Introduction
The repair of damaged cartilage is clinically
challenging as cartilage has a low capacity for self-repair
(1). Traditional therapies include
autologous tissue replacement and alloplastic implants, which can
result in foreign body reactions and local fibrosis (2). Recently, stem-cell-based tissue
engineering has come to be regarded as a potential ideal strategy
to address this problem (3).
Adipose-derived stem cells (ADSCs) are abundant and readily
available (4); furthermore, their
chondrogenic transformative ability makes them promising seed cells
for cartilage repair (5). However,
this strategy is limited as ischemia results in low survival rats
in transplant sites (6).
Transforming growth factor (TGF)-β3 is an important
cytokine that serves a role in the induction of chondro-lineage
differentiation in ADSCs (7). TGF-β3
is structurally and biologically distinct from TGF-β1 and TGF-β2
(8) and is able to promote cartilage
repair and accelerate cartilage differentiation. Associated studies
have demonstrated that TGF-β3 is cytokine important for the
differentiation of ADSCs into chondrocytes (9).
Whether TGF-β3 is a pro-apoptotic factor remains
controversial (10–12). To the best of our knowledge, the
effects of TGF-β3 on ischemia-induced apoptosis in ADSCs have not
previously been investigated. The aim of the present study was to
determine the effects and potential mechanisms of TGF-β3 on
ischemic-induced death in ADSCs.
Materials and methods
Culture and identification of human
ADSCs
Human ADSCs as passage two were purchased from
Cyagen Biosciences, Inc. (Santa Clara, CA, USA) and cultured in
complete Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere containing 5% CO2 at 37°C. The
medium was changed every 48 h. Once cells reached 80% confluence
they were passaged by digestion with 0.25% trypsin/EDTA (Gibco;
Thermo Fisher Scientific, Inc.) and seeded in 25 cm2
flasks at a density of 1×105 cells/cm2.
Cultured human ADSCs were analyzed using a flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Passage 3
ADSCs were digested with 0.25% trypsin/EDTA, washed in PBS and
resuspended in PBS at 5×106 cells/ml. After the cells
were blocked with 10% goat serum (Wuhan Boster Biological
Technology, Ltd., Wuhan, China) at room temperature for 30 min,
they were subsequently incubated for 15 min at 4°C in the dark with
the following antibodies: Anti-cluster of differentiation
(CD)29-fluorescein isothiocyanate (FITC), anti-CD44-FITC (cat. no.
560977), anti-CD105-phycoethrin (PE; cat. no. 560839), anti-CD14-PE
(cat. no. 557154), anti-CD34-FITC (cat. no. 560942) and
anti-CD45-FITC (cat. no. 555482; all BD Biosciences). All the above
antibodies were at a dilution of 1:500. Cells were washed twice
with PBS and analyzed using CellQuest software (version 0.9.13
alpha; BD Biosciences).
Differentiation properties of human
ADSCs
To assess osteogenic differentiation, ADSCs were
seeded (5×103 cells/cm2) in 6-wells plates
containing complete DMEM. Cells were cultured for 48 h, following
which the medium was changed to osteogenic induction medium from
the Osteogenesis Differentiation kit (Cyagen Biosciences, Inc.) and
cells were cultured for a further 20 days. The cells were
subsequently stained with a 1% Alizarin Red S solution at room
temperature for 15 min.
A total of 6 µl ADSCs (~1×107 cells) were
seeded in the bottom of a centrifuge tube with 5 ml DMEM containing
10% FBS. Cells were cultured for 48 h and the medium was changed to
chondrogenic induction medium using a Chondrogenesis
Differentiation kit (Cyagen Biosciences, Inc.). The medium was
changed every 3 days. Chondro-lineage differentiation was induced
for 15 days and the cells stained with 1% Alcian Blue at room
temperature for 15 min.
To induce adipogenic differentiation, ADSCs were
seeded on 6-wells plates at 1×104 cells/cm2
in complete DMEM. Following 24 h of culture, the medium was changed
to adipogenic inductive medium using an Adipogenesis
Differentiation kit (Cyagen Biosciences, Inc.) and the cells were
cultured for 15 days. Oil red O staining (0.3%) was used at room
temperature for 15 min to observe oil droplets in the induced
cells.
Colony-forming cell assay
ADSCs were seeded in 6-well plates at a density of
1×103 cells/well and cultured for 14 days. Colony
formation was observed using light microscopy (Olympus Corp.,
Tokyo, Japan) at a magnification of ×100. After staining with 1%
crystal violet at room temperature for 15 min and washing with PBS,
the number of colony-forming units (CFU) was counted and the
colony-forming rate was calculated as follows: no. CFUs/no.
starting cells/well ×100.
Cell migration assay
Uniform wound lines were introduced into ADSC
cultures using a culture insert (Ibidi, Munich, Germany). The
inserts were placed in individual wells of a 6-well plate and
removed following cell attachment. The gaps were washed with PBS
and 2 ml DMEM was added to each well. Cell migration into the wound
area was recorded using an inverted microscope (Olympus Corp.) at
0, 24, 48 and 72 h.
OSD treatment
ADSCs were seeded at a density of 2×104
cells/cm2 on glass coverslips for the TUNEL assay and in
6-well plates for western blotting and the Annexin V/propidium
iodide (PI) assay. A pilot study revealed that 10 ng/ml of TGF-β3
was sufficient to induce ADSC differentiation into chondrocytes
(data not shown), and so this concentration was selected for use in
the following experiments. To mimic ischemic conditions and analyze
the effects of every component of these conditions, cells were
divided into the oxygen-deprivation group (OD) and oxygen and serum
deprivation group (OSD). The OD group was cultured in DMEM
containing 10% FBS, and the OSD group was cultured in serum-free
DMEM. To analyze the effect of TGF-β3 on the OSD group, the culture
medium was replaced with serum-free DMEM with or without 10 ng/ml
TGF-β3 (the OSD+TGF-β3 group). Cells were cultured under hypoxic
conditions in an atmosphere containing 95% N2 and 5%
CO2 at 37°C for 48 h as previously reported (6). ADSCs cultured in complete DMEM in a 5%
CO2 humidified atmosphere at 37°C served as the negative
control (NC) group.
Flow cytometric Annexin V-FITC/PI
assay of ADSCs
An Annexin V/PI Apoptosis kit (Thermo Fisher
Scientific, Inc.) was used to assess the rate of apoptosis in ADSCs
following ischemia according to the manufacturer's protocol.
Briefly, ADSCs rom each group were digested with 0.25%
trypsin/EDTA. Cells were washed with PBS, re-suspended in binding
buffer and incubated for 15 min at room temperature in the dark
with 5 µl Annexin V-FITC and 10 µl PI. The Annexin V-FITC and
PI-labeled cells were analyzed using a flow cytometer (BD
Biosciences). Using flow cytometry and CellQuest software, dot
plots of PI on the y-axis against Annexin V-FITC on the x-axis were
used to distinguish viable cells, early apoptotic cells and late
apoptotic or necrotic cells.
