Introduction
Ankylosing spondylitis (AS) is a chronic,
progressive autoimmune disease that, theoretically, affects the
axial and sacroiliac joints. More importantly, the majority of
patients with AS eventually develop spine malformations, leading to
functional incapacity (1). Although,
the pathogenesis of AS remains unknown, an increasing amount of
research has been performed to elucidate it.
Previous studies have reported elevated percentages
of peripheral blood T helper (Th)1, Th17 and Th22 cells, and
decreased percentages of regulatory T cells in patients with AS
(2–4). However, to the best of our knowledge,
there have been only two studies regarding a novel, distinct
subgroup of cluster of differentiation (CD)4+ T cells,
known as follicular helper T cells (Tfh), in patients with AS
(5,6). A number of previous studies have
demonstrated that aberrant expression of circulating (c)Tfhs may
mediate the development of autoimmune diseases, such as systemic
lupus erythematosus (SLE), rheumatoid arthritis (RA), multiple
sclerosis and Sjogren's syndrome (7–10). Tfhs
are known as specialized providers of help to B cells and
persistently express C-X-C chemokine receptor type 5 (CXCR5), which
drives Tfh migration into B cell follicles in response to the
specific ligand CXCL13, which is expressed by follicular dendritic
cells (11). Morita et al
(12) have identified
CD4+CXCR5+ T cells as cTfhs as they share
similar functional properties with Tfh cells. Furthermore, Tfh
cells express programmed death 1 (PD-1), inducible T cell
costimulator (ICOS), CD40 ligand (CD40L) and interleukin (IL)-21,
which not only serve as excellent markers for the identification of
Tfh cells, but can also interact with B cell surface ligands to
promote the formation of germinal centers (GC), the differentiation
of B cells and antibody production (13,14).
In addition, other previous studies have reported
increased percentages of B cells and high levels of autoantibodies
in patients with AS (15,16). However, very few studies have focused
on the phenotypic and functional status of B cells in different
disease activities of AS. Several reports have demonstrated that
the abnormal distribution of B cell subtypes may participate in the
pathogenesis of autoimmune diseases, such as RA, SLE and IgA
nephropathy (17–19). A previous study of primary Sjogren's
syndrome (8) has reported that
abnormal increases of CD4+CXCR5+ Tfh cells
and B cell subsets in the salivary gland were significantly
correlated with serum antinuclear antibody titers. A further study
(20) revealed higher percentages of
activated B and Tfh cells in patients with RA as well as regulation
of B cell activation via active Tfh cells in the process of RA.
As such, the aim of the present study was to
investigate changes in the distribution of B cell subtypes and
whether Tfh is associated with the distribution of B cell subtypes
in patients with AS. The frequency of cTfhs and different stages of
differentiated B cells were investigated in 65 patients with AS as
well as in 20 gender and age-matched healthy participants. The
present findings suggest that certain subtypes of cTfh cells and B
cells may participate in the pathogenesis of AS due to their
distinct functions, and the percentages of cTfhs and B cell
subtypes may be useful as a valuable measure for evaluating disease
activity in patients with AS.
Materials and methods
Patients and controls
A total of 65 patients with AS were recruited
sequentially at the outpatient clinic of Guizhou Medical University
Hospital (Guiyang, China) from September 2014 to October 2015. All
patients fulfilled the 1984 modified New York criteria (21), which is the criterion for diagnosing
AS. A further 20 healthy age- and sex-matched individuals with no
history of inflammatory or autoimmune diseases were recruited
during the same period as healthy controls (HC). Patients with AS
were excluded if they had any other chronic inflammatory and
autoimmune disorders such as diabetes, multiple sclerosis or
inflammatory bowel disease, or if they were currently receiving
treatment with non-steroidal anti-inflammatory drugs, steroids, or
other immunosuppressants. The disease activity of individual
patients was measured using the Bath AS Disease Activity Index
(BASDAI) (22). The scores for each
criterion ranged from 0–10, with high activity defined as a BASDAI
score ≥4 and low activity defined as a BASDAI score <4. All
patients provided written informed consent prior to their inclusion
in the present study and the experimental protocol was approved by
the institutional ethics committee of Guizhou Medical University
Hospital. The characteristics of the study subjects are summarized
in Table I.
 | Table I.Characteristics of patients with AS
and HC. |
Table I.
Characteristics of patients with AS
and HC.
| Characteristic | AS patients
(n=65) | HC (n=20) |
|---|
| Age, years (mean ±
SD) | 27.8±8.5 | 31.4±7.4 |
| Sex
(male/female) | 55/10 | 15/5 |
| HLA-B27 positive,
n | 64 | 0 |
| BASDAI score (mean
± SD) |
4.38±1.73 | – |
Flow cytometry analysis
Peripheral venous blood samples were collected
following overnight fasting. Expression of human leukocyte antigen
(HLA)-B27 in T cells of patients with AS was characterized via flow
cytometry analysis. Fresh peripheral blood was incubated with mixed
fluorescein-labeled antibodies [4.2 µg/ml fluorescein
isothiocyanate (FITC)-anti-CD3 and 5.0 µg/ml phycoerythrin
(PE)-anti-HLA-B27; cat. no. 340183; BD Biosciences, Franklin Lakes,
NJ, USA] at room temperature for 15 min. Following lysis of
erythrocytes with hemolysin (cat. no. 349202; BD Biosciences) at
room temperature for 8 min and washing with PBS, white blood cells
were subjected to BD FACS Canto IIflow cytometry analysis using
FACSCanto Clinical software version 2.4 (BD Biosciences).
Peripheral venous blood in duplicate was incubated
with 12 µg/ml allophycocyanin (APC)-cyanine (CY)7-anti-CD4 (cat no.
341115; BD Biosciences), peridinin-chlorophyll-protein complex
(PERCP)-CY5.5-anti-CXCR5 (cat no. 562781; BD Pharmingen, San Diego,
CA, USA), 0.5 mg/ml FITC-anti-PD-1 (FITC-anti-CD279; cat no.
561035; BD Pharmingen; BD Biosciences) and 0.2 mg/ml PE-anti-ICOS
(PE-anti-CD278; cat no. 552146; BD Pharmingen; BD Biosciences)
antibodies in the dark at room temperature for 15 min. Control
cells were also from patients with AS and were incubated with 12
µg/ml APC-CY7-anti-CD4, 25 µg/ml PERCP-CY5.5-anti-immunoglobulin G
(IgG)1 (cat no. 347202; BD Biosciences), 50 µg/ml FITC-anti-IgG
(cat. no. 349041; BD Biosciences) and 50 µg/ml PE-anti-IgG1 (cat.
no. 349043; BD Bioscience) in the dark at room temperature for 15
min, which is a negative control of flow analysis. Controls were
isotyped, which helped eliminate non-specific fluorescent
interference in flow analysis. In addition, peripheral blood in
duplicate was stained with 0.05 mg/ml APC-anti-CD19 (cat. no.
