Introduction
Currently, glaucoma is an irreversible blinding eye
disease, ranking first in the world. As of 2020, more than 10
million people around the world have suffered from binocular
blindness due to glaucoma. The success rate of filtering operation
of refractory glaucoma is only 11–52%, which is much lower than
that of conventional glaucoma operation (70–90%) (1–4).
Since Molteno invented the glaucoma drainage device
in 1930, new progress has been made in the treatment of refractory
glaucoma (5). On the basis of its
drainage principle and structure, some new drainage devices have
also emerged; these implantation materials have common
characteristics in structure: A flexible silicone tube and an
implantation material in the equatorial part of the eyeball, whose
common mechanism is that the aqueous humor enters the fiber cavity
formed around the implantation material from anterior chamber via
silicone tube under the pressure action, and is then absorbed by
blood capillaries and lymphatic vessels (6). Ahmed glaucoma valve (AGV) is one of
such devices, which significantly improves the success rate of
refractory glaucoma operation, and has now become a recognized
effective treatment method of refractory glaucoma. However, it has
shortcomings of complex operation, low success rate and many
postoperative complications, so AGV is always applied
cautiously.
Ex-PRESS implant (Ex-PRESS) is a small, stainless
steel and splitless glaucoma drainage valve, as well as one of the
recently-developed minimally-invasive anti-glaucoma filtering
techniques (7). Ex-PRESS is placed
under the scleral flap, similar to the traditional trabeculectomy,
and it also avoids the removal of sclera and iris required in the
trabeculectomy (8). Its theoretical
advantages lie in the simplicity and repeatability of operation,
and reduction of eye tissue injury. In 2002, upon the approval of
Food and Drug Administration (FDA), Ex-PRESS glaucoma drainage
device began to be widely used in clinical practice. Ex-PRESS was
introduced to China in 2012.
Since Ex-PRESS was widely used in clinical practice,
many clinical studies in China and worldwide have compared the
treatment effect of Ex-PRESS implantation and trabeculectomy on
open-angle glaucoma (9–14), but there is a lack of clinical data
on curative effects on secondary glaucoma, refractory glaucoma, so
further clinical observation is needed. In this study, therefore,
the intraocular pressure (IOP) control, success rate of operation,
complications, after Ex-PRESS implantation and AGV implantation in
the treatment of refractory glaucoma were observed to evaluate the
effectiveness and safety of Ex-PRESS implantation in the treatment
of refractory glaucoma.
Patients and methods
General data
A total of 68 patients (76 eyes) with refractory
glaucoma treated in Zaozhuang Ophthalmologic Hospital (Zaozhuang,
China) from January to December 2016 were enrolled, and they were
informed of this study according to the Declaration of Helsinki and
signed the informed consent. They were randomly divided into
Ex-PRESS group [n=33 (38 eyes), implanted with Ex-PRESS implant]
and AGV group [n=35 (38 eyes), implanted with AGV]. The operation
was performed by the same treatment group. In Ex-PRESS group [n=33
(38 eyes)], there were 17 males (17 eyes) and 16 females (21 eyes)
aged 19–45 years with an average age of 51.61±13.76 years. In AGV
group [n=35 (38 eyes)], there were 20 males (23 eyes) and 15
females (15 eyes) aged 22–78 years with an average age of
55.27±13.34 years. This study was approved by the Ethics Committee
of Zaozhuang Ophthalmologic Hospital. Signed written informed
consents were obtained from the patients and/or guardians.
Inclusion and exclusion criteria
All patients were aged above 18 years with IOP
>21 mmHg. The postoperative follow-up time was less than 9
months, Ex-PRESS group: 11.84±1.77 months, AGV group: 12.88±1.88
months; IOP could not be controlled via systemic and local glaucoma
drugs, laser and surgical treatment. Patients with obvious
conjunctival scars, adhesions, receiving cyclophotocoagulation,
drainage nail or drainage valve implantation were excluded.
IOP measurement
IOP was measured using slit lamp Goldmann tonometer
for at least 3 times and the average was taken.
Visual acuity
Best corrected visual acuity (BCVA): Logarithmic
visual acuity was converted into LogMAR visual acuity (Table I).
 | Table I.Conversion table of logarithmic visual
acuity and LogMAR. |
Table I.
Conversion table of logarithmic visual
acuity and LogMAR.
| Logarithmic visual
acuity | LogMAR | Logarithmic visual
acuity | LogMAR |
|---|
| 0.01 | 2.0 | 0.25 | 0.6 |
| 0.02 | 1.7 | 0.3 | 0.5 |
| 0.03 | 1.5 | 0.4 | 0.4 |
| 0.05 | 1.3 | 0.5 | 0.3 |
| 0.1 | 1.0 | 0.6 | 0.2 |
| 0.15 | 0.8 | 0.8 | 0.1 |
| 0.2 | 0.7 | 1.0 | 0 |
Corneal endothelial cell count
The corneal endothelial cell counts
(n/mm2) before operation and at 3, 6 and 9 months after
operation were obtained using the non-contact corneal endothelial
cell counter.