TUNEL assay of the ischemic ADSCs
A TUNEL assay was performed to measure double
stranded DNA cleavage using one-step TUNEL kit (Beyotime Institute
of Biotechnology, Haimen, China). Cells were fixed with 10%
formalin at room temperature for 1 h and groups of slips were
incubated in permeabilisation solution (1% Triton X-100 in PBS,
freshly prepared) for 10 min on ice. The slides were washed three
times with PBS and incubated with TUNEL working solution in a
humidified chamber at 37°C for 1 h. Samples were counterstained
with DAPI in room temperature for 5 min. following the mounting of
the coverslips with glycerin (Beyotime Institute of Biotechnology),
the stained coverslips were examined under a fluorescence
microscope (Olympus Corp.). TUNEL positive cells in 4 random fields
were counted and analyzed with GraphPad Prism software (version
5.0; GraphPad Software, Inc., La Jolla, CA, USA) for each group
(magnification, ×200).
PARP gene silencing by siRNA
PARP was inhibited using small interfering RNA
(siRNA). Cells (2.5×105 cells/well) were seeded in
6-well plates and transfected with 20 nM PARP-targeting si-RNA
(5′-CCAACAGAAGTACGTGCAA-3′) or control si-RNA
(5′-CCCTCTGCACTAATCTGAA-3′; both Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) using Lipofectamine® 3000 (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol
and cultured for 72 h at 37°C in a 5% CO2 incubator.
PARP expression was subsequently measured by western blotting as
described below. Transfected ADSCs were cultured under OSD+TGF-β3
conditions and analyzed using western blotting (as described below)
and Annexin V/PI staining (as described above).
Western blotting
ADSCs were homogenized in radioimmunoprecipitation
assay buffer (Sigma Aldrich; Merck KGaA, Darmstadt, Germany) on ice
for 30 min. Proteins were quantified using a bicinchoninic acid
assay kit (Beyotime Institute of Biotechnology) and equal amounts
of proteins (20 µl/lane) were separated using 10% SDS-PAGE.
Proteins were transferred onto polyvinylidene fluoride membranes
and blocked in 5% non-fat milk in TBS-0.1% Tween-20 for 1 h at room
temperature. The membranes were subsequently incubated overnight at
4°C with primary antibodies against caspase 3 (cat. no. #9662;
1:500), activated caspase 3 (cat. no. #9664; 1:1,000), PARP (cat.
no. #9532; 1:1,000), activated PARP (cat. no. #5625; 1:1,000),
B-cell lymphoma 2 (Bcl-2; cat. no. #4223; 1:1,000),
Bcl-2-associated X protein (Bax; cat. no. #5023; 1:1,000),
activated caspase 9 (cat. no. #9505; 1:1,000) and GAPDH (cat. no.
#5174; 1:10,000). Following washing with TBST, membranes were
incubated with horseradish peroxidase-conjugated anti-rabbit
secondary antibodies (cat. no. #7074; 1:3,000) for 1 h at room
temperature. All the above antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Bands were
visualized using an enhanced chemiluminescence kit (EMD Millipore,
Billerica, MA, USA). Images of the bands were captured using a
Bio-Rad Gel Doc XR documentation system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Relative protein expression was
determined using densitometry and standardized to GAPDH levels
using ImageJ software (version 2.1.7.4; National Institutes of
Health, Bethesda, MA, USA).
Statistical analysis
Data are presented as the mean ± or + standard error
of the mean. All experiments were performed six times to provide
sufficient data. Differences between experimental conditions and
controls were analyzed using one-way analysis of variance followed
by Tukey's post hoc test and GraphPad Prism software. P<0.05 was
considered to indicate a statistically significant difference.
Results
Identification, differentiation
potential and stem cell properties analysis of ADSCs
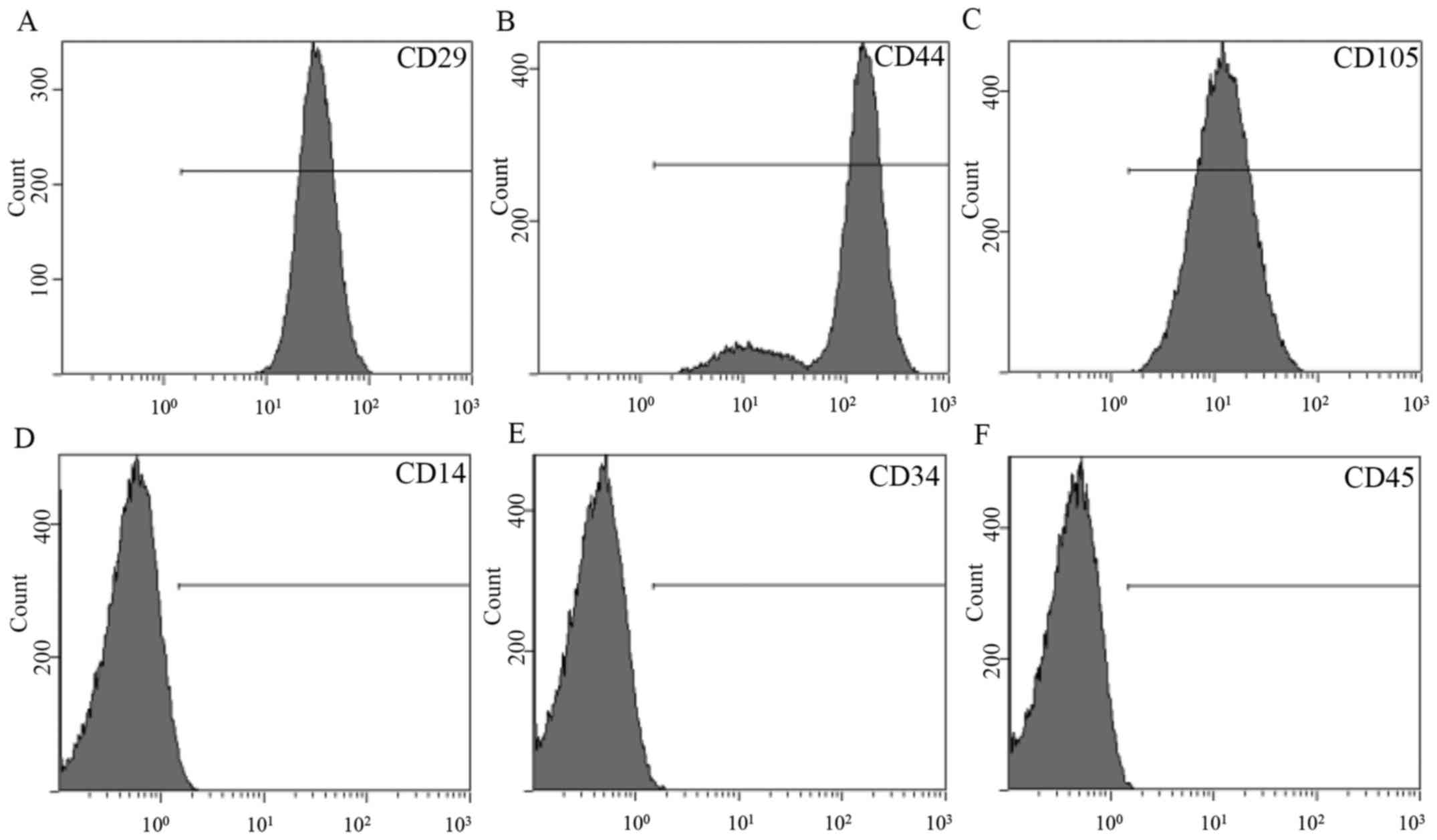
Flow cytometry revealed that ADSCs expressed stem
cell-associated markers CD29, CD44, CD105 and CD90 (Fig. 1A-C). However, expression of the
mononuclear phagocyte marker CD14, hematopoietic cell marker CD34
and leukocyte marker CD45 was not observed in ADSCs (Fig. 1D-F).
