Introduction
Autoimmune hepatitis (AIH) manifests as inflammation
of the liver with nonspecific symptoms, including fatigue, jaundice
and arthralgia, and some patients may develop cirrhosis, which
results in mortality due to liver failure or the need for liver
transplantation (1). AIH may occur
at all ages and is diagnosed more often in females, and it is
increasingly recognized as a global disease (2). Prednisone (pred) treatment or pred plus
azathioprine (AZA) treatment has been used as the conventional
treatment for AIH for >40 years (3). However, some patients with AIH do not
respond optimally to the standard treatment and those who do
respond may experience strong side effects, including infection and
diabetes mellitus, or a relapse following drug withdrawal (4). Thus, development of a novel alternative
treatment for AIH is required.
Several medications, including cyclosporine-A (CsA),
tacrolimus, mycophenolate mofetil, budesonide and ursodeoxycholic
acid (UDCA) are emerging as alternative frontline therapies for
patients with AIH (5–15). CsA, budesonide (bude) and UDCA have
been evaluated by randomized clinical trials (RCTs), and it has
been demonstrated that they are just as effective or more effective
than traditional treatment regimens (7,14,15).
Other advances in the pharmacotherapy of AIH have been derived
primarily from observational studies (5,8). Due to
the low incidence of the disease in the general populations (mean
annual incidence in white northern Europeans per 100,000 was
0.85–1.9 for AIH; point prevalence per 100,000 was 10.7–16.9 for
AIH) (16,17), RCTs on the treatment of AIH are
sparse and the number of patients included in RCTs is limited,
leading to some potential interferences on conclusions. Therefore,
the most effective treatment for AIH remains unclear.
The present study aimed to compare the efficacy of
eight treatments, including pred, pred + AZA, pred in titrated
doses given on alternate days (pred-T), placebo, AZA, bude + AZA,
UDCA + pred and CsA for adult patients with AIH. These eight
treatments were compared from six RCTs by network meta-analysis
(NMA), which calculates the relative effects for all treatments,
including treatments that have not been compared one by one, in the
evidence network in one simultaneous analysis (18). The aim was to provide hierarchies of
the comparative clinical and biochemical remission for eight
treatments.
Materials and methods
Search methods
Embase (www.embase.com), Pubmed (www.ncbi.nlm.nih.gov/pubmed) and the Cochrane Library
(www.cochranelibrary.com) databases were
searched for publications published between 1966 and April 2017.
Key words and Medical Subject Headings (the vocabulary thesaurus
used for indexing articles for PubMed) terms including ‘Autoimmune
Hepatitides’, ‘Hepatitides, Autoimmune’, ‘Autoimmune Chronic
Hepatitis’, ‘Autoimmune Chronic Hepatitides’, ‘Chronic Hepatitides,
Autoimmune’, ‘Hepatitis, Autoimmune Chronic,’ ‘Autoimmune
Hepatitis’, ‘Chronic Hepatitis, Autoimmune’ and ‘Hepatitides,
Autoimmune Chronic’, and ‘treatments and/or therapies’ were used
during the search. The reference list for any discounted papers was
also observed. Two reviewers independently made the selection of
studies to include in the present study based on titles and
abstracts. Any disagreement between reviewers was resolved by a
further reviewer.
Inclusion and exclusion criteria
The selected studies had to fulfill the following
inclusion criteria: i) Inclusion of patients with AIH; ii)
inclusion of patients receiving therapeutic intervention; and iii)
RCT study design that was randomized, placebo or an untreated
controlled trial. Prior to the proposed the definition of AIH by
the International Autoimmune Hepatitis Group in 1993, there were
evolving definitions of AIH (19).
Therefore, in order to check whether the search performed included
all published articles that were possibly associated with the
present meta-analysis, the reference lists of included papers were
also scrutinized.
Exclusion criteria included: i) Articles that were
not written in English, German, French or Spanish; ii) studies
including traditional Chinese medicine treatment; iii) studies
evaluating the efficacy of therapy for AIH in children; iv) studies
evaluating the efficacy of maintenance therapy for AIH; v) studies
with a follow-up of <6 months; and vi) case studies, case
series, review studies, letters and meeting proceedings.
Data collection
A total of two review authors screened papers,
removed ineligible references and contacted corresponding authors
if further information was required. If the article could not be
provided, the data was obtained from the author or other associated
articles. Information including the first author name, journal,
study date range, sample size, comparators, treatment plan,
country, study design, follow-up time and three outcomes
(remission, mortality rates and adverse events) were extracted for
each included study. Remission, mortality rates and adverse events
were used to estimate efficacy and tolerability of treatments for
the network meta-analysis.
Liver biopsy was not an outcome described in all
articles, therefore, clinical and biochemical remission was applied
as the primary outcome measure in order to achieve the most
appropriate definition of remission for the study. The definition
of clinical and biochemical remission was as follows, according to
previously published studies: Aspartate aminotransferase and
alanine transaminase (ALT) in the normal range and absence of any
clinical signs of deterioration (14,15). All
outcomes were extracted from the included studies and assessed at
maximum follow-up.
Study quality
The Cochrane Risk of Bias Tool was used to assess
the quality of included studies by two reviewers (20). The tool is based on assessing random
sequence generation, allocation concealment, blinding, incomplete
outcome data, selective reporting and other bias. The
classification of the judgment for each domain was low risk of
bias, unclear risk of bias or high risk of bias.
Data analysis
A traditional pairwise meta-analysis, which directly
compared different treatments, was first performed using Stata
software (version 13.0; StataCorp, College Station, TX, USA).
According to the Cochrane Handbook for Systematic Reviews of
Interventions Version 5.1.0, the pooled estimates of hazard ratios
(HRs), odds ratios (ORs) and 95% confidence intervals (CIs) of
remission were calculated using the DerSimonian and Laird
random-effects model. A χ2 square test and I2
test were used for testing heterogeneity among the studies. If the
OR and 95% CI were not close to 1, there was a statistical
difference between the two groups. P<0.05 indicated that the
difference between groups was statistically significant.
The network meta-analysis was also performed using
Stata software with the random effects models proposed by Chaimani
et al (21) (downloaded from
mtm.uoi.gr) according to the program. The ifplot command was used
to evaluate the consistency of direct and indirect estimates. A
funnel plot was used to identify possible publication bias if the
number of RCTs was >10 (22).
Posterior probabilities of outcomes were used to calculate
probabilities of treatment ranking and the cumulative ranking
probabilities (surface under the cumulative ranking curve; SUCRA)
were used to indicate the most effective treatment.
