Introduction
Osteosarcoma is one of the most common malignancies
in adolescents. The incidence rate of all people has been reported
to be 6–8/1 million (1–3), accounting for 2.4% of all malignancies
and 60% of all malignant bone tumors (4,5).
Furthermore, the incidence of osteosarcoma has been rising in
recent years (6). Osteosarcoma
originates from mesenchymal tissue and acquires the characteristics
of strong invasiveness and early metastasis, which are associated
with a poor prognosis (3,4). Although neoadjuvant chemotherapy and
surgical treatment are commonly used to treat osteosarcoma
(7,8), the curative effect and prognosis are
relatively poor; the 5-year survival rate for patients with
osteosarcoma without metastases is <70% (9). Some patients are insensitive to
chemotherapy, and others are not able to tolerate the side effects
of chemotherapy, including myelosuppression, kidney toxicity and
gastrointestinal reactions. Therefore, it is necessary to explore
the pathogenesis of osteosarcoma further and to develop novel
effective drugs with reduced side effects for these patients.
Calreticulin (CRT) is a multifunctional
Ca2+-binding protein and an effector of the unfolded
protein response, which participates in the regulation of
intracellular Ca2+ homeostasis, protein folding and
processing, antigen presentation, cellular differentiation and
apoptosis (10). It is a molecular
chaperone located in the endoplasmic reticulum (11). Previous studies have demonstrated
that CRT expression is closely associated with the occurrence,
development, diagnosis, prognosis and response to therapy of
various tumors (11–19); CRT has been shown to promote the
growth of certain tumors, but to inhibit the growth of others. A
previous study conducted by the present research team (20) identified that there was a strong
association between low expression levels of CRT and osteosarcoma
growth and metastasis, which suggests that CRT may serve as a
potential drug target for osteosarcoma.
Diallyl trisulfide (DATS) (21), a volatile oil-like bioactive compound
derived from allium vegetables, has a variety of biological
effects, including lipid-lowering, antibacterial, anti-inflammatory
and immune-enhancing effects (22).
DATS has previously exhibited strong anticancer effects, and is
widely used as an anticancer and chemopreventive agent for certain
tumors (23,24). However, the effect of DATS on
osteosarcoma and the underlying mechanism are largely unknown. A
previous proteomic study conducted by the present research team
(25) demonstrated that DATS
suppressed Saos-2 human osteosarcoma cell proliferation by blocking
cell cycle progression and inducing apoptosis in a dose- and
time-dependent manner. In addition, nine upregulated proteins were
detected as DATS-sensitive proteins, one of which was CRT. In the
present study, the effects of DATS on Saos-2 cells were observed,
and the level of CRT expression in the Saos-2 cells was
investigated.
Materials and methods
Materials
DATS (98% pure) was purchased from LKT Laboratories,
Inc. (Minneapolis, MN, USA). Dulbecco's modified Eagle's medium
(DMEM) and fetal bovine serum (FBS) were obtained from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Rabbit
anti-human polyclonal CRT antibody (cat. no. ab227444) and mouse
anti-human polyclonal β-actin antibody (cat. no. ab8227) were
purchased from Abcam (Cambridge, UK). Fluorescein isothiocyanate
(FITC)-labeled goat anti-rabbit immunoglobulin G antibody
(FITC-IgG; heavy and light chain; cat. no. E031220-01) was obtained
from EarthOx Life Sciences (Millbrae, CA, USA). Horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG antibodies (cat.
no. sc-2004) were obtained from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). The primer sequences were as follows: CRT
upstream, 5′-TTGGAAGAGATTGGGACT-3′ and downstream,
5′-GCCAAAGTTATCATAGGCATAGA-3′; β-actin upstream,
5′-CTCCCTGGAGAAGAGCTACGA-3′ and downstream,
5′-CGATCCACACGGAGTACTTGC-3′ (Takara Biotechnology Co., Ltd.,
Dalian, China).
Cell culture
Saos-2 cells were obtained from the Institute of
Biochemistry and Cell Biology at the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in DMEM supplemented
with 10% FBS at 37°C in humidified air containing 5%
CO2. Cells were used in the logarithmic phase of growth
throughout the study.
