Introduction
Intrahepatic cholangiocarcinoma (ICC) is a type of
malignant tumor with high incidence and an average age of onset of
50–70 years old, the etiology of which remains to be elucidated. A
previous study reported that patients with bile duct stones have a
high incidence of cholangiocarcinoma; however, the clinical
manifestations of ICC lack specificity and lead to delayed
diagnosis and treatment (1).
c-Met is a receptor of hepatocyte growth factor
(HGF), which serves a role in the regulation of cellular signaling
transduction and cytoskeleton rearrangement, as well as cell
division, proliferation, differentiation and migration (2). HGF is primarily produced by mesenchymal
cells acting in an autocrine and paracrine manner and (3) PF-2341066 is a c-Met inhibitor that was
approved by the United States Food and Drug Administration in 2011.
PF-2341066 exhibits antiproliferative and antiangiogenic effects,
suppressing the development of cancer by inhibiting the
phosphorylation of c-Met and it's downstream signaling (4). It has previously been reported that
c-Met signaling is important for the development of
cholangiocarcinoma (5) and so
PF-2341066 may be potential a candidate treatment for ICC. However,
this conjecture remains unproven.
COX-2 is primarily distributed in the nuclear
envelope of the nucleus and is able to promote cell proliferation,
inhibit apoptosis and promote blood vessel formation, contributing
to the pathogenesis of tumors (6,7).
Celecoxib is a selective inhibitor of COX-2 and has
anti-angiogenesis effects (8). A
previous study revealed that celecoxib is able to inhibit tumor
formation via the vascular endothelial growth factor (VEGF) pathway
(9). VEGF is one of the most potent
and specific vascular growth factors that induce angiogenesis in
the tumor microenvironment (10).
VEGF promotes tumor angiogenesis and provides a matrix for the
migration of vascular endothelial cells and the metastasis of tumor
cells (5).
A previous study revealed that c-Met affects COX-2;
HGF activates COX-2 expression via c-Met phosphorylation and the
extracellular signal-regulated kinase-2 cell signal transduction
pathway (11,12). Selective COX-2 inhibitors, including
celecoxib, may inhibit tumor growth by downregulating the
expression of COX-2 and c-Met (13).
However, whether c-Met inhibitors are able to regulate
COX-2-mediated signaling and the development of cholangiocarcinoma
remains to be elucidated. Little is known about the expression
profiles of COX-2 and c-Met in hepatobiliary calculus with
cholangiocarcinoma (HCWC).
The aim of the present study was to analyze the
expression of COX-2 and c-Met in normal tissue (NT), hepatobiliary
calculus tissue (HCT), paracarcinoma tissue (PT) and HCWC. The
effect of PF-2341066 and celecoxib, which are c-MET and COX-2
inhibitors, respectively, on proliferation and apoptosis in human
cholangiocarcinoma QBC939 cells was investigated. The results
suggest that combined treatment with PF-2341066 and celecoxib may
inhibit cell proliferation and promote cell apoptosis by
downregulating the expression of c-Met, COX-2 and VEGF.
Co-administration of c-MET and COX-2 may therefore have potential
for the treatment of cholangiocarcinoma.
Materials and methods
Tissue samples
The present study was performed in strict accordance
with the approval and recommendations of the Committee for Care and
Use in Clinical Study of Hunan Provincial People's Hospital
(C2016005). All patients provided written informed consent. A total
of 90 patients with cholangiocarcinoma aged 40.1–68.5 years were
recruited from January 2013 to January 2016 in the present study,
with a follow-up period of 36 months. The sex ratio of patients was
47:43 (male:female). NT was obtained by performing a hepatic
lobectomy in traumatic liver rupture. HCT was dissected from the
tissues, which were ≥4 cm from the bossing. PT was obtained 2 cm
away from the tumor tissue. HCWC was obtained directly from the
tumor tissue. All samples were double checked by the naked eye and
histological observation via hematoxylin and eosin (H&E)
staining. 1.5×1.5 cm tissues were frozen at −20°C via rapid
intraoperative freezing and then cut into 1.5 µm-thick sections for
H&E staining for 45 min at room temperature. Ten tissue samples
were obtained in each group. Tissues were, quickly stored in liquid
nitrogen for use in the following experiments.
Cell culture
The cholangiocarcinoma cell line QBC939 was
purchased from NanJing KeyGen Biotech Co., Ltd. (Nanjing, China)
and cultured with 90% RPMI 1640 medium supplemented with 10% fetal
bovine serum for 48 h at 37°C in an atmosphere containing 5%
CO2. When a monolayer in the culture flask formed, cells
were washed with PBS and digested with trypsin-EDTA (T4049;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 2 min. The cell
suspension was inoculated onto a culture plate for subsequent
experiments. Cells were divided into the following six groups:
Control group (no inhibitor treatment), 25 nM PF-2341066 (PZ0191;
Sigma-Aldrich; Merck KGaA) treatment group, 50 nM PF-2341066
treatment group, 100 nM PF-2341066 treatment group, 200 nM
PF-2341066 treatment group, 100 nM celecoxib (Y0001445;
Sigma-Aldrich; Merck KGaA) treatment group and 100 nM
PF-2341066+100 µM celecoxib treatment group. Cells were treated
with the appropriate agents at 37°C for 48 h and collected for
western blotting, reverse transcription-quantitative polymerase
chain reaction (RT-qPCR), flow cytometry and MTT assays.
