Introduction
Asthma is an inflammatory lung condition
characterized by an exaggerated response of the airways, which
remodelling is one of the most common medical pathologies of long
duration. Allergic asthma is an inflammatory lung disease
characterized by an abnormal response of lymphocytes T-helper 2
(TH2) after the inhalation of antigens (1,2).
It is well-established that a strong correlation
exists between the presence of eosinophils and the presence of Th2
cells in the asthmatic airways and that classical Th2 cell-derived
cytokines, namely interleukin (IL)-4, IL-5, IL-9 and IL-13, play
critical roles in orchestrating and amplifying allergic
inflammation in asthma (3).
More recently, roles for basophils, iNKT cells, Th17
cells, and a number of soluble mediators, including TSLP, IL-25,
and IL-33, have also been proposed (4,5).
MicroRNAs (miRNAs) are small non-coding RNAs that
regulate the function of the innate immune cells by controlling the
stability and translation of mRNA in health and disease (6). The emerging role of miRNAs as
biological agents in regulating immune and inflammatory responses
in the lung has been recently reviewed (7,8). The
main results indicate that these lung disorders can be attributed
to abnormal immune responses to environmental stimuli and
infections (9). Therefore,
understanding the host natural systems of innate defences and
regulating systems is essential for the development of new
therapeutic approaches. In this regard, there is growing interest
in the role of miRNAs in the regulation of host natural innate
immune defence responses and in the inflammatory sequels of the
respiratory disease.
The use of these miRNAs is opening a promising novel
biological approach to improve asthma processes (10). In fact, a subset of miRNAs has been
identified as potential therapeutic targets in asthma patients
(1,11,12).
Their role in regulating the response to corticosteroids and airway
hyper-responsiveness has also recently been verified. For example,
the microRNA miR-9 regulates the glucocorticoid receptor signalling
and the hyper-responsiveness of the airway resistant to steroids.
Very recently, it has also been proposed that modulating the
function of miR-9 could be a novel approach to the treatment of
asthma, even for the patients who are resistant to steroid therapy
(13).
Sublingual immunotherapy (SLIT) has been also
reported to be effective and safe in the treatment of allergic
rhinitis, in a systematic review type meta-analysis on the
treatment of asthma, although the magnitude of effect reported was
not very large (14). At the moment,
there is an increasingly broad consensus to promote SLIT, and to
consider SLIT as a safe alternative to subcutaneous therapy route
(15).
Materials and methods
The Bio Immune(G)ene Medicine
[BI(G)MED] as diagnostic method
As part of BI(G)MED therapeutic protocols, two
specific biological laboratory tests are essentially used and
systematically performed, regardless of diagnosis.
First a protein profile, as the blood protein
profile provides a good overview of the humoral immune status.
Second, a lymphocyte typing, as the characterization of lymphocytes
allows to evaluate the cellular immune status.
This modality helps the healthcare provider to look
at the overall response of the immune system of the patient and
thereby act upon biological evidence-based to perform subsequent
follow-up monitoring.
The two tests mentioned above are complemented by
studies to analyse the existence of pathogenic microorganisms
(bacterial, viral, fungal and parasitic). The existence of a
reactivation of microbial agents must be identified by serological
tests as a priority in all asthmatic process. Amongst the microbial
agents, two of them have to be considered as a priority:
Respiratory Syncytial Virus (RSV) and Rhinoviruses
(16) as well as Chlamydia
pneumoniae and Mycoplasma pneumoniae (17). It has to be taken into account that
most pathogens have the potential to trigger or worsen into more or
less a clinically latent asthmatic condition. In this context, we
think that the presence and reactivation of Epstein Barr
Virus (EBV) must always be explored.
Furthermore, in the case of bronchial asthma, it is
essential to assess the levels of immunoglobulin E (IgE). The
measure of IgE total and/or specific IgE allows to identify the
potential of allergens involved in triggering the asthmatic
process; nevertheless, the bacterial and viral serology tests are
the main tests that can help to clarify the pathogenesis of the
bronchial asthmatic process (18).
Only once a careful clinical and biological
diagnosis has been carried out, is it possible to identify which
BI(G)MED therapeutic protocol is best suited for modulating and
improving the cell imbalances found in the asthmatic patient, in
terms of: Th2 predominant polarity,
Th17/Tregs disbalance and/or regulation of the
intestinal barrier.
The BI(G)MED targets are several types of cells
belonging to the innate immune system (i.e., eosinophils,
basophils, CDs and macrophages), as well as the pathogens found in
serological tests, either bacteria and/or viruses (19).
The BI(G)MED as a nanobiotherapy
method
Nanotechnology is a growing sector. It uses
nanovectors capable to transport an active substance where it
should act in the organism, in order to increase its effectiveness
while minimizing side effects. The BI(G)MED-nanovectors are
so-called xylitol globules produced and analysed by Remedy Bank
(Hoboken, Belgium), that will be given on a sublingual way to reach
immediately the pharyngeal immune structures.
The BI(G)MED is included in this scientific field
and relies on different methods and concepts. There are five
fundamental pillars of BI(G)MED.
Nanomedicine refers to biomedical and pharmaceutical
applications of nanosized cargos of drugs/vaccine/DNA therapeutics
including nanoparticles, nanoclusters, and nanospheres. Such
particles have unique characteristics related to their size,
surface, drug loading, and targeting potential. This therapeutic
approach is already well known in oncology (20,21).
