Introduction
Diabetes mellitus is characterized by hyperglycemia
and a serious of chronic complications, and it is a common
metabolic disorder that occurs as a result of insulin deficiency or
insulin resistance. Clinical evidences showed that bone mineral
density (BMD) was below normal in patients with type 1 diabetes
mellitus, a subtype of diabetes caused by inability of pancreatic
beta-cells to secrete insulin (1).
In addition, the risk of fracture in those with type 1 diabetes was
6.3- to 6.9-fold higher than that in age-matched control subjects,
and the risk of fracture was increased to a less extent (1.4- to
1.7-fold) in those with type 2 diabetes (2). Diabetes-related decrease in BMD and
weakened bone structure, of complicated pathophysiology, is
considered to be one of the major factors that lead to bone
fragility and elevated incidence of fractures in diabetic patients
(1). Furthermore, some anti-diabetic
drugs (like thiazolidinediones) could also directly affect bone
metabolism and increase the risk of fracture (3).
Osteoporosis is a metabolic bone disease
characterized by loss of bone mass and deterioration of bone
microarchitecture. It mainly affects the elderly, especially
postmenopausal women, and results in hip and vertebral fractures
(4). Bone remodeling, referring to
continuous cycles of bone resorption by osteoclasts and bone
formation by osteoblasts, is in a homeostatic state under
physiological conditions (5). In
postmenopausal osteoporosis, estrogen deficiency leads to defective
bone formation and strong bone resorption, accounting for
progressive bone loss and an increased risk of fracture (5). Diabetic postmenopausal women, who
experience a high risk of fracture, have been paid special
attention to for the management of their bone health and prevention
of fractures (6–9).
Liraglutide, a glucagon-like peptide-1 (GLP-1)
receptor agonist (GLP-1 RA), is a novel anti-diabetic drug that
mimics the endogenous GLP-1 to potentiate insulin secretion
(10). A meta-analysis of fracture
incidence in diabetic patients shows a reduced risk of incident
fractures in the patients receiving liraglutide treatment (11). In addition, long-term administration
of liraglutide has an anabolic bone effect in weight-reduced obese
women (12). Further studies on
animal models demonstrated that liraglutide could prevent bone loss
and counter rapid bone deterioration in diabetic rodents (13,14).
Moreover, liraglutide could improve bone mass and architecture in
non-diabetic osteoporotic rodent models with ovariectomy (OVX)
(15,16). So far, the potential bone-protective
effect of liraglutide on the postmenopausal diabetic population has
not been assessed. In the present study, we explored the effects of
liraglutide on the osteoporotic bones in a rat model with
streptozotocin (STZ)-induced diabetes and bilateral OVX.
Furthermore, we investigated the modulatory effects of liraglutide
on the markers of bone turnover in the diabetic/OVX rats.
Materials and methods
Animal models and treatment
All the animal were housed under standard housing
conditions (12-h light/dark cycle, temperature of 22–24°C and
relative humidity of 50–60%, with access to food and water ad
libitum) in the animal facility center at our institute, and
all procedures on the animals were approved by the Institutional
Animal Care and Use Committee of The People's Hospital of Liaoning
Province (Shenyang, China). Nine-week-old female Sprague Dawley
rats were randomly divided into seven groups: Sham, STZ, STZ+L,
OVX, OVX+L, STZ+OVX, and STZ+OVX+L. To induce diabetes, the animals
were fasted overnight, and then received an intraperitoneal
injection of STZ (Solarbio, Beijing, China; dissolved in 0.1 M
citrate buffer, pH 4.5) at a dose of 60 mg/kg body weight. Those in
the Sham, OVX and OVX+L groups received an equal volume of the
solvent via the same administration route. Blood was sampled
through the tail vein 72 h later, and the rats with blood glucose
levels of 16.7 mM and above were considered to be successful
induction of the diabetic model. Thereafter, bilateral OVX was
performed on the rats in the OVX, OVX+L, STZ+OVX, and STZ+OVX+L
groups following the previously described two-incision procedures
(17). The same procedures were
performed on the rest animals except for the ligation and excision
of ovaries. One week later, liraglutide (Novo Nordisk, Copenhagen,
Demark) was given to the rats in the STZ+L, OVX+L and STZ+OVX+L
groups by subcutaneous injection at a dose of 0.6 mg/kg daily for a
total of eight weeks, and an equal volume of saline was given daily
by subcutaneous injection to the other rats during the same period.
The rats were monitored every day and the rats' body weights were
recorded every week during the course of the experiment. Eight
weeks after STZ administration, 1–2 out of 10 STZ-treated rats
became very lean and inactive, the rat was euthanized by overdose
anesthesia once it was no longer taking food (humane endpoint).
These rats were not included for the analyses.
Intraperitoneal glucose tolerance test
(IPGTT)
At the end of eight-week liraglutide treatment, rats
were fasted for 18 h. Blood was sampled through the tail vein prior
to (0 min) as well as 15, 30, 60 and 120 min after an
intraperitoneal injection of glucose solution (2 g/kg body weight).
A blood drop on a blood glucose test strip was read for glucose
concentration by the Safe-Accu Angel Blood Glucose Monitoring
System (Sannuo, Changsha, China).
Serum biochemistry
After eight-week liraglutide treatment, the rats
were euthanized with overdose chloral hydrate, and blood was
withdrawn from each rat via the inferior vena cava. ELISA was
performed to determine the levels of insulin, osteocalcin (OC),
c-terminal telopeptide of type 1 collagen (CTX-1), osteoprotegerin
(OPG) and receptor activator of nuclear factor-κB ligand (RANKL)
proteins in the serum using the commercially available ELISA kits
(OC: LSBio, Seattle, WA, USA; insulin, CTX-1, OPG and RANKL: USCN,
Wuhan, China) according to the manufacturer's instructions. The
ratio of serum RANKL/OPG was then calculated.