Immunofluorescent staining assay
The Saos-2 cells (~5×105) were treated
with different concentrations of DATS (0, 25, 50 and 100 µmol/l)
for 24 h, and with 50 µmol/l DATS for different time periods (0,
12, 24 and 36 h) in 24-well culture plates. Following treatment,
the Sao-2 cells were washed three times in PBS and then fixed in
PBS containing 4% formaldehyde for 30 min at room temperature. The
cells were then washed twice in PBS and incubated for 30 min in PBS
containing 0.1% Triton X-100. Next, the cells were blocked with 200
µl 10% normal goat serum (Gibco; Thermo Fisher Scientific, Inc.)
diluted with PBS per well and incubated for 30 min at room
temperature. Thereafter, the cells were incubated with 200 µl
primary CRT antibody (dilution, 1:75) per well in a moist chamber
at 4°C overnight. After washing three times with PBS, the cells
were incubated with goat anti-rabbit FITC-IgG antibody (dilution,
1:200) at room temperature for 30 min. Following this, the cells
were blocked with glycerol followed by incubation with DAPI for 5
min at room temperature.
The cells were then analyzed using a Leica DM4000B
microscope (Leica Microsystems GmbH, Wetzlar, Germany). Images were
captured and expression levels determined using the Image-Pro Plus
image analysis system 7.0 (Media Cybernetics, Inc., Rockville, MD,
USA). Cells in which the cytoplasm was stained green were
considered to be CRT-positive cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Following treatment with DATS as described for the
immunofluorescent staining assay, total RNA was extracted from the
Saos-2 cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and synthesized into cDNA using the PrimeScript
RT reagent kit (Takara Biotechnology Co., Ltd.) at 37°C for 15 min
and 85°C for 5 sec. qPCR analysis was then performed using SYBR
Premix Ex Taq II (Takara Biotechnology Co., Ltd.) with a
LightCycler 480 system (Roche Diagnostics, Basel, Switzerland). A
25-µl PCR reaction mixture contained 2 µl cDNA, 12.5 µl SYBR Premix
Ex Taq II, 1 µl each primer and 8.5 µl diethyl pyrocarbonate water.
Following an initial denaturation step at 95°C for 2 min, CRT and
ß-actin were amplified with 45 cycles at 95°C for 20 sec, 60°C for
30 sec and 68°C for 30 sec. The levels of β-actin were used as an
internal control. All reactions were run in triplicate. The data
output from the RT-qPCR experiments were then analyzed using the
2−ΔΔCq method (26).
Western blot analysis
Following treatment with DATS as described for the
immunofluorescent staining assay, protein was extracted from the
Saos-2 cells using radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Haimen, China) containing phenylmethane
sulfonyl fluoride. A BCA protein assay kit (Beyotime Institute of
Biotechnology) was used to evaluate the total protein
concentration. Protein samples (30 µg/lane) were separated by
SDS-PAGE (8%) and transferred to polyvinylidene difluoride
membranes. The membranes were incubated overnight at 4°C with
primary anti-CRT antibody (dilution, 1:1,000) followed by the
HRP-conjugated goat anti-rabbit IgG secondary antibody (dilution,
1:20,000) at room temperature for 1 h. The blots were then
visualized using a chemiluminescent detection kit (Amersham; GE
Healthcare Life Sciences, Chalfont, UK). A Typhoon PhosphorImager
with ImageQuant TL software version 7.0 (both GE Healthcare Life
Sciences) was used to quantify the protein. The antibody against
β-actin served as an internal reference. The expression level of
CRT protein in each sample was determined as follows: Expression
level of CRT protein = densitometric value of CRT
protein/densitometric value of β-actin.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean of three independent experiments. The statistical
significance of differences in the expression of CRT mRNA and
protein among multiple groups was determined using one-way analysis
of variance followed by Scheffe's post hoc test. SPSS 19.0 software
(IBM Corp., Armonk, NY, USA) was used for statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
DATS inhibits the growth of Saos-2
human osteosarcoma cells
Changes in the morphology and growth status of the
Saos-2 cells were observed under an inverted microscope following
culture with different concentrations of DATS (0, 25, 50 and 100
µmol/l) for 24 h, and with 50 µmol/l DATS for different exposure
times (0, 12, 24 and 36 h). In the DATS-treated cells,
morphological deformations, including the shrinkage of cell bodies,
loosening of intercellular gaps, cell lysis and condensation of
nuclei were observed. In addition, a number of cells became round,
floated and necrotic. Furthermore, these morphological changes
appeared to be concentration- and exposure time-dependent
phenomena. However, the cell morphology of the control group
appeared normal (data not shown). Images of the Saos-2 cells
following treatment with DATS under these conditions have been
presented by the present authors in a previous study (27).
