Introduction
The primary function of a tendon is to transmit a
mechanical force to move and stabilize joints (1). A tendon is characterized by a
relatively small number of tenocyte cells and an extensive
extracellular matrix (ECM), which includes collagens, proteoglycans
(PGs), glycoproteins and water (2).
Its dry mass is composed of ~86% collagen, 1–5% PGs and 0.2%
inorganic components (3).
In normal tendons, ~90% of collagen is collagen type
I (Col I) and the second most abundant is collagen type III (Col
III) (4). Col I fibers are
predominant structural components with dense, parallel arrays, and
are regarded as major contributors in the transmission of
mechanical force (5). The proportion
and structure of collagen fibrils are crucial for the function of
tendons and changes to the structure or proportion may result in
adaptation or injury (6). Collagen
molecules are produced by tenocytes, and are subsequently
aggregated end-to-end and side-to-side to produce collagen fibrils
(3). During this self-assembly
process, the diameter, length and organization of collagen fibrils
is regulated by PGs (7). Decorin,
biglycan and aggrecan are regarded as the principal PGs in tendons
(8). Subsequently, collagen fibrils,
organized into fibers, bundles, and fascicles, provide tendons with
structural integrity and mechanical function (9).
As tendons transmit mechanical force, tenocytes are
able to detect and convert mechanical signals through
mechanotransduction mechanisms into cellular biological events,
such as the synthesis and degradation of collagen (10). The tenocytes also secrete matrix
metalloproteinases (MMPs) and tissue inhibitor of
metalloproteinases (TIMPs) (10).
Previous studies have indicated that a balance of MMPs and TIMPs
regulates collagen synthesis and that an imbalance may result in
collagen degradation (11,12). Tsai et al (13) demonstrated that MMP-1 serves a major
role in the degradation of Col I. MMP-1 is the predominant
interstitial collagenase in humans; however, MMP-13 is predominant
in rodents (14). TIMP-1 inhibits
the MMP-mediated break-down of collagen matrix (15).
Furthermore, there is a vast difference in cellular
biological responses to mechanical loading conditions according to
the type, magnitude, frequency and intensity of these conditions
(16,17). Regarding intensity, it is recognized
that moderate mechanical loading may induce positive effects on
tendons, whereas strenuous mechanical loading may lead to injury
(18). Despite essential progress in
the field, the effect of different mechanical loading conditions on
tendons remains undefined (19). The
purpose of the present study was to investigate the effect of
different exercise intensity, characterized by very distinct
loading patterns, using treadmill running to assess the
alternations of collagens, PGs, MMP-13 and TIMP-1 in the ECM of rat
Achilles tendons, in order to gain insights to evaluate tendon
patho-physiology.
Materials and methods
Experimental animals and exercise
protocols
A total of 18 male Wistar rats (12–13 weeks old;
weight, 200–250 g) were purchased from the Central Laboratory of
Animal Science, Southern Medical University (Guangzhou, China)
(NFYY-2012-056). These rats were randomly and evenly assigned to
one of three groups as follows: i) sedentary control (CON, n=6),
ii) medium-intensity running (MIR, n=6) and iii) high-intensity
running (HIR, n=6). All animals were housed in cages with a
controlled humidity (40–60%) and temperature (22±1°C) under a 12h
light/dark cycle, with ad libitum access to food and water.
The protocol used in the present study was approved by the animal
Ethics Committee of Nanfang Hospital, Southern Medical University
(Guangzhou, China). The employed running protocol was described
previously (20). Briefly, rats in
the MIR and HIR groups were acclimatized to exercise for 1 week,
which consisted of running on a treadmill at a speed of 10 m/min
for 30 min/day, 5 days/week. Subsequently, animals in the MIR and
HIR groups were regularly trained for 8 weeks as described in
Table I. Rats in the CON group were
maintained in cages without any additional exercise. All
experiments were conducted in accordance with the institutional
guidelines for the care and use of experimental animals (20). At the end of the 8-week running
program, all rats were sacrificed via carbon dioxide asphyxiation
(flow rate: 30% volume/min) followed by cervical dislocation.