Synthetic Biology is a high biotechnology field
situated between molecular biology, organic chemistry, scientific
engineering, nanobiotechnology and information technology. The aim
of synthetic biology is to design and produce new biological parts,
devices and systems as well as to re-shape what already exists in
natural biological systems that have a proposed utility. By
genetically manipulating the biosynthetic machinery involved in the
assembly of natural products and exploiting Nature's strategies for
synthesizing structurally diverse metabolites, compounds with
enhanced biological features can be produced that were otherwise
inaccessible using traditional synthetic methods (22).
The concept of hormesis belongs to classical
pharmacology and helped to explain why the majority of substances
have a reserved effect when they are diluted; ‘Hormesis is now
generally accepted as a real and reproducible biological
phenomenon, being highly generalized and independent of biological
model, endpoint measured and chemical class/physical stressor. The
quantitative features of the hormetic dose response are generally
highly consistent, regardless of the model and mechanism, and
represent a quantitative index of biological plasticity at multiple
levels of biological organization’ (23,24).
Ab initio molecular dynamics simulation, improved to
its current stage where the analysis of existing processes and the
prediction of further chemical features and real-world processes
are feasible (25), explains the
dilution revitalization (dynamisation) process through molecular
acceleration which is the interaction source with the aqueous
substrate (26).
RNA interference is one of the most important
epigenetic processes, preserved during evolution and responsible,
through its post-transcriptional repression route, for the
suppression of gene expression (27). The most important feature today of
BI(G)MED is given by the therapeutic use of miRNAs.
The BI(G)MED scientific study described in this
manuscript applies all these basic concepts to allergy and
bronchial asthma at a diagnostic and therapeutic level. It works on
well-known key cellular events and signalling pathways.
Characteristics of patients who
participated in the study
We performed a multicentre study which involved 61
patients from private medical offices in several European
countries, including Germany, Austria, Belgium, France and Spain.
The sample included male and female patients of all ages in the
same proportion. That had a process of persistent bronchial asthma
or allergic asthma, whose evolution had started at least two years
ago. Patients who had asthma due to exercise have been specifically
excluded from this study.
In the selection of patients it has been taken into
consideration that they were not polymedicated by other diseases
and in particular, that they had not ruled on a routine basis in
the conventional treatment with β-mimetic agents but ruled only in
case of acute asthma attacks.
Inclusion criteria were as follows: Any age and
gender, regular asthma since at least 2 years prior to starting the
study, treatment with corticosteroids (oral or inhaled) for at
least two years.
Exclusion criteria, on the other hand, were as
follows: Other conventional treatments for asthma (i.e., treatment
with theophylline), coexistence of other illnesses of the immune
system or chronic infections, patients undergoing chronic treatment
with psychotropic drugs, patients unable to follow the study for
whatever reason both physical or mental (in order to make sure
there will be a good adhesion to the treatment).
Duration of the study
The study took place in 2016 over a 6-month period.
During this time patients included in the study followed a BI(G)MED
protocol treatment described later in detail. Four medical controls
were carried out during the study, one at study entry and then
follow-up checks were performed every 2 months.
BI(G)MED protocol and BIMUREGs used in
this study
There were five Bio Immune(G)ene Regulators
(BIMUREGs) used to improve the asthma process in this study. All
these BIMUREGs were prepared strongly according to the nanobiologic
method of dilution-dynamisation and all were certificated GPP.
Their composition is as described in Table I. The therapeutic protocol followed
for all patients of this study was as described in Table II.
 | Table I.BIMUREGs used in the study and their
composition. |
Table I.
BIMUREGs used in the study and their
composition.
| Compounds | Concentration
(Mol) |
|---|
| BIMUREG 1 |
|
| IL-4 |
1×10−10 |
| IL-5 | ″ |
| IL-9 | ″ |
|
IL-13 | ″ |
| EGF | ″ |
| PGD2 | ″ |
|
GM-CSF | ″ |
|
TNF-α | ″ |
| DNA (ADAM
33) | ″ |
| RNA
(miR-9, −19a, −155) | ″ |
| BIMUREG 2 |
|
| IL-4 |
1×10−10 |
| IL-5 | ″ |
|
IL-10 | ″ |
|
IL-25 | ″ |
|
TGF-β |
1×10−8 |
| Notch
gene | ″ |
|
CTLA-4 | ″ |
| DNA
(CTLA-4, Notch) | ″ |
| RNA
(miR-21, −106a, −126) | ″ |
| BIMUREG 3 |
|
| IL-3 |
1×10−10 |
| IL-5 | ″ |
| IL-9 | ″ |
| IL-6 |
1×10−8 |
|
IFN-γ | ″ |
|
TGF-β | ″ |
|
miR-126 | ″ |
| DNA
(IL-5) | ″ |
| RNA
(let-7, miR-145, −223) | ″ |
| BIMUREG 4 |
|
|
IL-4Rα |
1×10−10 |
|
IL-5 | ″ |
|
IFN-γ | ″ |
|
LTC4 | ″ |
|
PGD2 | ″ |
|
HISTAMINE | ″ |
|
miR-223 | ″ |
| DNA
(GATA-1) | ″ |
| RNA
(miR-132, miR-221, −222) | ″ |
| BIMUREG 5 |
|
|
IL-4 |
1×10−10 |
|
IL-10 | ″ |
|
TNF-α | ″ |
|
TGF-β |
1×10−8 |
|
IL-1RA | ″ |
| RNA
(miR-146a) | ″ |
 | Table II.Therapeutic protocol. |
Table II.
