Introduction
Severe ischemic brain damage belongs to a disease
type of cerebral infarction and is a global health problem
(1). According to statistics,
patients with this disease often suffer limb paralysis, fall into a
coma or even die. Its mortality rate is 20–35%, and the morbidity
rate and recurrence rate are also very high (2,3). At
present, the main drugs for the treatment of ischemic brain injury
are glutamate receptor antagonists, calcium channel blockers, free
radical scavengers, anti-inflammatory and anti-apoptotic drugs
(4,5). Although the relevant study has made
significant progress in many key mechanisms and processes of
injury, clinical trials of neurological protection in patients with
ischemic brain injury bring little effect (6). Over the past few years, it was found
that the neuroprotective drug monosialotetrahexosyl ganglioside
(GM1) promotes growth and repair the neurological impairment. As
reported, the exogenous GM1 has been used to promote the nervous
system cell regeneration and synapse formation (7,8).
However, the role of GM1 in patients with severe ischemic brain
injury is rarely reported. This study focused on the systematic
evaluation of the curative effect of GM1 in the treatment of
patients with severe ischemic brain injury.
Materials and methods
Data of patients
A total of 60 patients with severe ischemic brain
injury who were admitted to the Department of Emergency Medicine of
Jining Hospital (Jining, China) from June 2014 to March 2016 were
selected, and they were diagnosed by head computed tomography (CT)
and magnetic resonance imaging (MRI) and received routine
laboratory tests (such as erythrocyte sedimentation rate, white
blood cell count, urine detection), which are in line with the
relevant diagnostic criteria formulated in the Fourth National
Cerebrovascular Disease Conference. Under the condition that
patients or their family members signed the informed consent,
patients were randomly divided into the control group (n=30) and
the experimental group (n=30). In the control group, there were 14
males and 16 females with the average age of 54.9±5.4 years. In the
experimental group, there were 18 males and 12 females with the
mean age of 52.6±3.9 years. There were no statistically significant
differences between the two groups in terms of general data, and
the data were comparable. The study was approved by the Ethics
Committee of The First People's Hospital of Jining. Written
informed consents were signed by the patients and/or guardians.
Experimental grouping. Patients in the control group
received routine anti-infection and dehydration treatments to
reduce intracranial pressure and symptomatic and supportive
treatments were provided, thus preventing complications. Patients
in the experimental group were treated with intravenous infusion of
GM1 (Sai Dian; National Medicine Permission no. H20093980; 2 ml
each one; Beijing Science Sun Pharmaceutical Co., Ltd., Beijing,
China) with 2 ml each time and once a day for 14 days on the basis
of routine treatments.
Observational indexes
Detection of biochemical indexes
Ten milliliters whole blood of each patient was
taken intravenously before and after treatment. The blood was
coagulated at room temperature for 1 h. After centrifugation, the
serum was stored at 80°C, and the statistical monitoring was
conducted for all samples after collection.
Serum tumor necrosis factor-α (TNF-α) was measured
by horseradish peroxidase-labeled sandwich immunoassay. In short,
antibodies against TNF-α (75 kDa) were coated in each well of a
96-well plate and incubated after the addition of an appropriate
amount of serum. The content of enzyme and enzyme-bound TNF-α was
determined using tetramethyl-benzidine as the substrate. The
optical density (OD) values at the dual wavelengths of 450 and 600
nm were measured under the microplate reader, and the sample
concentration was calculated.
The experimental methods of Gao et al were
used for reference (9). The content
of superoxide dismutase (SOD) was determined by xanthine oxidase
assay, and the content of malondialdehyde (MDA) was detected by the
thiobarbituric acid method.
Observations of haemodynamics
The Medesonic Transpect thermal conductivity
detector (TCD) produced in the US was applied. Two megahertz pulsed
Doppler ultrasonography was used to detect peak velocity (Vp) and
mean velocity (Vm) from the temporal window at the depth of 50–65
mm, and the symmetry [peak velocity difference (DVp) and mean
velocity difference (DVm)] of both sides were observed.