Implantation materials
Ex-PRESS (Alcon Laboratories, Worth, TX, USA) is a
new type of aqueous drainage device consisting of a valve-free
casing pipe for drainage of aqueous fluid and a protective device
preventing the displacement of drainage device. The material is the
same stainless steel as the artificial cardiac valve, which has a
good biocompatibility with the body. P50 Ex-PRESS with an inner
diameter of 50 µm was used in the present study.
FP7 AGV is composed of drainage tube and drainage
disk. Silicone drainage tube is 0.63 mm in outer diameter, 0.30 mm
in inner diameter, and 25 mm in length; the area of drainage disk
is ~184 mm2 (13×18 mm) and it is oval and made of
polypropylene material. A silicone elastomer valve connects the
silicone tube and drainage disk. The silicone elastomer valve is a
one-way pressure-sensitive valve that can provide resistance to the
outflow of aqueous fluid [open pressure of 1.06–1.33 kPa (8–10
mmHg)].
Surgical methods
Ex-PRESS implantation procedures
Under subconjunctival infiltration anesthesia, the
conjunctival flap (diameter of 3 × 4 mm and thickness of l/2 of
scleral flap) with the dome as the base was made, the 0.4 mg/ml
mitomycin C (MMC) cotton was placed under the scleral flap and
conjunctival flap for 3 min, and then they were washed with 20 ml
normal saline. The 25G needle was punctured into anterior chamber
from the scleral flap corneal limbus gray line parallel to the iris
surface, part of aqueous fluid was replaced with viscoelastic
substance, and the Ex-PRESS drainage device was implanted. The two
angle ends and both sides of scleral flap were sutured using 10-0
suture in an adjustable way for a total of 4 needles; the bulbar
conjunctiva at the corneal limbus was sutured for 2 needles.
Tobradex eye drops and ointment were applied locally after
operation.
AGV implantation procedures
Under retrobulbar anesthesia with 1.5 ml 2%
lidocaine, the conjunctival flap with nasal or supertemporal
quadrant as the base was selected; the bulbar conjunctiva and
subconjunctival tissues were fully separated and the sclera was
exposed. Scleral flap (4×5 mm, 1/2 thickness) was made using
drainage tube into the anterior chamber. The 0.4 mg/ml MMC cotton
was placed under scleral flap and conjunctival flap for 3 min, and
then they were washed with 20 ml normal saline. Before AGV
implantation, the normal saline was injected via drainage tube to
ensure the smooth valve. The drainage disk was placed on the sclera
between two pieces of rectus muscles across the equatorial part of
eyeball. The anterior edge of drainage disk was ~13 mm away from
the corneal limbus. The drainage valve was sutured with 10-0 line
in sclera. The no. 7 needle was inserted into the anterior chamber
via corneoscleral limbus parallel to the iris. The drainage tube
was trimmed to incline 45° upward into the anterior chamber for 2–3
mm. It was parallel to the iris, but did not contact the corneal
endothelium and iris. The scleral flap was sutured using 10-0 line
for 2 needles and the bulbar conjunctiva at the corneal limbus was
sutured using 10-0 line for 2 needles. Tobradex eye drops and
ointment were applied locally after operation.
Treatment of complications
In case of complications, drug therapy was preferred
based on conditions; if failed, the remedial operative treatment
was performed for complications.
Evaluation criteria of operation
Based on literatures in China and worldwide, the
evaluation criteria of operation results were summarized as
follows: relative success: 5 mmHg <IOP <21 mmHg, with or
without local application of glaucoma drugs; complete success: 5
mmHg < IOP <21 mmHg, no application of any adjuvant drugs
after operation (1). Criteria of
operation failure: IOP >21 mmHg or IOP <6 mmHg; anti-glaucoma
surgery was needed again (cyclophotocoagulation or filtering
surgery and enucleation of eyeball), the drainage implant needed to
be removed or re-implanted, or severe complications (including
endophthalmitis, chronic ocular hypotension, malignant glaucoma,
retinal detachment and severe choroidal detachment), loss of light
sensation or atrophy of eyeball occurred.
Statistical analysis
SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) software
was used for statistical treatment. The independent-samples t-test
was used for the comparisons of age, preoperative IOP, preoperative
and postoperative BCVA, corneal endothelial cell count,
postoperative IOP and number of glaucoma drugs applied between the
two groups. Chi-square test was used for the comparisons of
glaucoma type, sex and postoperative complications between the two
groups. Besides, Kaplan-Meier survival curve and log-rank test were
adopted for the comparison of success rate of operation between the
two groups. A P<0.05 was considered to indicate a statistically
significant diference.