Subsequently, Achilles tendon tissues were surgically excised and
harvested from all rats.
 | Table I.Treadmill running protocols for rats
in the MIR and HIR groups, (n=6). Completed 5 days a week for 8
weeks. |
Table I.
Treadmill running protocols for rats
in the MIR and HIR groups, (n=6). Completed 5 days a week for 8
weeks.
| Group | Speed, m/min | Inclination, ° | Duration, min |
|---|
| MIR | 19.3 | 5 | 60 |
| HIR | 26.8 | 10 | 60 |
Picrosirius red staining
Achilles tendon tissues from each group were
obtained by surgery excision for histological staining, fixed in
10% buffered formalin (4°C, overnight) and embedded in paraffin.
Samples were cut into 4-mm thick sections, deparaffinized and
stained with 5% Picrosirius red to highlight collagen fiber
structure and improve its natural birefringence under a polarized
light microscope at a magnification ×20 (Axioskop 40 Pol; Carl
Zeiss AG, Oberkochen, Germany).
Immunohistochemistry
Immunohistochemistry for Col I and Col III was
performed as previously described (6). Briefly, Achilles tendon tissues were
decalcified with 9% formic acid for 10 min, washed with PBS for 1
min and embedded in paraffin. Then, 4-mm thick sections were cut
and deparaffinized with xylene and different concentrations of
ethanol (100, 95, 85 and 70%). Endogenous peroxidase activity was
quenched with 3% hydrogen peroxide for 20 min at room temperature.
Antigen retrieval was performed with citric acid (pH 6.0) using a
high pressure method (21). The
citric acid buffer was preheated for 5 min in an autoclave and
sections were boiled for 2 min, followed by cooling for 20 min.
Sections were blocked with 5% normal bovine serum albumin (BSA;
Merck KGaA, Darmstadt, Germany) for 20 min at room temperature and
sections were incubated with specific primary antibodies at 4°C
overnight. The mouse anti-rat primary antibodies anti-Col I (cat.
no. ab6308) and anti-Col III (cat. no. ab6310) were diluted by
1:100 (Abcam, Cambridge, UK). Sections were subsequently incubated
with horseradish peroxidase conjugated goat anti-mouse
Imunoglobulin G (1:200; cat. no. sc2005; Santa Cruz Biotechnology,
CA, USA) for 1 h at room temperature, developed with
3,3′-Diaminobenzidine tetrahydrochloride (DAKO; Agilent
Technologies, Inc., Santa Clara, CA, USA) and counter-stained in
hematoxylin. The primary antibody was replaced with 5% BSA at 4°C
overnight in the controls. To enable reproducibility and
comparability, all incubation times and conditions were strictly
controlled. The sections were examined under a color video camera
attached to a H600L light microscope and image analysis system
(Nikon Corporation, Tokyo, Japan). Images were captured using
Image-Pro Plus version 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Transmission electron microscopy
observation
Transmission electron microscopy (TEM) was completed
as previously described (22).
Achilles tendon tissues for TEM were fixed with 2.5%
glutaraldehyde/4% formaldehyde fixative for 2 h at 4°C, post-fixed
with 1% osmium tetroxide for 2 h, dehydrated with ethanol (50, 70,
90 and 100%; 20 min each step), embedded in Epon 812 and
polymerized at 60°C. Ultrathin (50–60 nm) cross-sections were
observed at 60 kV using a 7500 transmission electron microscope
(Hitachi, Ltd., Tokyo, Japan) and digital images were captured at a
magnification, ×60,000 with a Megaview III digital camera (Olympus
Soft Imaging Solutions GmbH, Münster, Germany). A total of 500
collagen fibrils in each group were randomly selected and diameters
were measured using Scion Image 4.0 Software (Scion Corporation,
Frederick, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Achilles tendon tissues for RT-qPCR were frozen in
liquid nitrogen and broken into pieces with a pestle and mortar.