Evaluation of clinical curative
effects
Study methods of Daousi et al were used for
reference for neurological deficit score (NDS) (10). The evaluation criteria for the
curative effect are based on the relevant criteria formulated in
the Fourth National Cerebrovascular Disease Conference (11), and judgments were made combined with
clinical symptoms and signs of patients. Total effective rate =
obviously effective rate + effective rate.
Statistical methods. The results were analyzed using
GraphPad Prism software Version 5.01 (GraphPad Software, San Diego,
Chile). Measurement data are expressed as mean ± SD, and
differences between indexes were detected using the paired t-test.
Count data were detected by the χ2 test. Pearson's
correlation coefficient was used to analyze the correlation between
TNF-α and NDS. P<0.05 was considered to indicate a statistically
significant difference.
Results
Detection of the expression level of
serum TNF-α by the enzyme-linked immunosorbent assay (ELISA)
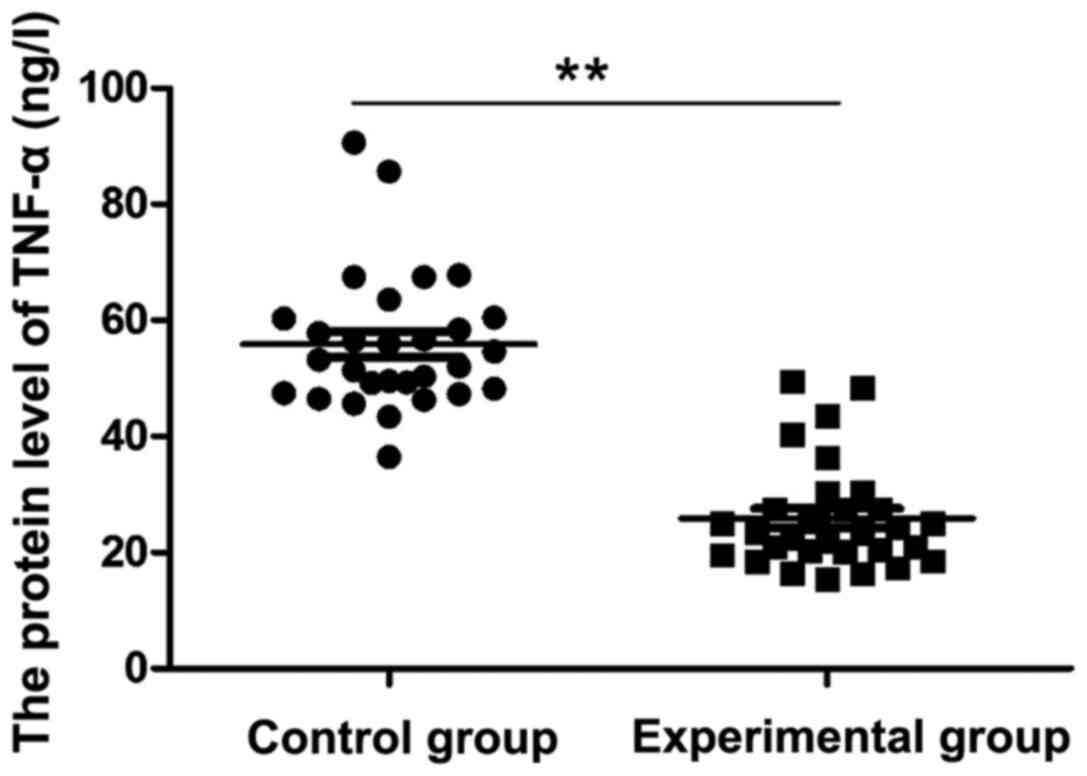
As shown in Fig. 1,
the serum TNF-α content in the experimental group was 25.89±9.157
ng/l after two weeks of GM1 treatment, while the serum TNF-α
content in the control group was 55.83±11.71 ng/l, which was
significantly higher than that in the experimental group
(P<0.05).