Western blotting
c-Met, COX-2 and VEGF proteins were detected using
western blotting as previously described (11). Briefly, the cells were lysed with
radioimmunoprecipitation assay lysis buffer (cat. no. P0013C;
Beyotime Institute of Biotechnology, Haimen, China), total proteins
were extracted and concentration was determined using a BCA assay
(cat. no. P0009; Beyotime Institute of Biotechnology). A total of
50 µg proteins were loaded per lane and subjected to 10% SDS-PAGE
and electrotransferred to polyvinylidene fluoride membranes.
Membranes were rinsed with TBS for 10–15 min and blocked with 5%
(w/v) skimmed milk powder at room temperature for 1 h. Membranes
were subsequently incubated with the following primary antibodies:
Anti-c-Met (cat. no. ab51067), anti-β-actin (cat. no. ab8227),
anti-COX-2 (cat. no. ab62331) and anti-VEGF (cat. no. ab46154; all
Abcam, Cambridge, UK; all 1:1,000) at room temperature for 2 h and
rinsed with TBST three times for 5–10 min. The membrane was then
with a horseradish peroxidase-conjugated secondary antibody
(SV0002; Wuhan Boster Biological Technology, Ltd., Wuhan, China;
1:10,000) at room temperature for 1 h and rinsed with TBST three
times for 5–10 min. The protein bands were scanned and quantified
as a ratio to β-actin. Target bands were visualized using a BeyoECL
Plus kit (cat. no. P0018; Beyotime Institute of Biotechnology)
according to the manufacturers' protocol and quantitatively
analyzed using Quantity One software (version 4.6.2; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Detection of c-Met, COX-2 and VEGF
mRNA expression by RT-qPCR
Cells were washed with RNase free PBS. Total RNA was
extracted using RNeasy Mini kit (Qiagen GmbH, Hilden, Germany)
according to the manufacturers' protocol and the concentration and
purity were determined using a Qubit Fluorometer (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturers' protocol. A total of 1 µg RNA was reverse
transcribed at 42°C using a Reverse Transcription System kit (cat.
no. A3500; Promega Corp., Madison, WI, USA) in accordance with the
manufacturers' instructions. qPCR was performed using the SYBR
Green PCR Master Mix (Qiagen AB, Sollentuna, Sweden). The primers
used are listed in Table I.
Thermocycling conditions were as follows: 95°C for 5 min, followed
by 40 cycles of 95°C for 30 sec, 60°C for 45 sec and 72°C for 30
sec. The relative mRNA expression levels were normalized to GAPDH
and calculated using the 2−∆∆Cq method (14).
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Direction | Primer sequence
(3′-5′) |
|---|
| c-Met | Forward |
AGCGTCAACAGAGGGACCT |
|
| Reverse |
GCAGTGAACCTCCGACTGTATG |
| Cyclooxygenase-2 | Forward |
GATTGCCCGACTCCCTTGG |
|
| Reverse |
AAAACTGATGCGTGAAGTGCTG |
| Vascular endothelial
growth factor | Forward |
AGCCCATGAAGTGGTGAA |
|
| Reverse |
TGCGGATCTTGGACAAAC |
| GAPDH | Forward |
TGACTTCAACAGCGACACCCA |
|
| Reverse |
CACCCTGTTGCTGTAGCCAAA |
Detection of cell apoptosis using flow
cytometry
Cell apoptosis was determined by flow cytometry
using an Annexin V-fluorescein isothiocyanate (FITC)/propidium
iodide (PI) apoptosis detection kit (KGA106; Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China) according to the manufacturer's
protocol. Briefly, the cell suspension was washed with PBS and
centrifuged at 1,000 × g for 5 min at 4°C. Cells were resuspended
with binding buffer as provided by the kit and incubated at room
temperature for 10–15 min. A total of 5 µl Annexin V-FITC was added
and mixed. Subsequently, 5 µl PI was added and incubated at room
temperature for 5–15 min. Results were detected using a flow
cytometer.
Cell proliferation detection
Cell proliferation was assessed using an MTT assay.
Cells were seeded in a 96-well plate at a density of
1×104 cells/well and plates were cultivated in an
incubator for 24 h at 37°C. A total of 50 µl 1X MTT (KGA311;
NanJing KeyGen Biotech Co., Ltd.) was added to each well and cells
were incubated for 4 h at 37°C. The culture medium was discarded
and 150 µl dimethyl sulfoxide was added to each well. The plates
were shaken for 10 min and the optical density (OD) was measured
using a microplate reader at 550 nm. The cell growth inhibition
rate=(1-mean OD value of treatment group/mean OD value of control
group) ×100.