340437; BD Biosciences), 8 µg/ml FITC-anti-CD27 (cat. no. 340424;
BD Biosciences), 25 µg/ml PE-CY7-anti-CD38 (cat. no. 335790; BD
Biosciences) or isotype-matched controls (BD Bioscience) at room
temperature for 15 min. Following lysis of erythrocytes with
hemolysin and washing with PBS, white blood cells were subjected to
FACS Canto II flow cytometry analysis using a FACSDiva software
version 6.1.3 (BD Biosciences).
Analysis of cytokine production
Peripheral blood in duplicate was stimulated with 50
ng/ml phorbol myristate acetate (PMA; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and 1 µg/ml ionomycin (Sigma-Aldrich; Merck
KGaA) with 10% fetal calf serum (Zhejiang Tianhang Biotechnology
Co., Ltd., Zhejiang, China) in RPMI 1640 medium (Boster Biological
Technology, Pleasanton, CA, USA) at 37°C in an incubator with 5%
CO2 for 1.5 h and subsequently cultured for a further
4.5 h in the presence of GlogiStop (cat. no. 554724; BD
Biosciences) at 37°C. As the expression of CD4 epitope would be
decreased following the stimulation of PMA and ionomycin (23), CD3+CD8−T was
used to represent CD3+CD4+ T cells. The
stimulated cells were incubated with 100 µg/ml FITC-anti-CD3 (cat.
no. 349201; BD Biosciences), 50 µg/ml PE-CY7-anti-CD8 (cat. no.
335822; BD Biosciences) and 3 µg/ml PE-anti-CD69 (cat. no. 341652;
BD Pharmingen; BD Bioscineces) antibodies in the dark at room
temperature for 15 min. Control cells were incubated with 100 µg/ml
FITC-anti-CD3, 50 µg/ml PE-CY7-anti-CD8 and 50 µg/ml PE-anti-IgG1
(cat. no. 349043; BD Biosciences) antibodies in the dark at room
temperature for 15 min. Following lysis of erythrocytes and washing
with PBS, the expression of CD69 in
CD3+CD8−(CD3+CD4+) T
lymphocytes was analyzed using the BD FACS Canto II flow cytometer.
When the proportion of
CD3+CD8−CD69+ T cells in
CD3+CD8− T lymphocytes was >90%, the
subsequent experiments were performed.
The stimulated cells (200 µl) in duplicate were
incubated with 100 µg/ml FITC-anti-CD3, 50 µg/ml PE-CY7-anti-CD8
and PERCP-CY5.5-anti-CXCR5 antibodies at room temperature for 15
min. The cells were subsequently fixed with 50 µl BD FACS
permeabilizing solution A (cat. no. 347692; BD Biosciences) at room
temperature for 5 min according to the manufacturer's protocol, and
then permeabilized with BD FACS permeabilizing solution B (cat. no.
347692; BD Biosciences) and incubated with PE-anti-IL-21 antibody
(cat. no. 562042; BD Pharmingen; BD Biosciences) or PE-anti-IgG1
antibody at room temperature for 20 min, followed by BD FACS Canto
II flow cytometry analysis of IL-21 expression.
Statistical analysis
Statistic analyses were performed using SPSS
(version 20.0; IBM Corp., Armonk, NY, USA). Normally distributed
data are expressed as the mean ± standard deviation, whereas skewed
data are presented as the median. Kolmogorov-Smirnov test was used
to assess the distribution of the data. In addition, Levene's test
was used to evaluate the homogeneity of variances. The significance
of the difference between multiple groups was performed using
one-way analysis of variance with a post-hoc Student-Newman-Keuls
test and the significance of difference between two groups was
evaluated with two-tailed Student's t-test. Spearman correlation
coefficient or Pearson correlation coefficient with two-tailed
P-values were determined in the analysis of correlations. Two-sided
P<0.05 was considered to indicate a statistically significant
difference.
Results
Increased percentages of
CD4+CXCR5+ cTfh cells in patients with
AS
To investigate the potential role of Tfh cells and
their surface molecules in the development of AS, the frequency of
peripheral blood CD4+ T and
CD4+CXCR5+ cTfh cells were characterized with
flow cytometry analysis (Fig. 1A).
It was demonstrated that there was a decreased frequency of
CD4+ T cells in patients with AS compared with HC
(P<0.01; Fig. 1B). conversely, it
was demonstrated that the percentages of
CD4+CXCR5+ cTfh cells,
CD4+CXCR5+PD-1+ and
CD4+CXCR5+ICOS+ T cells were
significantly increased in patients with AS compared with HC
(P<0.01 for all; Fig. 1C-E).
Furthermore, the percentage of CD4+CXCR5+
cTfh was significantly elevated in the high activity AS group
(BASDAI score ≥4; n=41) compared with that in the low activity AS
group (BASDAI score <4; n=24; P=0.001) and in HC (n=20;
P<0.001; Fig. 1C). However, there
was no significant difference observed in the frequency of
peripheral blood CD4+ T cells,
CD4+CXCR5+PD−1+ and
CD4+CXCR5+ICOS+ T cells between
the high and low activity AS groups (Fig. 1B, D and E).