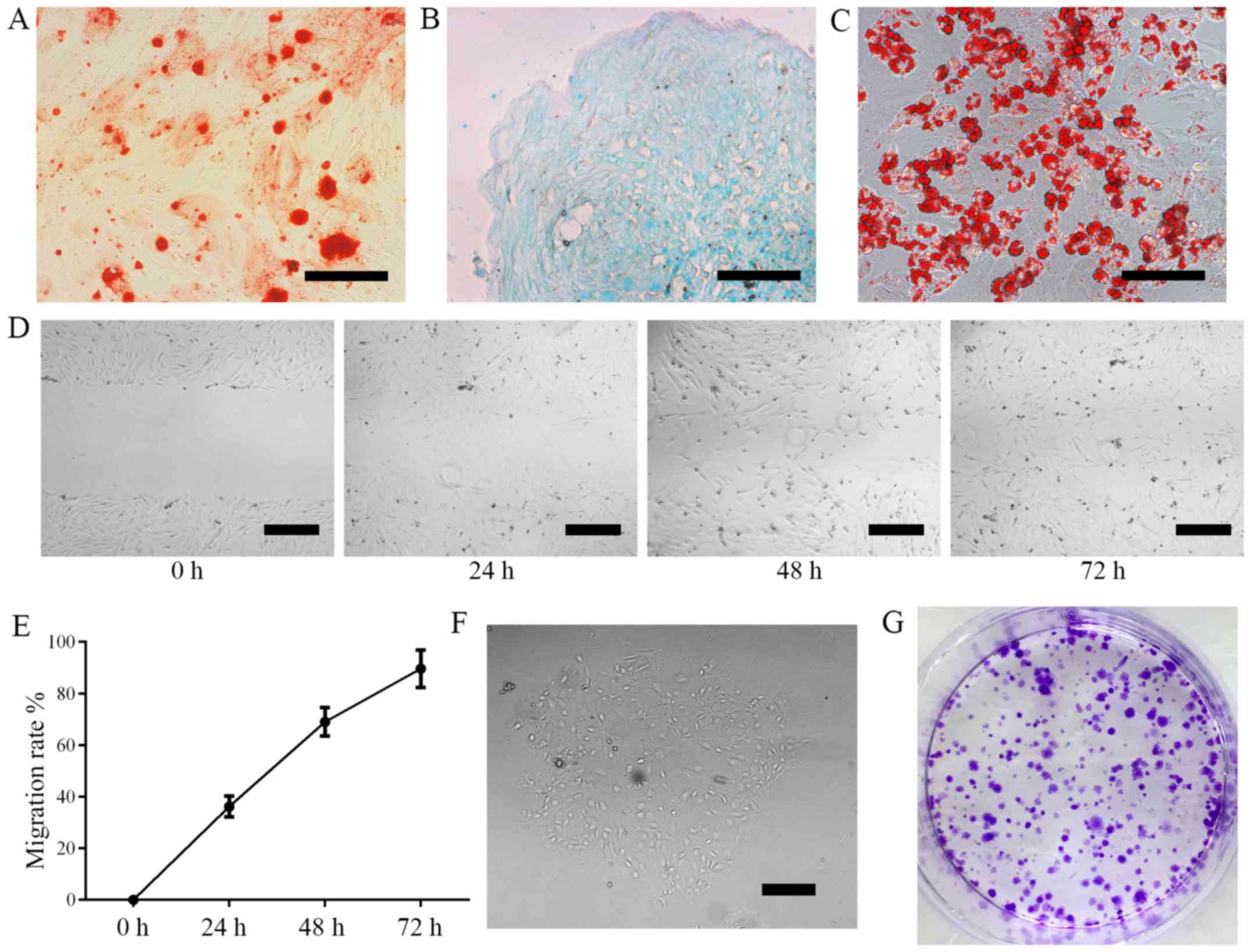
Cell differentiation was induced using 3-lineage
differentiation kits. ADSCs differentiated into osteoblasts
(Fig. 2A; Alizarin Red staining),
chondrocytes (Fig. 2B; Alcian Blue
staining) and adipocytes as confirmed by (Fig. 2C; Oil Red O staining). These results
demonstrate that ADSCs are capable of multi-lineage
differentiation.
The results of cell migration assays revealed that
the migration rate of ADSCs was 36.2±4.0% in 24 h, 69.0±5.5% in 48
h and 89.6±7.2% in 72 h (Fig. 2D-E).
Colony formation was measured following 6 days of culture. Colony
formation was 37±3.5%, demonstrating that cultured ADSCs have the
capacity for self-renewal (Fig.
2F-G).
ADSC apoptosis is induced by OSD and
alleviated by TGF-β3 treatment
To investigate the effect of TGF-β3 on
ischemia-associated apoptosis of ADSCs in vitro, ADSCs were
exposed to OD or OSD conditions for 48 h, with or without exogenous
TGF-β3. Apoptosis was assessed using an Annexin V/PI Apoptosis kit
(Fig. 3). The percentage of
apoptotic ADSCs in the NC, OSD, OD and OSD+TGF-β3 groups were
5.93±1.30, 9.22±3.63, 36.72±4.15 and 20.11±3.78%, respectively
(Fig. 3E). These results suggest
that OD alone did not result in apoptosis in ADSCs, which is
consistent with previous research (13). A previous study reported that low
oxygen conditions may improve ADSC proliferation ability (14). However, serum deprivation under
hypoxic conditions effectively induced apoptosis (Fig. 3E). Based on these results, it was
hypothesized that different components of ischemia exert their own
effects on ADSCs Adding TGF-β3 to the culture medium significantly
reduced the percentage of apoptotic cells. These results
demonstrate that TGF-β3 may reverse OSD-induced apoptosis by
preventing cells from entering the later stages of apoptosis.