Results
General conditions before
operation
There were no significant differences in sex, age,
preoperative IOP, preoperative BCVA, corneal endothelial cell
count, number of glaucoma drugs applied and history of eye
operation between Ex-PRESS group and AGV group (P>0.05). In
Ex-PRESS group, there were 13 eyes with neovascular glaucoma, 9
with traumatic glaucoma, 8 receiving failed glaucoma filtering
operation repeatedly, and 8 with secondary glaucoma to
inflammation. In AGV group, there were 15 eyes with neovascular
glaucoma, 4 with traumatic glaucoma, 14 receiving failed glaucoma
filtering operation repeatedly, 4 with secondary glaucoma to
inflammation and 1 receiving keratoplasty. There was no significant
difference in glaucoma type between the two groups of patients
(P>0.05). The results are shown in Table II.
 | Table II.Baseline demographic and clinical
characteristics of the patients. |
Table II.
Baseline demographic and clinical
characteristics of the patients.
| Parameters | Ex-PRESS group
(n=38) | AGV group (n-38) | P-value |
|---|
| Sex, n (%) |
|
| 0.192 |
| Male | 17 (44.7) | 23 (60.5) |
|
|
Female | 21 (55.3) | 15 (39.5) |
|
| Age (years) | 51.61±13.76 | 55.27±13.34 | 0.246 |
| Follow-up time
(months) | 11.84±1.77 | 12.08±1.84 | 0.57 |
| LogMAR | 0.87±0.53 | 1.12±0.56 | 0.063 |
| IOP (mmHg) | 36.33±8.02 | 34.13±7.35 | 0.216 |
| CECC | 2068±270 | 1947±277 | 0.058 |
| Glaucoma drugs | 3.53±0.86 | 3.24±0.88 | 0.152 |
| Diagnosis, n (%) |
|
| 0.197 |
| NVG | 13 (34.2) | 15 (39.5) |
|
| Traumatic
glaucoma | 9 (23.7) | 4 (10.5) |
|
| Uveitic
glaucoma | 8 (21.1) | 4 (10.5) |
|
| Filtration surgery
failed, n (%) |
|
|
|
| POAG | 8 (21.1) | 14 (36.8) |
|
| PKP
glaucoma | 0 (0) | 1 (2.6) |
|
| Previous surgery, n
(%) |
|
| 0.463 |
|
Trabeculectomy | 23 (60.5) | 27 (71.1) |
|
|
Vitrectomy | 6 (15.8) | 3 (7.9) |
|
|
Keratoplasty | 0 (0) | 1 (2.6) |
|
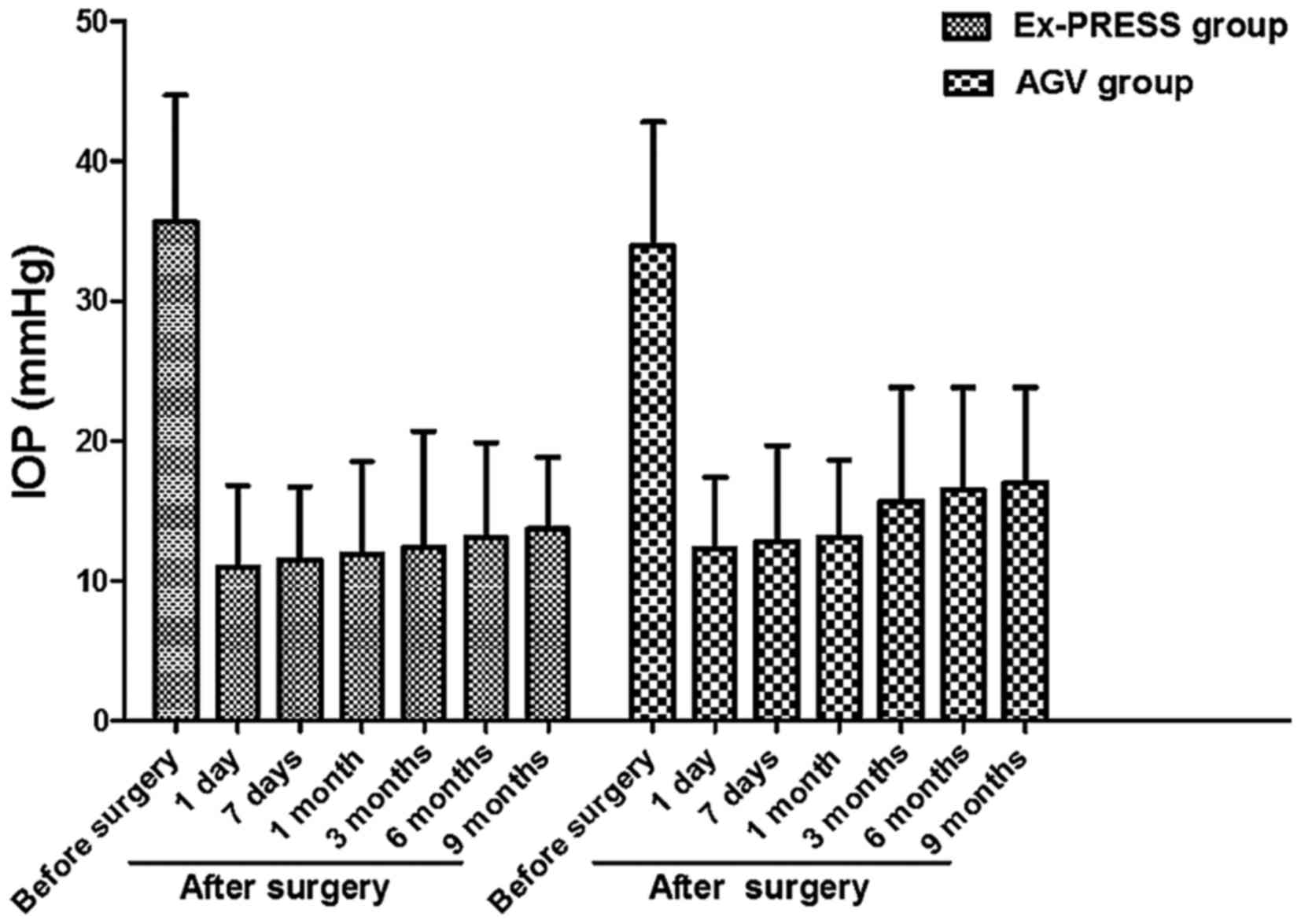
Comparison of average IOP between the