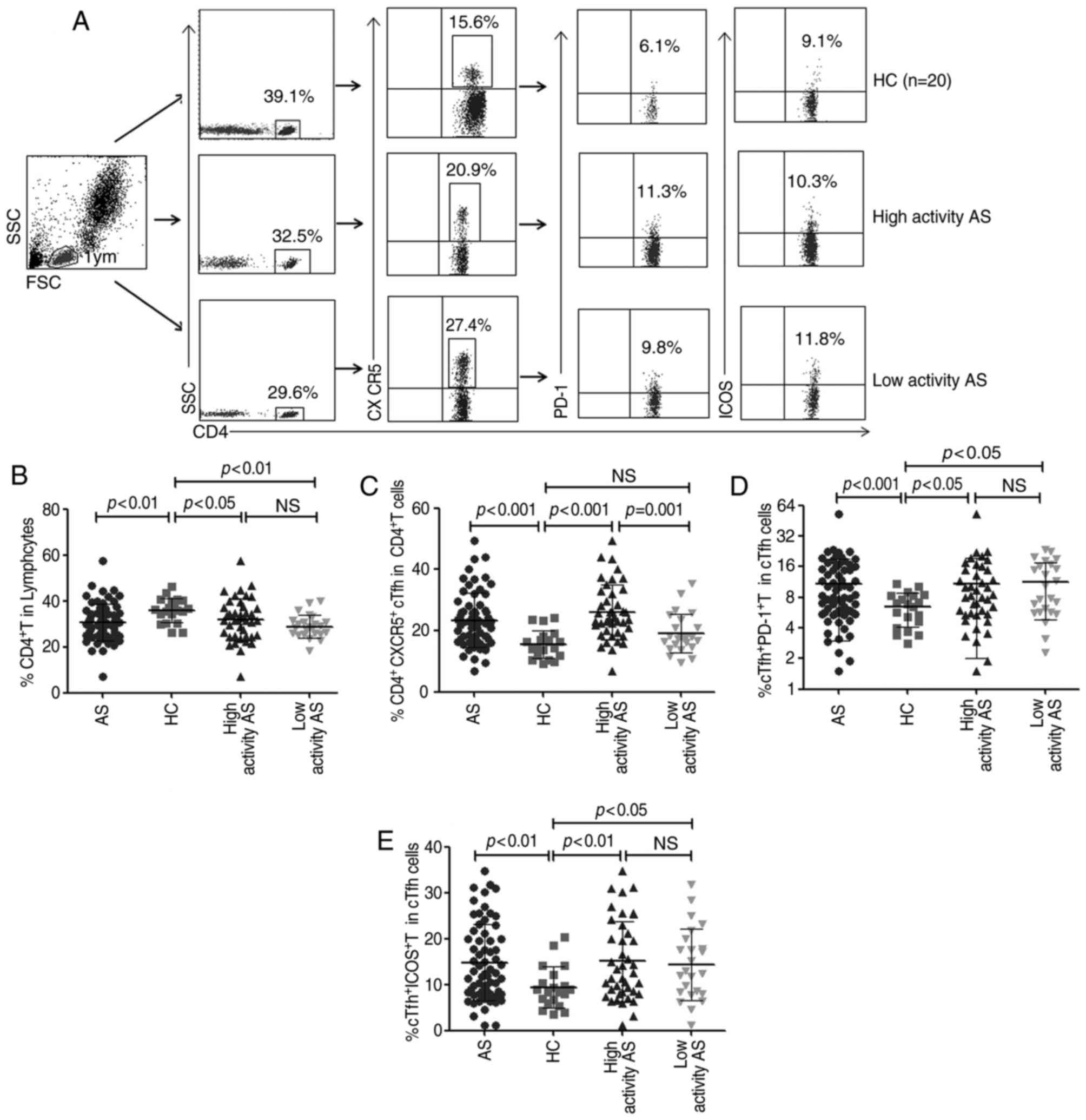 | Figure 1.Flow cytometry analysis of the
frequency of peripheral blood cTfh cells. Fresh peripheral blood
was stained with anti-CD4, anti-CXCR5, anti-PD-1 and anti-ICOS
antibodies. Following gating CD4+ T cells, frequencies
of CD4+CXCR5+ cTfh cells,
CD4+CXCR5+PD-1+ T cells and
CD4+CXCR5+ICOS+ T cells were
analyzed. (A) Representative images of flow cytometry analysis.
Percentages of (B) CD4+ T, (C)
CD4+CXCR5+ cTfh, (D)
CD4+CXCR5+PD-1+ T and (E)
CD4+CXCR5+ICOS+ T cells were
compared between patients with AS (n=65) and HC (n=20). cTfh,
circulating follicular helper T cells; CD, cluster of
differentiation; CXCR5, C-X-C chemokine receptor type 5; PD-1,
programmed death 1; ICOS, inducible T cell costimulator; NS, no
significant differences; HC, healthy controls; AS, ankylosing
spondylitis; SSC, side scatter data; FSC, forward scatter data. |
Increased percentage of
CD3+CD8−CXCR5+IL-21+ T
cells in patients with AS
To determine whether the lymphocytes were activated,
the expression of CD69 in stimulated T lymphocytes was measured
using flow cytometry, as presented in Fig. 2A. As such, these experiments were
performed only when the percentage of the
CD3+CD8−CD69+ T cells in
CD3+CD8− T cells was ≥90%.
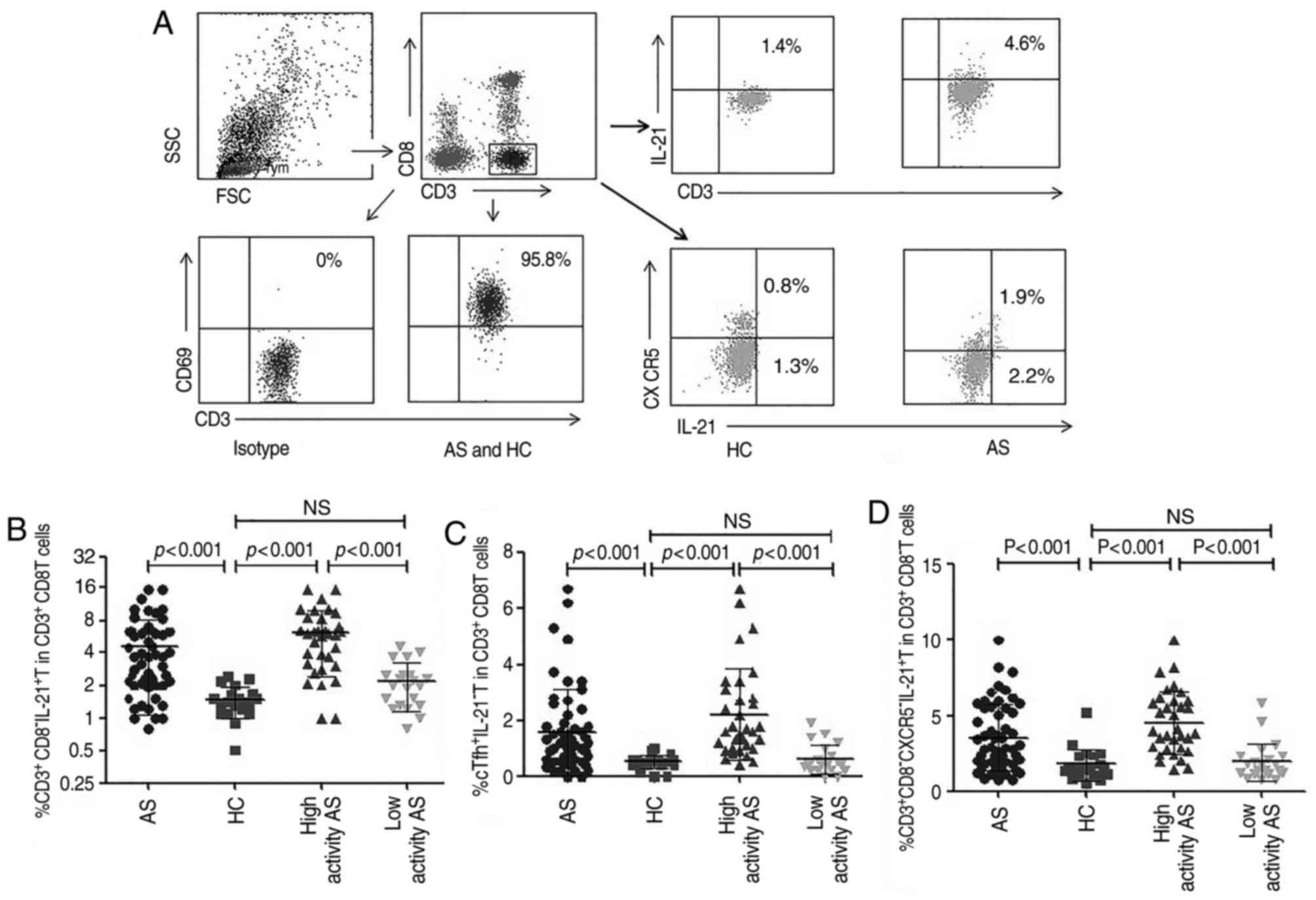 | Figure 2.Flow cytometry analysis of different
cellular sources of IL-21. The cells were characterized using flow
cytometry by gating living lymphocyte cells and then
CD3+CD8−T cells. The frequencies of
CD3+CD8−,
CD3+CD8−CXCR5−,
CD3+CD8−CXCR5+ T cells producing
IL-21 were then analyzed. (A) Representative flow cytometry
analysis. Percentages of (B) CD3+CD8−, (C)
CD3+CD8−CXCR5− and (D)
CD3+CD8−CXCR5+ T cells producing
IL-21 were compared between patients with AS (n=54; some patients
with AS failed the test and were not included in statistical
analysis) and HC (n=20). IL, interleukin; CD, cluster of
differentiation; CXCR5, C-X-C chemokine receptor type 5; NS, no
significant differences; HC, healthy controls; AS, ankylosing
spondylitis; SSC, side scatter data; FSC, forward scatter data. |
The intracellular production of IL-21 by
CD3+CD8−(CD3+CD4+) T
cells was initially assessed using flow cytometry (Fig. 2A), which revealed that
CD3+CD8−T cells producing IL-21 were
significantly increased in peripheral blood of patients with AS