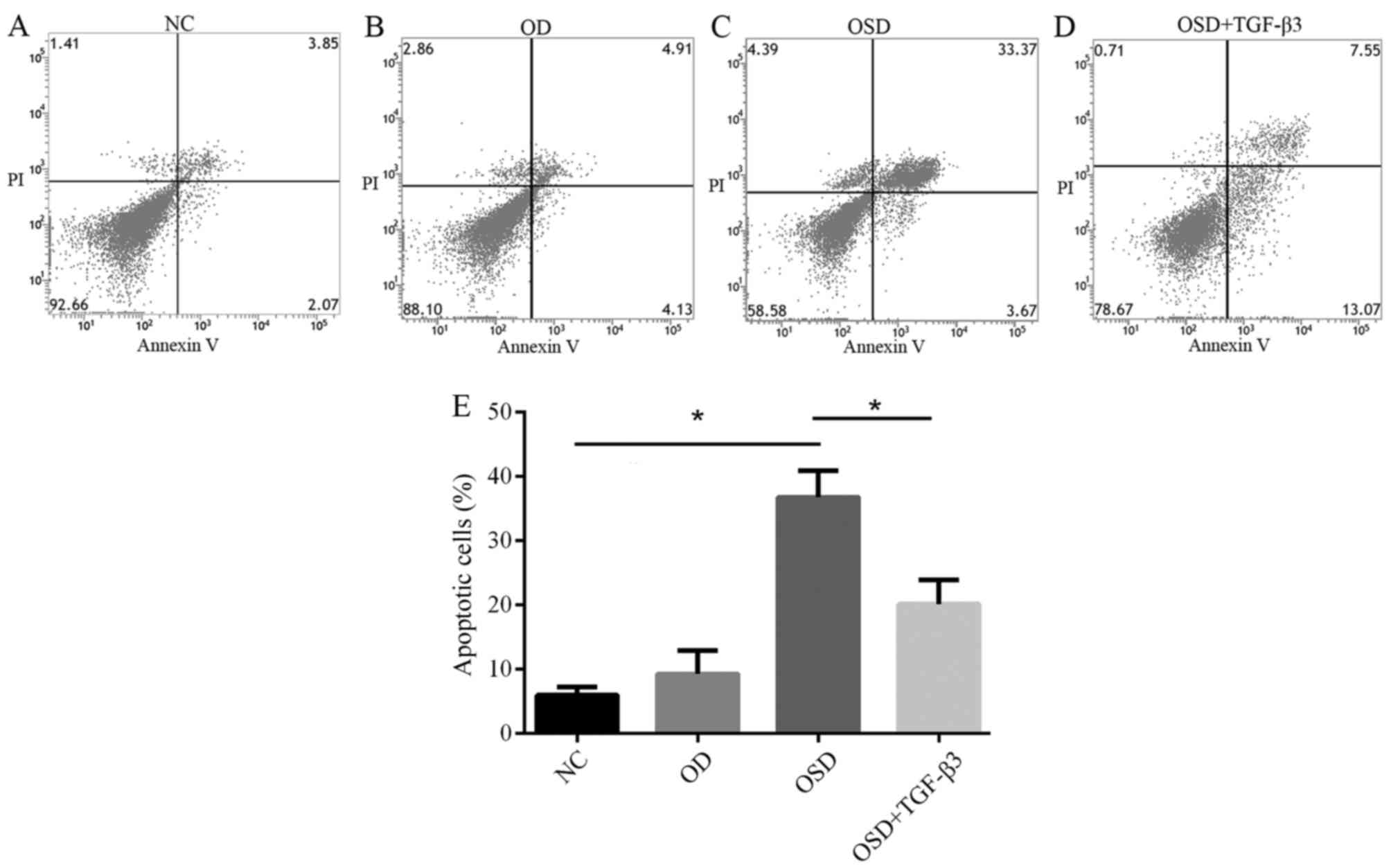 | Figure 3.Flow cytometric analysis of apoptosis
in the (A) NC, (B) OD, (C) OSD and (D) OSD + TGF-β3 groups. The
lower left quadrant represents normal cells, lower right quadrant
represents cells in the early stages of apoptosis and the upper
right quadrant represents cells in late apoptosis. (E) The number
of apoptotic cells of each group in the NC, OD, OSD and OSD +
TGF-β3 groups. *P<0.05. NC, negative control; OD, oxygen
deprivation; OSD, oxygen and serum deprivation; TGF, transforming
growth factor; PI, propidium iodide. |
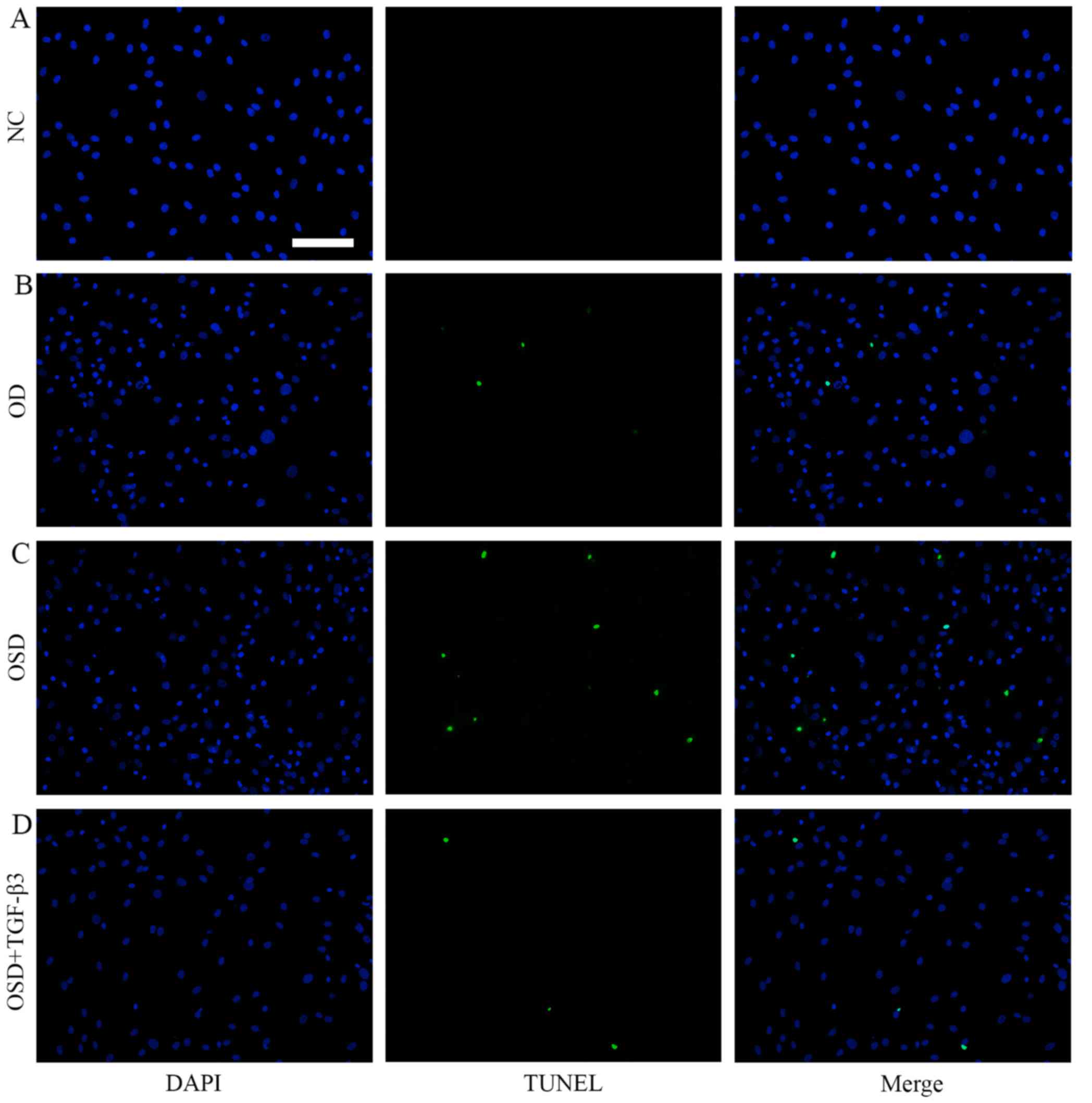
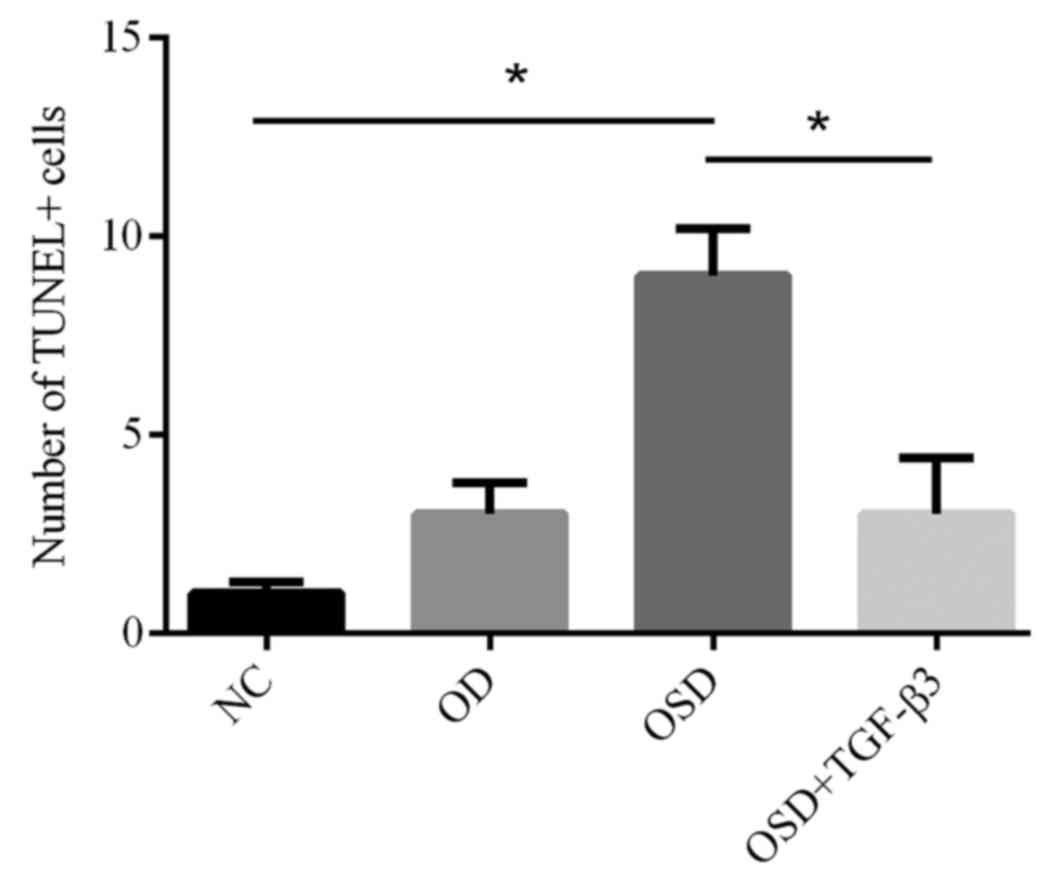
To further investigate apoptosis in ADSCs, TUNEL
staining was performed (Fig. 4).
Following treatment with OSD, nuclear condensation and cell
shrinkage were observed. The number of TUNEL-positive cells in the
OSD group was significantly increased compared with the NC group
(Fig. 5). The addition of TGF-β3
significantly reduced the number of TUNEL-positive cells compared
with the OSD group (Fig. 5). TUNEL
positive cells represent cells that underwent programmed breaks of
DNA double strands. Together, these results suggest that exogenous
TGF-β3 is able to improve OSD-resistance in ADSCs.
TGF-β3 attenuates OSD-induced
apoptosis in ADSCs
To analyze the mechanism by which TGF-β3 prevents