two groups before and after operation
At 9 months after operation, IOP was decreased from
35.6±9.1 to 13.7±5.1 mmHg in Ex-PRESS group, and from 33.9±8.9 to
17.0±6.8 mmHg in AGV group. IOP in both groups after operation was
significantly decreased compared with that before operation
(P<0.05). At 3, 6 and 9 months after operation, IOP in Ex-PRESS
group was obviously lower than that in AGV group (P<0.05)
(Fig. 1).
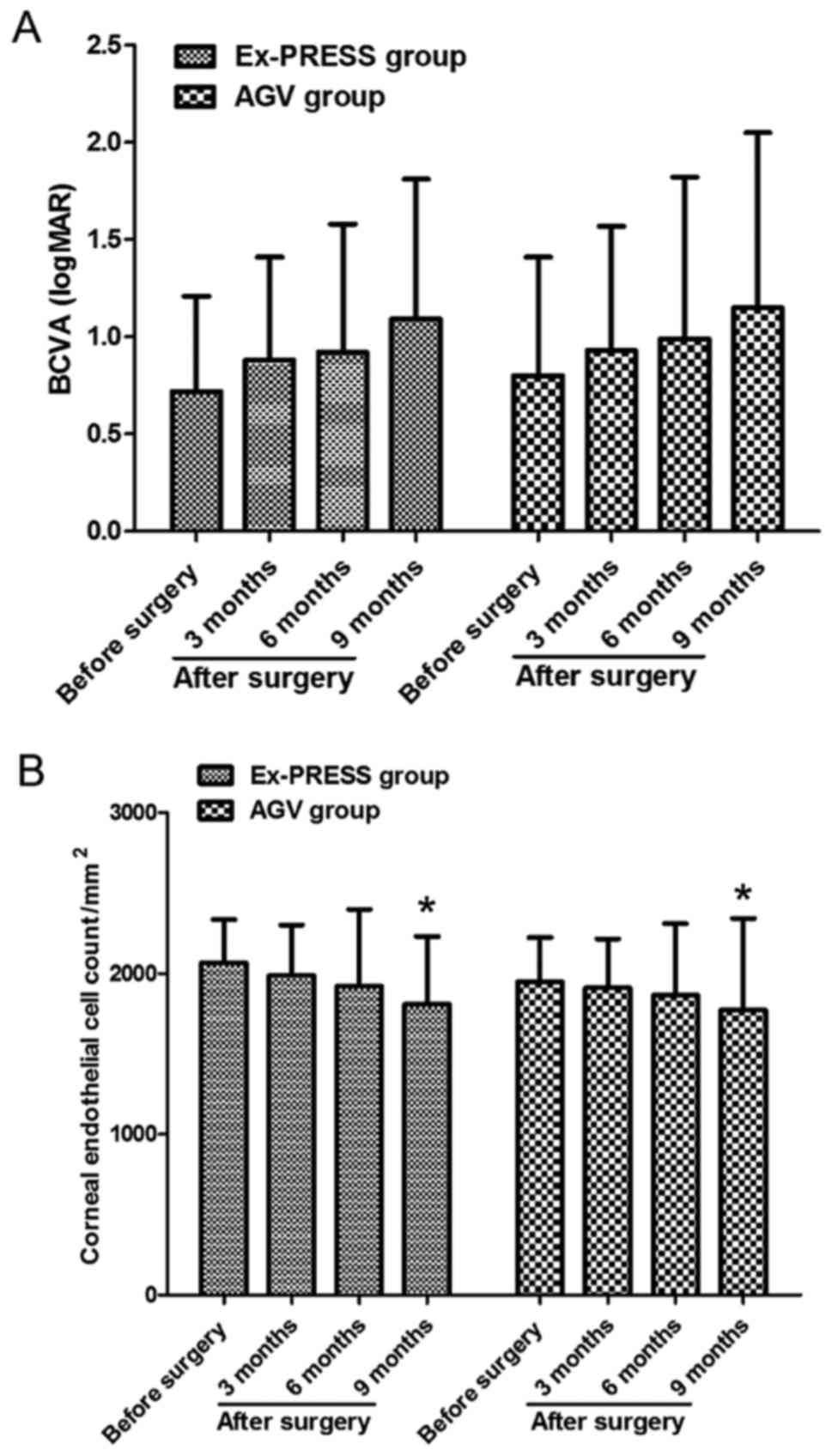
Comparisons of BCVA and corneal
endothelial cell counts between the two groups before and after
operation
In Ex-PRESS group, BCVA was 0.72±0.49 logMAR and the
corneal endothelial cell count was 2068±270/mm2 before
operation; BCVA was 1.09±0.72 logMAR and the corneal endothelial
cell count was 1809±423/mm2 at the end of follow-up. In
AGV group, BCVA was 0.80±0.61 logMAR and the corneal endothelial
cell count was 1947±277/mm2 before operation; BCVA was
1.15±0.90 logMAR and the corneal endothelial cell count was
1775±569/mm2 at the end of follow-up. BCVA in both
groups of patients at the end of follow-up was decreased compared
with that before operation, but the differences were not
statistically significant (P>0.05). Besides, the corneal
endothelial cell counts in both groups of patients at the end of
follow-up were also decreased compared with those before operation,
and the differences were statistically significant (P<0.05). At
the end of follow-up, BCVA and corneal endothelial cell count had
no statistically significant differences between the two groups of
patients (P>0.05) (Fig. 2).
Application of anti-glaucoma drugs in
the two groups before and after operation
At 9 months after operation, the number of
anti-glaucoma drugs applied was decreased from 3.53±0.86 to
0.55±0.60 in Ex-PRESS group, and from 3.24±0.88 to 0.89±0.73 in AGV
group; the number of anti-glaucoma drugs applied in Ex-PRESS group
was smaller than that in AGV at 9 months after operation (P=0.029;
Table III).