Therapeutic protocol.
| Time of day | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday |
|---|
| Morning | BIMUREG 2 |
| BIMUREG 2 |
| BIMUREG 2 |
|
| Afternoon |
| BIMUREG 3 |
| BIMUREG 3 |
| BIMUREG 3 |
| Evening | BIMUREG 1 | BIMUREG 1 | BIMUREG 1 | BIMUREG 1 | BIMUREG 1 | BIMUREG 1 |
All participants were instructed with
recommendations for dosage, time and way of intake of the drugs.
Opening the capsule that contains the BI(G)MED-formulas, then
pouring its contents under the tongue till it's fully absorbed by
the oral mucosa. Not to combine two BI(G)MED products at a time. To
wait at least for half an hour in between the two doses. To always
take BI(G)MED medication between meals, at least half an hour
before eating.
In case of acute asthma episode, the following
BI(G)MED-formulas were also used in combination: BIMUREG 4 +
BIMUREG 5, two or three times daily alternately. The parallel use,
or not, of Homeopathy and/or Phytotherapy, among other
complementary therapies, was assessed. And the eventual use of
β-mimetics and exceptionally corticosteroid, was also valued.
Monitoring and control parameters used
to assess clinical symptoms
The following clinical and biological parameters
were controlled regularly, i.e., at the beginning of the study, at
months 2 and 4 and at the end of the study (i.e., after 6 months of
treatment with BI(G)MED-formulas).
The clinical course was assessed by a BI(G)MED
standardized questionnaire for asthma (Table III) with evaluation of wheezing
(sibilances), cough, expectoration (cough-up), acute asthma crisis,
dyspnea (breathless), as well as value of total IgE and percentage
of eosinophils in peripheral blood.
 | Table III.BI(G)MED standardised questionnaire
for asthma multicentre study in reduced format. |
Table III.
BI(G)MED standardised questionnaire
for asthma multicentre study in reduced format.
To evaluate the progress of the clinical symptoms,
the standardised BI(G)MED questionnaire (Table III) was designed and translated
into different EU languages. Patient's consent for participating in
this study and for publication of material related was obtained
from all study participants. Patients names were not included, only
initials, age, sex and a number of questions for statistical
analysis purposes. Only a minimum set of key clinical tests was
included to facilitate the follow up in all cases.
It is important to specify here that all study
participants were informed according to the principles of the
Declaration of Helsinki, and that all doctors involved in this
study officially declare having obtained oral consent from all
patients.
Besides, due to the type of nanopreparations used as
treatment throughout the study, no harmful side effects were to be
expected, which was specified upfront to all the patients.
Finally, in the context of a study based on patients
coming from private practices only, no ethical committee (involved
in monitoring studies taking place in hospitals) could be
solicited.
Results
The statistical analysis has been carried out on the
XIstat programme, version 2015.2. The variables that have been
valued in this study are: Wheezing (sibilances), cough,
expectoration (cough-up), acute asthma crisis, dyspnea
(breathless), total IgE, percentage of eosinophils and inhaled use
of corticosteroids.
The data has been taken from 61 patients over 6
months. The variables described before have been valued
specifically at the start of the study (time 0), at 2 months (time
2), at 4 months (time 4), and at 6 months (time 6). Depending on
the nature of the variable, two types of analysis have been carried
out.
For the variables of the dichotomous type Yes/No
(i.e., wheezing, cough, expectoration, acute crisis) an analysis
was done to compare the percentage of YES recorded at each stage of
the study and the difference between these percentages have been
compared to evaluate when they are significantly different and when
they are not.
For the numeric type variables (i.e., dyspnea, total
IgE, eosinophils % and inhaled corticosteroids) a mixed model
analysis with repeated measurements has been carried out because
the data was obtained from the same patients at different times. In
this case, what has been evaluated is if the results obtained in
these variables were statistically different overtime, and in
particular, at what specific moments they were significantly
different.
The main difference between one analysis and another
is that in the first one percentages are compared while averages
are compared in the second one. No analysis has been carried out
for the variable oral corticosteroid because the great majority of
data is 0 and it doesn't make sense to analyse them by statistical
inference. In any case, we discuss the results obtained in the few
cases that patients do not have values 0. For all the comparisons
carried out a 5% level of error has been applied.