OSD-induced apoptosis in ADSCs, changes in the expression of
apoptotic genes was assessed using western blotting (Fig. 6). Caspase 3 is a key effector
molecule in the downstream signaling pathways associated with
apoptosis, which, together with other effector caspases, including
caspase 7 and caspase 6, orchestrates the dismantling of diverse
cell structures via cleavage of specific substrates (15). No significant difference in caspase 3
expression levels was observed between the NC and OD groups
(Fig. 3B). However, caspase 3 was
significantly downregulated and activated caspase 3 was
significantly upregulated under OSD conditions, which indicates
increased apoptosis (Fig. 6B and C).
The results also demonstrated that caspase 3 activation was
significantly decreased in the OSD+TGF-β3 group compared with the
OSD group (Fig. 6B and C). Bcl-2 and
Bax are important mitochondria-mediated apoptotic proteins; Bax
forms a complex with the anti-apoptotic protein Bcl-2 to balance
its apoptotic effects and, as such, the ratio of Bax/Bcl-2
determines whether cells will undergo apoptosis (16). Caspase 9 is an important protein in
the mitochondrial apoptotic pathway (17). Western blotting results demonstrated
that OSD conditions induced an increase in the Bax/Bcl-2 ratio and
caspase 9 activation (Fig. 6) and
both were significantly decreased with the addition of TGF-β3
(Fig. 6F-H).