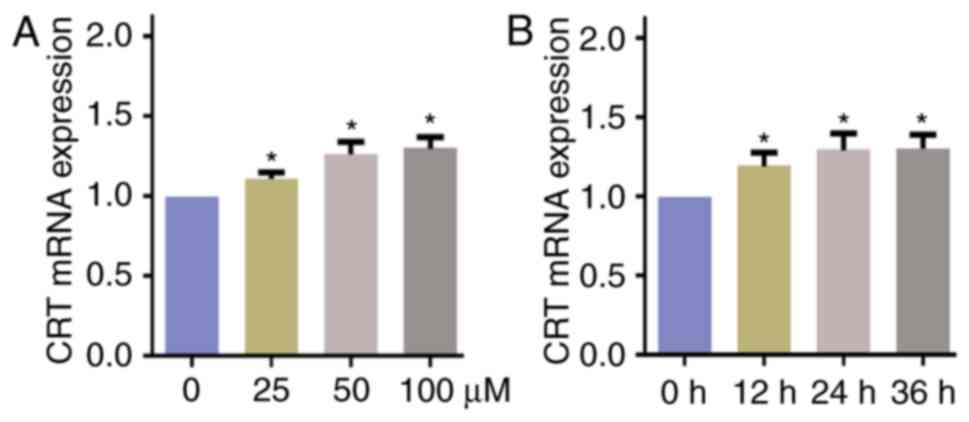
DATS induces the expression of CRT
mRNA in Saos-2 cells
The presence of CRT mRNA was detected in the Saos-2
cells treated with different concentrations of DATS for 24 h
(Fig. 1A) and 50 µmol/l DATS for
different exposure times (Fig. 1B).
The results demonstrated that the expression level of CRT mRNA was
significantly upregulated following culture with DATS, and the
upregulation appeared to be concentration- and exposure
time-dependent (Fig. 1).
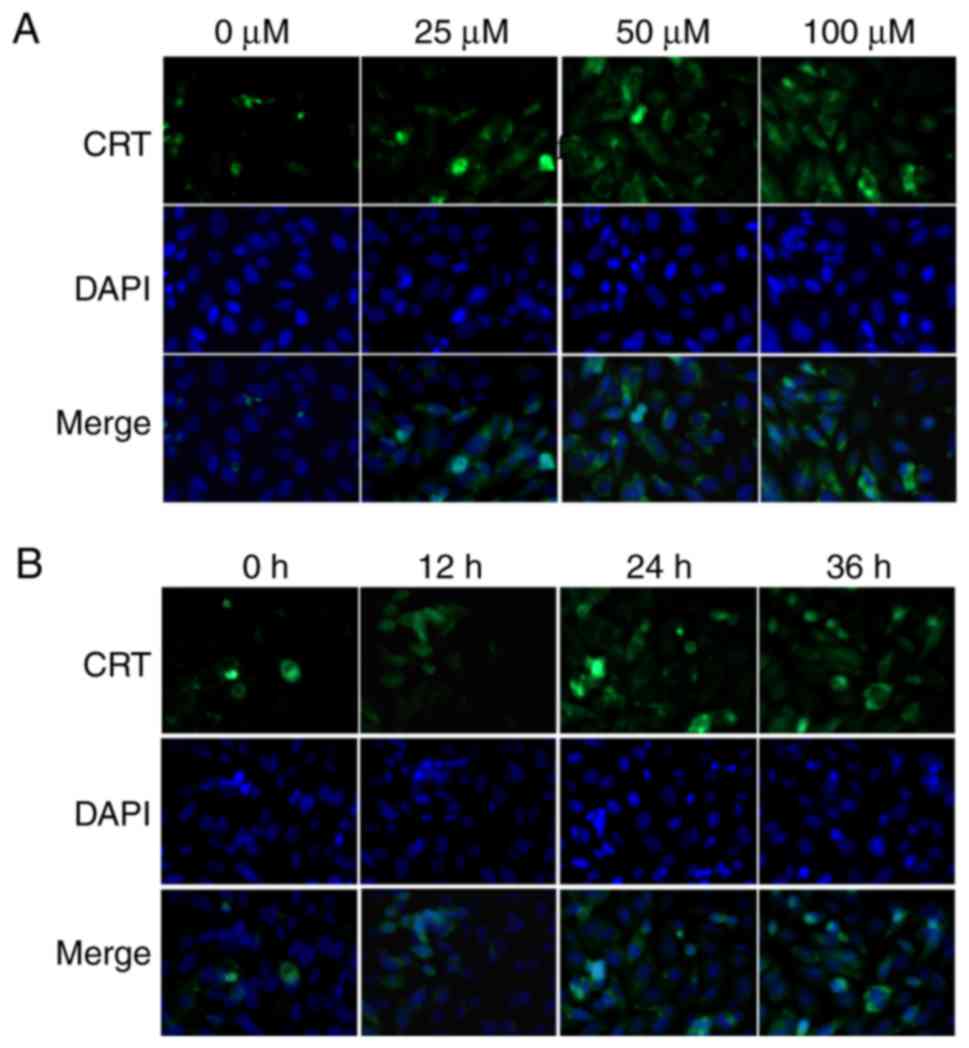
DATS increases CRT protein levels in
Saos-2 cells
To characterize the functional relevance of
increased CRT expression in osteosarcoma, analysis of CRT protein
levels in the Saos-2 cells following culture with different
concentrations of DATS for 24 h and with 50 µmol/l DATS for
different exposure times was conducted. In the immunofluorescent
staining assay, CRT exhibited prominent nuclear localization and
increased expression intensity following treatment with DATS
(Fig. 2). The expression intensity
of CRT appeared to increase as the concentration (Fig. 2A) and exposure time (Fig. 2B) increased.