compared with that in HC (P<0.001; Fig. 2B). As IL-21 is secreted by
CD4+ T cells, including Tfh cells, Th17 cells and
natural killer T cells (24), CXCR5
expression was used to dichotomize
CD3+CD8−IL-21+ T cells into
CD3+CD8−CXCR5+IL-21+ T
and
CD3+CD8−CXCR5−IL-21+ T
portions (Fig. 2A). Compared with
HC, the percentages of
CD3+CD8−CXCR5+IL-21+ T
and
CD3+CD8−CXCR5−IL-21+ T
cells were significantly increased in patients with AS (P<0.001
for all; Fig. 2C and D). The
percentage of
CD3+CD8−CXCR5+IL-21+ T
cells were significantly increased in the high activity AS group
(n=33; some patients with AS failed the test and were not included
in statistical analysis) compared with that in the low activity AS
group (n=21) and in HC (P<0.001 for all; Fig. 2C), and a similar increase was also
observed in the percentages of
CD3+CD8−IL-21+ T and
CD3+CD8−CXCR5−IL-21+ T
cells (P<0.001 for all; Fig. 2B and
D). This suggests that different cellular sources of IL-21 are
associated with the development of AS in this cellular
population.
Aberrant distribution of peripheral
blood B cell subtypes in patients with AS
As presented in Fig.
3, the distribution of B cell subtypes was also measured using
flow cytometry. The percentages of
CD19+CD27high plasmablasts and
CD19+CD38+ antibody-secreting B cells in
patients with AS were significantly higher than those in HC
(P<0.001 for all; Fig. 3C and F).
There was no significant difference in the frequency of
CD19+ B cells, CD19+CD27− naïve B
cells and CD19+CD27+ memory B cells between
patients with AS and HC (Fig. 3B, D and
E). The percentages of CD19+CD38+
antibody-secreting B cells and CD19+CD27−
naïve B cells were also significantly increased in the high
activity AS group (n=24) compared with the low activity AS group
(n=22) and HC groups (P<0.05; Fig. 3C
and D). In contrast, the frequency of
CD19+CD27+ memory B cells was significantly
decreased in the high activity patients with AS, compared with the
low activity AS group (P<0.05; Fig.
3E). Notably, the percentage of
CD19+CD38+ antibody-secreting B cells was
positively correlated with the BASDAI values in patients with AS
(r=0.329, P=0.007; Fig. 3G).
However, there was no significant correlation between the BASDAI
values and the frequency of other B cell subtypes in patients with
AS (data not shown).