Results
Study identification and
selection
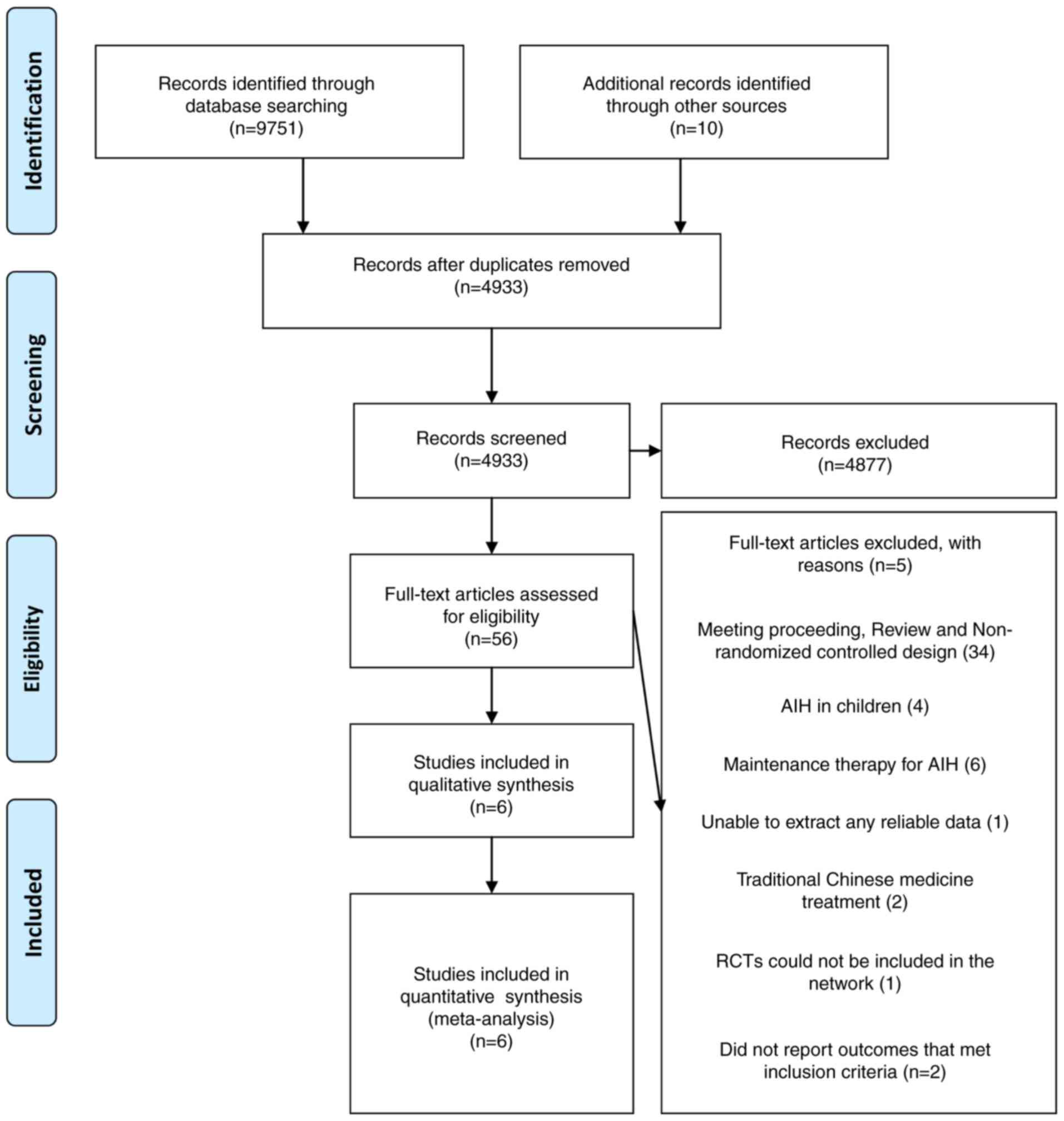
A PRISMA flow diagram of study selection is
presented in Fig. 1. The search was
performed on April 17 2017 and 9,761 references were identified.
Following removal of 4,828 duplicate references, 4,933 records were
screened. However, 4,877 articles were excluded following review of
the title and abstract (e.g., studies not associated with AIH). A
total of 56 studies were included in the narrative review and data
from six of these studies were included in the meta-analysis. A
total of 50 studies were excluded due to 34 studies not being RCT
studies, four studies evaluating the efficacy of therapy for AIH in
children (23–26), six studies evaluating maintenance
therapy for AIH (27–31), two studies evaluating traditional
Chinese medicine treatment (32,33), one
study being unable to extract any reliable data (34), two studies not reporting the outcome
that met inclusion criteria (9,11) and
one study where treatment duration was indefinite and may have
included the same relapsed patients with AIH as those included in
another study performed by the same author (6).
Study characteristics
Table I provides a
summary of the studies included in the present review. A total of
six studies were included, with a total of 517 patients. All
studies were RCTs directly comparing alternative treatments. A
total of four out of six studies were two-grouped studies, one was
a four-grouped study and one was a five-grouped study. These
studies were published between 1972 and 2013. The treatment
duration was between 6 months and 6 years and the mean age of
participants was 41.5 years. A total of eight treatments were
included in the network meta-analysis with no treatment considered
as placebo treatment. A total of six studies reported clinical and
biochemical remission as the outcome.
 | Table I.Characteristics of included
studies. |
Table I.
Characteristics of included
studies.
| Author, year | Journal | Study design | Patients | Age, years (mean ±
SD) | Intervention | Maximum
follow-up | Study size (n) | Remission (n) | (Refs.) |
|---|
| Soloway et al,
1972 |
Gastroenterology | RCT | Naïve or relapse
AIH | 44.0±17.1 | Pred: 60 mg/day for
1 week; 40 mg/day for 1 week; 30 mg/day for 2 weeks; 20 mg/day for
maintenance | 42 months | 18 | 8 | (10) |
|
|
|
|
|
| AZA: 100
mg/day |
| 14 | 1 |
|
|
|
|
|
|
| AZA (50 mg/day) +
Pred: |
| 14 | 3 |
|
|
|
|
|
|
| 30 mg/day for 1
week; |
|
|
|
|
|
|
|
|
|
| 20 mg/day for 1
week; |
|
|
|
|
|
|
|
|
|
| 15 mg/day for 2
weeks; |
|
|
|
|
|
|
|
|
|
| 10 mg/day for
maintenance |
|
|
|
|
|
|
|
|
|
| Placebo |
| 17 | 0 |
|
| Summerskill et al,
1975 | Gut | RCT | Naïve AIH | 40.0±3.6 | Pred: 60
mg/day | 36 months | 30 | 14 | (12) |
|
|
|
|
|
| for 1 week; 40
mg/day |
|
|
|
|
|
|
|
|
|
| for 1 week; 30
mg/day |
|
|
|
|
|
|
|
|
|
| for 2 weeks; 20
mg/day |
|
|
|
|
|
|
|
|
|
| for
maintenance |
|
|
|
|
|
|
|
|
| 44.0±3.5 | AZA (50 mg/day) +
Pred: |
| 30 | 17 |
|
|
|
|
|
|
| 30 mg/day for 1
week; |
|
|
|
|
|
|
|
|
|
| 20 mg/day for 1
week; |
|
|
|
|
|
|
|
|
|
| 15 mg/day for 2
weeks; |
|
|
|
|
|
|
|
|
|
| 10 mg/day for
maintenance |
|
|
|
|
|
|
|
|
| 38.0±3.5 | Placebo |
| – | – |
|
|
|
|
|
|
| AZA: 100
mg/day |
| – | – |
|
|
|
|
|
| 34.0±2.6 | Pred-T |
| 31 | 9 |
|
| Tage-Jensen et al,
1982 | Liver | RCT | naïve AIH | 67.0
(25.0–80.0)a | AZA: 10
mg/kg/week | 83 months | 37 | 6 | (13) |
|
|
|
|
|
| for first 2 weeks
and |
|
|
|
|
|
|
|
|
|
| then 5
mg/kg/week |
|
|
|
|
|
|
|
|
|
| Pred: <70 kg, 10
mg/day; |
| 47 | 21 |
|
|
|
|
|
|
| ≥70 kg 15
mg/day |
|
|
|
|
| Czaja et al,
1999 | Hepatology | RCT | Relapse AIH | 42.0±2.0 | UDCA (13–15
mg/kg/day) + | 6 months | 21 | 3 | (7) |
|
|
|
|
|
| Pred: 60 mg/day
for |
|
|
|
|
|
|
|
|
|
| 1 week; 40 mg/day
for |
|
|
|
|
|
|
|
|
|
| 1 week; 30
mg/day |
|
|
|
|
|
|
|
|
|
| for 2 weeks; 20
mg/day |
|
|
|
|
|
|
|
|
|
| for
maintenance |
|
|
|
|
|
|
|
|
| 50.0±4.0 | Pred: 60
mg/day |
| 16 | 3 |
|
|
|
|
|
|
| for 1 week; 40
mg/day |
|
|
|
|
|
|
|
|
|
| for 1 week; 30
mg/day |
|
|
|
|
|
|
|
|
|
| for 2 weeks; 20
mg/day |
|
|
|