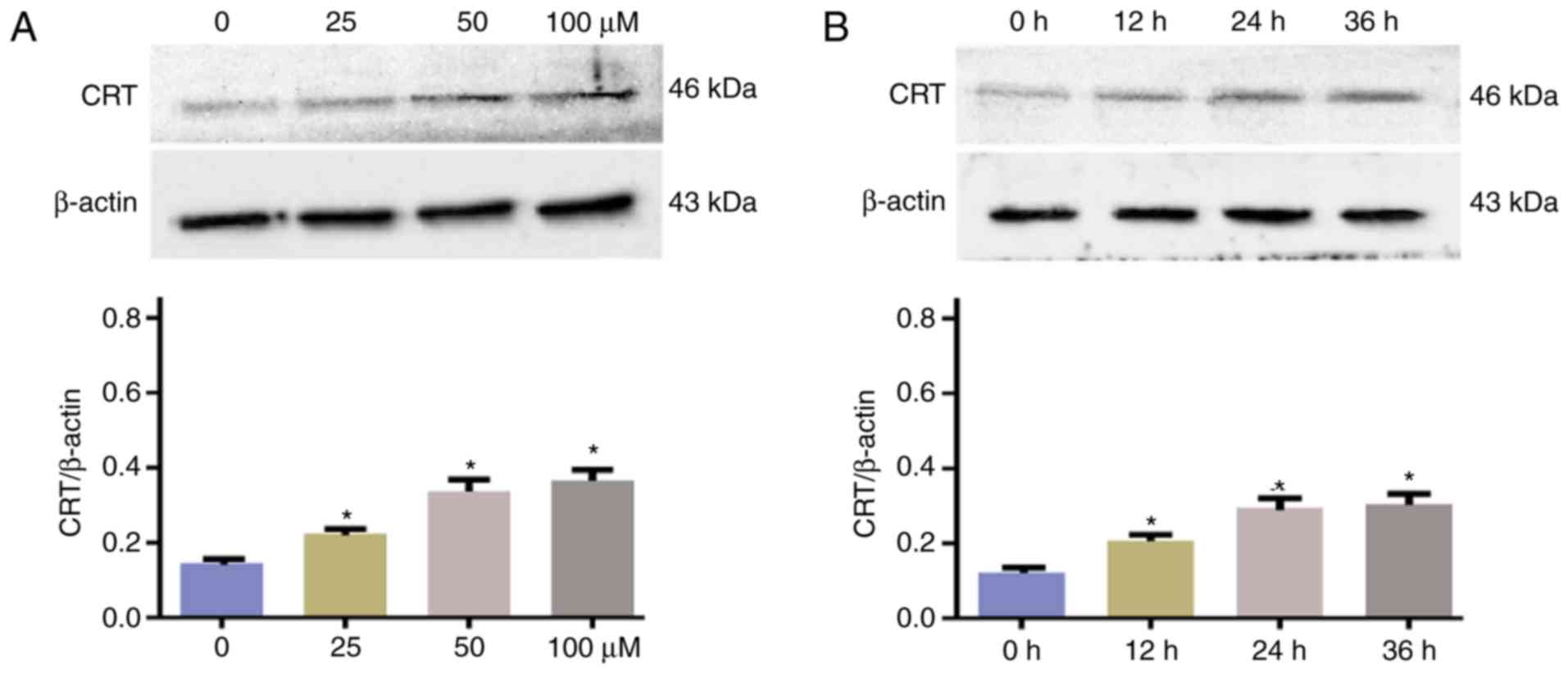
Western blotting was conducted to support further
evaluate the CRT protein content of the cells following culture
with different concentrations of DATS for 24 h (Fig. 3A) and with 50 µmol/l DATS for
different exposure times (Fig. 3B).