NDS
As shown in Table I,
the difference in the NDS between the experimental group and the
control group was not statistically significant before the
experiment (P>0.05). The NDS was 25.54±5.83 in the control group
and 16.34±8.41 in the experimental group after treatment, which was
significantly lower than that in the control group (P<0.05).
 | Table I.Comparison of the NDS between two
groups of patients. |
Table I.
Comparison of the NDS between two
groups of patients.
| Groups | No. | Before
experiment | After experiment | t-value | P-value |
|---|
| Control | 30 | 33.12±2.37 |
25.54±5.83a | 1.270 | 0.0431 |
| Experimental | 30 | 32.58±1.95 |
16.34±8.41a,b | 2.032 | 0.0092 |
| t-value |
| 0.652 | 1.745 |
|
|
| P-value |
| 0.347 | 0.0282 |
|
|
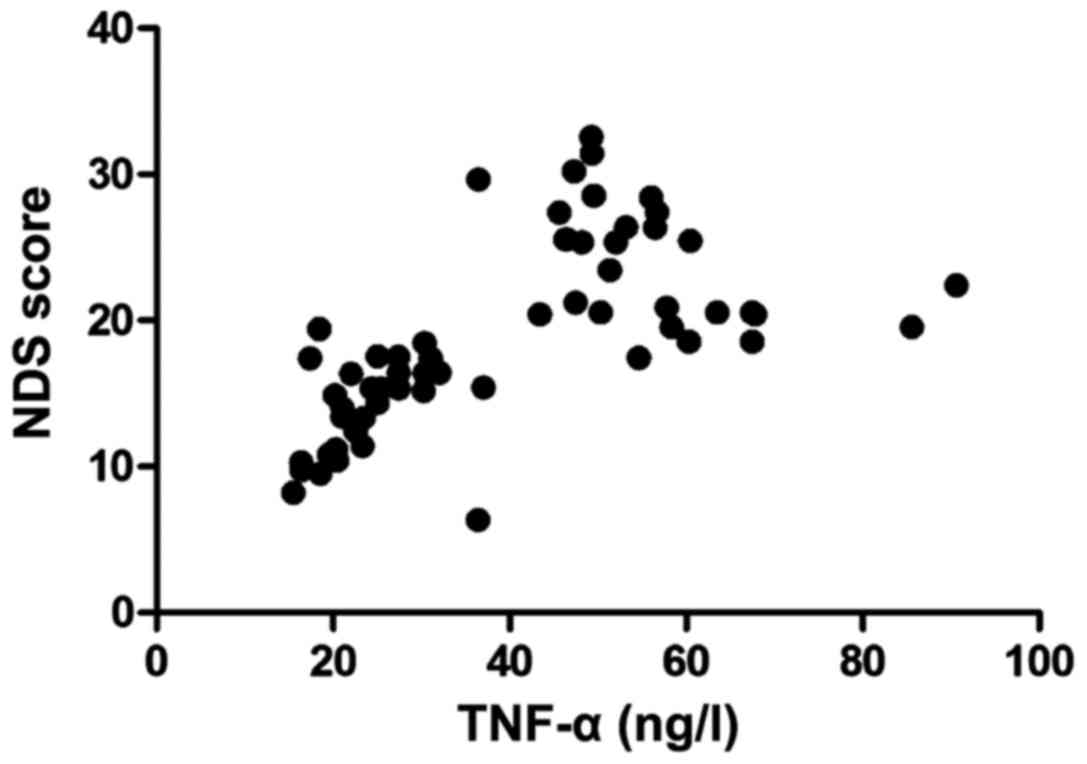
Correlation between the expression level of TNF-α
and NDS. Pearsons correlation coefficient (Fig. 2) showed that the content of TNF-α in
the patients was positively correlated with the NDS (r=4.321,
P<0.05).
Contents of serum MDA and SOD in two
groups of patients
As shown in Table
II, the contents of serum MDA and SOD in two groups of patients
were not statistically different before the experiment (P>0.05).