 | Table III.The number of anti-glaucoma drugs used
in patients before and after surgery. |
Table III.
The number of anti-glaucoma drugs used
in patients before and after surgery.
|
| Groups |
|
|---|
|
|
|
|
|---|
| Follow-up time | Ex-PRESS | AGV | P-value |
|---|
| Before surgery | 3.53±0.86 | 3.24±0.88 | 0.152 |
| 1 month after
surgery | 0.18±0.39 | 0.26±0.50 | 0.448 |
| 3 months after
surgery | 0.34±0.63 | 0.47±0.65 | 0.371 |
| 6 months after
surgery | 0.42±0.55 | 0.63±0.59 | 0.112 |
| 9 months after
surgery | 0.55±0.60 | 0.89±0.73 | 0.029 |
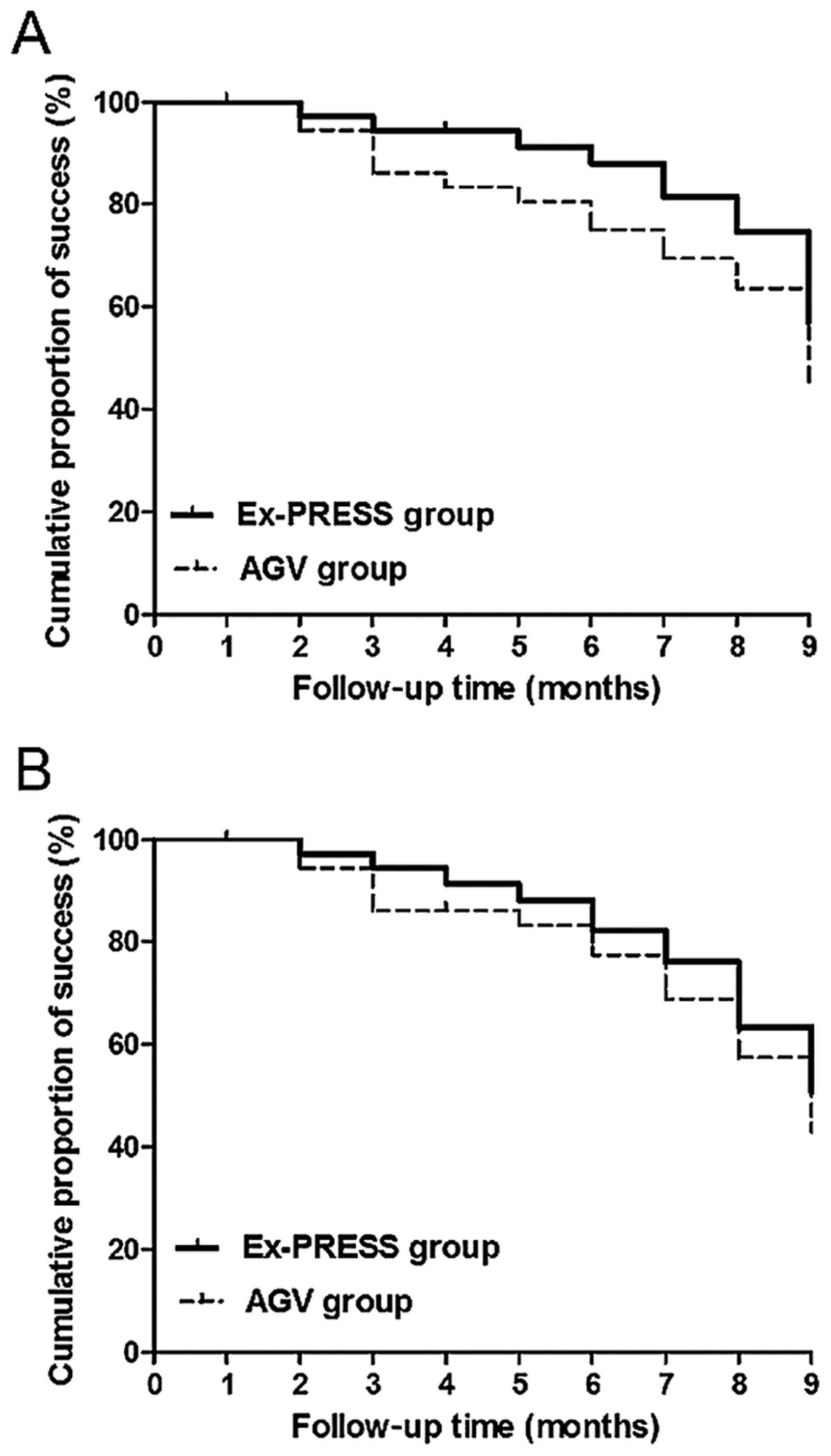
Comparison of success rate of
operation
Kaplan-Meier survival curve showed that there was no
statistically significant difference in relative success rate of
operation between Ex-PRESS and AGV group at the end of follow-up
(success rates, 65.8 and 52.6%, P=0.26, log-rank test) (Fig. 3A). The complete success rate of
operation had no statistically significant difference between the
two groups at the end of follow-up (success rates, 57.9 and 47.4%,
P=0.48, log-rank test) (Fig.
3B).
Complications
During and after operation, hyphema occurred in 7
eyes (18.4%) in Ex-PRESS and 10 eyes (26.3%) in AGV group, and the
blood was absorbed spontaneously within 2 weeks after operation.
Five eyes (13.2%) suffered from transient ocular hypertension in
each group within 7 days after operation. Shallow anterior chamber
occurred in 5 eyes (13.2%) in Ex-PRESS and 7 eyes (18.5%) in AGV
group after operation, among which 5 eyes were accompanied by
ocular hypotension in each group, and 2 eyes in AGV group suffered
from degree III shallow anterior chamber early after operation.
Moreover, 8 eyes (21.1%) in each group had the filtering bleb
capsule. Besides, malignant glaucoma occurred in 1 eye in AGV group
at 1 month after operation. The incidence rates of postoperative
complications had no statistically significant differences between
the two groups (P=0.599; Table
IV).
 | Table IV.Postoperative complications and