Subsequently, the fragments were mixed and placed in a vessel
containing 1 ml RNAiso Plus (Takara Biotechnology Co., Ltd.,
Dalian, China), followed by centrifugation at 13,362 × g for 15 min
at 4°C. Prior to mixing, 0.2 ml chloroform (analytical pure) was
added. The supernatant was removed following centrifugation at
13,362 × g for 15 min at 4°C, 500 ml isopropanol was added and the
samples were once again centrifuged at 13,362 × g for 15 min at
4°C. The supernatant was discarded; 75% ethanol and 500 ml diethyl
pyrocarbonate (DEPC)-treated H2O were added. The samples
were centrifuged at 4,547 × g for 5 min at 4°C. The supernatant was
discarded and the pellet was air dried. Subsequently, 30 µl of
DEPC-treated H2O was added. Reverse transcription of the
mRNA to template cDNA was completed using a PrimeScript RT reagent
kit (Takara Biotechnology Co., Ltd.). The enzyme mix and RT primer
mix were added to the mRNA sample and cDNA was generated by heating
at 37°C for 15 min and 85°C for 5 sec. Quantitative PCR was
performed using a 7500 Fast Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and a
SYBR Premix Ex Taq II kit (Takara Biotechnology Co., Ltd.);
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an
endogenous reference and each sample was normalized to its GAPDH
content. The PCR protocol used was as follows: 10 min heating at
95°C, followed by 45 cycles at 95°C for 10 sec, 55°C for 15 sec,
72°C for 30 sec. The mRNA expression of collagen (Col I and Col
III), principal PGs (decorin, biglycan and aggrecan), MMP-13 and
TIMP-1 in the Achilles tendon were detected via PCR. The sequences
of PCR primers pairs (BioTeke Corporation, Beijing, China) are
presented in Table II. The relative
gene expression was calculated using the 2−ΔΔCT method
(23). The assay was replicated in
triplicate.
 | Table II.Primer sequence used in reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primer sequence used in reverse
transcription-quantitative polymerase chain reaction.
| Primer | Forward | Reverse |
|---|
| GAPDH |
5′-GGCACAGTCAAGGCTGAGAATG-3′ |
5′-ATGGTGGTGAAGACGCCAGTA-3′ |
| COL I |
5′-CATCGGTGGTACTAAC-3′ |
5′-CTGGATCATATTGCACA-3′ |
| COL III |
5′-GATGGCTGCACTAAAC-3′ |
5′-CGAGATTAAAGCAAGAG-3′ |
| Decorin |
5′-ATGATTGTCATAGAACTGGGC-3′ |
5′-TTGTTGTTATGAAGGTAGAC-3′ |
| Biglycan |
5′-TCTACATCTCCAAGAACCACCTGG-3′ |
5′-GCTCTGGGCTCCTACTCCTT-3′ |
| Aggrecan |
5′-ATCGTGGGCCGCCCTAGGCA-3′ |
5′-TGGCCTTAGGGTTCAGAGGGG-3′ |
| MMP-13 |
5′-TACAACTTGTTCCTTGTCGC-3′ |
5′-CTGGGCCATAGAGAGACT-3′ |
| TIMP-1 |
5′-CAGCGAGGAGTTTCTGG-3′ |
5′-GGTAAACACTGTGCACCC-3′ |
Statistical methods
Results are expressed as the mean ± standard
deviation. Statistical analysis was carried out using one-way
analysis of variance and Tukey's test for post hoc analysis. Data
analysis was performed using SPSS 16.0 (SPSS, Inc., Chicago, IL,
USA) and P<0.05 was considered to indicate a statistically
significant difference.