|
|
|
|
|
|
| for
maintenance |
|
|
|
|
| Manns et al,
2010 |
Gastroenterology | RCT | Naïve or relapse
AIH | 36.0±17.0 | Bude: 3 mg three
times daily or 3 mg twice daily, after biochemical remission + AZA
1–2 mg/kg/day | 6 months | 100 | 60 | (14) |
|
|
|
|
| 38.0±19.0 | Pred: Starting dose
40 mg/day tapered to 10 mg/day + AZA 1–2 mg/kg/day |
| 103 | 40 |
|
| Siavosh et al,
2013 | Middle East Journal
of Digestive Diseases | RCT | Naïve AIH | 33.6±12.7 | Pred: 50
mg/day | 12 months | 24 | 12 | (15) |
|
|
|
|
| 30.0±10.3 | Cyclosporine-A: 2
mg/kg body weight in two divided doses administered orally 12 h
apart |
| 15 | 7 |
|
Risk of bias in included studies
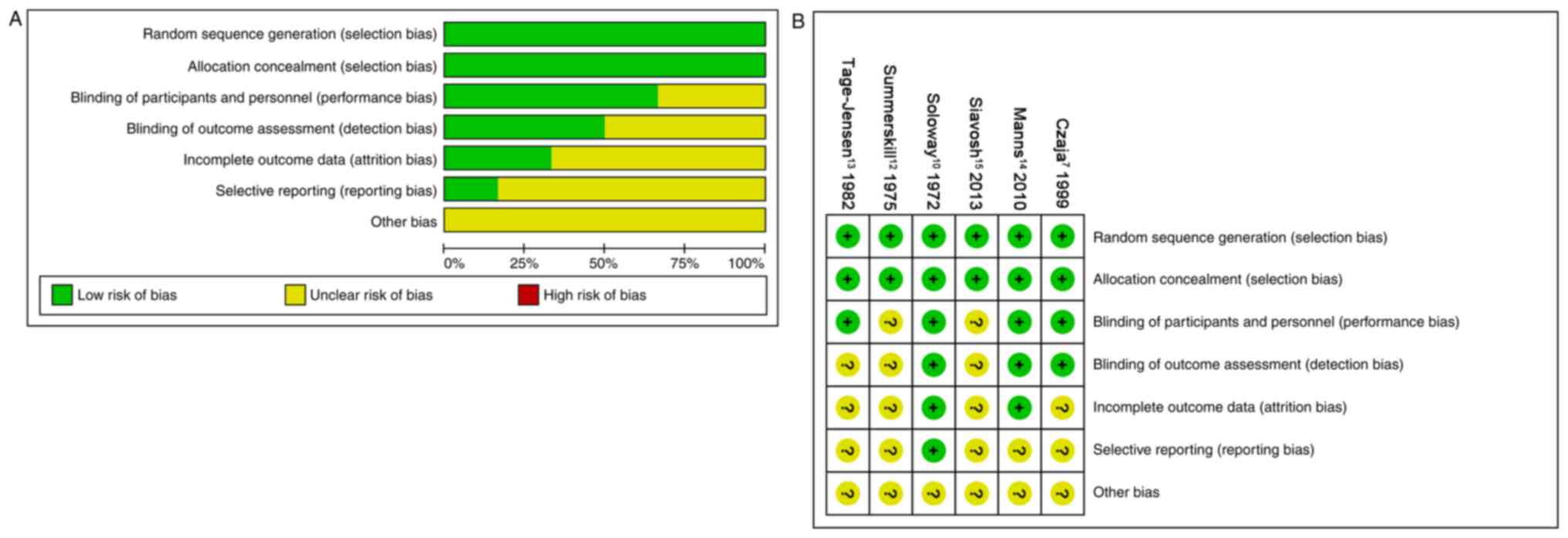
The risk of bias in all six studies is presented in
Fig. 2. A total of six studies
(100%) described random sequence generation and adequate allocation
concealment (100%). A total of four studies (67%) described
blinding of participants and personnel, and three studies (50%) had
low risk of blinding of outcome assessment. A total of two studies
(33%) had a low risk of incomplete outcome data and one study (16%)
had low risk of selectively reporting results. Although some
studies had dropout, the effect of intervention was not affected
due to the small scale of dropout.
Effects of treatments on
remission
A total of 147 patients were assigned to pred + AZA
treatment, 135 to pred therapy, 100 to bude + AZA therapy, 51 to
AZA therapy, 31 to pred-T therapy, 21 to UDCA + pred therapy, 17 to
placebo therapy and 15 to CsA therapy.
The direct comparisons on remission were performed
in Table II. The result indicated
that patients treated with pred had significantly increased rates
of remission compared with patients treated with AZA (OR, 0.20; 95%
CI, 0.08–0.53) or placebo (OR, 0.04; 95% CI, 0.00–0.68) and
patients treated with pred + AZA had a significantly increased rate
of remission compared with those treated with pred-T (OR, 0.31; 95%
CI, 0.11–0.90). In addition, patients treated with bude + AZA had a
significantly increased rate of remission compared with those
treated with pred + AZA (OR, 2.36; 95% CI, 1.35–4.15).
 | Table II.Direct comparisons or network
meta-analysis of the remission of different treatments.. |
Table II.
Direct comparisons or network
meta-analysis of the remission of different treatments..
| Treatment | Pred | Pred + AZA | Pred-T | Placebo | AZA | Bude + AZA | UDCA + Pred | CsA |
|---|
| Pred | – | 0.89 (0.31,
2.56) | 0.47 (0.16,
1.35) | 0.04 (0.00,
0.68)a | 0.20 (0.08,
0.53)a | – | 0.72 (0.13,
4.17) | 0.88 (0.24,
3.19) |
| Pred + AZA | 0.90 (0.28,
2.88) | – | 0.31 (0.11,
0.90) | 0.09 (0.00,
1.99) | 0.28 (0.03,
3.11) | 2.36 (1.35,
4.15)a | – | – |
| Pred-T | 0.36 (0.09,
1.44) | 0.40 (0.10,
1.59) | – | – | – | – | – | – |
| Placebo | 0.06 (0.00,
1.16) | 0.06 (0.00,
1.39) | 0.16 (0.01,
4.03) | – | 3.89 (0.15,
103.19) | – | – | – |
| AZA | 0.21 (0.06,
0.71)a | 0.23 (0.05,
1.10) | 0.57 (0.09,
3.42) | 3.56 (0.16,
78.85) | – | – | – | – |
| Bude + AZA | 2.14 (0.43,
10.65) | 2.36 (0.78,
7.20) | 5.87 (1.00,
34.44) | 36.66 (1.40,
962.49)a | 10.30 (1.50,
70.70)a | – | – | – |
| UDCA + Pred | 0.72 (0.10,
5.33) | 0.80 (0.08,
8.60) | 1.98 (0.18,
22.44) | 12.40 (0.34,
452.42) | 3.49 (0.33,
36.50) | 0.34 (0.03,
4.40) | – | – |
| CsA | 0.88 (0.17,
4.38) | 0.97 (0.13,
7.04) | 2.40 (0.29,
19.97) | 15.02 (0.50,
448.64) | 4.22 (0.56,
32.12) | 0.41 (0.04,
3.99) | 1.21 (0.09,
15.78) | – |
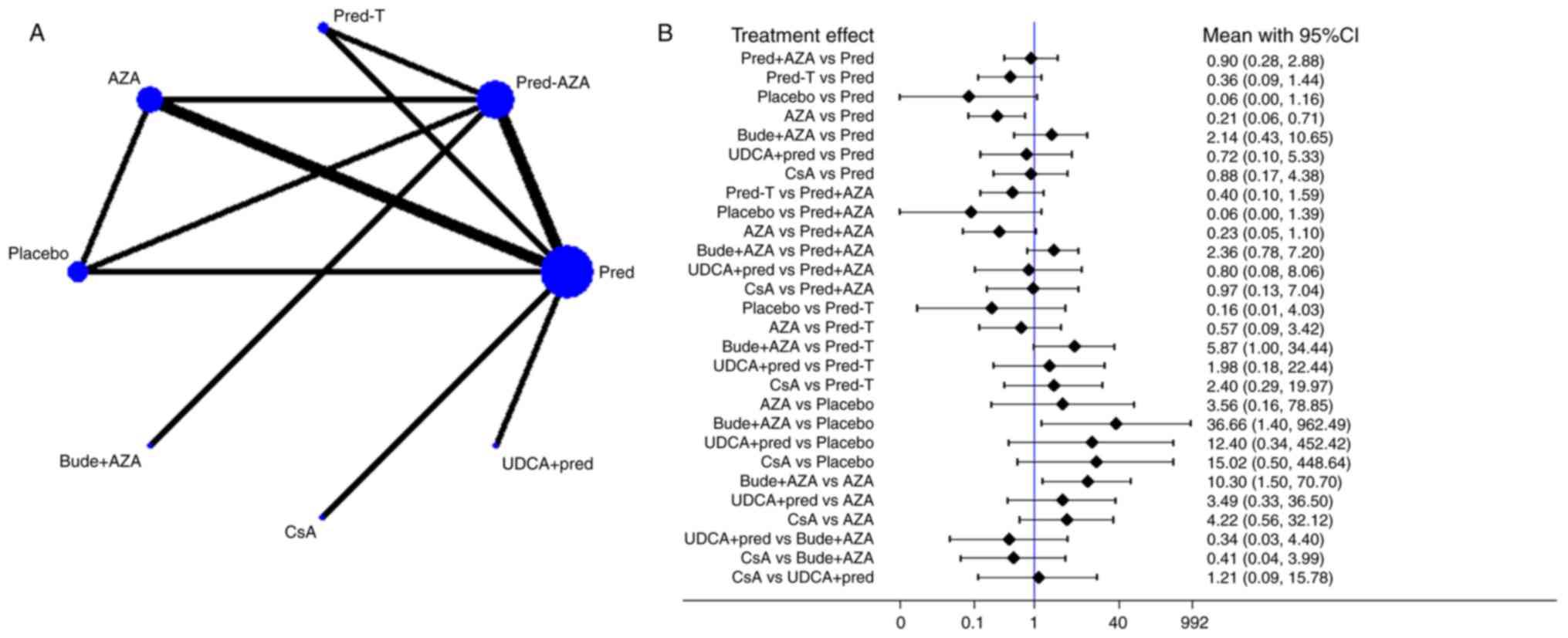
The network of comparisons on remission is presented
in Fig. 3 and Table II. Treatment with pred significantly
increased remission compared with treatment with AZA (OR, 0.21; 95%
CI, 0.06–0.71) and bude + AZA treatment significantly increased
remission compared with placebo treatment (OR, 36.66; 95% CI,
1.40–962.49) or AZA (OR, 10.30; 95% CI, 1.50–70.70). A ranking
graph of distribution of probabilities on remission is presented in
Fig. 4A. Based on SUCRA, bude + AZA
treatment (89.4) was ranked first, pred (69.1) was ranked second,
pred + AZA (63.2) and CsA (62.8) were ranked fourth and placebo was
ranked last (7.8; Table III).