The content of serum MDA of patient in the experimental group was
lower than that in the control group, while the content of SOD was
significantly higher than that in the control group after treatment
(P<0.05).
 | Table II.Detection of contents of serum MDA and
SOD in two groups of patients (mean ± SD). |
Table II.
Detection of contents of serum MDA and
SOD in two groups of patients (mean ± SD).
| Detection time | No. | Groups | MDA (nmol/100
mg) | SOD (nmol/100
mg) |
|---|
| Before
experiment | 30 | Control | 87.18±5.92 | 184.25±10.04 |
|
|
| Experimental | 90.51±7.33 | 178.23±8.93 |
| After experiment | 30 | Control |
72.34±6.45a |
158.73±12.57b |
|
|
| Experimental |
50.81±10.88c,d |
120.75±6.82c,d |
Changes in hemodynamic parameters
before and after GM1 treatment
After GM1 treatment, the Vp and Vm in the blood of
patients in the experimental group were significantly increased
compared with those in the control group (P<0.05), while the DVp
and DVm in the experimental group were significantly decreased
compared with those in the control group (P<0.05) (Table III).
 | Table III.Detection of changes in hemodynamic
parameters of two groups of patients before and after GM1 treatment
(mean ± SD, mm/sec). |
Table III.
Detection of changes in hemodynamic
parameters of two groups of patients before and after GM1 treatment
(mean ± SD, mm/sec).
| Detection time | No. | Groups | Vp | Vm | DVp | DVm |
|---|
| Before
experiment | 30 | Control | 47.36±5.62 | 32.17±3.57 | 26.05±3.89 | 15.04±2.16 |
|
|
| Experimental | 48.92±4.77 | 33.23±3.61 | 25.58±2.80 | 14.43±2.22 |
| After experiment | 30 | Control |
67.23±11.40b |
38.76±4.03a |
15.63±3.52a |
7.32±1.42a |
|
|
| Experimental |
85.04±8.68c,d |
44.37±5.36a,e |
10.25±4.63b,e |
3.65±0.77b,d |
Comparison of the clinical recovery
time between two groups of patients
Main clinical manifestations of patients with severe
ischemic brain injury are disturbance of consciousness and
abnormalities in muscle tension and original reflexes and with the
alleviation of the disease, these symptoms will also improve.
Results of this study (Table IV)
revealed that the reflex recovery time, muscle tension recovery
time and consciousness recovery time in the experimental group were
significantly shorter than those in the control group (P<0.05),
and the differences were statistically significant (P<0.05).
 | Table IV.Comparison of the clinical recovery
time between two groups of patients (mean ± SD). |
Table IV.
Comparison of the clinical recovery
time between two groups of patients (mean ± SD).
| Groups | No. | Reflex recovery
time (day) | Muscle tension
recovery time (day) | Consciousness
recoverytime (day) |
|---|
| Control | 30 | 10.34±1.87 | 10.50±1.42 | 9.47±1.37 |
| Experimental | 30 |
6.83±3.26b |
8.62±2.73a |
5.22±1.99a |
| t-value |
| 3.34 | 1.85 | 4.36 |
| P-value |
| 0.028 | 0.041 | 0.023 |
Comparison of the clinical curative
effect between two groups of patients
After 2 months of treatment, the total effective
rate of patients in the control group was 66.67% and that of
patients in the experimental group was 87.18%. The results
(Table V) showed that the clinical
curative effect of the experimental group was better than that of
the control group (P<0.05).
 | Table V.Comparison of the clinical curative
effect between two groups of patients. |
Table V.