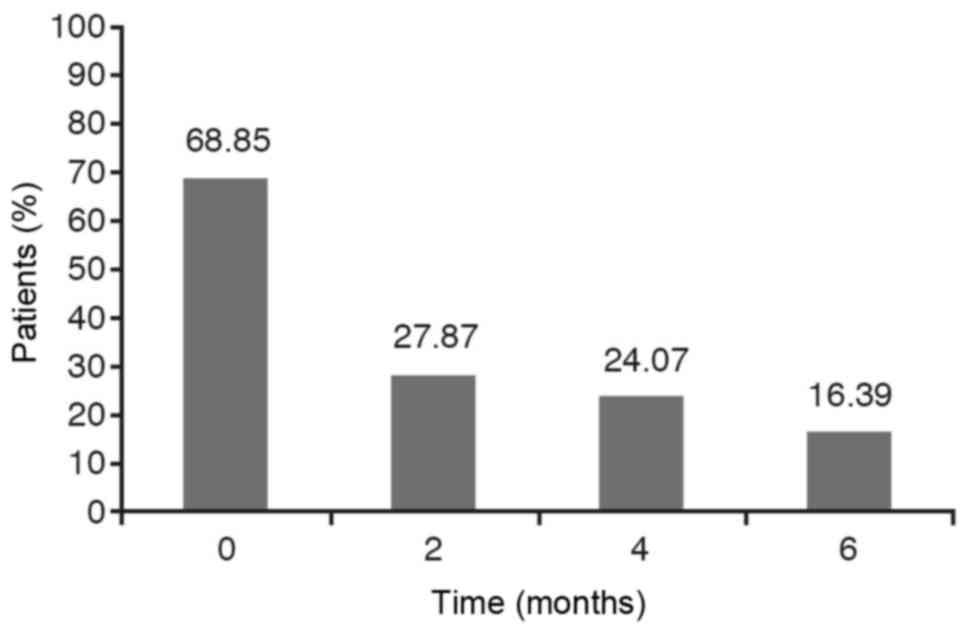
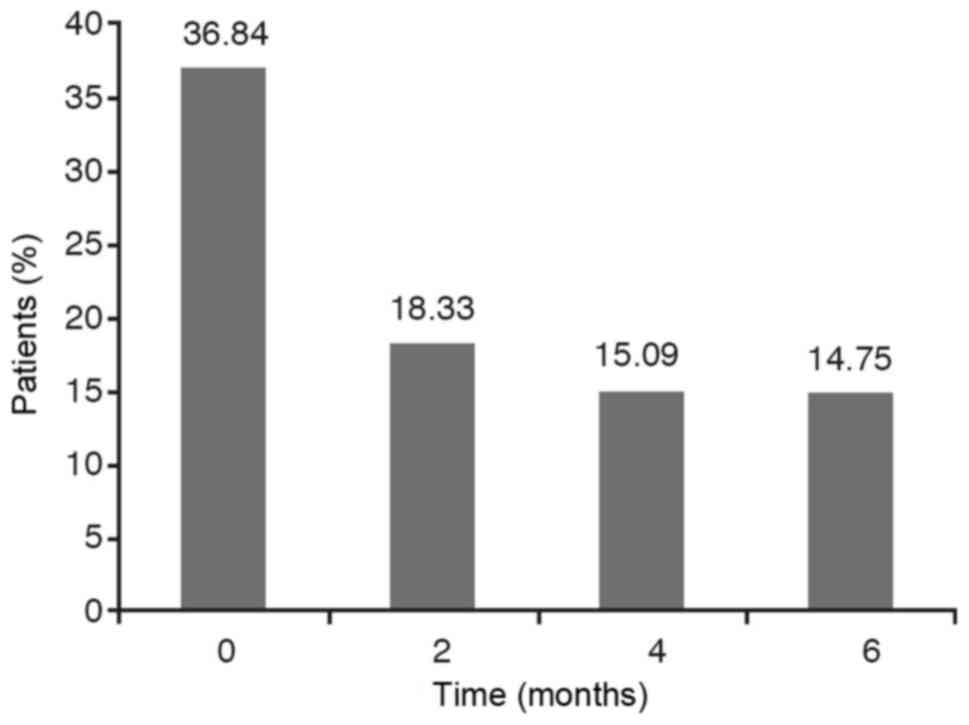
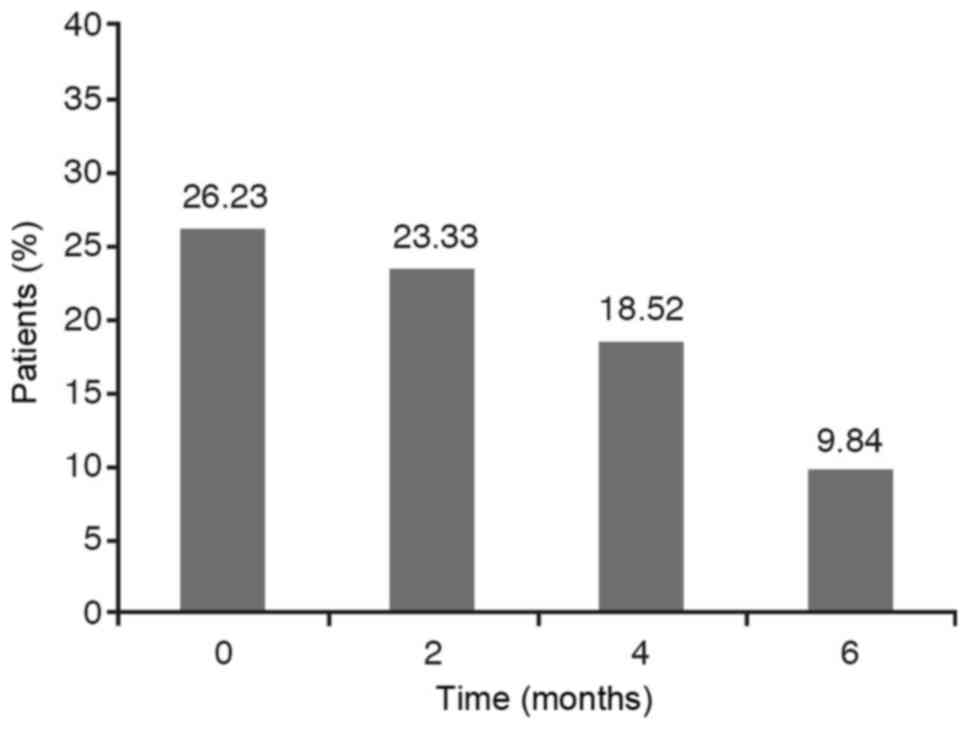
If we take into account the dichotomous variables
such as wheezing, cough and expectoration it has been observed that
the percentage of YES diminishes over time. However, we have to
analyse if this reduction is statistically significant.