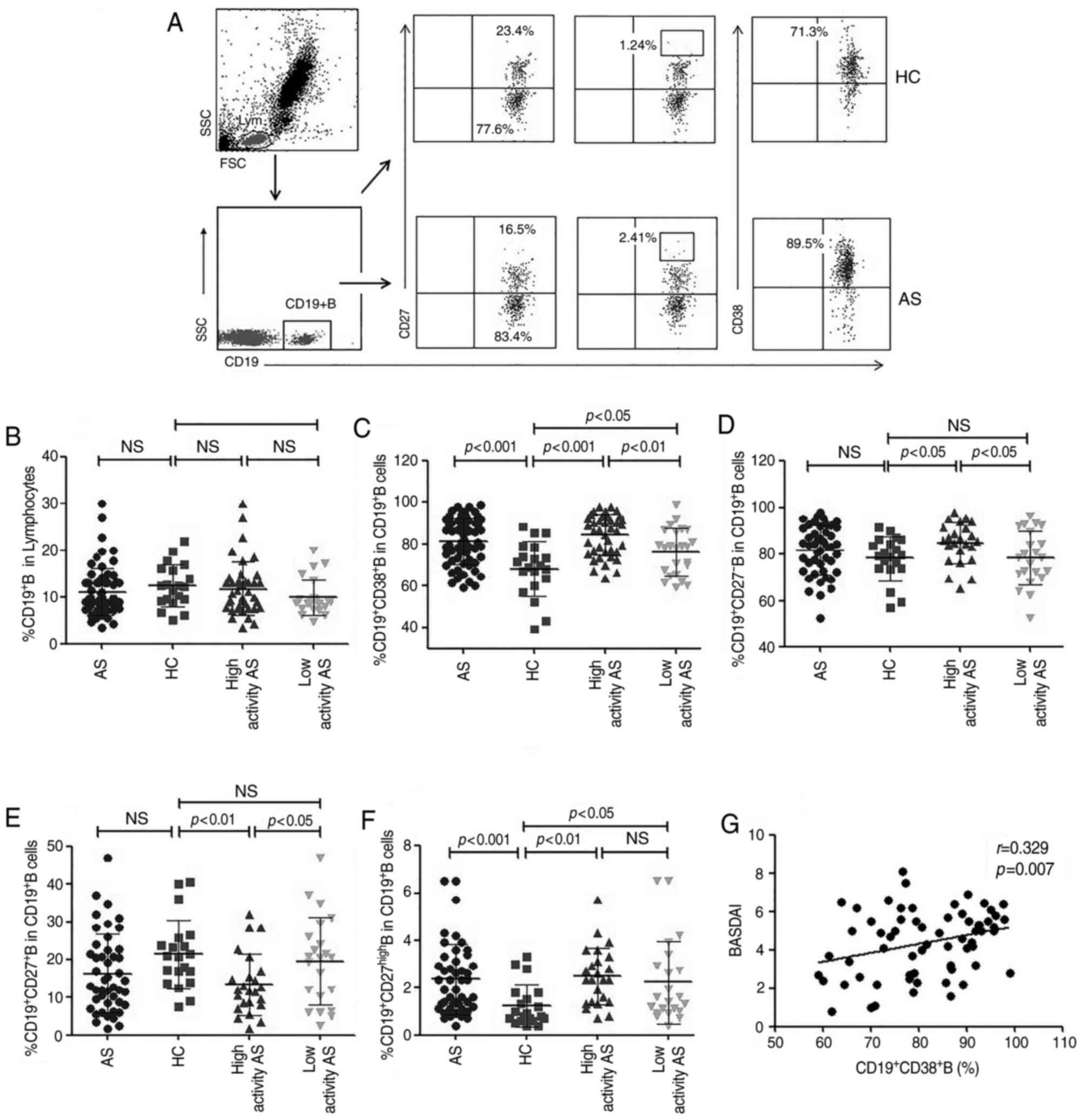 | Figure 3.Flow cytometry analysis of different
subtypes of B cells. Peripheral blood in duplicate was stimulated
with anti-CD19, anti-CD27 and anti-CD38 antibodies. After gating
CD19+B cells, frequencies of
CD19+CD27− naïve B cells,
CD19+CD27+ memory B cells,
CD19+CD27high plasmablast B cells and
CD19+CD38+ antibody-secreting B cells were
analyzed. (A) Representative flow cytometry analysis. Percentages
of (B) CD19+B and (C) CD19+CD38+
antibody-secreting B cells were compared between patients with AS
(n=65) and HC (n=20). Percentages of (D)
CD19+CD27− naïve B cells, (E)
CD19+CD27+ memory B cells and (F)
CD19+CD27high plasmablast B cells were
compared between patients with AS (n=46) and HC (n=20). (G)
Correlation analysis (n=65). CD, cluster of differentiation; NS, no
significant differences; HC, healthy controls; AS, ankylosing
spondylitis; SSC, side scatter data; FSC, forward scatter data;
BASDAI, Bath AS Disease Activity Index. |
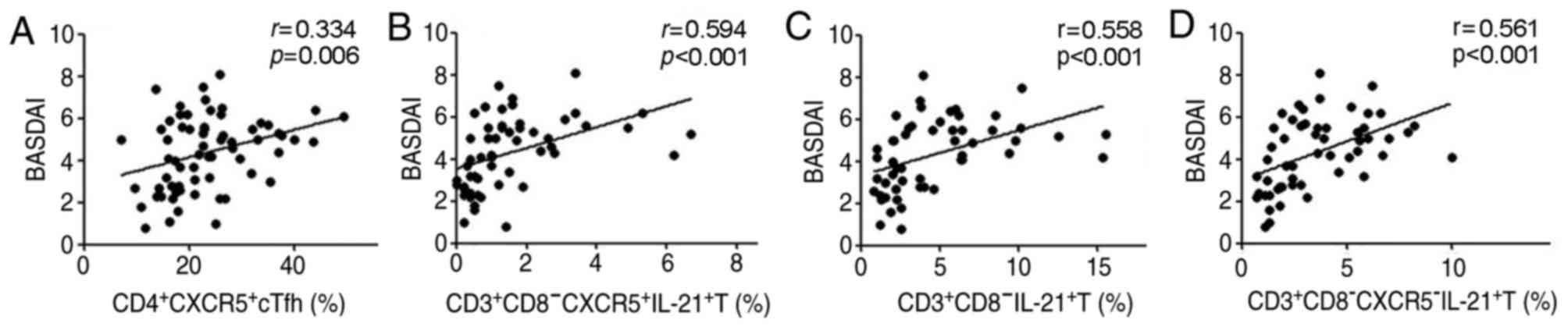
Increase in peripheral blood cTfh
cells was positively correlated with disease activities in patients
with AS
The percentage of CD4+CXCR5+
cTfh and
CD3+CD8−CXCR5+IL-21+ T
cells was positively correlated with the values of BASDAI
(r=0.334, P=0.006; r=0.594, P<0.001; Fig. 4A and B). A similar correlation was
observed in the percentages of
CD3+CD8−IL-21+ T and
CD3+CD8−CXCR5−IL-21+ T
cells with the BASDAI values (r=0.558, P<0.001;
r=0.561, P<0.001; Fig. 4C and
D). However, there was no significant correlation between
percentages of other cTfh cell phenotypes
(CD4+CXCR5+PD-1 and
CD4+CXCR5+ICOS+ T cells) and the
BASDAI values in patients with AS (data not shown).