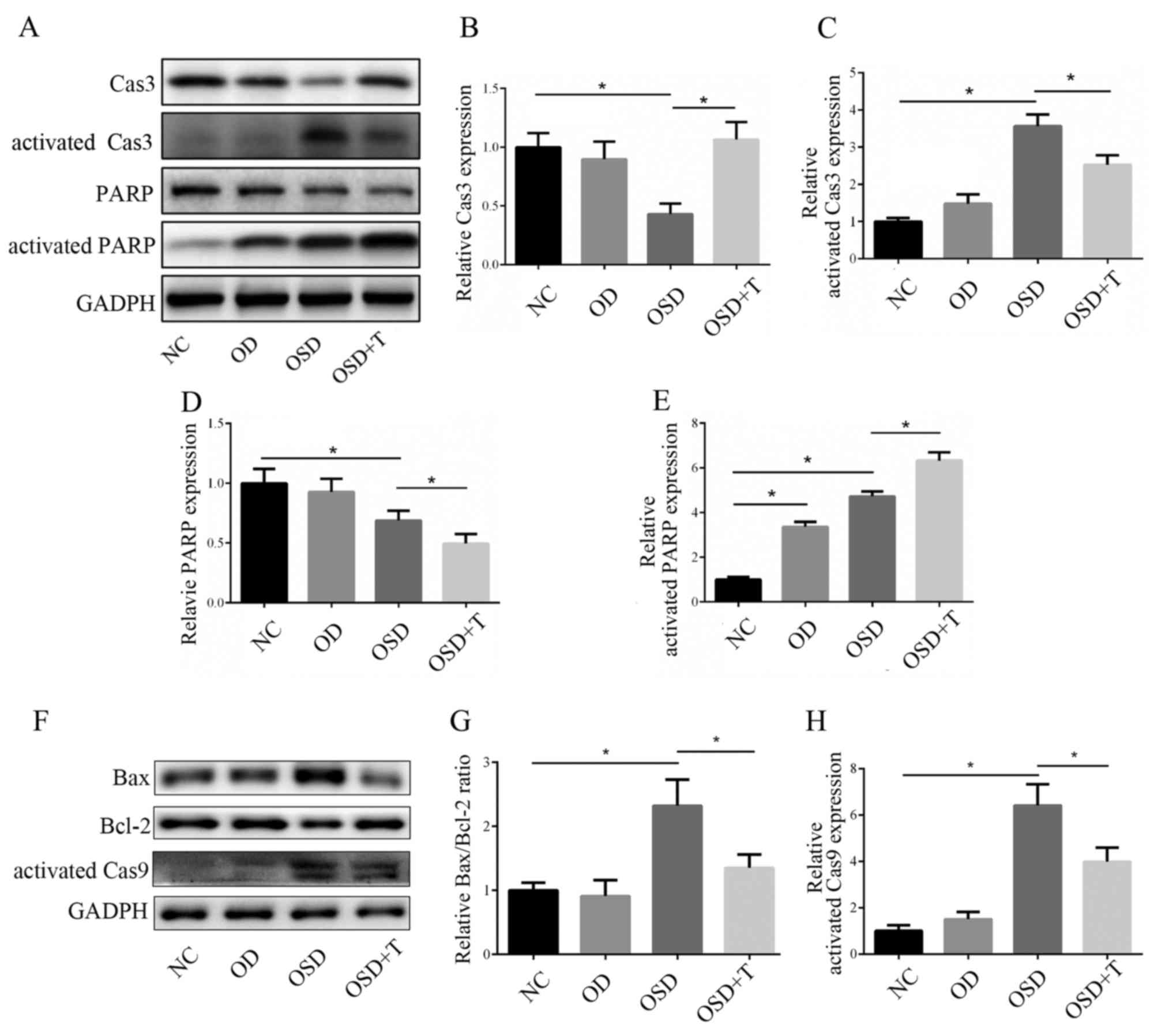 | Figure 6.(A) Western blotting was performed to
measure the activation of Cas3 and PARP. The expression of (B)
Cas3, (C) activated Cas3, (D) PARP and (E) activated PARP was
analyzed (F) Western blotting was performed to measure the
expression of apoptosis-associated proteins. The expression of (G)
Bax/Bcl-2 and (H) activated Cas9 was analyzed. *P<0.05. Cas,
caspase; PARP, poly ADP-ribose polymerase; Bax, B-cell lymphoma
2-associated X protein; Bcl-2, B-cell lymphoma 2; NC, negative
control; OD, oxygen deprivation; OSD, oxygen and serum deprivation;
T, transforming growth factor-β3. |
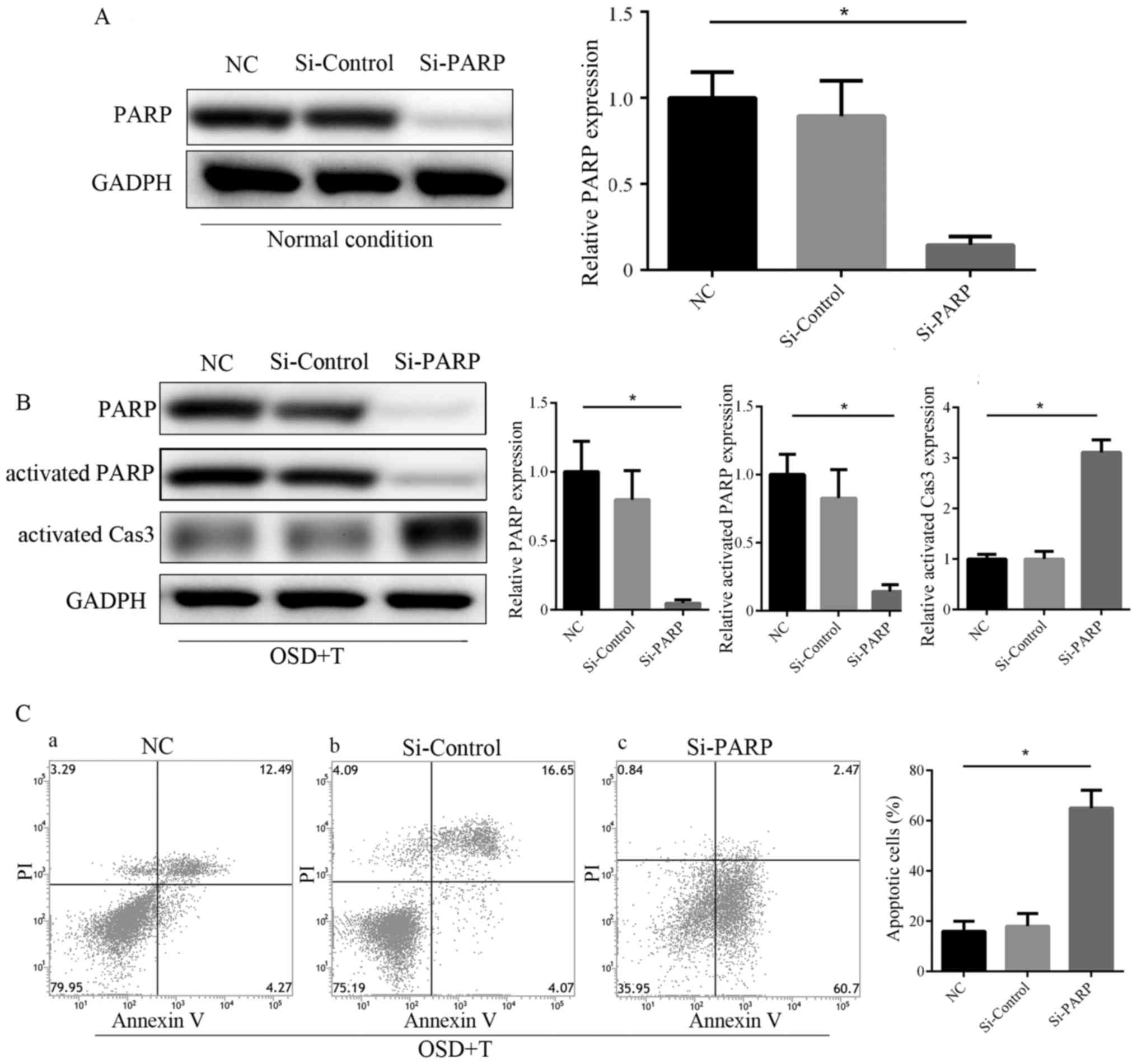
PARP has been reported to modulate various DNA
damage response (DDR) processes to ensure genomic integrity and
induce DNA repair or cell death (18). PARP is cleaved by activated caspase
3, and so changes in activated PARP are often consistent with
activate caspase 3 levels (19). The
results of western blotting revealed that activated PARP was
upregulated in the OSD+TGF-β3 group compared with the OD and OSD
groups (Fig. 6D and E). These
results demonstrate that OSD conditions induce increased apoptosis
and DDR in ASDCs compared with OD alone. The addition of TGF-β3 is
able to alleviate OSD-induced apoptosis, which may be achieved via
enhancement of the PARP-associated DNA repairing effect. To further
confirm this, PARP knockdown ADSCs were constructed via siRNA
transfection (Fig. 7A). The results
of western blotting and Annexin V/PI staining revealed that PARP
knockdown significantly abolished the protective effect of TGF-β3
in ADSCs under OSD conditions (Fig. 7B
and C).
Discussion
The results of the present study revealed that OD
alone does not induce apoptosis in ADSCs, whereas OSD does. It was
also demonstrated that the addition of TGF-β3 is able to attenuate
OSD-induced apoptosis by enhancing activated PARP. A previous in
vivo study revealed that a low partial pressure of oxygen is
optimal for ADSCs (20), which
explains why OD did not induce apoptosis in the present study.
TGF-β3 is a member of the TGF family, which mainly functions
through the Smad pathway (21). A
previous study reported that TGF-β3 is an important initiator of
chondrocyte lineage differentiation (22). It has also been demonstrated that
TGF-β3 is associated with DNA mismatch repair genes (23). During embryonic development, TGF-β3
functions as a pro-apoptotic cytokine in some cells, including
finger puff cells and is associated with palatogenesis (23,24).
TGF-β3 also serves an anti-apoptotic role in the repair of tissue
damage (25). TGF-β3 is able to
alleviate intestinal epithelial radiation damage and oral mucosal
epithelial scratches, as well as increasing the survival rates of
mice subjected to radiation injury (12).
In the present study, the addition of exogenous
TGF-β3 in OSD-treated ADSCs resulted in a decrease in apoptosis.