Determination of BMD
Prior to STZ administration, and four and eight
weeks after liraglutide treatment, BMD was measured at the proximal
femurs in vivo using a GE-Lunar dual energy X-ray
absorptiometer (GE healthcare, Madison, WI, USA). Each femur was
measure three times for an average value.
Hematoxylin and eosin (H&E) and TRAP
staining. H&E and TRAP staining were performed nine weeks
after STZ administration and/or OVX procedure. The right femur of
each rat was fixed in 10% neutral buffered formalin and decalcified
in 10% ethylenediamine tetraacetic acid disodium (EDTA-2Na) (pH
7.2) for 30 days. The decalcified femurs were dehydrated, embedded
in paraffin, and sectioned with a Leica RM2235 microtome (Germany).
The tissue sections were stained with H&E following routine
procedures, followed by microscopic examination (magnification,
×400) for histopathological evaluation and osteoblast counts. In
order to characterize osteoclasts, the bone sections were subjected
to TRAP staining as previously described (18). Six random fields on each H&E- or
TRAP-stained sections were photographed at 400×, and
osteoblast/osteoclast number and bone perimeter/area on each
microscopic image were determined using Image Pro Plus 6.0 (Media
Cybernetics, Rockville, MD, USA). The average number of osteoblasts
per bone perimeter (Ob.N/B.Pm) and the number of osteoclasts per
tissue area (Oc.N/T.Ar, mm−2) were reported according to
the guidelines by the American Society for Bone and Mineral
Research Nomenclature Committee (19).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expressions of OPG and RANKL mRNAs
in the proximal femur were measured by qRT-PCR. The proximal femur
was pulverized in liquid nitrogen, and total RNA was extracted from
the bone tissues using a Total RNA Fast Extraction kit (BioTeke,
Beijing, China) according to the manufacturer's instructions. The
extracted RNA was reverse transcribed into cDNA using Super M-MLV
Reverse Transcriptase (BioTeke). RT-qPCR was performed in
Exicycler™ 96 Realtime Quantitative Thermal Block
(BIONEER, Daejeon, Korea) using SYBR-Green Master Mix (Solarbio)