Statistical analysis
Data are presented as the mean + standard deviation
and were analyzed using SPSS 20.0 software (IBM Corp., Armonk, NY,
USA). One-way analysis of variance was used to evaluate the
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
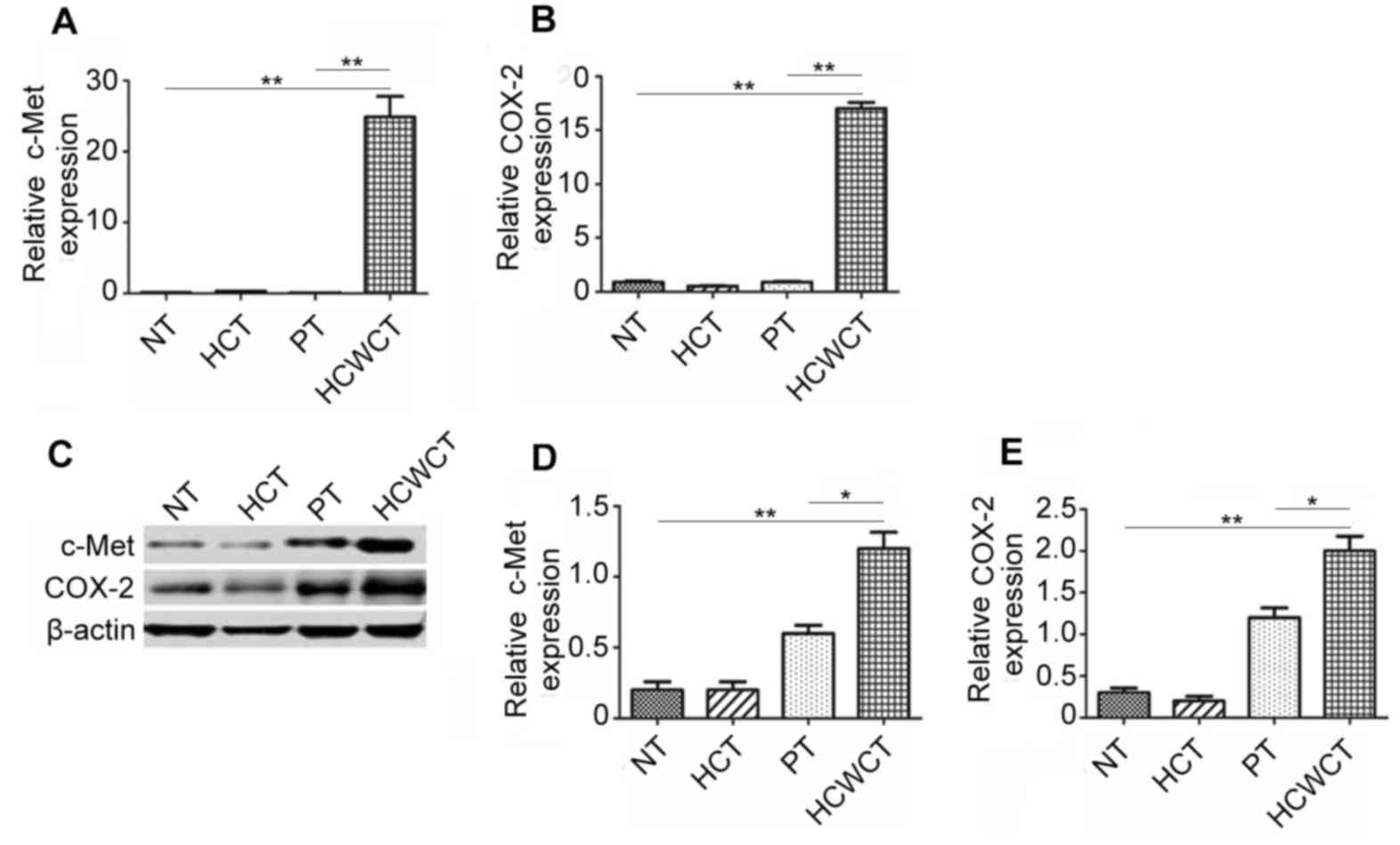
c-Met and COX-2 were highly expressed
in hepatobiliary calculi with cholangiocarcinoma tissue
To investigate the expression of c-Met and COX-2 in
cholangiocarcinoma, gene expression in NT, HCT, PT and HCWCT was
measured using RT-qPCR. The results demonstrated that c-Met and
COX-2 expression was significantly higher in HCWCT compared with NT
and PT (Fig. 1A and B). Western
blotting was performed to measure the expression of c-Met and COX-2
at the protein level and it was demonstrated that they were
significantly upregulated in HCWCT compared with NT and PT
(Fig. 1C-E). These results indicate
that c-Met and COX-2 may serve a role in the development of
cholangiocarcinoma.
