo D, Posthauer ME, Ratliff C and Taler
G: National Pressure Ulcer Advisory Panel: National Pressure Ulcer
Advisory Panel's updated pressure ulcer staging system. Adv Skin
Wound Care. 20:269–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mathus-Vliegen EM: Clinical observations:
Nutritional status, nutrition, and pressure ulcers. Nutr Clin
Pract. 16:286–291. 2001. View Article : Google Scholar
|
|
18
|
Morifuji M, Sakai K, Sanbongi C and
Sugiura K: Dietary whey protein downregulates fatty acid synthesis
in the liver, but upregulates it in skeletal muscle of
exercise-trained rats. Nutrition. 21:1052–1058. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gautam BP, Kishore N and Gondwal M:
Adverse effect in human beings associated with excess dietary
protein intakeBiomedical Applications of Natural Proteins. Kumar D
and Kundapur RR: Springer Briefs; pp. 115–128. 2015, View Article : Google Scholar
|
|
20
|
Posthauer ME, Banks M, Dorner B and Schols
JM: The role of nutrition for pressure ulcer management: National
pressure ulcer advisory panel, European pressure ulcer advisory
panel, and pan pacific pressure injury alliance white paper. Adv
Skin Wound Care. 28:175–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Malafarina V, Úriz-Otano F,
Fernández-Catalán C and Tejedo-Flors D: Nutritional status and
pressure ulcers. Risk assessment and estimation in older adults. J
Am Geriatr Soc. 62:1209–1210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guan X, Wang L and Wang Z: Study of Braden
scale prediction of diagnosis boundary value of pressure ulcer and
its risk factors. J Nurs Adm. 14:R4722014.
|
|
23
|
Anthony D, Rafter L, Reynolds T and
Aljezawi M: An evaluation of serum albumin and the sub-scores of
the Waterlow score in pressure ulcer risk assessment. J Tissue
Viability. 20:89–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thomas GM: Raising hemoglobin: An
opportunity for increasing survival? Oncology. 63 Suppl 2:19–28.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bogie K and Dan B: Susceptibility of
Spinal Cord-Injured Individuals to Pressure Ulcers. Springer;
Berlin, Heidelberg, New York: pp. 73–88. 2005
|
|
26
|
Thomson RL and Buckley JD: Protein
hydrolysates and tissue repair. Nutr Res Rev. 24:191–197. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palermo J and Szabo F: 50 Years ago in The
Journal of Pediatrics: Hypoproteinemia and edema in infants with
cystic fibrosis of the pancreas. J Pediatr. 164:6382014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li F, Yuan MZ, Wang L, Wang XF and Liu GW:
Characteristics and prognosis of pulmonary infection in patients
with neurologic disease and hypoproteinemia. Expert Rev Anti Infect
Ther. 13:521–526. 2015.PubMed/NCBI
|
|
29
|
Mayr FB, Yende S and Angus DC:
Epidemiology of severe sepsis. Virulence. 5:4–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tobalem M, Lévigne D, Modarressi A, Atashi
F, Villard F, Hinz B and Pittet-Cuénod B: Hyperglycemia interacts
with ischemia in a synergistic way on wound repair and
myofibroblast differentiation. Plast Reconstr Surg Glob Open.
3:e4712015. View Article : Google Scholar : PubMed/NCBI
|