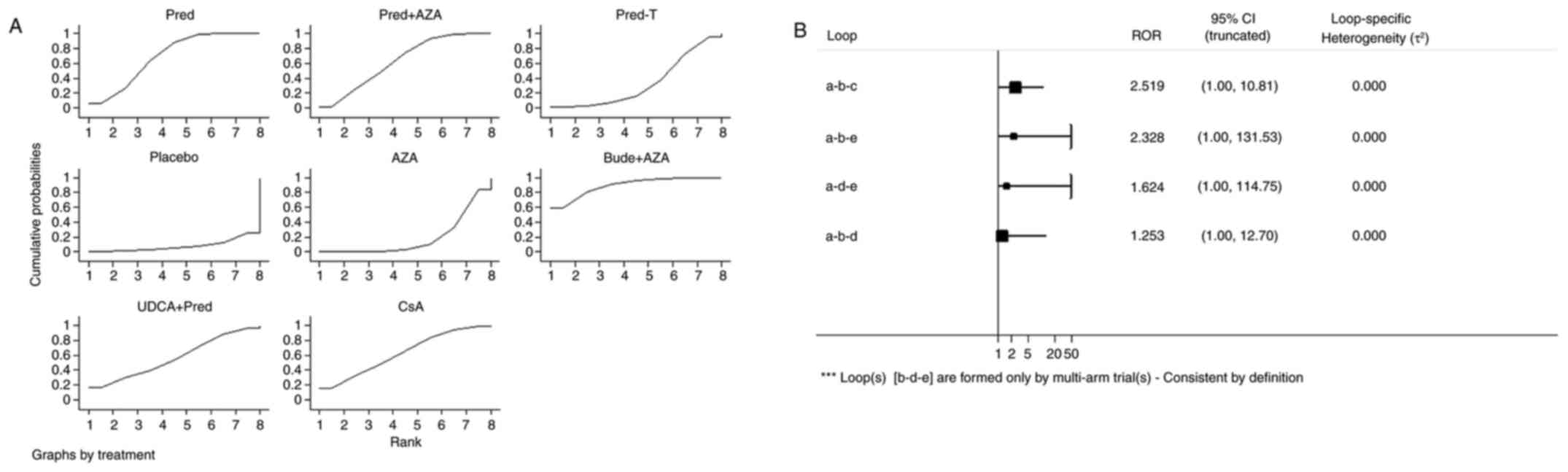 | Figure 4.Ranking of treatment strategies. (A)
Ranking of treatment strategies based on the probability of their
effects on outcome of remission. (B) Inconsistency plot for primary
efficacy outcome of remission. Pred, prednisone; AZA, azathioprine;
pred-T, prednisone in titrated doses given on alternate days; bude,
budesonide; UDCA, ursodeoxycholic acid; CsA, cyclosporine-A; CI,
confidence interval; a, pred; b, prednisone + AZA; c, pred-T; d,
AZA; e, placebo; RoR, ratio of two odds ratios. |
 | Table III.SUCRA and rank results. |
Table III.
SUCRA and rank results.
| Treatment | SUCRA | PrBest | Mean rank |
|---|
| Pred | 69.1 | 6.3 | 3.2 |
| Pred + AZA | 63.2 | 1.6 | 3.6 |
| Pred-T | 33.0 | 0.6 | 5.7 |
| Placebo | 7.8 | 0.5 | 7.5 |
| AZA | 18.6 | 0.0 | 6.7 |
| Bude + AZA | 89.4 | 59.2 | 1.7 |
| UDCA + pred | 56.1 | 16.4 | 4.1 |
| Cyclosporine-A | 62.8 | 15.4 | 3.6 |
Inconsistency test
Inconsistency refers to differences between direct
and various indirect effect estimates for the same comparison. The
ratio of two odds ratios (RoR) from direct and indirect evidence in
each loop was calculated to estimate the inconsistency. RoR values
close to 1 mean that the direct and indirect evidence were in
agreement. Fig. 4B demonstrated that
in a total of four loops there were none with statistically
significant inconsistency as all CIs for RoRs were compatible with
zero inconsistency (RoR=1). These results indicate that direct and
indirect estimates were consistent with one another.
Publication biases
Publication bias was not assessed because the number
of RCTs was limited (<10).
Other outcomes
The frequencies and percentages of adverse events
were not recorded in the majority of articles (Table IV). Adverse events associated with
pred and bude treatment were as follows: Cushingoid appearances,
including moon face, acne and hirsutism; diabetes mellitus;
hypertension; and cataracts. The adverse events following AZA
therapy were gastrointestinal bleeding, leucopenia, trombopenia and
arthralgia. Combination therapy with pred + AZA exhibited a
markedly lower reported frequency of adverse events compared with
pred monotherapy (data not shown). In addition, bude treatment had
less adverse events compared with pred treatment (data not
shown).
 | Table IV.Adverse events and mortality. |
Table IV.
Adverse events and mortality.