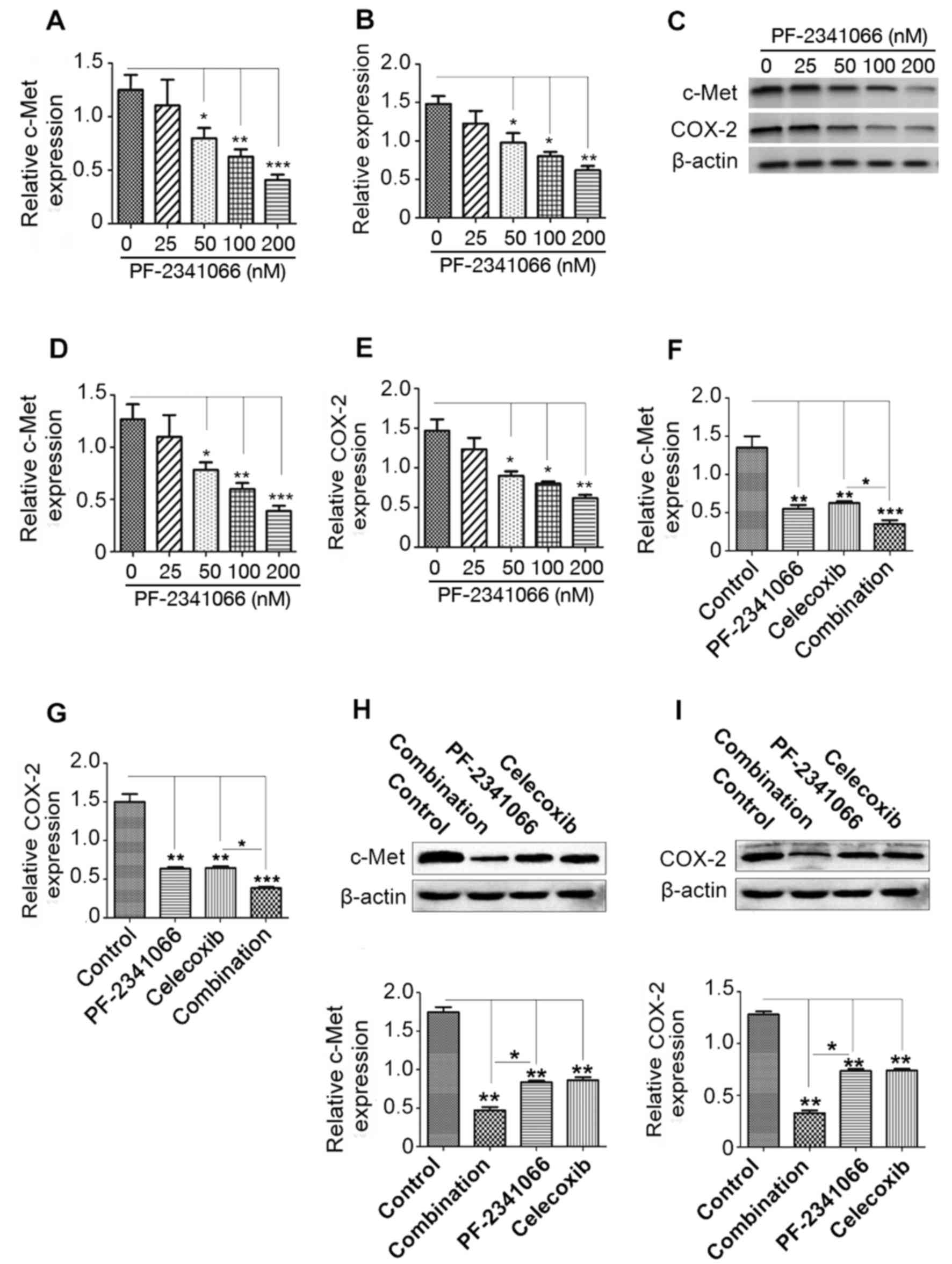
PF-2341066 and celecoxib inhibit the
expression of c-Met and COX-2 in a concentration-dependent manner
in QBC939 cells
Human cholangiocarcinoma QBC939 cells were treated
with PF-2341066 and celecoxib. The results demonstrated that
treatment with PF-2341066 at a dose of 50 nM or higher
significantly inhibited the expression of c-Met and COX-2 at the
mRNA level (Fig. 2A and B) in a
dose-dependent manner. Western blotting revealed a similar effect
at the protein level; treatment with ≥50 nM PF-2341066
significantly downregulated c-Met and COX-2 expression compared
with 0 nM (Fig. 2C-E). Combined
treatment with PF-2341066 and celecoxib significantly suppressed
the expression of COX-2 and c-Met, and this suppression was
significantly greater than that observed with PF-2341066 treatment
along (Fig. 2F-I). These results
suggest that combined treatment with PF-2341066 and celecoxib
inhibits the expression of c-Met and COX-2 at the transcription and
translation levels and inhibition is improved by combined
treatment.