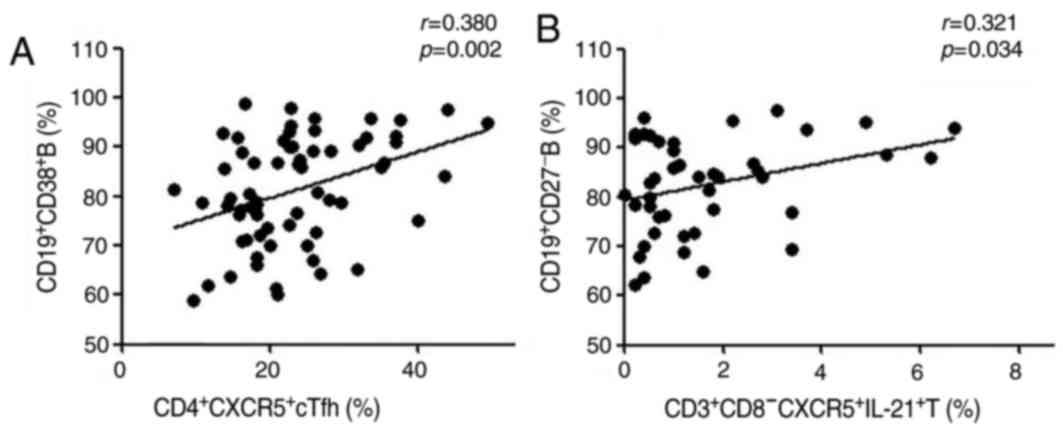
Association between cTfh cells and B
cells in patients with AS
The percentage of CD4+CXCR5+
cTfh cells was positively correlated with the frequency of
CD19+CD38+ antibody-secreting B cells in
patients with AS (r=0.38, P=0.002; Fig. 5A). Similarly, the percentage of
CD3+CD8−CXCR5+IL-21+ T
cells was also positively correlated with the frequency of
CD19+CD27− naïve B cells (r=0.321,
P=0.034; Fig. 5B). However, there
was no significant association between the percentages of other
phenotypes of cTfh cells and the subtypes of B cells evaluated in
these patients (data not shown). These data suggest that different
phenotypes of cTfh cells may have variable functions in regulating
the differentiation of B cells during AS development.
Discussion
Tfh cells have been recognized in recent years as a
crucial regulator of GC formation, B cell development and long-term
humoral memory generation (25–27), and
have a significant role in the pathogenesis of autoimmune diseases.
Previous studies (5,6) have characterized the frequency of
circulating Tfh cells in AS. However, the mechanisms by which cTfh
cells and associated molecules regulate B cell differentiation in
humans remain largely unknown. In the present study, it was
observed that the frequency of peripheral blood
CD4+CXCR5+ cTfh cells was significantly
higher in patients with AS than in HC. Furthermore, the frequency
of CD4+CXCR5+ cTfh cells was positively
correlated with BASDAI values in patients with AS. These results
improve upon those of previous studies by Xiao et al
(5) and Wu et al (6), which did not reveal the correlation of
CD4+CXCR5+ cTfh cells with BASDAI values in
patients with AS.
ICOS and PD-1 are expressed by Tfh cells (28) and are closely associated with the
function of Tfh cells (29–31). ICOS is known as a positive regulator
of Tfh cells and previous studies using a mouse model of AS
indicated that ICOS overexpression induces overproduction of
CD4+CXCR5+ Tfh cells and exuberant GC
responses, and promotes antibody production (32,33),
whereas PD-1 is a potent negative regulator of humoral immune
responses (31,34). The present study demonstrated
increased percentages of
CD4+CXCR5+PD-1+ T and
CD4+CXCR5+ICOS+ T cells in
patients with AS compared with those in HC. According to BASDAI
values, which are important for the evaluation of AS disease
activity, patients were categorized into high activity and low
activity AS groups. It was observed that higher percentages of
CD4+CXCR5+ICOS+ T cells were
present in the high activity AS group compared with the low
activity AS group. Conversely, lower percentages of
CD4+CXCR5+PD-1+ T cells were
detected in the high activity AS group compared with the low
activity AS group. However, differences between these two
comparisons were not significant. As expected, there was no
correlation between BASDAI values and the percentages of
CD4+CXCR5+ICOS+ T cells or
CD4+CXCR5+PD-1+ T cells. These
data suggest that high percentages of
CD4+CXCR5+ cTfh cells and expression of ICOS
and PD-1 may be associated with the pathogenesis of AS; however,
further research is required to elucidate the precise function of
ICOS and PD-1 in AS.
Subsequently, to better understand the role of Tfh
in the pathogenesis of AS, the main effector cytokines of Tfh cells
were investigated, including IL-21, which exists with IL-6,
regulates the differentiation of Tfh cells and induces B cell
proliferation and class switch recombination (24,35).
Xiao et al (5) previously
observed a higher frequency of peripheral blood IL-21 positive Tfh
cells in patients with AS-a result which the present findings
expand upon. The present study demonstrated that there were
significantly higher percentages of CD3+CD8−
IL-21+,
CD3+CD8−CXCR5+IL-21+
and
CD3+CD8−CXCR5−IL-21+ T
cells in patients with AS compared with HC. Notably, the
percentages of CD3+CD8− IL-21+,
CD3+CD8−CXCR5+IL-21+
and
CD3+CD8−CXCR5−IL-21+ T
cells were positively correlated with BASDAI values in patients
with AS. These findings suggest that regardless of cellular sources
of IL-21, they may participate in the development of AS.
The activation and differentiation of B cells
require help from Tfh cells. Different subtypes of B cell express
unique profiles of immunomodulatory factors and thereby evolve
distinct functions. CD19+CD27− B cells
display no maturity and are defined as naïve B cells (36). Upon gain of expression of CD27
(CD19+CD27+ B cells), the cells become
activated memory B cells (37).
CD19+CD27high B cells are defined as
plasmablasts, whereas upon the expression of CD38, the cells lead
to the antibody-secreting phenotype
(CD19+CD38+) (17,18). In
the present study, it was demonstrated that the percentages of
CD19+CD27high plasmablasts and
CD19+CD38+ antibody-secreting B cells were
increased in patients with AS, relative to HC, although there was
no significant difference in the frequency of CD19+ B
cells, CD19+CD27− naïve B cells, and
CD19+CD27+ memory B cells between patients
with AS and HC. These results suggest that, following antigen
stimulation, the distribution of B cell subtypes was changed and
the proportions of the plasmablasts and antibody-secreting B cells
were highly increased compared with other B cell subtypes in
patients with AS. This was likely due to a higher presence of
plasmablasts and antibody-secreting B cells following the high
differentiation of bone marrow stem cells in order to produce more
antibodies against a foreign antigen. The present findings were
partially consistent with a previous study by Lin et al
(15), which demonstrated that,
compared with controls, the percentages of CD19+ B cells
and subsets (CD19+CD27−,
CD19+CD27dim and
CD19+CD27high) were not significantly
different in patients with AS. These disparities may be due to a
number of reasons, including varying genetic backgrounds, disease
duration and cohort size.