| Author, year | Journal | Intervention | Treatment
duration | Patients (n) | Adverse events
(n) | Mortalities
(n) | (Refs.) |
|---|
| Soloway et al,
1972 |
Gastroenterology | Pred: 60 mg/day
for | 3 months-3.5
years | 18 | Cushingoid
appearance (13), | 1 | (10) |
|
|
| 1 week; 40 mg/day
for |
|
| diabetes requiring
insulin (1), |
|
|
|
|
| 1 week; 30 mg/day
for |
|
| GI-bleeding
(1), spinal |
|
|
|
|
| 2 weeks; 20
mg/day |
|
| collapse, aseptic
necrosis of |
|
|
|
|
| maintenance |
|
| hip or cataracts
(3) |
|
|
|
|
| AZA: 100
mg/day |
| 14 | Cushingoid
appearance (2), | 5 |
|
|
|
|
|
|
| GI-bleeding
(3), spinal |
|
|
|
|
|
|
|
| collapse, aseptic
necrosis |
|
|
|
|
|
|
|
| of hip or cataracts
(1), |
|
|
|
|
|
|
|
|
leucopenia/thrombocytopenia |
|
|
|
|
|
|
|
| (2), ascites + 2 × increase in |
|
|
|
|
|
|
|
| bilirubin (>6
mg/100 ml) (2) |
|
|
|
|
| AZA (50 mg/day) +
Pred: |
| 14 | Cushingoid
appearance (10) | 1 |
|
|
|
| 30 mg/day for 1
week; |
|
|
|
|
|
|
|
| 20 mg/day for 1
week; |
|
|
|
|
|
|
|
| 15 mg/day for 2
weeks; |
|
|
|
|
|
|
|
| 10 mg/day for
maintenance |
|
|
|
|
|
|
|
| Placebo |
| 17 | None | 7 |
|
| Summerskill et al,
1975 | Gut | Pred: 60 mg/day
for | 36 months | 30 | Severe cosmetic
changes (5), | 3 | (12) |
|
|
| 1 week; 40 mg/day
for |
|
| diabetes (6), cataracts (5), |
|
|
|
|
| 1 week; 30 mg/day
for |
|
| hypertension
(3), duodenal |
|
|
|
|
| 2 weeks; 20
mg/day |
|
| ulcer (1), steroid psychosis (2), |
|
|
|
|
| maintenance |
|
| aseptic necrosis of
hip (1), |
|
|
|
|
|
|
|
| vertebral collapse
(1), |
|
|
|
|
|
|
|
| haematemesis
(1) |
|
|
|
|
| AZA (50 mg/day) +
Pred: |
| 30 | Diabetes mellitus
(3), | 2 |
|
|
|
| 30 mg/day for 1
week; |
|
| haematemesis
(1), skin |
|
|
|
|
| 20 mg/day for 1
week; |
|
| rash (2), cacinoma (bladder) (1), |
|
|
|
|
| 15 mg/day for 2
weeks; |
|
| thrombocytopenia
(3) |
|
|
|
|
| 10 mg/day for
maintenance |
|
|
|
|
|
|
|
| AZA: 100
mg/day |
| 13 | – | 6 |
|
|
|
| Placebo |
| 16 | None | 6 |
|
|
|
| Pred-T |
| 31 | Severe cosmetic
changes, diabetic mellitus cataracts, hypertension | 2 |
|
| Tage-Jensen et al,
1982 | Liver | AZA: 10 mg/kg/week
for the first 2 weeks and then 5 mg/kg/week | 38 (12–83)
months | 37 | – | 10 | (13) |
|
|
| Pred: <70 kg 10
mg/day, ≥70 kg 15 mg/day |
| 47 | – | 13 |
|
| Czaja et al,
1999 | Hepatology | UDCA (13–15
mg/kg/day) + | 6 months | 21 | – | 1 | (7) |
|
|
| Pred: 60 mg/day
for |
|
|
|
|
|
|
|
| 1 week; 40 mg/day
for |
|
|
|
|
|
|
|
| 1 week; 30 mg/day
for |
|
|
|
|
|
|
|
| 2 weeks; 20
mg/day |
|
|
|
|
|
|
|
| for
maintenance |
|
|
|
|
|
|
|
| Pred: 60 mg/day
for |
| 16 | – | 0 |
|
|
|
| 1 week; 40 mg/day
for |
|
|
|
|
|
|
|
| 1 week; 30 mg/day
for |
|
|
|
|
|
|
|
| 2 weeks; 20
mg/day |
|
|
|
|
|
|
|
| for
maintenance |
|
|
|
|
|
| Manns et al,
2010 |
Gastroenterology | Bude: 3 mg three
times daily or 3 mg twice daily, after biochemical remission + AZA
1–2 mg/kg/day | 6 months | 100 | Moon face (10), acne (8), hirsutism (9), skin striae (2), buffalo hump (1), diabetes (4) | 0 | (14) |
|
|
| Pred: starting dose
40 mg/day tapered to 10 mg/day+AZA 1–2 mg/kg/day |
| 103 | Moon face (43), acne (15), hirsutism (3), skin striae (4), buffalo hump (4) | 0 |
|
| Nasseri-Moghaddam
et al, 2013 | Middle East Journal
of Digestive Diseases | Pred: 50
mg/day | 12 months | 24 | – | 2 | (15) |
|
|
| CsA: 2 mg/kg body
weight in two divided doses administered orally 12 h apart |
| 15 | – | 0 |
|
Table IV revealed
the mortalities for all included studies. The direct comparisons on
mortality were presented in Table V.
The results indicated that patients treated with pred had
significantly decreased rates of mortality compared with patients
treated with the placebo (OR, 0.14; 95% CI, 0.04–0.52). No
significant differences in the mortality rates were identified
between patients treated with pred and those treated with pred-T
(OR, 1.61; 95% CI, 0.25–10.40), AZA (OR, 0.29; 95% CI, 0.06–1.51),
UDCA + pred (OR, 0.41; 95% CI, 0.02–10.85) and CsA (OR, 3.44; 95%
CI, 0.15–76.81). In addition, patients treated with pred + AZA had
significantly decreased rates of mortality compared with patients
treated with the placebo (OR, 0.12; 95% CI, 0.03–0.46) and AZA (OR,
0.10; 95% CI, 0.02–0.42). No significant differences in the
mortality rates were identified between patients treated with pred
+ AZA and those treated with and pred-T (OR, 1.04; 95% CI,
0.14–7.87), bude + AZA (OR, 0.97; 95% CI, 0.06–15.74). No
statistical differences of mortality rate were identified between
AZA therapy and placebo treatment (data not shown).
 | Table V.Mortality rates of different
treatments. |
Table V.
Mortality rates of different
treatments.
| Treatment | Pred-T | Placebo | AZA | Bude + AZA | UDCA + Pred | CsA |
|---|
| Pred | 1.61 (0.25,
10.40) | 0.14 (0.04,
0.52)a | 0.29 (0.06,
1.51) | – | 0.41 (0.02,
10.85) | 3.44 (0.15,
76.81) |
| Pred + AZA | 1.04 (0.14,
7.87) | 0.12 (0.03,
0.46)a | 0.10 (0.02,
0.42)a | 0.97 (0.06,
15.74) | – | – |
Discussion
The network meta-analysis performed in the present
study provided hierarchies of treatments for clinical and
biochemical remission in adult patients with AIH. The meta-analysis
demonstrated that the remission of patients was significantly
increased following treatment with pred compared with AZA and was
significantly increased following treatment with bude + AZA
compared with placebo or AZA. Direct comparison demonstrated that
remission of patients was significantly increased following
treatment with pred compared with AZA or placebo treatment and
similarly, remission of patients was significantly increased
following treatment with pred + AZA compared with pred-T treatment.
In addition, direct comparison indicated that patient remission was
significantly increased following treatment with bude + AZA
compared with pred + AZA. Treatments were ranked for increasing
remission as follows: Bude + AZA, pred, pred + AZA or CsA, UDCA +
pred, pred-T, AZA and placebo. Direct comparisons indicated that
the frequency of adverse events in patients treated with pred + AZA
combination therapy was decreased compared with that in patients
treated with pred monotherapy, and patients treated with bude
experienced fewer adverse events compared with those treated with
pred alone. Furthermore, direct comparisons also demonstrated that
the mortality of patients who underwent AZA or placebo treatment
was increased compared with that of patients who underwent
treatment with pred or pred + AZA; however, there was no notable
difference between other treatments and pred or pred + AZA
treatment.
The present study has a range of strengths: i) The
search strategy was comprehensive in order to decrease the
possibilities of publication bias; ii) only RCTs without high risk
of bias were included in the current study; iii) network
meta-analysis was used in the current study, which produces direct
and indirect evidence in a network of trials that compare multiple
interventions; and iv) the eight treatments included in the study
were ranked based on the posterior probabilities of outcomes and
SUCRA.
However, there are also limitations to the current
study. The results were affected by study characteristics,
including sex, detection bias and performance bias. In addition,
the primary outcome measures did not include histological remission
and may have an impact on the assessment of treatment efficacy, and
an evolving set of diagnostic criteria may affect the objectivity
of the results.