surgical treatment. |
Table IV.
Postoperative complications and
surgical treatment.
|
| Groups |
|
|---|
|
|
|
|
|---|
| Items | Ex-PRESS n,
(%) | AGV n, (%) | P-value |
|---|
| Postoperative
complications |
|
| 0.599 |
|
Hyphema | 7 (18.4) | 10 (26.3) |
|
| Shallow
anterior chamber | 5 (13.2) | 7 (18.5) |
|
| Bleb
dysfunction | 8 (21.1) | 8 (21.1) |
|
|
Transient intraocular
hypertension | 5 (13.2) | 5 (13.2) |
|
|
Malignant glaucoma | 0 (0) | 1 (13.2) |
|
| Surgical
treatment |
|
| 0.426 |
|
Bleb-plasty and 5-FU
subconjunctival injection | 8 (21.1) | 8 (21.1) |
|
|
Gonioplasty | 3 (7.9) | 5 (13.2) |
|
|
Cyclophotocoagulation | 3 (7.9) | 5 (13.2) |
|
| Implant
removal | 0 (0) | 2 (5.3) |
|
Postoperative intervention
Eight eyes (21.1%) in each group had the filtering
bleb capsule, and the subconjunctival injection of filtering bleb
needle combined with 5-fluorouracil was needed. Three patients
(7.9%) with shallow anterior chamber in each group required
anterior chamber angioplasty. Malignant glaucoma occurred in 1 eye
in AGV group at 1 month after operation, and anterior chamber
angioplasty, vitreous cavity drainage and drug therapy were
required. In AGV group, 3 eyes had cilio-choroidal detachment, and
treatment was performed, such as atropine for mydriasis, local or
systemic application of hormones, binocular bandaging, oral
administration of methazolamide and intravenous infusion of
mannitol; as a result, 2 eyes returned to normal within 7 days, and
drug therapy failed in 1 eye with degree III shallow anterior
chamber, and the eye was recovered after suprachoroidal drainage
and anterior chamber angioplasty. Transient ocular hypertension in
both groups declined to normal after blood-absorption drug therapy
and adjustable suture release. Three cases (7.9%) in Ex-PRESS and 5
cases (13.2%) in AGV group finally received cyclophotocoagulation,
and the implantation materials were removed from 2 cases (5.3%) due
to exposure (Table IV).