The results are obtained from carrying out a
hypothesis testing to compare the four YES percentages obtained at
each stage. As the P-value for wheezing and cough is less than
0.0001 and, for the expectoration variable is less than 0,05 with
the chi-square test as well as with the Montecarlo method, the
conclusion is that these percentages are not statistically equal,
that is to say, they are statistically significant. The Marascuilo
procedure has been used to analyse between what stages we find the
significant differences and at what other stages we don't. In these
cases, we find that the YES percentage at the initial stage (0) is
significantly different to the YES percentages obtained at 2 months
(time 2), 4 months (time 4) and 6 months (time 6) while no
significant differences are observed between the percentages
obtained at 2 months, 4 months and 6 months (Figs. 1–3).
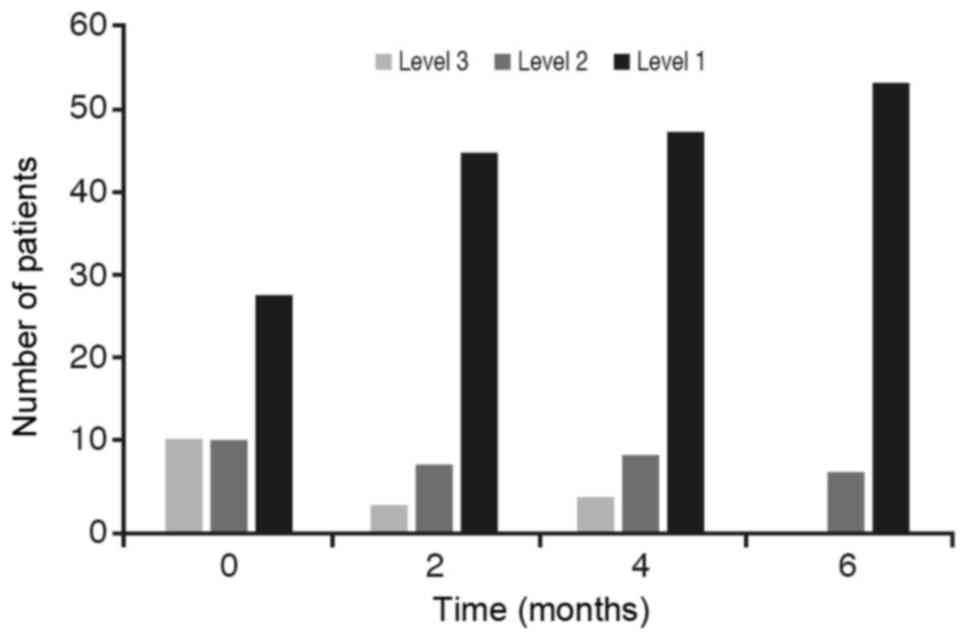
With respect to the contrast in the acute asthma
crisis (with a P-value slightly above 0.05) the conclusion is that
these percentages are statistically different between initial stage
and after 6 months of treatment (Fig.
4).
On the other hand, if we take into account the
quantitative variables such as dyspnea, total IgE, percentage of
eosinophils and the corticosteroids dose we obtain results from
carrying out a hypothesis testing to compare the four mean averages
of each of these variables obtained at each stage. As the P-value
(in the case of dyspnea and the corticosteroids dose) is less than
0.0001 and (in the case of the total IgE and the percentage of
eosinophils) is less than 0.05, the conclusion is that these
averages are not statistically the same. The Tukey post-hoc
contrast has been used to analyse at what stages we find the
significant differences and at what stages we don't.
In the case of dyspnea we find that the mean average
of this variable is significantly different at the initial stage
from the measures obtained at 2 months, 4 months and 6 months. The
means obtained after 2 months and 6 months are also significantly
different, whilst no significant differences are observed between
the 2 months and 4 months measures or between the 4 months and 6
months measures. We could say that we go from dyspnea occurring
with a normal lifestyle (phase 2 of dyspnea) to dyspnea when doing
exercise (phase 1), that is to say, one degree less of dyspnea than
before the treatment was started (Fig.
5).