Comparison of the clinical curative
effect between two groups of patients.
| Groups | No. | Obviously
effective | Effective | Ineffective | Total effective
rate |
|---|
| Control | 30 | 12 | 9 | 11 | 63.33% |
| Experimental | 30 | 24 | 4 | 2 | 93.33%a |
| t-value |
|
|
|
| 3.61 |
| P-value |
|
|
|
| 0.017 |
Discussion
A pathological study has shown that ischemic injury
can lead to different types of neuronal cell primary and necrotic
cell death (11). The hippocampal
CA1 pyramidal neuron is found to be the most vulnerable and most
common injury, which is often the leading cause of memory
impairment in patients (12,13), and the cerebellar Purkinje cell
injury leads to torso ataxia of patients (14). With the prolongation of ischemic
time, the pyramidal cell layer, mitral neurons and striatum neurons
of the thalamus are damaged to varying degrees (15,16). In
this study, the NDS of patients was significantly decreased after
GM1 treatment, and the reflex recovery time, muscle tension
recovery time and consciousness recovery time were significantly
shorter than those in the control group, which suggested that GM1
significantly protects the nervous system of patients with severe
cerebral ischemic injury.
Under normal physiological conditions, oxygen free
radicals (oxygen free radicals, H2O2 and OH)
in the cells are produced in the cytoplasm and mitochondria, and
then are rapidly removed under the action of endogenous
anti-oxidants (SOD) (17). During
the hypoxia-ischemia, the amount of generated oxygen free radicals
is more than the nervous system can protect, so they cause brain
injuries by attacking polyunsaturated fats. If oxygen free radicals
cannot be removed in time, they will cause lipid peroxidation, thus
leading to the formation of lipid peroxides (MDA) so as to further
damage brain tissues (18). In the
present study, it was found that GM1 significantly increased the
level of SOD in patients while reducing MDA content, indicating
that GM1 can restore the balance of oxygen free radical reaction
and lipid peroxidation in patients.
Inflammatory mediators play a key role in the
pathogenesis of severe ischemic brain injury (19,20).
TNF-α is a proinflammatory cytokine. In previous studies, TNF-α
messenger ribonucleic acid (mRNA) has been confirmed to be
expressed in the brain at 1–4 h after hypoxia-ischemia. TNF-α at
high concentration causes neuronal apoptosis by mediating the
caspase-8 pathway, but reducing TNF-α receptors can decrease
neuronal injuries (21,22). The inhibition of TNF-α is beneficial
in maintaining neurological function and protecting nerve cells
from neurotoxicity. Consistent with these findings, the results of
this study showed that the level of serum TNF-α in the experimental
group was significantly decreased compared with that in the control
group and was positively correlated with the NDS, suggesting that
GM1 inhibits the expression of TNF-α and alleviates the
neurological function of ischemic brain injury, and TNF-α is also a
clinical index to evaluate the neurological function of patients
with ischemic brain injury.
In conclusion, this study revealed that good
clinical benefits were achieved using GM1 in the treatment of
severe ischemic brain injury. Other studies have shown that GM1
inhibits TNF-α level in patients, reduces systematic NDS, improves
patient blood flow, regulates the balance of oxygen free radical
responses and lipid peroxidation in patients and significantly
shortens the clinical recovery time of patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL and YiZ designed, conducted the study and
analyzed the data. FL and YiZ wrote the manuscript. FL and YuZ
collected and analyzed the fundemental data of patients. XS
interpreted the biochemical indexes, haemodynamics and evaluation
of clinical curative effects. GZ conducted the measurements of
serum TNF-α and YL evaluated the neurological defcit score (NDS).
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First People's Hospital of Jining (Jining). Written informed
consents were signed by the patients and/or guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Broughton BRS, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kahlert P, Knipp SC, Schlamann M,
Thielmann M, Al-Rashid F, Weber M, Johansson U, Wendt D, Jakob HG,
Forsting M, et al: Silent and apparent cerebral ischemia after
percutaneous transfemoral aortic valve implantation: A
diffusion-weighted magnetic resonance imaging study. Circulation.
121:870–878. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beck H and Plate KH: Angiogenesis after
cerebral ischemia. Acta Neuropathol. 117:481–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hossmann KA: Treatment of experimental
cerebral ischemia. J Cereb Blood Flow Metab. 2:275–297. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maher J and Hachinski V: Hypothermia as a
potential treatment for cerebral ischemia. Cerebrovasc Brain Metab
Rev. 5:277–300. 1993.PubMed/NCBI
|
|
6
|
Ginsberg MD: Current status of
neuroprotection for cerebral ischemia: Synoptic overview. Stroke.