Discussion
In this study, the effect and safety of Ex-PRESS and
AGV implantation in the treatment of refractory glaucoma were
studied, and it was found that the postoperative IOP in both groups
was significantly decreased and controlled well. At 3 months after
operation, IOP in Ex-PRESS group was significantly lower than that
in AGV group. Moreover, the number of glaucoma drugs applied after
operation in both groups was significantly reduced; the number of
glaucoma drugs applied at 9 months after operation in Ex-PRESS
group was obviously smaller than that in AGV group.
The reason for the better control of IOP in Ex-PRESS
group may be due to the inconsistent structure of the two drainage
materials. Firstly, the aqueous drainage mechanisms of the two
kinds of implantation materials were different. Ex-PRESS drainage
device is a valve-free casing tube, so the communication between
intraocular and extraocular aqueous fluid has no obstacle; due to
the fixed diameter (50 µm), the postoperative aqueous effluent is
fixed, there is less ocular hypotension and the postoperative IOP
is stable (15); AGV silicone
elastic valve is a one-way pressure-sensitive valve that can
provide the resistance to the outflow of aqueous fluid to prevent
the excessive drainage of anterior aqueous fluid before the capsule
is formed on the surface of the drainage disk, and it can only open
when IOP reaches or exceeds the preset value of 8–10 mmHg, and
close when IOP is <8 mmHg to prevent the outflow of aqueous
fluid (16). Such a pressure
structure results in higher IOP after AGV implantation. Secondly,
the drainage capsule and fiber proliferation were different after
the two types of implantation. The study of Bae et al
(17) showed that the filtering bleb
fibrous layer in AGV group was significantly thickened compared
with trabeculectomy. Similar to trabeculectomy, the aqueous fluid
outflow pathway in Ex-PRESS group comes from the sclera, and the
intraoperative application of MMC can effectively reduce the fiber
proliferation between and around the sclera, so that the filtering
bleb fiber proliferation is significantly reduced. As posterior
filtration in AGV, AGV was placed under the conjunctival flap and
Tenon's capsule to drain the anterior aqueous fluid to the rear of
Tenon's capsule; the limited capsule around the drainage valve can
significantly reduce the outflow of aqueous fluid. Susanna et
al (18) studied and also showed
that the application of MMC in AGV implantation does not
significantly improve the success rate of operation. Overall,
compared with AGV, Ex-PRESS aqueous fluid drainage mechanism and
postoperative filtration channel formation mechanism are more
conducive to the IOP control.
Many studies have shown that the success rate of
Ex-PRESS implantation is similar to that of trabeculectomy, but the
postoperative complications are significantly reduced compared with
trabeculectomy (7–14,19). The
1-year success rate of Ex-PRESS implantation in the treatment of
open-angle glaucoma is more than 80%. As far as we know, there are
still few clinical reports on Ex-PRESS implantation in the
treatment of refractory glaucoma (20). In this study, after Ex-PRESS and AGV
implantation for refractory glaucoma, Kaplan-Meier survival curves
showed that there were no statistically significant differences in
the relative and complete success rates of operation at the end of
follow-up between Ex-PRESS group and AGV group; the success rate of
AGV implantation was consistent with that in previous studies
(6,21), but that of Ex-PRESS implantation was
lower than that in previous studies (9–14). This
is mainly because of the different types of glaucoma. This study
mainly aimed at the refractory glaucoma. It still can be seen from
the previous and this study that Ex-PRESS implantation and AGV
implantation are undoubtedly effective treatment methods for
refractory glaucoma, compared with the drug and laser therapy,
although the success rate of operation is decreased over time. This
study showed no statistically significant differences in incidence
rates of postoperative complications and surgical intervention
rates between the two groups.