Annexin-V/PI flow cytometric analysis revealed that the number of
cells in the early stages of apoptosis increased in the OSD+TGF-β3
group compared with OSD alone, however the total number of cells in
the late stages of apoptosis was decreased. These results suggest
that TGF-β3 may inhibit apoptosis in OSD-treated ADSCs, preventing
ADSCs from entering the later stages of apoptosis. This result was
further confirmed by a TUNEL array. The addition of exogenous
TGF-β3 significantly reduced the number TUNEL-positive cells, which
represented a decrease of the programmed induction of DNA double
strand breaks. It can then be hypothesized that when ADSCs are used
as seed cells for cartilage tissue engineering, additional TGF-β3
supplement may be necessary to alleviate OSD-associated cell
death.
The results of western blotting revealed that the
anti-apoptotic effects of TGF-β3 may be achieved via the activation
of PARP-associated DNA repair. Caspase 3 activation was upregulated
in the OSD group and downregulated in the OSD+TGF-β3 group. PARP
knockdown significantly counteracts the protective effect of TGF-β3
in ADSCs under OSD conditions. Among the molecular functions
attributed to PARPs, their role in the DDR has been widely
documented (18). In particular,
PARP is associated with several cellular processes that respond to
DNA lesions, including DDR, DNA signaling and DNA repair (18).
The results of the present study provide an insight
into the role of TGF-β3 in ADSCs under OSD conditions and may be
used to develop and improve ADSCs-associated stem cell therapy with
the use of exogenous TGF-β3. However, the present study is not
without limitations. Tissue engineering represents an attractive
strategy for regenerative medicine, but cell survival following
transplantation remains poor due to a combination of mechanical,
metabolism and host factors, limiting the efficacy of stem cell
therapy (26). OSD was used in the
present study to mimic in vivo hypoxia based on previous
studies (27–29). However, other factors, including
glucose metabolism disorder and lactic acid accumulation, may also
have an impact on cell survival in vivo (30) and were not considered in he present
study. Future research should focus on the effect of TGF-β3 on
ADSCs in vivo under ischemic condition.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Planning Project of Guangdong Province, China (grant no.
2012B061700104), the Key Laboratory of Malignant Tumor Molecular
Mechanism and Translational Medicine of Guangzhou Bureau of Science
and Information Technology [grant no. (2013) 163] and the Key
Laboratory of Malignant Tumor Gene Regulation and Target Therapy of
Guangdong Higher Education Institutes (grant no. KLB09001).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FW and HY cultured the cells, performed the
experiments and were major contributors in writing the manuscript.