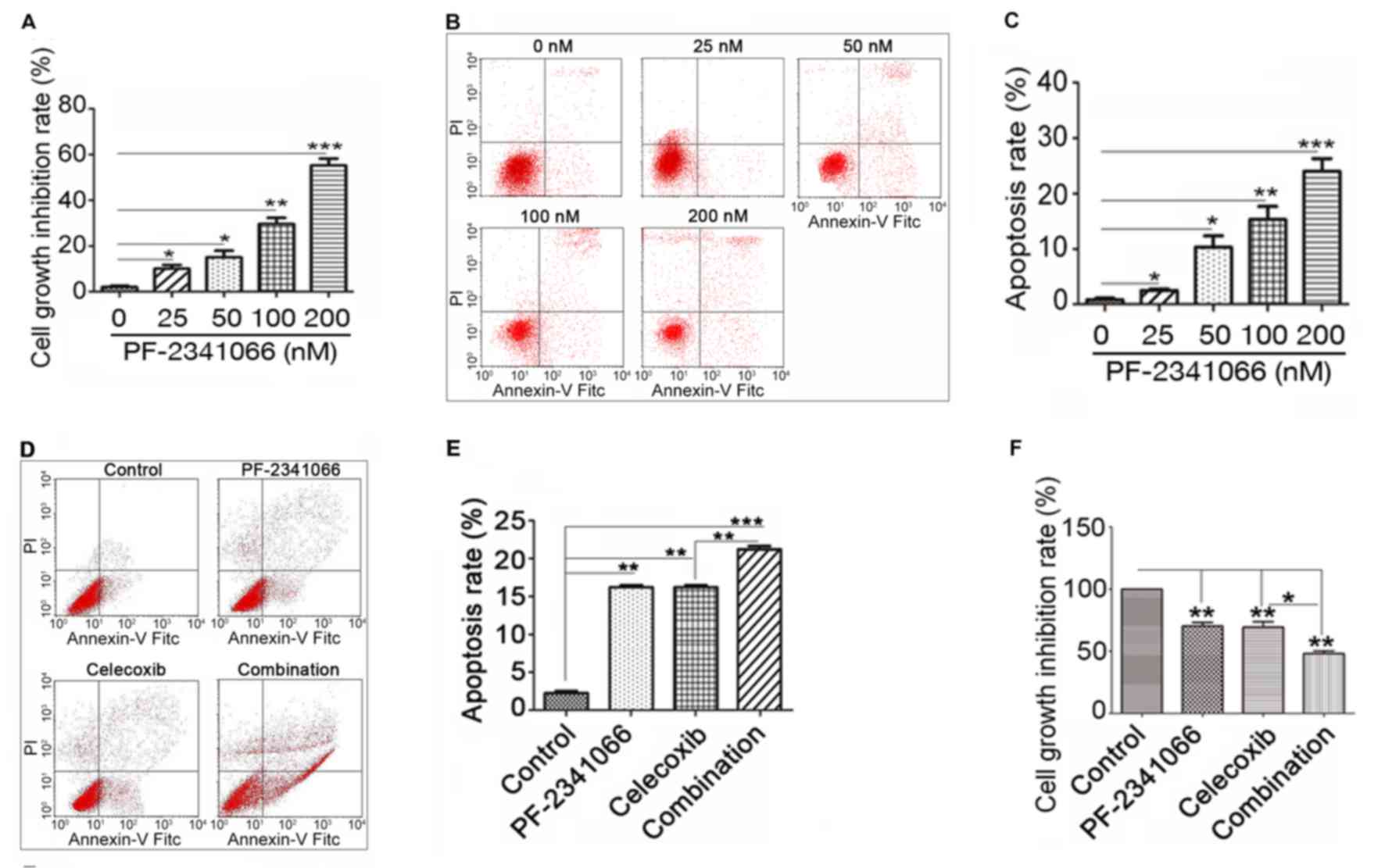
PF-2341066 and celecoxib inhibit cell
proliferation and promote cell apoptosis
The results described above indicated that
PF-2341066 and celecoxib may inhibit cholangiocarcinoma by
downregulating the expression of c-Met and COX-2. To further
investigate this, the proliferation and apoptosis of QBC939 cells
were measured following treatment with PF-2341066 and celecoxib.
The results demonstrated that PF-2341066 significantly inhibits
cell proliferation in a concentration-dependent manner (Fig. 3A) and promotes apoptosis in a
dose-dependent manner (Fig. 3B and
C). It was also observed that the increase in cell apoptosis
was significantly greater with combined PF-2341066 and celecoxib
treatment compared with PF-2341066 alone (Fig. 3D and E). In addition, combined
treatment with PF-2341066 and celecoxib significantly increased
cell growth inhibition compared with PF-2341066 alone (Fig. 3F). These results suggest that
PF-2341066 and celecoxib inhibit proliferation and promote
apoptosis in QBC939 cells and thereby may have potential as a
therapeutic treatment for hepatobiliary calculus with
cholangiocarcinoma.
VEGF expression was inhibited by
PF-2341066 and celecoxib treatment
VEGF promotes tumor neovascularization, thereby
providing a matrix for the migration of vascular endothelial cells
and promoting tumor metastasis (15). To investigate whether PF-2341066 and
celecoxib serve a role in inhibiting angiogenesis and metastasis in
cholangiocarcinoma, the expression of VEGF in QBC939 cells was
measured following treatment with PF-2341066 and celecoxib. VEGF
mRNA and protein expression was downregulated by PF-2341066 in a
dose-dependent manner (Fig. 4A and
B). Furthermore, this inhibition was significantly increased by
combined treatment with PF-2341066 and celecoxib (Fig. 4C and D). These results suggest that
PF-2341066 and celecoxib may restrict the development of
cholangiocarcinoma by suppressing VEGF-mediated angiogenesis and
tumor metastasis.