and the following primers: OPG forward,
5′-GACCCCAGAGCGAAACACG-3′, and reverse, 5′-GGCACAGCAAACCTGAAGAA-3′;
RANKL forward, 5′-CATCGGGTTCCCATAAAG-3′, and reverse,
5′-GAAGCAAATGTTGGCGTA-3′; ACTB forward,
5′-GGAGATTACTGCCCTGGCTCCTAGC-3′, and reverse,
5′-GGCCGGACTCATCGTACTCCTGCTT-3′. The expression levels of OPG and
RANKL mRNAs were normalized to the expression level of ACTB
mRNA, an internal reference gene encoding β-actin, and the values
were expressed as relative values to the Sham group. The ratio of
RANKL/OPG was calculated accordingly.
Statistical analysis
All the data are presented as means ± SD.
Statistical analysis was performed using GraphPad Prism 5.0
(GraphPad Software Inc., La Jolla, CA, USA). Comparisons between
groups were conducted using one-way analysis of variance (ANOVA)
followed by Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Liraglutide attenuated STZ-induced
diabetic conditions in rats
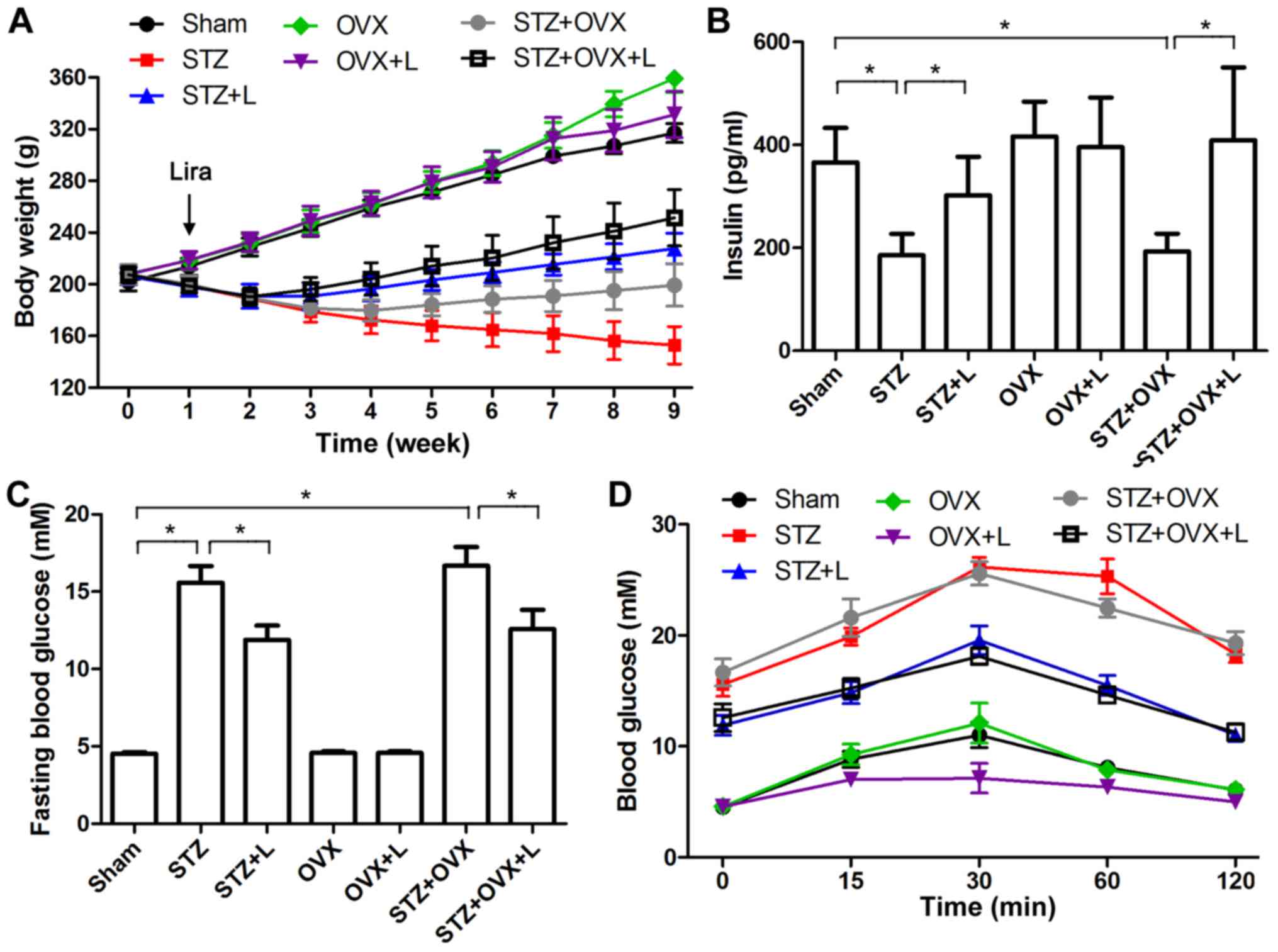
One week after STZ administration and/or OVX, the
rats were treated with liraglutide for eight weeks. As shown in
Fig. 1A, rat body weight dropped
over time following STZ administration, and liraglutide attenuated
the weight loss in STZ-treated rats. In addition, rat body weight
increased after OVX as compared with sham-operated rats. As a GLP-1
analog that can potentiate insulin secretion (10), liraglutide significantly increased
serum insulin concentrations in STZ and STZ+OVX rats after eight
week treatment (Fig. 1B), and it did
not affect serum estradiol levels in rats with STZ-induced diabetes
and/or OVX procedure (data not shown). Moreover, fasting blood
glucose (Fig. 1C) and glucose
tolerance (Fig. 1D) were measured,
and the results confirmed the anti-hyperglycemic effect of
liraglutide in STZ-induced diabetes.
Liraglutide increased BMD and
modulated bone metabolism in rats with STZ-induced diabetes and
OVX
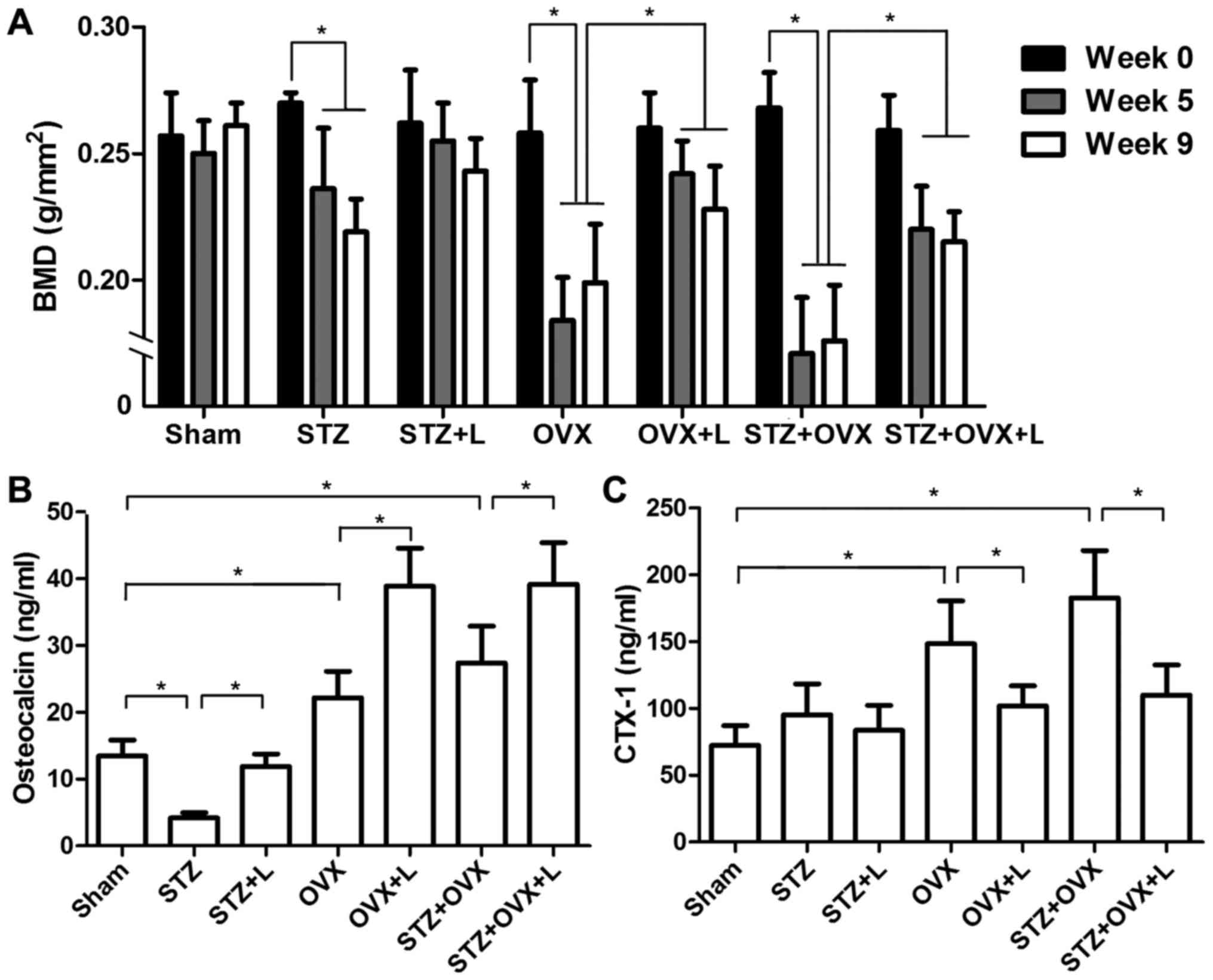
Femoral BMD was measured before STZ administration
and/or OVX procedure, as well as four and eight weeks after
liraglutide treatment. As shown in Fig.