40 Suppl 3:S111–S114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ledeen RW and Wu G: The multi-tasked life
of GM1 ganglioside, a true factotum of nature. Trends Biochem Sci.
40:407–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Q, Song YH, Tang Z, Wang ZP, Xu Q and
Bao N: Effects of ganglioside GM1 and neural growth factor on
neural stem cell proliferation and differentiation. Genet Mol Res.
Aug 5–2016.(Epub ahead of print). doi: 10.4238/gmr.15038376.
|
|
9
|
Gao M, Ding H, Zhong G, Lu J, Wang H, Li Q
and Wang Z: The effects of transrectal radiofrequency hyperthermia
on patients with chronic prostatitis and the changes of MDA, NO,
SOD, and Zn levels in pretreatment and posttreatment. Urology.
79:391–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Daousi C, Benbow SJ, Woodward A and
MacFarlane IA: The natural history of chronic painful peripheral
neuropathy in a community diabetes population. Diabet Med.
23:1021–1024. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu X: Summary of the Fourth National
Cerebrovascular Disease Conference. Stroke Nerv Dis:. 4:105–109.
1997.
|
|
12
|
Niizuma K, Yoshioka H, Chen H, Kim GS,
Jung JE, Katsu M, Okami N and Chan PH: Mitochondrial and apoptotic
neuronal death signaling pathways in cerebral ischemia. Mol Basis
Dis. 1802:92–99. 2010. View Article : Google Scholar
|
|
13
|
Wang JY, Xia Q, Chu KT, Pan J, Sun LN,
Zeng B, Zhu YJ, Wang Q, Wang K and Luo BY: Severe global cerebral
ischemia-induced programmed necrosis of hippocampal CA1 neurons in
rat is prevented by 3-methyladenine: A widely used inhibitor of
autophagy. J Neuropathol Exp Neurol. 70:314–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang F and Chen J: Leptin protects
hippocampal CA1 neurons against ischemic injury. J Neurochem.
107:578–587. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee JJ, Li L, Jung H and Zuo Z:
Postconditioning with isoflurane reduced ischemia-induced brain
injury in rats. Anesthesiology. 108:1055–1062. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
del Zoppo GJ and Zoppo G: Inflammation and
the neurovascular unit in the setting of focal cerebral ischemia.
Neuroscience. 158:972–982. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei L, Fraser JL, Lu ZY, Hu X and Yu SP:
Transplantation of hypoxia preconditioned bone marrow mesenchymal
stem cells enhances angiogenesis and neurogenesis after cerebral
ischemia in rats. Neurobiol Dis. 46:635–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Niatsetskaya ZV, Sosunov SA, Matsiukevich
D, Utkina-Sosunova IV, Ratner VI, Starkov AA and Ten VS: The oxygen
free radicals originating from mitochondrial complex I contribute
to oxidative brain injury following hypoxia-ischemia in neonatal
mice. J Neurosci. 32:3235–3244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar A, Mittal R, Khanna HD and Basu S:
Free radical injury and blood-brain barrier permeability in
hypoxic-ischemic encephalopathy. Pediatrics. 122:e722–e727. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Candelario-Jalil E, Yang Y and Rosenberg
GA: Diverse roles of matrix metalloproteinases and tissue
inhibitors of metalloproteinases in neuroinflammation and cerebral
ischemia. Neuroscience. 158:983–994. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iadecola C and Alexander M: Cerebral
ischemia and inflammation. Curr Opin Neurol. 14:89–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Günther C, Buchen B, He GW, Hornef M,
Torow N, Neumann H, Wittkopf N, Martini E, Basic M, Bleich A, et
al: Caspase-8 controls the gut response to microbial challenges by
TNF-α-dependent and independent pathways. Gut. 64:601–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|