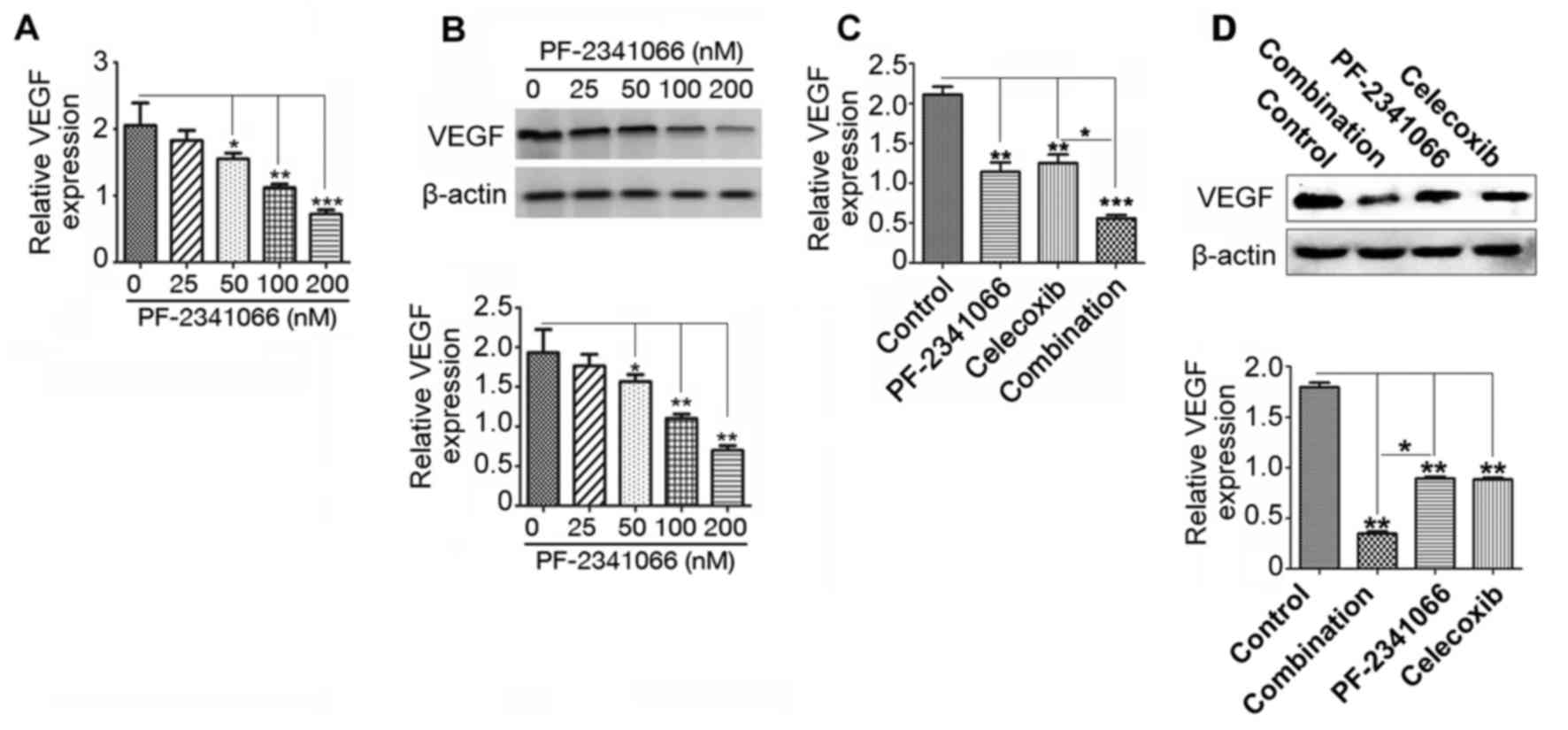 | Figure 4.Expression of VEGF in QBC939 cells
following treatment with PF-2341066 and celecoxib. Following
treatment with 0, 25, 50, 100 and 200 nM PF-2341066, VEGF (A) mRNA
and (B) protein expression was measured using RT-qPCR and western
blotting, respectively. Cells were treated with control, 100 nM
PF-2341066, 100 µM celecoxib or 100 nM PF-2341066 + 100 µM
celecoxib and VEGF (C) mRNA and (D) protein expression was assessed
using RT-qPCR and western blotting, respectively. *P<0.05,
**P<0.01 and ***P<0.001. VEGF, vascular endothelial growth
factor; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; Combination, treatment with 100 nM PF-2341066 + 100
µM celecoxib. |
Discussion
Previous studies have demonstrated that c-Met is
expressed in colon cancer, gastric cancer, esophageal cancer,
pancreatic cancer, thyroid cancer and medulloblastoma (16,17).
c-Met expression is positively correlated with HGF, tumor
angiogenesis and poor clinical outcome in patients with tumors
(18). c-Met expression was
increased in tumor tissues compared with NT and may activate the
downstream signal transduction pathway to affect proliferation,
differentiation and apoptosis in tumor cells (19). In the present study it was revealed
that c-Met is highly expressed in HCWC compared with NT, which
suggests that c-Met may serve an important role in the development
of HCWC. A previous study reported that c-Met is an important
treatment target for cholangiocarcinoma (5), which supports the results herein. The
c-Met inhibitor PF-2341066 is an ATP competitive small molecule
compound; it has good biological availability and high water
solubility (4). PF-2341066 is able
to inhibit the c-Met signaling pathway by suppressing c-Met
phosphorylation (4). In the present
study, it was also demonstrated to inhibit c-Met expression at the
mRNA and protein levels in QBC939 cells. According to the results
of the present study, PF-2341066 significantly inhibited cell
proliferation and promoted cell apoptosis. Thus suggests that
PF-2341066 may restrict the development of HCWC by downregulating
c-Met and its downstream signaling.