For the IgE total, the mean average at the initial
stage is significantly different to the mean average obtained at 6
months. It is also quite pronounced (without being statistically
significant at 5%) between the average at the initial stage and at
4 months. The remaining comparisons are not at all significant
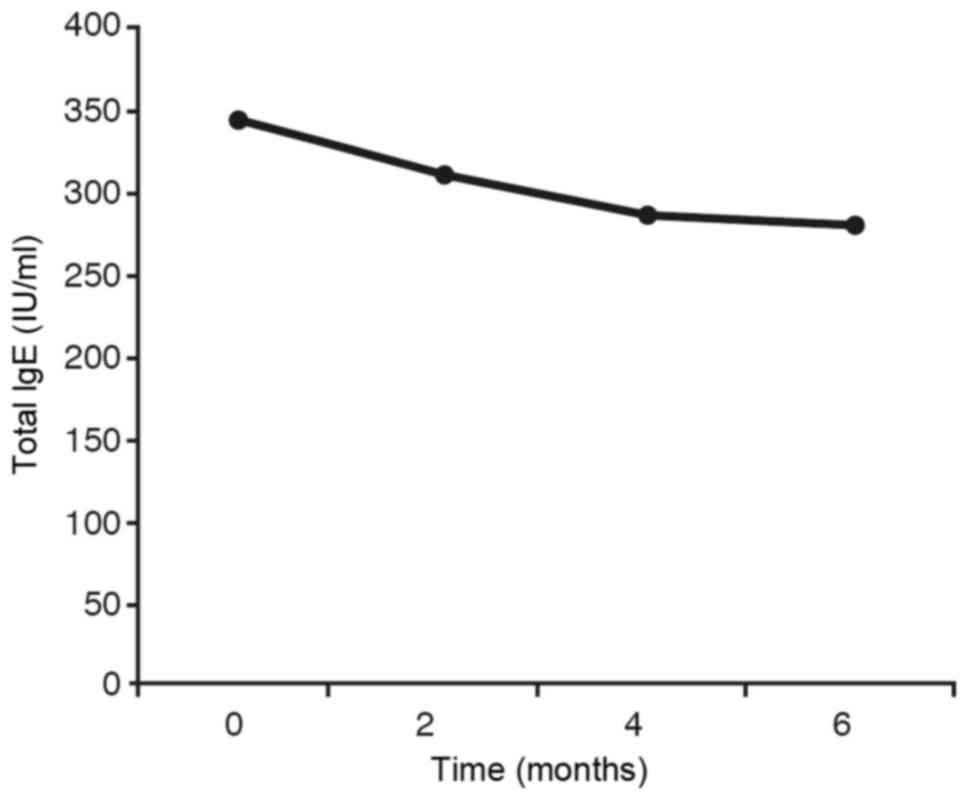
(Fig. 6).
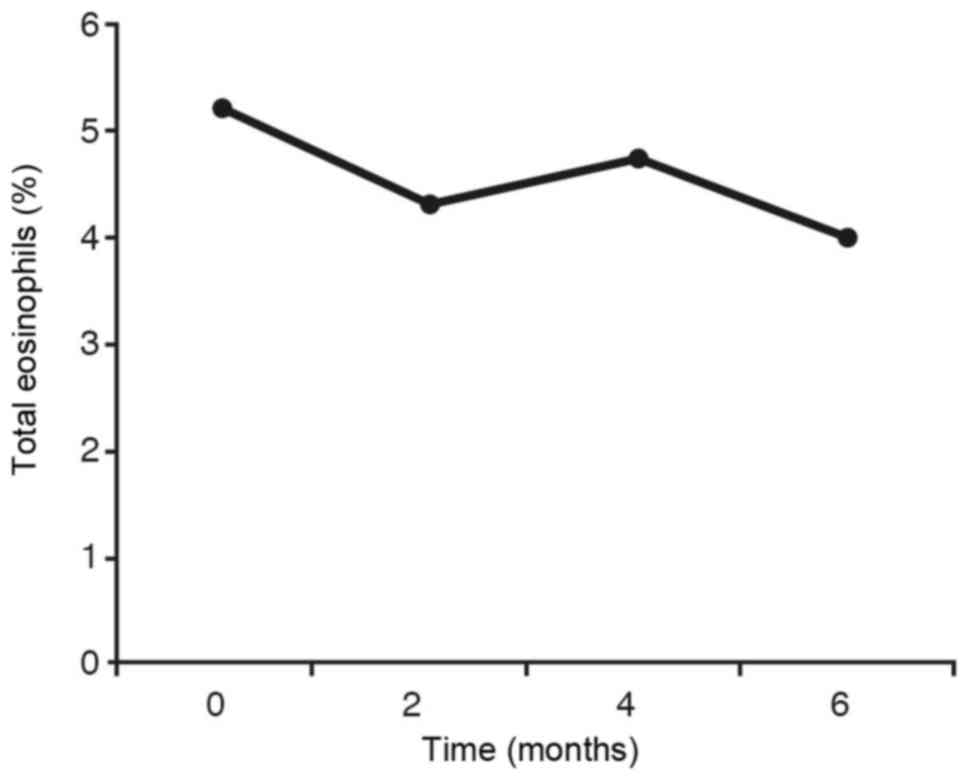
For the eosinophils (Fig.