2A, administration of STZ led to gradual reduction of BMD in
the femur. In contrast, OVX caused a sudden decline in BMD as an
early response, and BMD was slightly increased in the OVX rats at a
later adaptive stage. In STZ+OVX rats, BMD declined quickly and
kept at the low level. Liraglutide treatment maintained BMD after
STZ induction. Moreover, liraglutide significantly attenuated the
reduction of BMD in the rats with OVX or OVX+STZ.
Next, we assessed the serum levels of OC and CTX-1,
the markers of bone formation and resorption, respectively. As
shown in Fig. 2B, STZ decreased the
level of OC in the serum, whereas OVX increased serum OC
concentration. Liraglutide significantly increased serum OC
concentrations in all three osteoporotic models, demonstrating an
anabolic bone effect of liraglutide. On the other hand, OVX
markedly elevated the level of serum CTX-1, while STZ had little
effect on serum CTX-1 though (Fig.
2C). Liraglutide significantly reduced the elevation of serum
CTX-1 in OVX and STZ+OVX rats as compared with the corresponding
counterparts without liraglutide treatment. These results
demonstrated that liraglutide promoted bone formation and markedly
inhibited bone resorption in the rats with OVX and STZ-induced
diabetes.
Liraglutide prevented bone destruction
and reduced osteoclast numbers in STZ+OVX rats