Treatment with DATS significantly reduced the CRT protein levels.
The levels of CRT protein detected by western blotting were
consistent those indicated by immunofluorescent staining.
Discussion
Developments in neoadjuvant chemotherapy, surgical
treatment and comprehensive treatments for osteosarcoma have
provided significant benefits to the treatment of osteosarcoma in
recent years. Neoadjuvant chemotherapy combined with surgery is
currently the preferred treatment for osteosarcoma (28), following more than 40 years of
development and improvement (29).
This type of treatment has great advantages, including the ability
to limit or shrink the primary lesion, increase the likelihood of
surgical success, reduce the difficulty of surgery and increase the
5-year survival rate of patients with osteosarcoma (28,29).
However, it has numerous side effects and complications, including
myelosuppression, hepatorenal toxicity, serious gastrointestinal
reactions and drug resistance (30).
The development of neoadjuvant chemotherapy has slowed. Therefore,
the exploration of new effective biomarkers, sensitive
chemotherapeutic agents with reduced side effects and molecular
therapeutic targets is necessary.
A large amount of research has been conducted on
plant extracts, and many satisfactory results have been achieved.
Garlic extract has attracted considerable attention due to its
advantages of readily absorbed, non-polluting to the environment
and widely available compared with traditional chemotherapeutic
medicines. However, although the term garlic extract usually refers
to a complex mixture of heterogeneous allyl organic sulfides, the
present study investigated a specific component of garlic extract,
namely DATS. DATS has been demonstrated to have good anticancer
effects, with the ability to inhibit the proliferation and
metastasis of various types of cancer, including prostate cancer
(31), gastric cancer (32), lung cancer (33), breast cancer (34) and colon cancer (35). Oommen et al (36) reported that DATS promoted the
apoptosis of SiHa cells by increasing the activity of caspase-3, −8
and −9, and downregulating the expression of poly (ADP-ribose)
polymerase. Xu et al (37)
revealed that C-Jun N-terminal kinase (JNK) activation and
mitochondrial Bax translocation were involved in the apoptosis of
SKOV3 cells induced by another component of garlic extract,
allicin. Li et al (38)
demonstrated that allicin inhibited the proliferation of Caco-2
cells by downregulating the expression of nuclear factor-κB p65 and
blocking the p38 and JNK signaling pathway. However, few studies
investigating the effect of DATS on osteosarcoma and the underlying
mechanism have been conducted.
A previous study conducted by the present research
team demonstrated that DATS inhibited the proliferation of
osteosarcoma cells and promoted their apoptosis in a dose- and
time-dependent manner in vitro (25). Another study by the present research
team (20) observed that the
expression of CRT in patients with osteosarcoma was higher in the
normal tissues surrounding the tumors than in the tumor tissues. In
addition, CRT expression was greater in patients without metastasis
than in those with metastasis, and in patients following
chemotherapy than in those prior to chemotherapy. These results
suggest that the CRT expression level is negatively associated with
osteosarcoma malignancy. Therefore, further experiments to
elucidate the mechanism of DATS on growth of human osteosarcoma
were conducted in the present study.
The observation of Saos-2 cells under an inverted
microscope following treatment with DATS revealed that DATS damaged
the normal structure of the cells and inhibited their growth. These
morphological changes appeared to be positively associated with the
DATS concentration and exposure time, and indicate that DATS is
able to inhibit the growth of osteosarcoma, consistent with
previous study (27).
Immunofluorescence staining, RT-qPCR and western blot analyses were
conducted in the present study to detect CRT expression following
the exposure of Saos-2 cells to DATS. The experimental results
consistently demonstrated that DATS upregulated the expression of
CRT in these cells, in an apparently concentration- and exposure
time-dependent manner. Therefore, it is possible that the
inhibition of osteosarcoma cell growth by DATS may occur via the
upregulation of CRT expression. Furthermore, upregulating the
expression of CRT via DATS treatment may be a possible therapeutic
strategy for the effective management of osteosarcoma.