7) the same statistical result is obtained as in IgE (Fig. 6). In the graph showing the inhaled
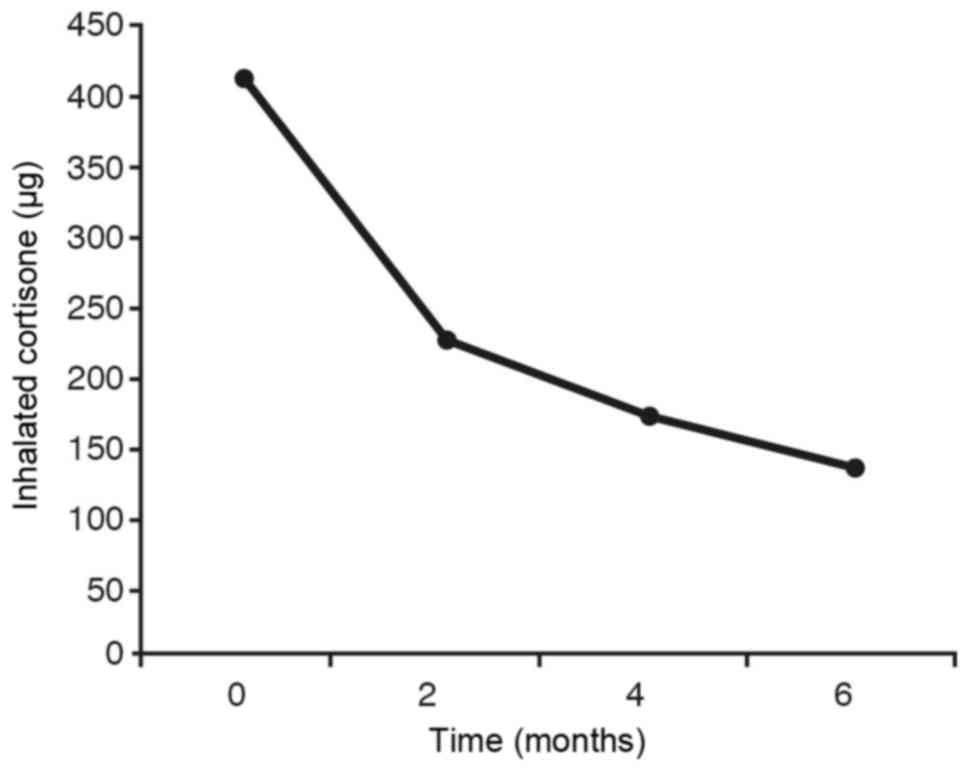
corticosteroids (Fig. 8) we observe
that the average of this variable is significantly different at the
initial stage from the averages obtained at 2 months, 4 months and
6 months. The difference is also quite pronounced (without being
statistically significant) between 2 months and 6 months, whilst no
significant differences are observed between the rest of
comparisons.
When we interpret the results, we can conclude that
there is a noticeable improvement of the variables in the study as
a whole. The use of the BI(G)MED protocol clearly improved the
clinical symptoms and the biological parameters tested on one hand
and on the other hand there is a significant reduction of the dose
of inhaled corticosteroids in the long-term treatment of asthmatic
patients that participated in this study. It is probably useful to
specify here that no participant in the study followed oral
corticosteroid therapy as a disease-modifying treatment of asthma,
all used corticosteroids in inhaled form as basic treatment. In
addition, only a few of them have exceptionally used oral
corticosteroid therapy in the case of a particularly severe acute
asthma crisis. For this reason, we have not introduced doses of
oral corticosteroids as an evolutionary parameter in our study.
Discussion
The BI(G)MED can improve the evolution of chronic
asthma and reduce the dose of corticosteroid treatment in asthmatic
patients of long evolution. It even allows suppressing
corticosteroids in some cases. Thus opening up new therapeutic
possibilities, promoting self-regulation of immune-genetic
mechanisms and restoring the homeostatic balance at genomic,
proteomic and cellular levels. Furthermore, it becomes a new
alternative for those patients who respond poorly to corticosteroid
therapy, even at high doses.
The Bio Immune-regulatory ‘BI(G)MED’-formulas,
so-called ‘BIMUREGs’, used in this study have managed to stabilize
first, and thereafter to slow down and to stop the development and
evolution of bronchial chronic asthmatic processes. Additionally,
it improves other allergic manifestations. With, the added value
that it has been proven to be a safe, innocuous and lacking in side
effects nanotherapy.
And thanks to the therapeutic effects in line with
the laws from quantum physics, hormesis and nanobiotechnology, it
becomes possible to prevent some patients from the damaging effects
that the long-term corticosteroid treatment for chronic asthma
causes, and thereby open up new possibilities for a predictive,
preventive and personalised medicine.
In conclusion, we can state that the BI(G)MED is
actually a bio-medical nanotherapy approach of the highest order in
the sense that it mimics nature in its most intimate essence, and
our study demonstrates its efficacy in a disabling illness such as
chronic bronchial asthma.
Acknowledgements
The author would like to thank Professor Maria Rosa
Fenoll Brunet for her most appreciated contribution as regards to
revising and formatting this manuscript, Josep Maria Mateo Sanz for
developing the statistical analysis of this study and Monica Romero
for all her matchless coordination work, so as all colleagues who
have participated to this study by providing one or more
cases-Linda Gryp, Monika Hartmann, Christian Hönemann, Stipe MALES,
Beate Manderla, Martin Musch, Ernst Oelmann, Renate Quaißer, Andrea
Roschlau, Ingeborg Spreng, Roland Ullrich, Ulrike van Campenhausen,
Monika Wöstehoff, Gabriele Merkel, Christine Lang, Gerlinde Meyer,
Ingrid Mayer, Rigoberto Lopez, Carme Pares Santillari, Horst
Malsch. Dr. Gilbert Glady, main author of this manuscript and
coordinator of the mentioned scientific study, is the president of
the European Bio Immune(G)ene Medecine Association (https://translate.google.co.uk/translate?hl=en&sl=fr&u=http://www.ebma-europe.com/&prev=search).
Glossary
Abbreviations
Abbreviations:
|
BI(G)MED
|
Bio Immune(G)ene Medicine
|
|
EBMA
|
European Bio Immune(G)ene Medecine
Association
|
References
|
1
|
Mattes J, Collison A, Plank M, Phipps S
and Foster PS: Antagonism of microRNA-126 suppresses the effector
function of TH2 cells and the development of allergic airways
disease. Proc Natl Acad Sci USA. 106:18704–18709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stott B, Lavender P, Lehmann S, Pennino D,
Durham S and Schmidt-Weber CB: Human IL-31 is induced by IL-4 and
promotes TH2-driven inflammation. J Allergy Clin Immunol Aug.