COX is a major rate limiting enzyme in the synthesis
of prostaglandin and is able to metabolize arachidonic acid into a
variety of prostaglandin products, including prostaglandin E2, to
induce a number of biological effects. It has previously been
reported that high COX-2 expression is associated with the
occurrence of malignant tumors and precancerous lesions (20,21),
including breast cancer (22), colon
cancer (23), lung cancer (24), gastric cancer (25) and esophageal cancer (26). The selective COX-2 inhibitor
celecoxib is a non-steroidal anti-inflammatory agent that is able
to inhibit tumor cell proliferation and induce apoptosis (27,28). The
results of the present study revealed that celecoxib significantly
downregulates the expression of COX-2 at the mRNA and protein
levels. When QBC939 cells were co-treated with celecoxib and
PF-2341066, the effects on cell proliferation and apoptosis were
greater than those observed with PF-2341066 alone. It may therefore
by hypothesized that combined treatment with celecoxib and
PF-2341066 may inhibit the development of HCWC by regulating the
c-Met and COX-2-mediated common pathway or the cross-talk between
these two pathways.
It is possible to inhibit the growth and metastasis
of tumors by targeting and reducing tumor angiogenesis. Tumor
angiogenesis is a multi-step process and blocking any one of the
steps in this process, including inhibiting angiogenesis factor
binding, destruction of the adhesion model or blocking the signal
transduction pathway may prevent the formation of blood vessels in
the tumor (29). VEGF was first
identified in 1989 by Go Spodarwia; it was similar to vascular
permeability factor and was able to promote the mitosis of vascular
endothelial cells (30). VEGF
promotes the proliferation of vascular endothelial cells, increases
vascular permeability and maintains the integrity of blood vessels
by binding to its receptor (15,31,32). In
the present study, it was demonstrated that PF-2341066 alone and in
combination with celecoxib may inhibit the expression of VEGF at
the transcription and translation levels in QBC939 cells. The
results of the present study suggest that PF-2341066 and celecoxib
may be an effective treatment for HCWC, which functions by
restricting tumor angiogenesis.
Acknowledgements
The authors thank the nurses at the Hunan Provincial
People's Hospital (Changsha, China) for their assistance in the
collection of samples.
Funding
This work was supported by the research fund of
Hunan provincial health and Family Planning Commission (grant no.
C2016005) and the scientific research fund of Changsha Municipal
Science and Technology Bureau (grant no. k15zd006-34).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CCh designed the research, performed the
experiments, analyzed the data and wrote the manuscript. DY
designed and directed the research. QZ, LL and CCa performed the
experiments and analyzed data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was performed in strict accordance
with the approval and recommendations of the Committee for Care and
Use in Clinical Study of Hunan Provincial People's Hospital
(C2016005). All patients provided written informed consent.
Consent for publication
The patients have provided written informed consent
for the publication of all data and accompanying images.
Competing interests
The authors declare no conflict of interest.
References
|
1
|
Haga H, Yan IK, Takahashi K, Wood J,
Zubair A and Patel T: Tumour cell-derived extracellular vesicles
interact with mesenchymal stem cells to modulate the
microenvironment and enhance cholangiocarcinoma growth. J Extracell
Vesicles. 4:249002015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang WG, Martin TA, Parr C, Davies G,
Matsumoto K and Nakamura T: Hepatocyte growth factor, its receptor,
and their potential value in cancer therapies. Crit Rev Oncol
Hematol. 53:35–69. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sandler AB and Dubinett SM: COX-2
inhibition and lung cancer. Semin Oncol. 31 2 Suppl 7:S45–S52.
2004. View Article : Google Scholar
|
|
4
|
Zou HY, Li Q, Lee JH, Arango ME, McDonnell
SR, Yamazaki S, Koudriakova TB, Alton G, Cui JJ, Kung PP, et al: An
orally available small-molecule inhibitor of c-met, PF-2341066,
exhibits cytoreductive antitumor efficacy through antiproliferative
and antiangiogenic mechanisms. Cancer Res. 67:4408–4417. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Socoteanu MP, Mott F, Alpini G and Frankel
AE: c-Met targeted therapy of cholangiocarcinoma. World J
Gastroenterol. 14:2990–2994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gandou C, Harada K, Sato Y, Igarashi S,
Sasaki M, Ikeda H and Nakanuma Y: Hilar cholangiocarcinoma and
pancreatic ductal adenocarcinoma share similar histopathologies,
immunophenotypes, and development-related molecules. Hum Pathol.
44:811–821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrucci A, Moschetta M, Frassanito MA,
Berardi S, Catacchio I, Ria R, Racanelli V, Caivano A, Solimando
AG, Vergara D, et al: A HGF/cMET autocrine loop is operative in
multiple myeloma bone marrow endothelial cells and may represent a
novel therapeutic target. Clin Cancer Res. 20:5796–5807. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klenke FM, Gebhard MM, Ewerbeck V,
Abdollahi A, Huber PE and Sckell A: The selective Cox-2 inhibitor
Celecoxib suppresses angiogenesis and growth of secondary bone
tumors: An intravital microscopy study in mice. BMC Cancer.
6:92006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou YY, Hu ZG, Zeng FJ and Han J:
Clinical profile of cyclooxygenase-2 inhibitors in treating
non-small cell lung cancer: A meta-analysis of nine randomized
clinical trials. PLoS One. 11:e01519392016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clapéron A, Mergey M, Nguyen Ho-Bouldoires
TH, Vignjevic D, Wendum D, Chrétien Y, Merabtene F, Frazao A,
Paradis V, Housset C, et al: EGF/EGFR axis contributes to the
progression of cholangiocarcinoma through the induction of an
epithelial-mesenchymal transition. J Hepatol. 61:325–332. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hung TH, Li YH, Tseng CP, Lan YW, Hsu SC,
Chen YH, Huang TT, Lai HC, Chen CM, Choo KB and Chong KY: Knockdown
of c-MET induced apoptosis in ABCB1-overexpressed
multidrug-resistance cancer cell lines. Cancer Gene Ther.