Although a number of studies on the association
between CRT and malignant tumors have been conducted, the findings
concerning the level and significance of CRT expression in cancer
are not consistent in different types of tumors. Previous studies
have demonstrated that CRT is upregulated in oral squamous cell
carcinoma (11), pancreatic cancer
(14), breast cancer (15), hepatocellular carcinoma (17), adrenocortical carcinomas (18) and gastric cancer (19). However, the results of a previous
study by the present authors indicated that CRT was downregulated
in osteosarcoma cells (20). In the
present study, immunofluorescent staining, RT-qPCR and western
blotting were conducted to detect the expression of CRT in Saos-2
osteosarcoma cells following culture with DATS at different
concentrations and exposure times.
CRT has been demonstrated to serve an important role
in inhibiting neovascularization and decreasing the blood supply to
tumor tissue. In addition, CRT recruits macrophages to tumor cells
to engulf and destroy the tumor cells, induces the production of
killer lymphocytes and improves the ability of the immune system to
kill tumor cells (39). Furthermore,
CRT binds to the cluster of differentiation 47 receptor on the
surface of certain innate immune cells, such as macrophages and
dendritic cells, and increases the immune response to tumor cells
(40). These previous observations
and the results of the present study suggest that CRT may serve as
a potential target for the development of therapeutics and
preventive strategies for osteosarcoma.
The present study demonstrated that the treatment of
Saos-2 osteosarcoma cells with DTS was associated with an increase
in CRT expression. The possible mechanisms for this are as follows.
Firstly, DATS may upregulate CRT expression by antagonizing the
peroxisome proliferator-activated receptors that control the
metabolic processes in various cells (37,41).
Secondly, DATS acts as a calcium antagonist and is able to
influence the calcium channels on cell membranes (42). Therefore, it may upregulate CRT
expression by regulating the calcium concentration in osteosarcoma
cells.
However, the present study is a preliminary study in
which only in vitro experiments were conducted. Further
in-depth experiments using relevant animal models are required to
clarify the curative effect and mechanism of DATS. In addition, the
biological roles of CRT require further investigation in
DATS-treated osteosarcoma cells in which CRT expression has been
knocked down or overexpressed. In addition, the results require
confirmation using other osteosarcoma cell lines, for example, HOS,
U2OS and MG63 cells. Therefore, the investigation of the
CRT-dependent molecular targets and signaling pathways will be the
focus of future studies by the present research team.
In conclusion, the present study revealed a novel
mechanism and potential molecular target of DATS in osteosarcoma
cells. The results indicate that the known inhibitory effect of
DATS on the growth of Saos-2 osteosarcoma cells may be mediated via
upregulation of the expression of CRT. The present study provides a
basis for the clinical use of DATS and suggests a novel molecular
target for the treatment of osteosarcoma.
Acknowledgements
The authors thank Professor Yanli Ban, Qilu Hospital
of Shandong University (Jinan, China), for linguistic advice.
Funding
The present study was funded by the National Science
Foundation of Shandong Province (grant no. ZR2014HQ034) and the
Medical Science Development Project of Shandong Province (grant no.
2014WS0432).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WPX and YZ performed most of the experiments and
were major contributors in writing the manuscript. YKZ guided the
design and implementation of the experiment. GL and JX analyzed and
interpreted the experimental data. RXB and CJL conducted the
immuofluorescence staining and processed the figures in the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of
interest.
Glossary
Abbreviations
Abbreviations:
|
CRT
|
calreticulin
|
|
DATS
|
diallyl trisulfide
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
Chen N, Zhang R, Konishi T and Wang J:
Upregulation of NRF2 through autophagy/ERK 1/2 ameliorates ionizing
radiation induced cell death of human osteosarcoma U-2 OS. Mutat
Res. 813:10–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bielack SS, Hecker-Nolting S, Blattmann C
and Kager L: Advances in the management of osteosarcoma. F1000 Res.
5:27672016. View Article : Google Scholar
|
|
3
|
Friebele JC, Peck J, Pan X, Abdel-Rasoul M
and Mayerson JL: Osteosarcoma: A meta-analysis and review of the
literature. Am J Orthop (Belle Mead NJ). 44:547–553.
2015.PubMed/NCBI
|
|
4
|
Liu K, Sun X, Zhang Y, Liu L and Yuan Q:
MiR-598: A tumor suppressor with biomarker significance in
osteosarcoma. Life Sci. 188:141–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taran SJ, Taran R and Malipatil NB:
Pediatric osteosarcoma: An updated review. Indian J Med Paediatr
Oncol. 38:33–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen L, Pei H, Lu SJ, Liu ZJ, Yan L, Zhao
XM, Hu B and Lu HG: SPOP suppresses osteosarcoma invasion via
PI3K/AKT/NF-κB signaling pathway. Eur Rev Med Pharmacol Sci.