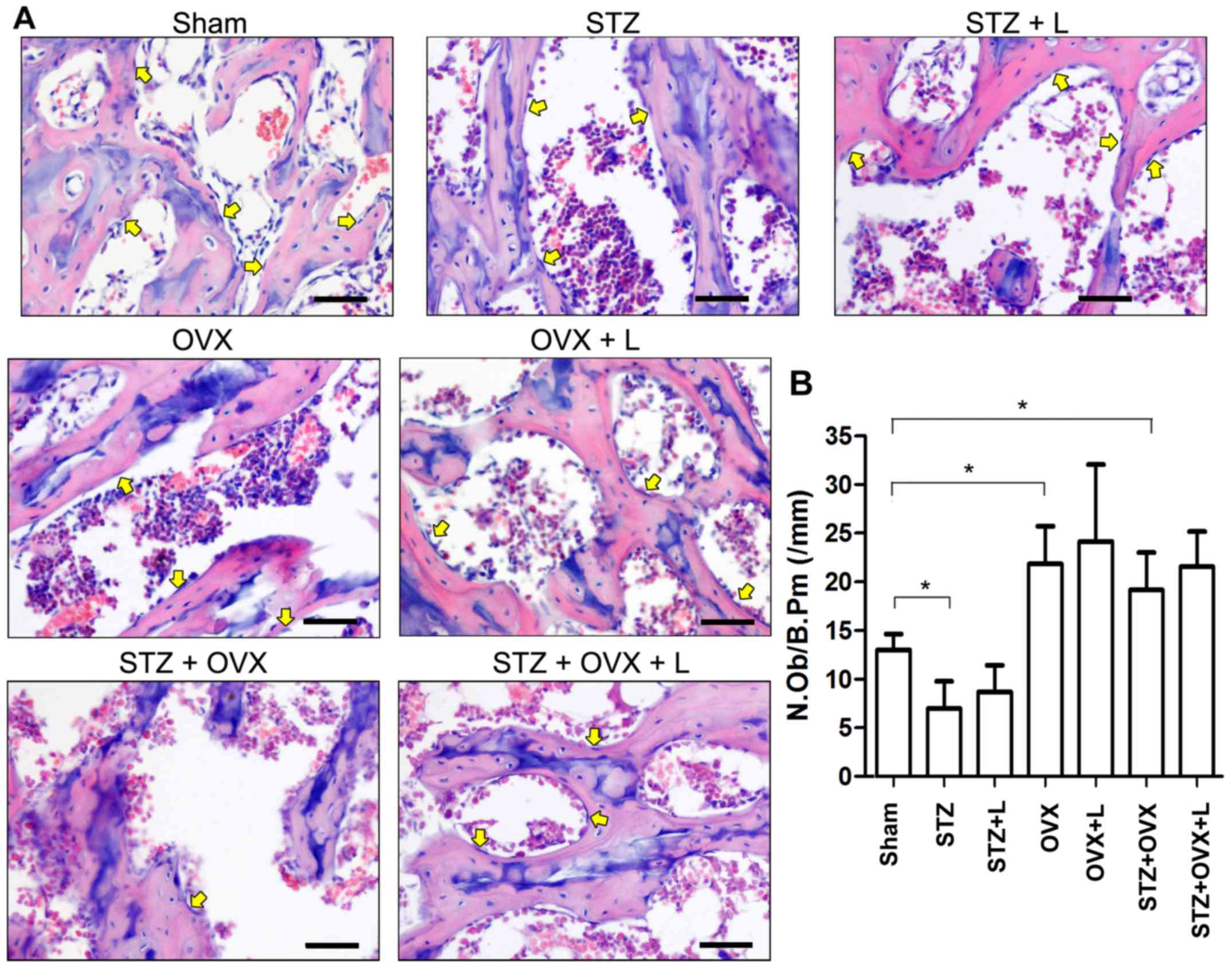
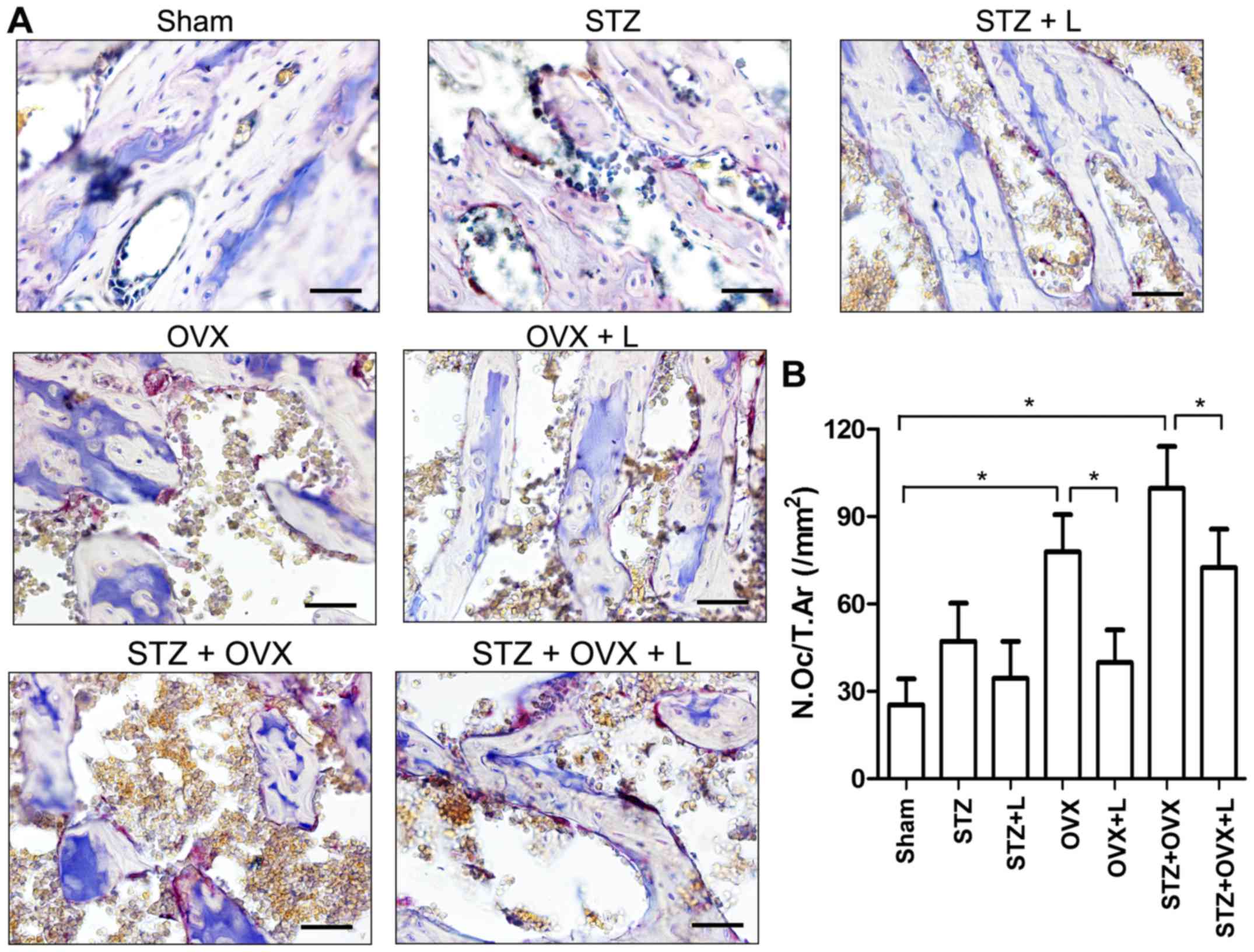
Histological changes in the proximal femur were
examined with H&E staining. As shown in Fig. 3, STZ or OVX resulted in thinning of
trabeculae and increased numbers and sizes of empty bone lacunae in
the proximal femur, while STZ+OVX exaggerated the pathological
alterations compared with either single treatment. Compared to
STZ+OVX group, STZ+OVZ+L group showed significant increases in the
thickness of trabeculae and total bone mass in the proximal
femur.
Next, the numbers of osteoblasts and osteoclasts
were counted based on H&E- and TRAP-stained sections,
respectively (Figs. 3 and 4). STZ decreased, while OVX and STZ+OVX
increased, the number of osteoblasts in the trabecular bone
(Fig. 3B). Liraglutide treatment,
however, did not show any significant effect on the osteoblasts at
week 9 in rats with STZ-induced diabetes and/or OVX. On the other
hand, STZ slightly while OVX markedly increased the number of
osteoclasts in the proximal femur, whereas liraglutide treatment
significantly reduced osteoclast number in the rats received OVX or
co-treated with STZ+OVX (Fig. 4B).
These data suggest that liraglutide may preserve bone mass and
architecture in the OVX+STZ rats mainly by inhibiting
osteoclast-mediated bone resorption.