Pred alone or in combination with AZA was
demonstrated to be more effective compared with AZA or placebo
monotherapy in early studies, and therefore has become the standard
therapy used to treat patients with AIH (35). Furthermore, treatment with pred + AZA
had fewer adverse events compared with pred treatment alone
(35,36), which was consistent with the results
of the current study. Bude therapy in combination with AZA has now
emerged as an alternative frontline therapy for classical therapy
in AIH (37–39). Through random experiments,
Woynarowski et al (24) also
discovered that bude may be an alternative to standard pred therapy
in children. However, Czaja and Lindor (40) demonstrated that bude therapy had a
low frequency of remission in ten treatment-dependent patients with
AIH. Peiseler et al (41)
also demonstrated that the efficacy of bude therapy may be lower
compared with that of pred therapy alone; however, this effect may
have been caused by the different study populations used.
Danielsson and Prytz (42) indicated
that oral bude therapy decreased liver inflammation in patients
with non-cirrhotic AIH only. The network analysis in the current
study ranked bude + AZA as higher than pred + AZA. Furthermore,
descriptive analysis indicated that patients undergoing bude
treatment experienced fewer adverse events compared with those who
underwent pred treatment alone. The study population of included
RCTs focusing on bude + AZA treatment was patients with
non-cirrhotic AIH, and other studies did not distinguish between
cirrhotic and non-cirrhotic patients. Therefore, further
large-scale studies with a longer duration of follow-up history and
a focus on dose response are required to investigate the use of
bude + AZA as frontline therapy in adults with AIH. However, the
results of the current study suggest that bude + AZA may be the
most appropriate candidate for treatment of non-cirrhotic patients,
which is consistent with the results demonstrated by Czaja
(43).
The results of the current study demonstrated that
treatment with CsA, UDCA + pred or pred-T resulted in increased
remission rates compared with that observed in patients who
received placebo treatment. Treatment with CsA, UDCA + pred or
pred-T also had the same mortality rate as standard therapy. Thus,
these therapies may be used as alternative treatment in patients
with AIH who cannot undergo classical therapy. No statistical
differences of mortality rate were identified between AZA therapy
and placebo treatment; therefore, AZA is not recommended for
treatment of patients with AIH, which is consistent with the
conclusions provided by Gleeson (35).
It has been demonstrated that mycophenolate mofetil
(MMF) (5,44–51),
methotrexate (52), 6-mercaptopurine
(53), allopurinol (54,55),
tacrolimus (8,45,56–59) and
everolimus (60) are effective and
well-tolerated in patients with AIH. Torisu et al (61) indicated that UDCA monotherapy may be
considered for treatment of patients with AIH with a serum ALT
level of <200 IU/l. Among these treatments, MMF and tacrolimus
were the most studied. Efe et al (62) also demonstrated that tacrolimus was
more effective compared with MMF as therapy for difficult-to-treat
AIH. In addition, MMF (63),
calcineurin inhibitors (64,65), sirolimus (66,67),
denosumab (68), rituximab (69) and infliximab (70) have been used successfully as salvage
therapies in small observational studies. Although these therapies
have been effective as treatment for patients with AIH, their
superiority to standard treatment has not been evaluated.
Therefore, clinical RCTs are required. Cytotoxic T lymphocyte
antigen-4 (71), non-mitogenic
antibodies to cluster of differentiation 3 (72), regulatory T cell promoters (73,74),
natural killer T cell modulators (75), anti-fibrotic agents (76), monoclonal antibodies or nanobodies to
chemokines (77) and anti-apoptotic
agents (78) have been proposed for
the treatment of autoimmune disease and may be novel alternative
treatments for AIH; however, this requires further study.
In conclusion, the results of the present study
based on relatively small numbers suggest that treatment with pred
alone or in combination with AZA remain the standard treatment for
AIH. Bude in combination with AZA may be the most appropriate
treatment for non-cirrhotic patients. However, bude + AZA as
frontline therapy in adults with AIH requires additional
large-scale studies with a longer duration of follow-up histology
and a focus on dose-response. Additionally, development of other
prospective treatments, which may be used as alternative therapies
or first line therapies, and their subsequent evaluation in
clinical RCTs is required.
Acknowledgements
The authors wish to thank members of Wenzhou Key
Laboratory of Hepatology and the Hepatology Institute of Wenzhou
Medical University for technical assistance. The present study was
supported by the National Natural Science Foundation of China
(grant nos. 81570514 and 81500477); Natural Science Foundation of
Zhejiang Province (grant nos. LY15H030017 and LQ15H030006); Public
Welfare Science and Technology Project of Wenzhou (grant no.
Y20150014); Medical Award Fund (Beijing, China; grant no.
YJHYXKYJJ-162); Scientific and Technological Innovation Team of the
Early Warning and Intervention to End-stage Liver Disease of
Wenzhou (grant no. C20150005); and the National Major Scientific
and Technological Special Project during the Twelfth Five-year Plan
Period (China; grant nos. 2013ZX10005002-001-008, 2013ZX10002003
and 2012ZX10002004).
Glossary
Abbreviations
Abbreviations:
|
AIH
|
autoimmune hepatitis
|
|
pred
|
prednisone
|
|
AZA
|
azathioprine
|
|
pred-T
|
prednisone in titrated doses given on
alternate days
|
|
bude + AZA
|
budesonide + azathioprine
|
|
UDCA
|
ursodeoxycholic acid
|
|
CsA
|
cyclosporine-A
|
|
RCTs
|
randomized controlled trials
|
References
|
1
|
Manns MP, Czaja AJ, Gorham JD, Krawitt EL,
Mieli-Vergani G, Vergani D and Vierling JM: American Association
for the Study of Liver Diseases: Diagnosis and management of
autoimmune hepatitis. Hepatology. 51:2193–2213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heneghan MA, Yeoman AD, Verma S, Smith AD
and Longhi MS: Autoimmune hepatitis. Lancet. 382:1433–1444. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gleeson D and Heneghan MA: British Society
of Gastroenterology: British Society of Gastroenterology (BSG)
guidelines for management of autoimmune hepatitis. Gut.
60:1611–1629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Selvarajah V, Montano-Loza AJ and Czaja
AJ: Systematic review: Managing suboptimal treatment responses in
autoimmune hepatitis with conventional and nonstandard drugs.
Aliment Pharmacol Ther. 36:691–707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zachou K, Gatselis N, Papadamou G,
Rigopoulou EI and Dalekos GN: Mycophenolate for the treatment of
autoimmune hepatitis: Prospective assessment of its efficacy and
safety for induction and maintenance of remission in a large cohort
of treatment-naïve patients. J Hepatol. 55:636–646. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Czaja AJ, Wang KK, Shiels MT and Katzmann
JA: Oral pulse prednisone therapy after relapse of severe
autoimmune chronic active hepatitis. A prospective randomized
treatment trial evaluating clinical, biochemical, and lymphocyte
subset responses. J Hepatol. 17:180–186. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Czaja AJ, Carpenter HA and Lindor KD:
Ursodeoxycholic acid as adjunctive therapy for problematic type 1
autoimmune hepatitis: A randomized placebo-controlled treatment
trial. Hepatology. 30:1381–1386. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Thiel DH, Wright H, Carroll P,
Abu-Elmagd K, Rodriguez-Rilo H, McMichael J, Irish W and Starzl TE:
Tacrolimus: A potential new treatment for autoimmune chronic active
hepatitis: Results of an open-label preliminary trial. Am J
Gastroenterol. 90:771–776. 1995.PubMed/NCBI
|
|
9
|
Cook GC, Mulligan R and Sherlock S:
Controlled prospective trial of corticosteroid therapy in active
chronic hepatitis. Q J Med. 40:159–185. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soloway RD, Summerskill WH, Baggenstoss
AH, Geall MG, Gitnićk GL, Elveback IR and Schoenfield LJ: Clinical,
biochemical, and histological remission of severe chronic active
liver disease: A controlled study of treatments and early
prognosis. Gastroenterology. 63:820–833. 1972.PubMed/NCBI
|
|
11
|
Murray-Lyon IM, Stern RB and Williams R:
Controlled trial of prednisone and azathioprine in active chronic
hepatitis. Lancet. 1:735–737. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Summerskill WH, Korman MG, Ammon HV and
Baggenstoss AH: Prednisone for chronic active liver disease: Dose
titration, standard dose, and combination with azathioprine
compared. Gut. 16:876–883. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tage-Jensen U, Schlichting P, Aldershvile
J, Andersen P, Dietrichson O, Hardt F, Mathiesen LR and Nielsen JO:
Azathioprine versus prednisone in non-alcoholic chronic liver
disease (CLD). Relation to a serological classification. Liver.