The present study also demonstrated that, compared
with the low activity AS group, the high activity AS group
exhibited increased percentages of CD19+CD38+
antibody-secreting B cells and CD19+CD27− naïve B cells,
but a decreased percentage of CD19+CD27+
memory B cells. These results indicate that there may be an
association between B cell subtypes and BASDAI values. However, the
present results have only revealed a significant positive
correlation between the percentage of
CD19+CD38+ antibody-secreting B cells and
BASDAI values in patient with AS, which is in accordance with a
previous study (38). This suggests
that particular B cell subtypes may be associated with different
stages of AS disease pathogenesis and the percentage of
CD19+CD38+ antibody-secreting B cells may be
a potential biomarker to evaluate the disease activity of AS.
It has been reported previously that Tfh cells are
able to regulate B cell activation and promote B cell maturation in
autoimmune disease (8,20). In the present study, it was observed
that the percentage of CD4+CXCR5+ cTfh cells
was positively correlated with the percentage of
CD19+CD38+ antibody-secreting B cells in
patients with AS. The percentage of
CD3+CD8−CXCR5+IL-21+ T
cells was also correlated positively with the frequency of
CD19+CD27− naïve B cells in patients with AS.
These findings may be a clinical display of a previous in
vitro finding by Morita et al (12), which demonstrated that culturing
naïve B cells with CXCR5+CD4+ T cells yielded
higher numbers of CD38+CD19lo cells than with
CXCR5−CD4+ T cells. This indicated that
CXCR5+CD4+ T cells were more efficient at
inducing naive B cells to differentiate into CD38+B
cells. In addition, the study by Morita et al (12) also demonstrated that blood
CXCR5+CD4+ T cells secreted IL-21 upon
contact with naïve B cells. High percentages of
CXCR5+CD4+ T cells secrete more IL-21
following interaction with naïve B cells in patients with AS, as
the increased IL-21 promotes naïve B and memory B cells
differentiation into antibody-secreting B cells (39). This may explain why
antibody-secreting B cell levels markedly increased and more naive
B cells were released by bone marrow. Furthermore, this may be the
underlying reason for the significant positive correlations between
cTfh and B subtypes. These findings support the hypothesis that Tfh
cells may regulate the distribution of B cell subtypes via
different functional molecules; however, further research is
required to elucidate the precise molecular and immunological
mechanism.
In conclusion, the present findings suggest that
the percentages of cTfh cells and activated B cell subtypes are
significantly increased and positively correlated with disease
activity in patients with AS, and the percentage of cTfh cells is
positively correlated with particular B cell subtypes. The
limitations of the present study, which must be considered when
interpreting these results, include the relatively small sample
size and the lack of cell culture experiments on cTfh cells and B
cells. Further studies are required to address the relationship
between circulating Tfh cells and B cell subtypes in patients with
AS.
Acknowledgements
The authors would like to thank Dr Fred Bogott at
the Austin Medical Center (Austin, MN, USA) and Dr Joshua Liao at
Hormel Institute of University of Minnesota (Austin, MN, USA) for
their English language editing of this manuscript.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LM and SQL designed and directed the research. SQL
performed the experiments, analyzed and interpreted data, and
drafted the manuscript. DSW collected the experimental data and SWS
collected the clinical data. All authors read and approved the
final for publication.
Ethics approval and consent to
participate
All patients provided written informed consent
prior to their inclusion in the present study and the experimental
protocol was approved by the institutional ethics committee of
Guizhou Medical University Hospital (Guiyang, China).
Consent for publication
All patients provided written informed consent
prior to their inclusion in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Westerveld LA, Verlaan JJ and Oner FC:
Spinal fractures in patients with ankylosing spinal disorders: A
systematic review of the literature on treatment, neurological
status and complications. Eur Spine J. 18:145–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang C, Liao Q, Hu Y and Zhong D: T
lymphocyte subset imbalance in patients contribute to ankylosing
spondylitis. Exp Ther Med. 9:250–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang L, Li YG, Li YH, Qi L, Liu XG, Yuan
CZ, Hu NW, Ma DX, Li ZF, Yang Q, et al: Increased frequencies of
Th22 cells as well as Th17 cells in the peripheral blood of
patients with ankylosing spondylitis and rheumatoid arehritis. PLoS
One. 7:e310002012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu Y, Ren M, Yang R, Liang X, Ma Y, Tang
Y, Huang L, Ye J, Chen K, Wang P and Shen H: Reduced
immunomodulation potential of bone marrow-derived mesenchymal stem
cells induced CCR4+CCR6+Th/Treg cell subset imbalance in ankylosing
spondylitis. Arthritis Res Ther. 13:R292011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao F, Zhang HY, Liu YJ, Zhao D, Shan YX
and Jiang YF: Higher frequency of peripheral blood interleukin 21
positive follicular helper T cells in patients with ankylosing
spondylitis. J Rheumatol. 40:2029–2037. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu S, Yang T, Pan F, Xia G, Hu Y, Liu L,
Fan D, Duan Z, Ding N, Xu S, et al: Increased frequency of
circulating follicular helper T cells in patients with ankylosing
spondylitis. Mod Rheumatol. 25:110–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi JY, Ho JH, Pasoto SG, Bunin V, Kim
ST, Carrasco S, Borba EF, Gonçalves CR, Costa PR, Kallas EG, et al:
Circulating follicular helper-like T cells in systemic lupus
erythematosus: Association with disease activity. Arthritis
Rheumtol. 67:988–999. 2015. View Article : Google Scholar
|
|
8
|
Jin L, Yu D, Li X, Yu N, Li X and Wang Y
and Wang Y: CD4+CXCR5+ follicular helper T cells in salivary gland
promote B cells maturation in patients with primary Sjogren's
syndrome. Int J Clin Exp Pathol. 7:1988–1996. 2014.PubMed/NCBI
|
|
9
|
Ma J, Zhu C, Ma B, Tian J, Baidoo SE, Mao
C, Wu W, Chen J, Tong J, Yang M, Jiao Z, et al: Increased frequency
of circulating follicular helper T cells in patients with
rheumatoid arthritis. Clin Dev Immunol. 2012:8274802012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan X, Jin T, Zhao S, Liu C, Han J, Jiang
X and Jiang Y: Circulating CCR7+ICOS+ memory T follicular helper
cells in patients with multiple sclerosis. PLoS One.