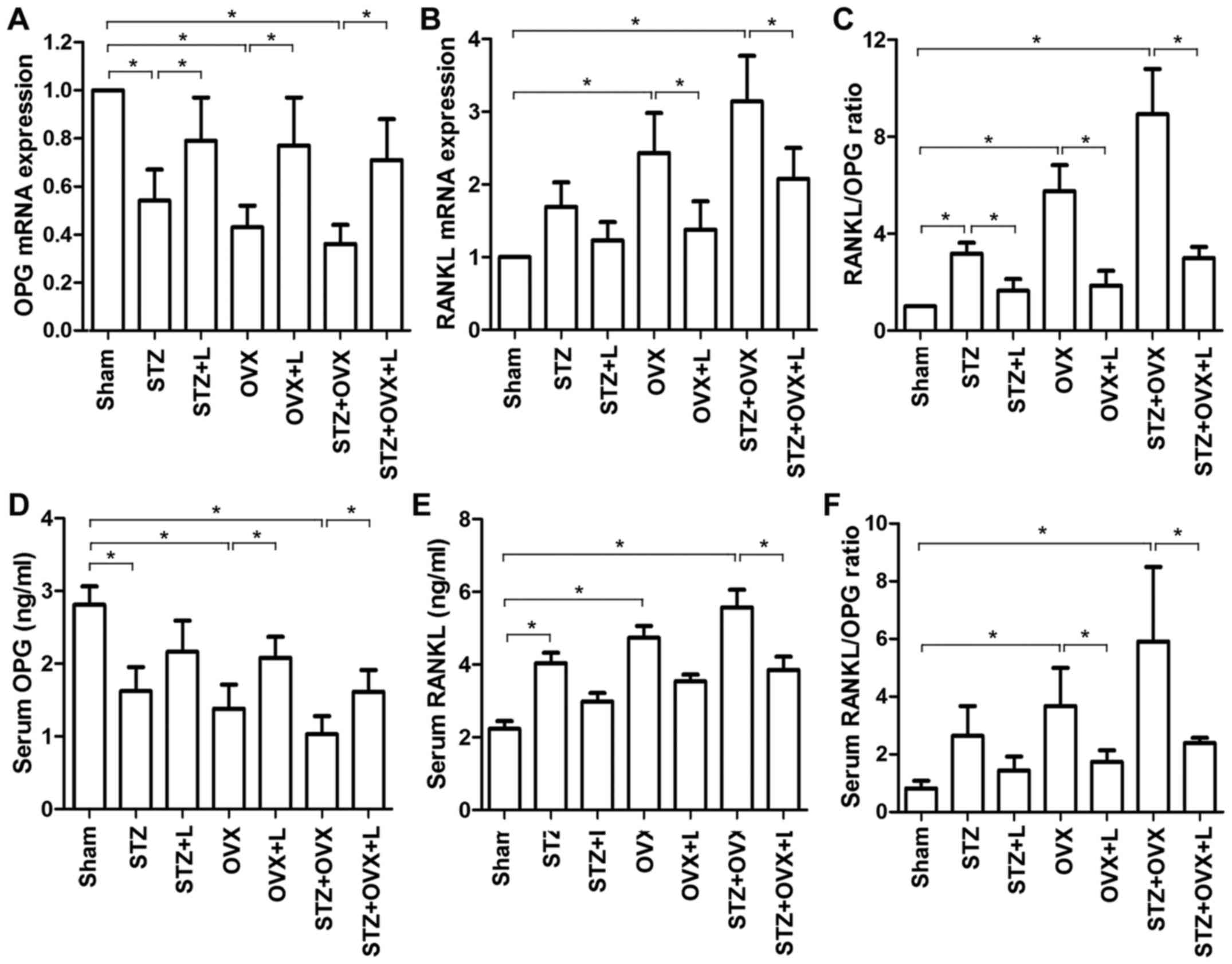
Liraglutide decreased the RANKL/OPG
ratio in osteoporotic rats
RANKL is an essential factor that drives osteoclast
differentiation and activation (20), and OPG is a natural antagonist of
RANKL (21). Hence, the RANKL/OPG
ratio determines the commitment to osteoclast differentiation. The
expression of OPG and RANKL mRNAs in the femoral
tissue was assessed by RT-qPCR. Compared with the sham-operated
animals, either STZ or OVX significantly reduced the expression of
OPG mRNA in the femur, and OVX markedly increased femoral
expression of RANKL mRNA (Fig. 5A
and B). Liraglutide increased OPG expression and
decreased RANKL expression in the osteoporotic rat models as
compared with the untreated counterparts. Moreover, STZ and OVX led
to increased ratios of RANKL/OPG mRNA in the femur, which
favored osteoclast differentiation. Liraglutide treatment, by
contrast, significantly decreased the RANKL/OPG ratio as
compared to the corresponding untreated groups (Fig. 5C). Consistently, STZ and/or OVX
significantly decreased serum OPG level and increased serum RANKL
level, resulting in increased ratios of serum RANKL/OPG (Fig. 5D-F). Liraglutide reversed the
alterations in serum OPG and RANKL levels, and reduced the serum
RANKL/OPG ratio in the osteoporotic models. Thus, these results
indicated that STZ- and OVX-induced osteoclastogenesis was
inhibited by liraglutide treatment.
Discussion
GLP-1 is an incretin hormone that is synthesized and
secreted by gut L cells in response to food intake, and it
stimulates insulin release by pancreatic beta-cells and suppresses
glucagon secretion from alpha-cells (22). As GLP-1 is rapidly degraded in
circulation, stable GLP-1 RAs are developed as a new class of
anti-diabetic medications that mimic incretin activities in the
body (10). Since expression of
functional GLP-1 receptors was identified on osteoblasts (23), the potential beneficial effects of
GLP-1 RAs has been evaluated experimentally on diabetic and
non-diabetic models (11,13,15,16,24,25).
Previous studies have demonstrated that liraglutide treatment could
increase BMD, improve bone micro-architecture and restore
mechanical properties of the bone in diabetic rodents (13,14), and
it also exerts beneficial effects on the bone in non-diabetic
osteoporotic models (15,16). In the present study, we constructed a
rat model of osteoporosis induced by both diabetes and OVX, and
showed that long-term liraglutide treatment was able to ameliorate
the defects in the bone of the severely osteoporotic rats. Our
study supports the bone-protective effect of liraglutide, and
provides preliminary evidence for the potential benefits of
liraglutide on the bone health of postmenopausal diabetic
patients.
The bone is a metabolically active tissue that
undergoes constant turnover through bone formation and resorption.