2:95–103. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manns MP, Woynarowski M, Kreisel W, Lurie
Y, Rust C, Zuckerman E, Bahr MJ, Günther R, Hultcrantz RW, Spengler
U, et al: Budesonide induces remission more effectively than
prednisone in a controlled trial of patients with autoimmune
hepatitis. Gastroenterology. 139:1198–1206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nasseri-Moghaddam S, Nikfam S, Karimian S,
Khashayar P and Malekzadeh R: Cyclosporine-a versus prednisolone
for induction of remission in auto-immune hepatitis: Interim
analysis report of a randomized controlled trial. Middle East J Dig
Dis. 5:193–200. 2013.PubMed/NCBI
|
|
16
|
Boberg KM, Aadland E, Jahnsen J, Raknerud
N, Stiris M and Bell H: Incidence and prevalence of primary biliary
cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis
in a Norwegian population. Scand J Gastroenterol. 33:99–103. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Werner M, Prytz H, Ohlsson B, Almer S,
Björnsson E, Bergquist A, Wallerstedt S, Sandberg-Gertzén H,
Hultcrantz R, Sangfelt P, et al: Epidemiology and the initial
presentation of autoimmune hepatitis in Sweden: A nationwide study.
Scand J Gastroenterol. 43:1232–1240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caldwell DM, Ades AE and Higgins JP:
Simultaneous comparison of multiple treatments: Combining direct
and indirect evidence. BMJ. 331:897–900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnson PJ and Mcfarlane IG: Meeting
report: International Autoimmune Hepatitis Group. Hepatology.
18:998–1005. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L and Sterne JA:
Cochrane Bias Methods Group; Cochrane Statistical Methods Group:
The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chaimani A, Higgins JP, Mavridis D,
Spyridonos P and Salanti G: Graphical tools for network
meta-analysis in STATA. PLoS One. 8:e766542013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen LX, Li YL, Ning GZ, Li Y, Wu QL, Guo
JX, Shi HY, Wang XB, Zhou Y and Feng SQ: Comparative efficacy and
tolerability of three treatments in old people with osteoporotic
vertebral compression fracture: A network meta-analysis and
systematic review. PLoS One. 10:e01231532015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sogo T, Fujisawa T, Inui A, Komatsu H,
Etani Y, Tajiri H, Waki K, Shimizu Y, Nakashima S, Imagawa T and
Yokota S: Intravenous methylprednisolone pulse therapy for children
with autoimmune hepatitis. Hepatol Res. 34:187–192. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Woynarowski M, Nemeth A, Baruch Y,
Koletzko S, Melter M, Rodeck B, Strassburg CP, Pröls M, Woźniak M
and Manns MP: European Autoimmune Hepatitis-Budesonide Study Group:
Budesonide versus prednisone with azathioprine for the treatment of
autoimmune hepatitis in children and adolescents. J Pediatr.
163:1347–1353.e1. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cuarterolo M, Ciocca M, López S, López V,
Araujo M and Alvarez F: Initial treatment of autoimmune hepatitis
in children: Neoral cyclosporine versus prednisone plus
azathioprine. J Pediat Gastroenterol Nutr. 63:S562016.
|
|
26
|
Waisbourd-Zinman O, Hilmara D, Lin HC and
Rand E: Steroid free treatment of autoimmune hepatitis in selected
children. Hepatol. 63:354A–355A. 2016.
|
|
27
|
Stern RB, Wilkinson SP, Howorth PJ and
Williams R: Controlled trial of synthetic D-penicillamine and
prednisone in maintenance therapy for active chronic hepatitis.
Gut. 18:19–22. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hegarty JE, Aria Nouri KT, Eddleston AL
and Williams R: Controlled trial of a thymic hormone extract
(Thymostimulin) in ‘autoimmune’ chronic active hepatitis. Gut.
25:279–283. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stellon AJ, Hegarty JE, Portmann B and
Williams R: Randomised controlled trial of azathioprine withdrawal
in autoimmune chronic active hepatitis. Lancet. 1:668–670. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stellon AJ, Keating JJ, Johnson PJ,
McFarlane IG and Williams R: Maintenance of remission in autoimmune
chronic active hepatitis with azathioprine after corticosteroid
withdrawal. Hepatology. 8:781–784. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mucenic M, Mello ES and Cançado EL:
Chloroquine for the maintenance of remission of autoimmune
hepatitis: Results of a pilot study. Arq Gastroenterol. 42:249–255.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chi XL, Xiao HM, Xie YB, Cai GS, Jiang JM,
Tian GJ, Shi MJ, Wu SD, Zhao PT and Chen HJ: Protocol of a
prospective study for the combination treatment of Shu-Gan-jian-Pi
decoction and steroid standard therapy in autoimmune hepatitis
patients. BMC Complement Altern Med. 16:5052016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai X, Weng HH and Miao LY: Yinzhihuang
injection for treatment of patients with autoimmune hepatitis:
Clinical efficacy and impact on hepatic fibrosis indexes. World
Chin J Digestol. 25:726–731. 2017. View Article : Google Scholar
|
|
34
|
Imanieh MH, Khatami G and Ghavanini AA:
Comparison of prednisolone alone and in combination with
azathioprine regimens in treatment of autoimmune hepatitis: A
prospective study. Iranian J Med Sci. 25:67–71. 2000.
|
|
35
|
Gleeson D: Standard treatment in adults:
Which steroid? Or without steroids? Digest Dis (Basel,
Switzerland). 33 Suppl 2:S75–S82. 2015. View Article : Google Scholar
|
|
36
|
Cropley A and Weltman M: The use of
immunosuppression in autoimmune hepatitis: A current literature
review. Clin Mol Hepatol. 23:22–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Czaja AJ: Current and future treatments of
autoimmune hepatitis. Expert Rev Gastroenterol Hepatol. 3:269–291.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Czaja AJ: Drug choices in autoimmune
hepatitis: Part A-steroids. Expert Rev Gastroenterol Hepatol.
6:603–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Czaja AJ: Nonstandard drugs and feasible
new interventions for autoimmune hepatitis: Part I. Inflamm Allergy
Drug Targets. 11:337–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Czaja AJ and Lindor KD: Failure of
budesonide in a pilot study of treatment-dependent autoimmune
hepatitis. Gastroenterology. 119:1312–1316. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peiseler M, Liebscher T, Pannicke N,
Sebode M, Zenouzi R, Hartl J, Ehlken H, Weiler-Normann C, Lohse AW
and Schramm C: Budesonide for autoimmune hepatitis: Response rate
and limitations in a large real life cohort. J hepatol. 62 Suppl
2:S2332015. View Article : Google Scholar
|
|
42
|
Danielsson A and Prytz H: Oral budesonide
for treatment of autoimmune chronic active hepatitis. Aliment
Pharmacol Ther. 8:585–590. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Czaja AJ: Current and prospective
pharmacotherapy for autoimmune hepatitis. Expert Opin Pharmacother.
15:1715–1736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Anderka MT, Lin AE, Abuelo DN, Mitchell AA
and Rasmussen SA: Reviewing the evidence for mycophenolate mofetil
as a new teratogen: Case report and review of the literature. Am J
Med Genet A. 149A:1241–1248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Efe C, Hagström H, Bhanji RA, Müller NF,
Wang Q, Purnak T, Muratori L, Werner M, Marschall HU, Muratori P,
et al: Tacrolimus or mycophenylate mofetil as a second-line
therapeutic options in patients with Autoimmune Hepatitis: An
international multicentre observational study. AASLD.
62:1095522015.
|
|
46
|
Zachou K, Gatselis N, Gabeta S, Saitis A,
Koukoulis G and Dalekos GN: P1138: Long-term outcome of patients
with autoimmune hepatitis receiving mycophenolate mofetil (MMF) as
first line treatment. J Hepatol. 62 Suppl 2:S778–S779. 2015.