132:446–454.e5. 2013. View Article : Google Scholar
|
|
3
|
Nakajima H and Takatsu K: Role of
cytokines in allergic airway inflammation. Int Arch Allergy
Immunol. 142:265–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barrett NA and Austen KF: Innate cells and
T helper 2 cell immunity in airway inflammation. Immunity.
31:425–437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moreira AP, Cavassani KA, Ismailoglu UB,
Hullinger R, Dunleavy MP, Knight DA, Kunkel SL, Uematsu S, Akira S
and Hogaboam CM: The protective role of TLR6 in a mouse model of
asthma is mediated by IL-23 and IL-17A. J Clin Invest.
121:4420–4432. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taft RJ, Pang KC, Mercer TR, Dinger M and
Mattick JS: Non-coding RNAs: Regulators of disease. J Pathol.
220:126–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Foster PS, Plank M, Collison A, Tay HL,
Kaiko GE, Li J, Johnston SL, Hansbro PM, Kumar RK, Yang M and
Mattes J: The emerging role of microRNAs in regulating immune and
inflammatory responses in the lung. Immunol Rev. 253:198–215. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deshpande DA, Dileepan M, Walseth TF,
Subramanian S and Kannan MS: MicroRNA regulation of airway
inflammation and airway smooth muscle function: Relevance to
asthma. Drug Dev Res. 76:286–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simpson LJ, Patel S, Bhakta NR, Choy DF,
Brightbill HD, Ren X, Wang Y, Pua HH, Baumjohann D, Montoya MM, et
al: A microRNA upregulated in asthma airway T cells promotes TH2
cytokine production. Nat Immunol Dec. 15:1162–1170. 2014.
View Article : Google Scholar
|
|
10
|
Brook PO, Perry MM, Adcock IM and Durham
AL: Epigenome-modifying tools in asthma. Epigenomics. 7:1017–1032.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Collison A, Herbert C, Siegle JS, Mattes
J, Foster PS and Kumar RK: Altered expression of microRNA in the
airway wall in chronic asthma: miR-126 as a potential therapeutic
target. BMC Pulm Med. 11:292011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Collison A, Mattes J, Plank M and Foster
PS: Inhibition of house dust mite-induced allergic airways disease
by antagonism of microRNA-145 is comparable to glucocorticoid
treatment. J Allergy Clin Immunol. 128:160–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li JJ, Tay HL, Maltby S, Xiang Y, Eyers F,
Hatchwell L, Zhou H, Toop HD, Morris JC, Nair P, et al: MicroRNA-9
regulates steroid-resistant airway hyperresponsiveness by reducing
protein phosphatase 2A activity. J Allergy Clin Immunol.
136:462–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calamita Z, Saconato H, Pelá AB and
Atallah AN: Efficacy of sublingual immunotherapy in asthma:
Systematic review of randomized-clinical trials using the cochrane
collaboration method. Allergy. 61:1162–1172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Compalati E, Braido F and Canonica GW: An
update on allergen immunotherapy and asthma. Curr Opin Pulm Med.
20:109–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moser S, Peroni DG, Comberiati P and
Piacentini GL: Asthma and viruses: Is there a relationship? Front
Biosci (Elite Ed). 6:46–54. 2014.PubMed/NCBI
|
|
17
|
Metz G and Kraft M: Effects of atypical
infections with Mycoplasma and Chlamydia on asthma. Immunol Allergy
Clin North Am. 30(575–585): vii–viii. 2010.
|
|
18
|
Kudo M, Ishigatsubo Y and Aoki I:
Pathology of asthma. Front Microbiol. 4:2632013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Assa'ad AH and Rothenberg ME: Eosinophilic
asthma: Insights into the effects of reducing IL-5
receptor-positive cell levels. J Allergy Clin Immunol.
132:1097–1085. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paliwal R, Babu RJ and Palakurthi S:
Nanomedicine scale-up technologies: Feasibilities and challenges.
AAPS PharmSciTech. 15:1527–1534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen LS, Wang AX, Dong B, Pu KF, Yuan LH
and Zhu YM: A new prospect in cancer therapy: Targeting cancer stem
cells to eradicate cancer. Chin J Cancer. 31:564–572. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Winter JM and Tang Y: Synthetic biological
approaches to natural product biosynthesis. Curr Opin Biotechnol.
23:736–743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Calabrese EJ: Hormesis is central to
toxicology, pharmacology and risk assessment. Hum Exp Toxicol.
29:249–261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Calabrese EJ: The hormesis concept is the
most fundamental dose-response in the biomedical and toxicological
sciences. Br J Clin Pharmacol. 66:594–617. 2008.PubMed/NCBI
|
|
25
|
Kirchner B, di Dio PJ and Hutter J:
Real-world predictions from ab initio molecular dynamics
simulations. Top Curr Chem. 307:109–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schrauwers A and Poolman B: Synthetische
Biologie - Der Mensch als Schöpfer? Springer Spektrum; Heidelberg:
2013, View Article : Google Scholar
|
|
27
|
Melo CA and Melo SA: Biogenesis and
Physiology of MicroRNAsNon-Coding RNAs and Cancer. Fabbri M:
Springer Science+Business Media, LLC; New York, NY: pp. 5–24.
2013
|