In conclusion, the results of this study suggested
that Ex-PRESS implantation is a safe and effective treatment method
of refractory glaucoma, characterized by simple operation, small
trauma and small volume of implantation materials, so it is easier
to be popularized and applied clinically. However, this study had
some limitations. First of all, Ex-PRESS has been applied in China
for a short time, so only a small number of cases were included in
this study; secondly, there was a certain bias. In order to
minimize the bias, a detailed comparative analysis was performed
for the homogeneity of patients' general conditions before data
statistics, and patients were followed up for at least 9 months, so
that the short-term effect of Ex-PRESS implantation could be
described and analyzed more reliably. The long-term curative effect
of Ex-PRESS implantation on refractory glaucoma remains to be
further demonstrated via further large-sample multi-center
randomized controlled clinical research.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WB designed the study, DD collected data, FS
analysed the data, WB and FS prepared the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Zaozhuang Ophthalmologic Hospital (Zaozhuang, China). Signed
written informed consents were obtained from the patients and/or
guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ansari E: An update on implants for
minimally invasive glaucoma surgery (MIGS). Ophthalmol Ther.
6:233–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bikbov MM and Khusnitdinov II: The results
of the use of ahmed valve in refractory glaucoma surgery. J Curr
Glaucoma Pract. 9:86–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gandham SB, Costa VP, Katz LJ, Wilson RP,
Sivalingam A, Belmont J and Smith M: Aqueous tube-shunt
implantation and pars plana vitrectomy in eyes with refractory
glaucoma. Am J Ophthalmol. 116:189–195. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishida K: Update on results and
complications of cyclophotocoa gulation. Curr Opin Ophthalmol.
24:102–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Molteno AC: New implant for drainage in
glaucoma. Clinical trial. Br J Ophthalmol. 53:606–615. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taglia DP, Perkins TW, Gangnon R, Heatley
GA and Kaufman PL: Comparison of the Ahmed glaucoma valve, the
Krupin eye valve with disk, and the double-plate Molteno implant. J
Glaucoma. 11:347–353. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nyska A, Glovinsky Y, Belkin M and Epstein
Y: Biocompatibility of the Ex-PRESS miniature glaucoma drainage
implant. J Glaucoma. 12:275–280. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dahan E and Carmichael TR: Implantation of
a miniature glaucoma device under a scleral flap. J Glaucoma.
14:98–102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moisseiev E, Zunz E, Tzur R, Kurtz S and
Shemesh G: Standard trabeculectomy and Ex-PRESS miniature glaucoma
shunt: A comparative study and literature review. J Glaucoma.
24:410–416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Jong LA: The Ex-PRESS glaucoma shunt
versus trabeculectomy in open-angle glaucoma: A prospective
randomized study. Adv Ther. 26:336–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Good TJ and Kahook MY: Assessment of bleb
morphologic features and postoperative outcomes after Ex-PRESS
drainage device implantation versus trabeculectomy. Am J
Ophthalmol. 151:507–13.e1. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Jong L, Lafuma A, Aguadé AS and
Berdeaux G: Five-year extension of a clinical trial comparing the
EX-PRESS glaucoma filtration device and trabeculectomy in primary
open-angle glaucoma. Clin Ophthalmol. 5:527–533. 2011.PubMed/NCBI
|
|
13
|
Dahan E, Ben Simon GJ and Lafuma A:
Comparison of trabeculectomy and Ex-PRESS implantation in fellow
eyes of the same patient: A prospective, randomised study. Eye
(Lond). 26:703–710. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seider MI, Rofagha S, Lin SC and Stamper
RL: Resident-performed Ex-PRESS shunt implantation versus
trabeculectomy. J Glaucoma. 21:469–474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salim S: Ex-PRESS glaucoma filtration
device-surgical technique and outcomes. Int Ophthalmol Clin.
51:83–94. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wishart PK, Choudhary A and Wong D: Ahmed
glaucoma valves in refractory glaucoma: A 7-year audit. Br J
Ophthalmol. 94:1174–1179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bae K, Suh W and Kee C: Comparative study
of encapsulated blebs following Ahmed glaucoma valve implantation
and trabeculectomy with mitomycin-C. Korean J Ophthalmol.
26:265–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Susanna R Jr: Latin American Glaucoma
Society Investigators: Partial Tenons capsule resection with
adjunctive mitomycin C in Ahmed glaucoma valve implant surgery. Br
J Ophthalmol. 87:994–998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maris PJ Jr, Ishida K and Netland PA:
Comparison of trabeculectomy with Ex-PRESS miniature glaucoma
device implanted under scleral flap. J Glaucoma. 16:14–19. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wamsley S, Moster MR, Rai S, Alvim HS and
Fontanarosa J: Results of the use of the Ex-PRESS miniature
glaucoma implant in technically challenging, advanced glaucoma
cases: A clinical pilot study. Am J Ophthalmol. 138:1049–1051.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yalvac IS, Eksioglu U, Satana B and Duman
S: Long-term results of Ahmed glaucoma valve and Molteno implant in
neovascular glaucoma. Eye (Lond). 21:65–70. 2007. View Article : Google Scholar : PubMed/NCBI
|