22:262–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marti P, Stein C, Blumer T, Abraham Y,
Dill MT, Pikiolek M, Orsini V, Jurisic G, Megel P, Makowska Z, et
al: YAP promotes proliferation, chemoresistance, and angiogenesis
in human cholangiocarcinoma through TEAD transcription factors.
Hepatology. 62:1497–1510. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang YK, Muro K, Ryu MH, Yasui H, Nishina
T, Ryoo BY, Kamiya Y, Akinaga S and Boku N: A phase II trial of a
selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a
second- or third-line therapy in the patients with metastatic
gastric cancer. Invest New Drugs. 32:355–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rizvi S and Gores GJ: Pathogenesis,
diagnosis, and management of cholangiocarcinoma. Gastroenterology.
145:1215–1229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patel MB, Pothula SP, Xu Z, Lee AK,
Goldstein D, Pirola RC, Apte MV and Wilson JS: The role of the
hepatocyte growth factor/c-MET pathway in pancreatic stellate
cell-endothelial cell interactions: Antiangiogenic implications in
pancreatic cancer. Carcinogenesis. 35:1891–1900. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park HJ, Kim K, Paik JH, Chie EK, Kim S,
Jang JY, Kim SW, Han SW, Oh DY, Im SA, et al: Is c-Met oncoprotein
expression an adverse prognosticator in extrahepatic bile duct
cancer treated with curative resection followed by adjuvant
chemoradiotherapy? Clin Transl Oncol. 18:625–631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu GS, Zou SQ, Liu ZR, Tang ZH and Wang
JH: Celecoxib inhibits proliferation and induces apoptosis via
prostaglandin E2 pathway in human cholangiocarcinoma cell lines.
World J Gastroenterol. 9:1302–1306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hosomi Y, Yokose T, Hirose Y, Nakajima R,
Nagai K, Nishiwaki Y and Ochiai A: Increased cyclooxygenase 2
(COX-2) expression occurs frequently in precursor lesions of human
adenocarcinoma of the lung. Lung Cancer. 30:73–81. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soslow RA, Dannenberg AJ, Rush D, Woerner
BM, Khan KN, Masferrer J and Koki AT: COX-2 is expressed in human
pulmonary, colonic, and mammary tumors. Cancer. 89:2637–2645. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ristimäki A, Sivula A, Lundin J, Lundin M,
Salminen T, Haglund C, Joensuu H and Isola J: Prognostic
significance of elevated cyclooxygenase-2 expression in breast
cancer. Cancer Res. 62:632–635. 2002.PubMed/NCBI
|
|
23
|
Tsujii M, Kawano S and DuBois RN:
Cyclooxygenase-2 expression in human colon cancer cells increases
metastatic potential. Proc Natl Acad Sci USA. 94:3336–3340. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hida T, Yatabe Y, Achiwa H, Muramatsu H,
Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T and Takahashi
T: Increased expression of cyclooxygenase 2 occurs frequently in
human lung cancers, specifically in adenocarcinomas. Cancer Res.
58:3761–3764. 1998.PubMed/NCBI
|
|
25
|
Ristimäki A, Honkanen N, Jänkälä H,
Sipponen P and Härkönen M: Expression of cyclooxygenase-2 in human
gastric carcinoma. Cancer Res. 57:1276–1280. 1997.PubMed/NCBI
|
|
26
|
Zimmermann KC, Sarbia M, Weber AA,
Borchard F, Gabbert HE and Schrör K: Cyclooxygenase-2 expression in
human esophageal carcinoma. Cancer Res. 59:198–204. 1999.PubMed/NCBI
|
|
27
|
Alshafie GA, Abou-Issa HM, Seibert K and
Harris RE: Chemotherapeutic evaluation of Celecoxib, a
cyclooxygenase-2 inhibitor, in a rat mammary tumor model. Oncol
Rep. 7:1377–1381. 2000.PubMed/NCBI
|
|
28
|
Leahy KM, Ornberg RL, Wang Y, Zweifel BS,
Koki AT and Masferrer JL: Cyclooxygenase-2 inhibition by celecoxib
reduces proliferation and induces apoptosis in angiogenic
endothelial cells in vivo. Cancer Res. 62:625–631. 2002.PubMed/NCBI
|
|
29
|
Balansky R, Ganchev G, Iltcheva M, Nikolov
M, La Maestra S, Micale RT, D'Agostini F, Steele VE and De Flora S:
Modulation by licofelone and celecoxib of experimentally induced
cancer and preneoplastic lesions in mice exposed to cigarette
smoke. Curr Cancer Drug Targets. 15:188–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Couffinhal T, Kearney M, Witzenbichler B,
Chen D, Murohara T, Losordo DW, Symes J and Isner JM: Vascular
endothelial growth factor/vascular permeability factor (VEGF/VPF)
in normal and atherosclerotic human arteries. Am J Pathol.
150:1673–1685. 1997.PubMed/NCBI
|
|
31
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee SH, Jeong D, Han YS and Baek MJ:
Pivotal role of vascular endothelial growth factor pathway in tumor
angiogenesis. Ann Surg Treat Res. 89:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|