22:609–615. 2018.PubMed/NCBI
|
|
7
|
He X, Gao Z, Xu H, Zhang Z and Fu P: A
meta-analysis of randomized control trials of surgical methods with
osteosarcoma outcomes. J Orthop Surg Res. 12:52017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun J, Mu H, Dai K and Yi L: Calreticulin:
A potential anti-cancer therapeutic target. Pharmazie. 72:503–510.
2017.PubMed/NCBI
|
|
11
|
Harada K, Takenawa T, Ferdous T, Kuramitsu
Y and Ueyama Y: Calreticulin is a novel independent prognostic
factor for oral squamous cell carcinoma. Oncol Lett. 13:4857–4862.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fucikova J, Truxova I, Hensler M, Becht E,
Kasikova L, Moserova I, Vosahlikova S, Klouckova J, Church SE,
Cremer I, et al: Calreticulin exposure by malignant blasts
correlates with robust anticancer immunity and improved clinical
outcome in AML patients. Blood. 128:3113–3124. 2016.PubMed/NCBI
|
|
13
|
Obakan-Yerlikaya P, Arisan ED,
Coker-Gurkan A, Adacan K, Ozbey U, Somuncu B, Baran D and
Palavan-Unsal N: Calreticulin is a fine tuning molecule in
epibrassinolide-induced apoptosis through activating endoplasmic
reticulum stress in colon cancer cells. Mol Carcinog. 56:1603–1619.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsukuma S, Yoshimura K, Ueno T, Oga A,
Inoue M, Watanabe Y, Kuramasu A, Fuse M, Tsunedomi R, Nagaoka S, et
al: Calreticulin is highly expressed in pancreatic cancer stem-like
cells. Cancer Sci. 107:1599–1609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zamanian M, Hamadneh Qader LA,
Veerakumarasivam A, Rahman Abdul S, Shohaimi S and Rosli R:
Calreticulin mediates an invasive breast cancer phenotype through
the transcriptional dysregulation of p53 and MAPK pathways. Cancer
Cell Int. 16:562016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fucikova J, Becht E, Iribarren K, Goc J,
Remark R, Damotte D, Alifano M, Devi P, Biton J, Germain C, et al:
Calreticulin expression in human non-small cell lung cancers
correlates with increased accumulation of antitumor immune cells
and favorable prognosis. Cancer Res. 76:1746–1756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng R, Ye J, Zhou C, Qi L, Fu Z, Yan B,
Liang Z, Li R and Zhai W: Calreticulin down-regulation inhibits the
cell growth, invasion and cell cycle progression of human
hepatocellular carcinoma cells. Diagn Pathol. 10:1492015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang MS, Wang HS, Wang BS, Li WH, Pang ZF,
Zou BK, Zhang X, Shi XT, Mu DB, Zhang DX, et al: A comparative
proteomic study identified calreticulin and prohibitin up-regulated
in adrenocortical carcinomas. Diagn Pathol. 8:582013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen CN, Chang CC, Su TE, Hsu WM, Jeng YM,
Ho MC, Hsieh FJ, Lee PH, Kuo ML, Lee H and Chang KJ: Identification
of calreticulin as a prognosis marker and angiogenic regulator in
human gastric cancer. Ann Surg Oncol. 16:524–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang XH, Zhang Y, Xie WP, Sun DS, Zhang
YK, Hao YK and Tan GQ: Expression and significance of calreticulin
in human osteosarcoma. Cancer Biomark. 18:405–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mikaili P, Maadirad S, Moloudizargari M,
Aghajanshakeri S and Sarahroodi S: Therapeutic uses and
pharmacological properties of garlic, shallot, and their
biologically active compounds. Iran J Basic Med Sci. 16:1031–1048.
2013.PubMed/NCBI
|
|
22
|
Horn N, Miller G, Ajuwon KM and Adeola O:
Garlic diallyl disulfide and diallyl trisulfide mitigates effects
of pro-oxidant induced cellular stress and has immune modulatory
function in LPS-stimulated porcine epithelial cells. J Anim Sci.
95:4045–4051. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antony ML and Singh SV: Molecular
mechanisms and targets of cancer chemoprevention by garlic-derived
bioactive compound diallyl trisulfide. Indian J Exp Biol.
49:805–816. 2011.PubMed/NCBI
|
|
24
|
Seki T, Hosono T, Hosono-Fukao T, Inada K,
Tanaka R, Ogihara J and Ariga T: Anticancer effects of diallyl
trisulfide derived from garlic. Asia Pac J Clin Nutr. 17 Suppl
1:S249–S252. 2008.
|
|
25
|
Zhang YK, Zhang XH, Li JM, Sun DS, Yang Q
and Diao DM: A proteomic study on a human osteosarcoma cell line
Saos-2 treated with diallyl trisulfide. Anticancer Drugs.