View Article : Google Scholar
|
|
47
|
Gazzola A, Lim R, Strasser S, Nicoll A,
Mitchell J, Siow W, Khoo T, Hamarneh Z, Weltman M, Janko N, et al:
Mycophenolate in autoimmune hepatitis not responsive or intolerant
to standard therapy: The TAPESTRY study. J Gastroenterol Hepatol
(Australia). 31:100–101. 2016.
|
|
48
|
Gazzola A, Lim R, Strasser SI, Nicoll A,
Mitchell J, Siow W, Khoo TS, Hamarneh Z, Weltman M, Janko N, et al:
Mycophenolate mofetil in autoimmune hepatitis patients not
responsive or intolerant to standard therapy: The Australian
tapestry study. Hepatology. 63:817A2016.
|
|
49
|
Kostyrko O, Shumilov P and Shigoleva N:
Mycophenolate mofetil for the treatment of autoimmune hepatitis in
children. J hepatol. 64:S6402016. View Article : Google Scholar
|
|
50
|
Park SW, Um SH, Lee HA, Kim SH, Sim Y, Yim
SY, Seo YS and Ryu HS: Mycophenolate mofetil as an alternative
treatment for autoimmune hepatitis. Clin Mol Hepatol. 22:281–285.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zachou K, Gatselis NK, Arvaniti P, Gabeta
S, Rigopoulou EI, Koukoulis GK and Dalekos GN: A real-world study
focused on the long-term efficacy of mycophenolate mofetil as
first-line treatment of autoimmune hepatitis. Aliment Pharmacol
Ther. 43:1035–1047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Haridy J, Nicoll A and Sood S:
Methotrexate therapy for autoimmune hepatitis. Clin Gastroenterol
Hepatol. Jul 12–2017.(Epub ahead of print).
|
|
53
|
Hübener S, Oo YH, Than NN, Hübener P,
Weiler-Normann C, Lohse AW and Schramm C: Efficacy of
6-mercaptopurine as second-line treatment for patients with
autoimmune hepatitis and azathioprine intolerance. Clin
Gastroenterol Hepatol. 14:445–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Deswal S and Srivastava A: Role of
allopurinol in optimizing thiopurine therapy in patients with
autoimmune hepatitis: A review. J Clin Exp Hepatol. 7:55–62. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Terrabuio DR, De Moraes Falcao LT, Ono SK,
Diniz MA, Carrilho FJ and Cancado E: Allopurinol is safe and
effective to achieve biochemical and histological remission in
patients with autoimmune hepatitis with incomplete therapeutic
response. J Hepatol. 64 Suppl:S4352016. View Article : Google Scholar
|
|
56
|
Marlaka JR, Papadogiannakis N, Fischler B,
Casswall TH, Beijer E and Németh A: Tacrolimus without or with the
addition of conventional immunosuppressive treatment in juvenile
autoimmune hepatitis. Acta Paediatr. 101:993–999. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Than NN, Wiegard C, Mann J, Fussel K,
Hirschfield G, Lohse AW, Adams D, Schramm C and Oo YH: Tacrolimus
is safe and effective in patients with resistant type 1 autoimmune
hepatitis. J Hepatol. 62:S805–S806. 2015. View Article : Google Scholar
|
|
58
|
Than NN, Wiegard C, Weiler-Normann C,
Weiler-Normann C, Füssel K, Mann J, Hodson J, Hirschfield GM, Lohse
AW, Adams DH, et al: Long-term follow-up of patients with difficult
to treat type 1 autoimmune hepatitis on Tacrolimus therapy. Scand J
Gastroenterol. 51:329–336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Al Taii H, Hanouneh MA, Hanouneh I, Lopez
R, Zein N and Alkhouri N: The use of tacrolimus in refractory
autoimmune hepatitis in children and adults: A single center
experience. Scand J Gastroenterol. 52:157–158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ytting H and Larsen FS: Everolimus
treatment for patients with autoimmune hepatitis and poor response
to standard therapy and drug alternatives in use. Scand J
Gastroenterol. 50:1025–1031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Torisu Y, Nakano M, Takano K, Nakagawa R,
Saeki C, Hokari A, Ishikawa T, Saruta M and Zeniya M: Clinical
usefulness of ursodeoxycholic acid for Japanese patients with
autoimmune hepatitis. World J Hepatol. 9:57–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Efe C, Hagstrom H, Ytting H, Bhanji RA,
Müller NF, Wang Q, Purnak T, Muratori L, Werner M, Marschall HU, et
al: Efficacy and Safety of Mycophenolate Mofetil and Tacrolimus as
Second-line Therapy for Patients With Autoimmune Hepatitis. Clin
Gastroenterol Hepatol. 15:1950–1956. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Baven-Pronk AM, Coenraad MJ, van Buuren
HR, de Man RA, van Erpecum KJ, Lamers MM, Drenth JP, van den Berg
AP, Beuers UH, den Ouden J, et al: The role of mycophenolate
mofetil in the management of autoimmune hepatitis and overlap
syndromes. Aliment Pharmacol Ther. 34:335–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Czaja AJ: Autoimmune hepatitis: Focusing
on treatments other than steroids. Can J Gastroenterol. 26:615–620.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Czaja AJ: Drug choices in autoimmune
hepatitis: Part B-Nonsteroids. Expert Rev Gastroenterol Hepatol.
6:617–635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kurowski J, Melin-Aldana H, Bass L, Alonso
EM and Ekong UD: Sirolimus as rescue therapy in pediatric
autoimmune hepatitis. J Pediatr Gastroenterol Nutr. 58:e4–e6. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chatrath H, Allen L and Boyer TD: Use of
sirolimus in the treatment of refractory autoimmune hepatitis. Am J
Med. 127:1128–1131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ikejima K, Yaginuma R, Kon K, Aoyama T,
Uchiyama A, Yamashina S and Watanabe S: Efficacy of denosumab on
progression of osteoporosis in autoimmune liver diseases. Hepatol
(Baltimore, Md). 63:201A2016.
|
|
69
|
Gautam N, Than NN, Nizamuddin M, Adams D
and Oo YH: PTU-123 use of rituximab in resistant autoimmune
hepatitis-Birmingham experience. Gut. 63:A932014. View Article : Google Scholar
|
|
70
|
Weiler-Normann C, Schramm C, Quaas A,
Wiegard C, Glaubke C, Pannicke N, Möller S and Lohse AW: Infliximab
as a rescue treatment in difficult-to-treat autoimmune hepatitis. J
Hepatol. 58:529–534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dhirapong A, Yang GX, Nadler S, Zhang W,
Tsuneyama K, Leung P, Knechtle S, Ansari AA, Coppel RL, Liu FT, et
al: Therapeutic effect of cytotoxic T lymphocyte antigen
4/immunoglobulin on a murine model of primary biliary cirrhosis.
Hepatology. 57:708–715. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Killestein J: Anti-CD3 monoclonal antibody
in new-onset type 1 diabetes mellitus. N Engl J Med. 347:1116–1117.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Czaja AJ: Challenges in the diagnosis and
management of autoimmune hepatitis. Can J Gastroenterol.
27:531–539. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lapierre P, Béland K, Yang R and Alvarez
F: Adoptive transfer of ex vivo expanded regulatory T cells in an
autoimmune hepatitis murine model restores peripheral tolerance.
Hepatology. 57:217–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Blumenfeld HJ, Tohn R, Haeryfar SM, Liu Y,
Savage PB and Delovitch TL: Structure-guided design of an invariant
natural killer T cell agonist for optimum protection from type 1
diabetes in non-obese diabetic mice. Clin Exp Immunol. 166:121–133.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Czaja AJ: The prevention and reversal of
hepatic fibrosis in autoimmune hepatitis (Review). Aliment
Pharmacol Ther. 39:385–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yellin M, Paliienko I, Balanescu A,
Ter-Vartanian S, Tseluyko V, Xu LA, Tao X, Cardarelli PM, Leblanc
H, et al: A phase II, randomized, double-blind, placebo-controlled
study evaluating the efficacy and safety of MDX-1100, a fully human
anti-CXCL10 monoclonal antibody, in combination with methotrexate
in patients with rheumatoid arthritis. Arthritis Rheum.
64:1730–1739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Anstee QM, Concas D, Kudo H, Levene A,
Pollard J, Charlton P, Thomas HC, Thursz MR and Goldin RD: Impact
of pan-caspase inhibition in animal models of established steatosis
and non-alcoholic steatohepatitis. J Hepatol. 53:542–550. 2010.
View Article : Google Scholar : PubMed/NCBI
|