In the present study, although both STZ and OVX led to bone loss in
rats, their action mechanisms are apparently different. In the
diabetic rats, serum OC and osteoblast count were markedly reduced,
and these observations are consistent with previous reports
(13,26). In the meanwhile, serum CTX-1 and
osteoclast count were only slightly increased in STZ-induced
diabetes. These data imply that STZ-induced osteopenia was mainly
attributed to impaired bone formation. In the OVX rats, by
contrast, serum markers of bone formation (OC) and bone resorption
(CTX-1) were both elevated, while both osteoblasts and osteoclasts
were increased. These findings, in line with previous studies
(27,28), suggest that OVX-induced bone loss was
due to an increase rate of bone turnover. Liraglutide was
implicated to have an anabolic bone effect because it increased the
bone formation marker (N-terminal propeptide of type 1 procollagen)
in weight loss-induced bone mass reduction (12). This is similar with the phenomenon
observed in our study that liraglutide treatment led to increased
serum OC levels in STZ, OVX and STZ+OVX rats. Liraglutide and other
GLP-1 RA have been demonstrated to exert osteogenic actions in
animal models (14,25). In a spontaneous diabetic rat model,
liraglutide stimulates the expression of Runx2, a transcription
factor that drives the expression of osteoblast-specific genes, as
well as a number of bone formation markers such as alkaline
phosphatase, collagen 1 and OC in the bone (14). Intriguingly, liraglutide elevated the
level of bone formation marker but showed no effect on osteoblast
counts in rats with STZ- and OVX-induced osteoporosis. Our results
suggest that liraglutide promotes bone formation in the rats with
STZ- and OVX-induced osteoporosis probably by enhancing the
expression osteoblast-specific genes rather than promoting the
proliferation or differentiation of osteoblasts.
In the STZ+OVX rats with high rates of bone
formation and resorption, liraglutide treatment significantly
reduced serum CTX-1 and osteoclast number, suggesting that
liraglutide inhibited osteoclastogenesis and consequently
attenuated bone resorption in the osteoporotic rats. It was
reported that mice with GLP-1 receptor deficiency exhibited
osteopenia, increased osteoclast numbers and elevated bone
resorption (29), confirming an
inhibitory role of GLP-1 signaling in osteoclastogenesis. During
osteoclastogenesis, RANKL drives osteoclast differentiation
(20) while OPG antagonizes RANKL
action (21), and the RANKL/OPG
ratio determines osteoclastogenesis in the bone. Here, we showed
that both STZ and OVX increased RANKL/OPG ratio, which is
consistent with previous studies (30–33).
Liraglutide significantly reduced RANKL/OPG ratio compared to the
untreated counterparts, indicating that liraglutide can inhibit
osteoclastogenesis in STZ, OVX and STZ+OVX rats. GLP-1 and GLP-1 RA
exendin-4 have been shown to reverse hyperlipidic-related
osteopenia and decrease tibia RANKL/OPG ratio (24). Our data are consistent with the
previous findings and suggest that liraglutide or GLP-1 signaling
may suppress osteoclastogenesis via modulating the RANKL/OPG
ratio.
In conclusion, the present study demonstrates a
bone-preserving effect of liraglutide in a rat osteoporotic model
with STZ-induced diabetes and OVX, and this effect is mainly
associated with liraglutide-mediated suppression of
osteoclastogenesis. Our data suggest a potential value of
liraglutide in protecting the bone health of postmenopausal
diabetic population, yet this idea needs to be assessed in future
clinical studies.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Liaoning Province (no. 2015020658) and the
Diagnostic and Therapeutic Capability Construction Project for Key
Clinical Departments of Liaoning Provincial Hospital Reform (no.
LNCCC-D35-2015).
References
|
1
|
Vestergaard P: Discrepancies in bone
mineral density and fracture risk in patients with type 1 and type
2 diabetes-a meta-analysis. Osteoporos Int. 18:427–444. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Janghorbani M, Van Dam RM, Willett WC and
Hu FB: Systematic review of type 1 and type 2 diabetes mellitus and
risk of fracture. Am J Epidemiol. 166:495–505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grey A: Skeletal consequences of
thiazolidinedione therapy. Osteoporos Int. 19:129–137. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaidi M: Skeletal remodeling in health and
disease. Nat Med. 13:791–801. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berberoglu Z, Yazici AC and Demirag NG:
Effects of rosiglitazone on bone mineral density and remodelling
parameters in Postmenopausal diabetic women: A 2-year follow-up
study. Clin Endocrinol (Oxf). 73:305–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heilmeier U, Carpenter DR, Patsch JM,
Harnish R, Joseph GB, Burghardt AJ, Baum T, Schwartz AV, Lang TF
and Link TM: Volumetric femoral BMD, bone geometry and serum
sclerostin levels differ between type 2 diabetic postmenopausal
women with and without fragility fractures. Osteoporos Int.
26:1283–1293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Henriksen DB, Alexandersen P, Hartmann B,
Adrian CL, Byrjalsen I, Bone HG, Holst JJ and Christiansen C:
Four-month treatment with GLP-2 significantly increases hip BMD: A
randomized, placebo-controlled, dose-ranging study in
postmenopausal women with low BMD. Bone. 45:833–842. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nicodemus KK and Folsom AR: Iowa Women's
Health Study: Type 1 and type 2 diabetes and incident hip fractures
in postmenopausal women. Diabetes Care. 24:1192–1197. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nauck MA: Incretin-based therapies for
type 2 diabetes mellitus: Properties, functions and clinical
implications. Am J Med. 124 Suppl 1:S3–S18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su B, Sheng H, Zhang M, Bu L, Yang P, Li
L, Li F, Sheng C, Han Y, Qu S and Wang J: Risk of bone fractures
associated with glucagon-like peptide-1 receptor agonists'
treatment: A meta-analysis of randomized controlled trials.