20:702–712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang YK, Li JM, Wang DL and Chen YQ:
Effects of diallyl trisulfide on cell cycle and apoptosis of human
osteosarcoma cell line Saos-2. Tumor. 33:214–222. 2013.(In
Chinese).
|
|
28
|
Yuan G, Chen J, Wu D and Gao C:
Neoadjuvant chemotherapy combined with limb salvage surgery in
patients with limb osteosarcoma of Enneking stage II: A
retrospective study. Onco Targets Ther. 10:2745–2750. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rosen G, Marcove RC, Caparros B, Nirenberg
A, Kosloff C and Huvos AG: Primary osteogenic sarcoma: The
rationale for preoperative chemotherapy and delayed surgery.
Cancer. 43:2163–2177. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Masood S: Neoadjuvant chemotherapy in
breast cancers. Womens Health (Lond). 12:480–491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Borkowska A, Knap N and Antosiewicz J:
Diallyl trisulfide is more cytotoxic to prostate cancer cells PC-3
than to noncancerous epithelial cell line PNT1A: A possible role of
p66Shc signaling axis. Nutr Cancer. 65:711–717. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pan Y, Lin S, Xing R, Zhu M, Lin B, Cui J,
Li W, Gao J, Shen L, Zhao Y, et al: Epigenetic upregulation of
metallothionein 2A by diallyl trisulfide enhances chemosensitivity
of human gastric cancer cells to docetaxel through attenuating
NF-κB activation. Antioxid Redox Signal. 24:839–854. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li W, Tian H, Li L, Li S, Yue W, Chen Z,
Qi L, Hu W, Zhu Y, Hao B, et al: Diallyl trisulfide induces
apoptosis and inhibits proliferation of A549 cells in vitro and in
vivo. Acta Biochim Biophys Sin (Shanghai). 44:577–583. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim SH, Kaschula CH, Priedigkeit N, Lee AV
and Singh SV: Forkhead box Q1 is a novel target of breast cancer
stem cell inhibition by diallyl trisulfide. J Biol Chem.
291:13495–13508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lai KC, Hsu SC, Yang JS, Yu CC, Lein JC
and Chung JG: Diallyl trisulfide inhibits migration, invasion and
angiogenesis of human colon cancer HT-29 cells and umbilical vein
endothelial cells, and suppresses murine xenograft tumour growth. J
Cell Mol Med. 19:474–484. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oommen S, Anto RJ, Srinivas G and
Karunagaran D: Allicin (from garlic) induces caspase-mediated
apoptosis in cancer cells. Eur J Pharmacol. 485:97–103. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu L, Yu J, Zhai D, Zhang D, Shen W, Bai
L, Cai Z and Yu C: Role of JNK activation and mitochondrial bax
translocation in allicin-induced apoptosis in human ovarian cancer
SKOV3 cells. Evid Based Complement Alternat Med. 2014:3786842014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li C, Lun W, Zhao X, Lei S, Guo Y, Ma J
and Zhi F: Allicin alleviates inflammation of
trinitrobenzenesulfonic acid-induced rats and suppresses P38 and
JNK pathways in Caco-2 cells. Mediators Inflamm. 2015:4346922015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tufi R, Panaretakis T, Bianchi K, Criollo
A, Fazi B, Di Sano F, Tesniere A, Kepp O, Paterlini-Brechot P,
Zitvogel L, et al: Reduction of endoplasmic reticulum
Ca2+ levels favors plasma membrane surface exposure of
calreticulin. Cell Death Differ. 15:274–282. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chao MP, Jaiswal S, Weissman-Tsukamoto R,
Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB,
Raveh T, Park CY, et al: Calreticulin is the dominant
pro-phagocytic signal on multiple human cancers and is
counterbalanced by CD47. Sci Transl Med. 2:63–94. 2010. View Article : Google Scholar
|
|
41
|
No authors listed: Calreticulin inhibits
commitment to adipocyte differentiation. J Cell Biol. 208:249–250.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Martín N, Bardisa L, Pantoja C, Barra E,
Demetrio C, Valenzuela J, Barrios M and Sepúlveda MJ: Involvement
of calcium in the cardiac depressant actions of a garlic dialysate.
J Ethnopharmacol. 55:113–118. 1997. View Article : Google Scholar : PubMed/NCBI
|