JL and YX analyzed and interpreted the data. HZ and HX participated
in the design of the study and analysis of data, and agree to be
accountable for all aspects of work in ensuring that questions
related to the accuracy of the study. ML and YZ designed the
experiments, were involved in revising the manuscript for important
intellectual content and participated in the analysis and
interpretation of data. SC designed and directed the
experiments.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Sun Yat-sen Memorial Hospital (Guangzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hunziker EB: Articular cartilage repair:
Basic science and clinical progress. A review of the current status
and prospects. Osteoarthritis Cartilage. 10:432–463. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodriguez-Merchan EC: Regeneration of
articular cartilage of the knee. Rheumatol Int. 33:837–845. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimomura K, Ando W, Fujie H, Hart DA,
Yoshikawa H and Nakamura N: Scaffold-free tissue engineering for
injured joint surface restoration. J Exp Orthop. 5:22018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sterodimas A, de Faria J, Nicaretta B and
Pitanguy I: Tissue engineering with adipose-derived stem cells
(ADSCs): Current and future applications. J Plast Reconstr Aesthet
Surg. 63:1886–1892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng DY, Dan Y, Yang SH, Liu GH, Shao ZW,
Yang C, Xiao BJ, Liu X, Wu S, Zhang T and Chu PK: Controlled
chondrogenesis from adipose-derived stem cells by recombinant
transforming growth factor-β3 fusion protein in peptide scaffolds.
Acta Biomater. 11:191–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cui Z, Shen L, Lin Y, Wang S, Zheng D and
Tan Q: Inhibition of oxygen-glucose deprivation-induced apoptosis
of human adipose-derived stem cells by genetic modification with
antiapoptotic protein bcl-2. Aesthetic Plast Surg. 38:779–787.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu S, Chen P, Wu Y, Xiong S, Sun H, Xia
Q, Shi L, Liu H and Ouyang HW: Programmed application of
transforming growth factor β3 and rac1 inhibitor NSC23766 committed
hyaline cartilage differentiation of adipose-derived stem cells for
osteochondral defect repair. Stem Cells Transl Med. 3:1242–1251.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Laverty HG, Wakefield LM, Occleston NL,
O'Kane S and Ferguson MW: TGF-beta3 and cancer: A review. Cytokine
Growth Factor Rev. 20:305–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen B, Wei A, Tao H, Diwan AD and Ma DD:
BMP-2 enhances TGF-beta3-mediated chondrogenic differentiation of
human bone marrow multipotent mesenchymal stromal cells in alginate
bead culture. Tissue Eng Part A. 15:1311–1320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dünker N, Schmitt K and Krieglstein K:
TGF-beta is required for programmed cell death in interdigital webs
of the developing mouse limb. Mech Dev. 113:111–120. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fogli M, Carlo-Stella C, Curti A, Ratta M,
Tazzari PL, Ragazzi E, Colla S, Santucci AM, Tura S and Lemoli RM:
Transforming growth factor beta3 inhibits chronic myelogenous
leukemia hematopoiesis by inducing Fas-independent apoptosis. Exp
Hematol. 28:775–783. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Booth D and Potten CS: Protection against
mucosal injury by growth factors and cytokines. J Natl Cancer Inst
Monogr. 16–20. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mylotte LA, Duffy AM, Murphy M, O'Brien T,
Samali A, Barry F and Szegezdi E: Metabolic flexibility permits
mesenchymal stem cell survival in an ischemic environment. Stem
Cells. 26:1325–1336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang DW, Fermor B, Gimble JM, Awad HA and
Guilak F: Influence of oxygen on the proliferation and metabolism
of adipose derived adult stem cells. J Cell Physiol. 204:184–191.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
D'Amelio M, Cavallucci V and Cecconi F:
Neuronal caspase-3 signaling: Not only cell death. Cell Death
Differ. 17:1104–1114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharifi S, Barar J, Hejazi MS and Samadi
N: Roles of the Bcl-2/Bax ratio, caspase-8 and 9 in resistance of
breast cancer cells to paclitaxel. Asian Pac J Cancer Prev.
15:8617–8622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sousa FG, Matuo R, Soares DG, Escargueil
AE, Henriques JA, Larsen AK and Saffi J: PARPs and the DNA damage
response. Carcinogenesis. 33:1433–1440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang LJ, Chen Y, He J, Yi S, Wen L, Zhao S
and Cui GH: Effects of gambogic acid on the activation of caspase-3
and downregulation of SIRT1 in RPMI-8226 multiple myeloma cells via
the accumulation of ROS. Oncol Lett. 3:1159–1165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yue Y, Zhang P, Liu D, Yang JF, Nie C and
Yang D: Hypoxia preconditioning enhances the viability of ADSCs to
increase the survival rate of ischemic skin flaps in rats.
Aesthetic Plast Surg. 37:159–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Zhang X, Xie F, Zhang Z, van Dam
H, Zhang L and Zhou F: The regulation of TGF-β/SMAD signaling by
protein deubiquitination. Protein Cell. 5:503–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bian L, Zhai DY, Tous E, Rai R, Mauck RL
and Burdick JA: Enhanced MSC chondrogenesis following delivery of
TGF-β3 from alginate microspheres within hyaluronic acid hydrogels
in vitro and in vivo. Biomaterials. 32:6425–6434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He F, Xiong W, Wang Y, Li L, Liu C,
Yamagami T, Taketo MM, Zhou C and Chen Y: Epithelial Wnt/β-catenin
signaling regulates palatal shelf fusion through regulation of
Tgfβ3 expression. Dev Biol. 350:511–519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dunker N, Schmitt K and Krieglstein K:
TGF-beta is required for programmed cell death in interdigital webs
of the developing mouse limb. Mech Dev. 113:111–120. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu BX, Li A, Lei L, Kaneko S, Wallace C,
Li X and Li Z: Glycoprotein A repetitions predominant (GARP)
positively regulates transforming growth factor (TGF) β3 and is
essential for mouse palatogenesis. J Biol Chem. 292:18091–18097.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haleem AM and Chu CR: Advances in tissue
engineering techniques for articular cartilage repair. Oper Tech
Orthop. 20:76–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng J, Han Y, Yan C, Tian X, Tao J, Kang
J and Li S: Overexpressing cellular repressor of E1A-stimulated
genes protects mesenchymal stem cells against hypoxia- and serum
deprivation-induced apoptosis by activation of PI3K/Akt. Apoptosis.
15:463–473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu W, Chen J, Cong X, Hu S and Chen X:
Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem
cells. Stem Cells. 24:416–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou M, Liu J, Liu F, Liu K and Yu B: C1q
tumor necrosis factor-related protein-3 protects mesenchymal stem
cells against hypoxia- and serum deprivation-induced apoptosis
through the phosphoinositide 3-kinase/Akt pathway. Int J Mol Med.
33:97–1044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deschepper M, Oudina K, David B, Myrtil V,
Collet C, Bensidhoum M, Logeart-Avramoglou D and Petite H: Survival
and function of mesenchymal stem cells (MSCs) depend on glucose to
overcome exposure to long-term, severe and continuous hypoxia. J
Cell Mol Med. 15:1505–1514. 2011. View Article : Google Scholar : PubMed/NCBI
|