10:e01345232015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hardtke S, Ohl L and Förster R: Balanced
expression of CXCR5 and CCR7 on follicular T helper cells
determines their transient positioning to lymph node follicles and
is essential for efficient B-cell help. Blood. 106:1924–1931. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morita R, Schmitt N, Bentebibel SE,
Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S,
Sabzghabaei N, et al: Human blood CXCR5(+)CD4(+) T cells are
counterparts of T follicular cells and contain specific subsets
that differentially support antibody secretion. Immunity.
34:108–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
King C: New insights into the
differentiation and function of T follicular helper cells. Nat Rev
Immunol. 9:757–766. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crotty S: T follicular helper cell
differentiation, function, and roles in disease. Immunity.
41:529–542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin Q, Gu JR, Li TW, Zhang FC, Lin ZM,
Liao ZT, Wei QJ, Cao SY and Li L: Value of the peripheral blood
B-cells subsets in patients with ankylosing spondylitis. Chin Med J
(Engl). 122:1784–1789. 2009.PubMed/NCBI
|
|
16
|
Wright C, Sibani S, Trudgian D, Fischer R,
Kessler B, LaBaer J and Bowness P: Detection of multiple
autoantibodies in patients with ankylosing spondylitis using
nucleic acid programmable protein arrays. Mol Cell Proteomics.
11:M9.003842012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marston B, Palanichamy A and Anolik JH: B
cells in the pathogenesis and treatment of rheumatoid arthritis.
Curr Opin Rheumatol. 22:307–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stasi R: Rituximab in autoimmune
hematologic diseases: Not just a matter of B cells. Semin Hematol.
47:170–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang YY, Zhang L, Zhao PW, Ma L, Li C, Zou
HB and Jiang YF: Functional implications of regulatory B cells in
human IgA nephropathy. Scand J Immunol. 79:51–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Shan Y, Jiang Z, Feng J, Li C, Ma
L and Jiang Y: High frequencies of activated B cells and T
follicular helper cells are correlated with disease activity in
patients with new-onset rheumatoid arthritis. Clin Exp Immunol.
174:212–220. 2013.PubMed/NCBI
|
|
21
|
Van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–368. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cardiel MH, Londoño JD, Gutiérrez E,
Pacheco-Tena C, Vázquez-Mellado J and Burgos-Vargas R: Translation,
cross-cultural adaptation, and validation of the Bath Ankylosing
Spondylitis Functional Index (BASFI), the Bath Ankylosing
Spondylitis Disease Activity Index (BASDAI) and the Dougados
Functional Index (DFI) in a Spanish speaking population with
spondyloarthropathies. Clin Exp Rheumatol. 21:451–458.
2003.PubMed/NCBI
|
|
23
|
Petersen CM, Christensen EI, Andresen BS
and Møller BK: Internalization, lysosomal degradation and new
synthesis of surface membrane CD4 in phorbol ester-activated
T-lymphocytes and U-937 cells. Exp Cell Res. 201:160–173. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Spolski R and Leonard WJ: Interleukin-21:
Basic biology and implications for cancer and autoimmunity. Annu
Rev Immunol. 26:57–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McHeyzer-Williams LJ, Pelletier N, Mark L,
Fazilleau N and McHeyzer-Williams MG: Follicular helper T cells as
cognate regulators of B cell immunity. Curr Opin Immunol.
21:266–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Linterman MA and Vinuesa CG: T follicular
helper cells during immunity and tolerance. Prog Mol Biol Transl
Sci. 92:207–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Crotty S: Follicular helper CD4 T cells
(TFH). Annu Rev Immunol. 29:621–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kerfoot SM, Yaari G, Patel JR, Johnson KL,
Gonzalez DG, Kleinstein SH and Haberman AM: Germinal center B cell
and T follicular helper cell development initiates in the
interfollicular zone. Immunity. 34:947–960. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bossaller L, Burger J, Draeger R,
Grimbacher B, Knoth R, Plebani A, Durandy A, Baumann U, Schlesier
M, Welcher AA, et al: ICOS deficiency is associated with a severe
reduction of CXCR5+CD4 germinal center Th cells. J Immunol.
177:4927–4932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tafuri A, Shahinian A, Bladt F, Yoshinaga
SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan
G, et al: ICOS is essential for effective T-helper-cell responses.
Nature. 409:105–109. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Good-Jacobson KL, Szumilas CG, Chen L,
Sharpe AH, Tomayko MM and Shlomchik MJ: PD-1 regulates
germinalcenter B cell survival and the formation and affinity of
longlived plasma cells. Nat Immunol. 11:535–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fazilleau N, Mark L, McHeyzer-Williams LJ
and McHeyzer-Williams MG: Follicular helper T cells: Lineage and
location. Immunity. 30:324–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu D, Rao S, Tsai LM, Lee SK, He Y,
Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, et al:
The transcriptional repressor Bcl-6 directs T follicular helper
cell lineage commitment. Immunity. 31:457–468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu H, Wang X, Lackner AA and Veazey RS:
PD-1(HIGH) follicular CD4 T helper cell subsets residing in lymph
node germinal centers correlate with B cell maturation and IgG
production in rhesus macaques. Front Immunol. 5:852014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eto D, Lao C, DiToro D, Barnett B, Escobar
TC, Kageyama R, Yusuf I and Crotty S: IL-21 and IL-6 are critical
for different aspects of B cell immunity and redundantly induce
optimal follicular helper CD4 T cell (Tfh) differentiation. PloS
One. 6:e177392011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Potter KN, Mockridge CI, Rahman A, Buchan
S, Hamblin T, Davidson B, Isenberg DA and Stevenson FK:
Disturbances in peripheral blood B cell subpopulations in
autoimmune patients. Lupus. 11:872–877. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Agematsu K, Nagumo H, Yang FC, Nakazawa T,
Fukushima K, Ito S, Sugita K, Mori T, Kobata T, Morimoto C and
Komiyama A: B cell subpopulations separated by CD27 and crucial
collaboration of CD27+ B cells and helper T cells in immunoglobulin
production. Eur J Immunol. 27:2073–2079. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Niu XY, Zhang HY, Liu YJ, Zhao D, Shan YX
and Jiang YF: Peripheral B-cell activation and exhaustion markers
in patients with ankylosing spondylitis. Life Sci. 93:687–692.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ettinger R, Sims GP, Fairhurst AM, Robbins
R, da Silva YS, Spolski R, Leonard WJ and Lipsky PE: IL-21 induces
differentiation of human naive and memory B cells into
antibody-secreting plasma cells. J Immunol. 175:7867–7879. 2005.
View Article : Google Scholar : PubMed/NCBI
|