Endocrine. 48:107–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iepsen EW, Lundgren JR, Hartmann B,
Pedersen O, Hansen T, Jørgensen NR, Jensen JE, Holst JJ, Madsbad S
and Torekov SS: GLP-1 receptor agonist treatment increases bone
formation and prevents bone loss in weight-reduced obese women. J
Clin Endocrinol Metab. 100:2909–2917. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mansur SA, Mieczkowska A, Bouvard B, Flatt
PR, Chappard D, Irwin N and Mabilleau G: Stable incretin mimetics
counter rapid deterioration of bone quality in type 1 diabetes
mellitus. J Cell Physiol. 230:3009–3018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun HX, Lu N, Luo X, Zhao L and Liu JM:
Liraglutide, the glucagon-like peptide-1 receptor agonist, has
anabolic bone effects in diabetic Goto-Kakizaki rats. J Diabetes.
7:584–588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu N, Sun H, Yu J, Wang X, Liu D, Zhao L,
Sun L, Zhao H, Tao B and Liu J: Glucagon-like peptide-1 receptor
agonist Liraglutide has anabolic bone effects in ovariectomized
rats without diabetes. PLoS One. 10:e01327442015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pereira M, Jeyabalan J, Jorgensen CS,
Hopkinson M, Al-Jazzar A, Roux JP, Chavassieux P, Orriss IR,
Cleasby ME and Chenu C: Chronic administration of Glucagon-like
peptide-1 receptor agonists improves trabecular bone mass and
architecture in ovariectomised mice. Bone. 81:459–467. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lasota A and Danowska-Klonowska D:
Experimental osteoporosis-different methods of ovariectomy in
female white rats. Rocz Akad Med Bialymst. 49 Suppl 1:S129–S131.
2004.
|
|
18
|
Moon YJ, Yun CY, Choi H, Ka SO, Kim JR,
Park BH and Cho ES: Smad4 controls bone homeostasis through
regulation of osteoblast/osteocyte viability. Exp Mol Med.
48:e2562016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dempster DW, Compston JE, Drezner MK,
Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR
and Parfitt AM: Standardized nomenclature, symbols and units for
bone histomorphometry: A 2012 update of the report of the ASBMR
histomorphometry nomenclature committee. J Bone Miner Res. 28:2–17.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong YY, Yoshida H, Sarosi I, Tan HL,
Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G,
Itie A, et al: OPGL is a key regulator of osteoclastogenesis,
lymphocyte development and lymph-node organogenesis. Nature.
397:315–323. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kazafeos K: Incretin effect: GLP-1, GIP,
DPP4. Diabetes Res Clin Pract. 93 Suppl 1:S32–S36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nuche-Berenguer B, Portal-Núñez S, Moreno
P, González N, Acitores A, López-Herradón A, Esbrit P, Valverde I
and Villanueva-Penacarrillo ML: Presence of a functional receptor
for GLP-1 in osteoblastic cells, independent of the cAMP-linked
GLP-1 receptor. J Cell Physiol. 225:585–592. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nuche-Berenguer B, Lozano D,
Gutierrez-Rojas I, Moreno P, Marinoso ML, Esbrit P and
Villanueva-Penacarrillo ML: GLP-1 and exendin-4 can reverse
hyperlipidic-related osteopenia. J Endocrinol. 209:203–210. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nuche-Berenguer B, Moreno P, Portal-Nuñez
S, Dapía S, Esbrit P and Villanueva-Penacarrillo ML: Exendin-4
exerts osteogenic actions in insulin-resistant and type 2 diabetic
states. Regul Pept. 159:61–66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nuche-Berenguer B, Moreno P, Esbrit P,
Dapia S, Caeiro JR, Cancelas J, Haro-Mora JJ and
Villanueva-Penacarrillo ML: Effect of GLP-1 treatment on bone
turnover in normal, type 2 diabetic and insulin-resistant states.
Calcif Tissue Int. 84:453–461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tantikanlayaporn D, Wichit P,
Weerachayaphorn J, Chairoungdua A, Chuncharunee A, Suksamrarn A and
Piyachaturawat P: Bone sparing effect of a novel phytoestrogen
diarylheptanoid from Curcuma comosa Roxb. in ovariectomized rats.
PLoS One. 8:e787392013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takano-Yamamoto T and Rodan GA: Direct
effects of 17 beta-estradiol on trabecular bone in ovariectomized
rats. Proc Natl Acad Sci USA. 87:2172–2176. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamada C, Yamada Y, Tsukiyama K, Yamada K,
Udagawa N, Takahashi N, Tanaka K, Drucker DJ, Seino Y and Inagaki
N: The murine glucagon-like peptide-1 receptor is essential for
control of bone resorption. Endocrinology. 149:574–579. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng J, Hui K, Hao C, Peng Z, Gao QX, Jin
Q, Lei G, Min J, Qi Z, Bo C, et al: Low bone turnover and reduced
angiogenesis in streptozotocin-induced osteoporotic mice. Connect
Tissue Res. 57:277–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu SG, Zhang CJ, Xu XE, Sun JH, Zhang L
and Yu PF: Ursolic acid derivative ameliorates
streptozotocin-induced diabestic bone deleterious effects in mice.
Int J Clin Exp Pathol. 8:3681–3690. 2015.PubMed/NCBI
|
|
32
|
Ma B, Zhang Q, Wu D, Wang YL, Hu YY, Cheng
YP, Yang ZD, Zheng YY and Ying HJ: Strontium fructose
1,6-diphosphate prevents bone loss in a rat model of postmenopausal
osteoporosis via the OPG/RANKL/RANK pathway. Acta Pharmacol Sin.
33:479–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sato T, Watanabe K, Masuhara M, Hada N and
Hakeda Y: Production of IL-7 is increased in ovariectomized mice,
but not RANKL mRNA expression by osteoblasts/stromal cells in bone
and IL-7 enhances generation of osteoclast precursors in vitro. J
Bone Miner Metab. 25:19–27. 2007. View Article : Google Scholar : PubMed/NCBI
|