Results
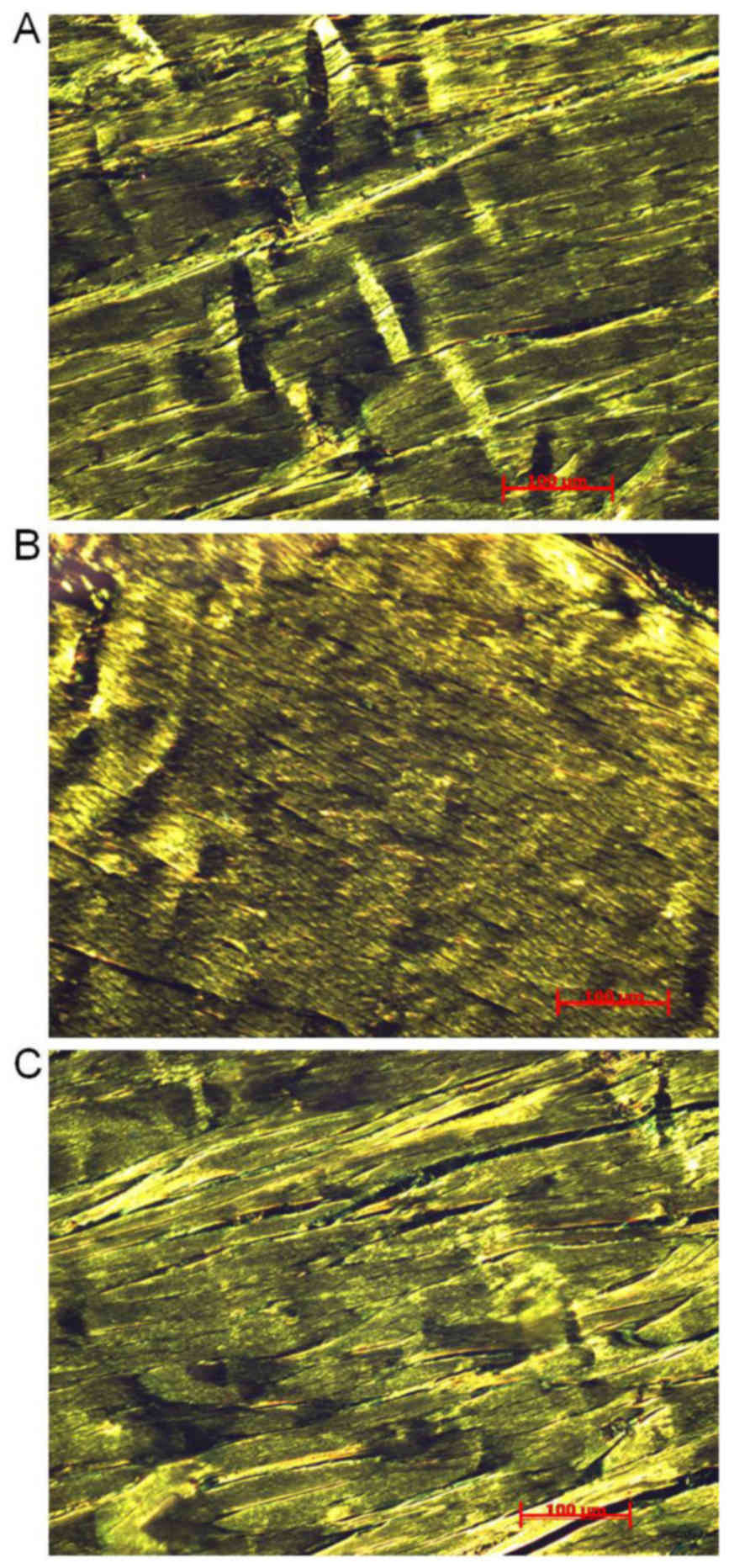
Picrosirius red staining
Following 8 weeks of treadmill running programs,
Achilles tendons sections were stained with Picrosirius red to
observe structural features of collagen fibrils using a polarized
light microscope. Fig. 1 presents
observed structural features in the three groups. Collagen fibers
were organized in parallel with crimps in the CON group (Fig. 1A). Regularly and densely organized
collagen fibrils were recorded in the MIR group (Fig. 1B). However, irregular and loosely
organized collagen fibrils were observed in the HIR group (Fig. 1C).
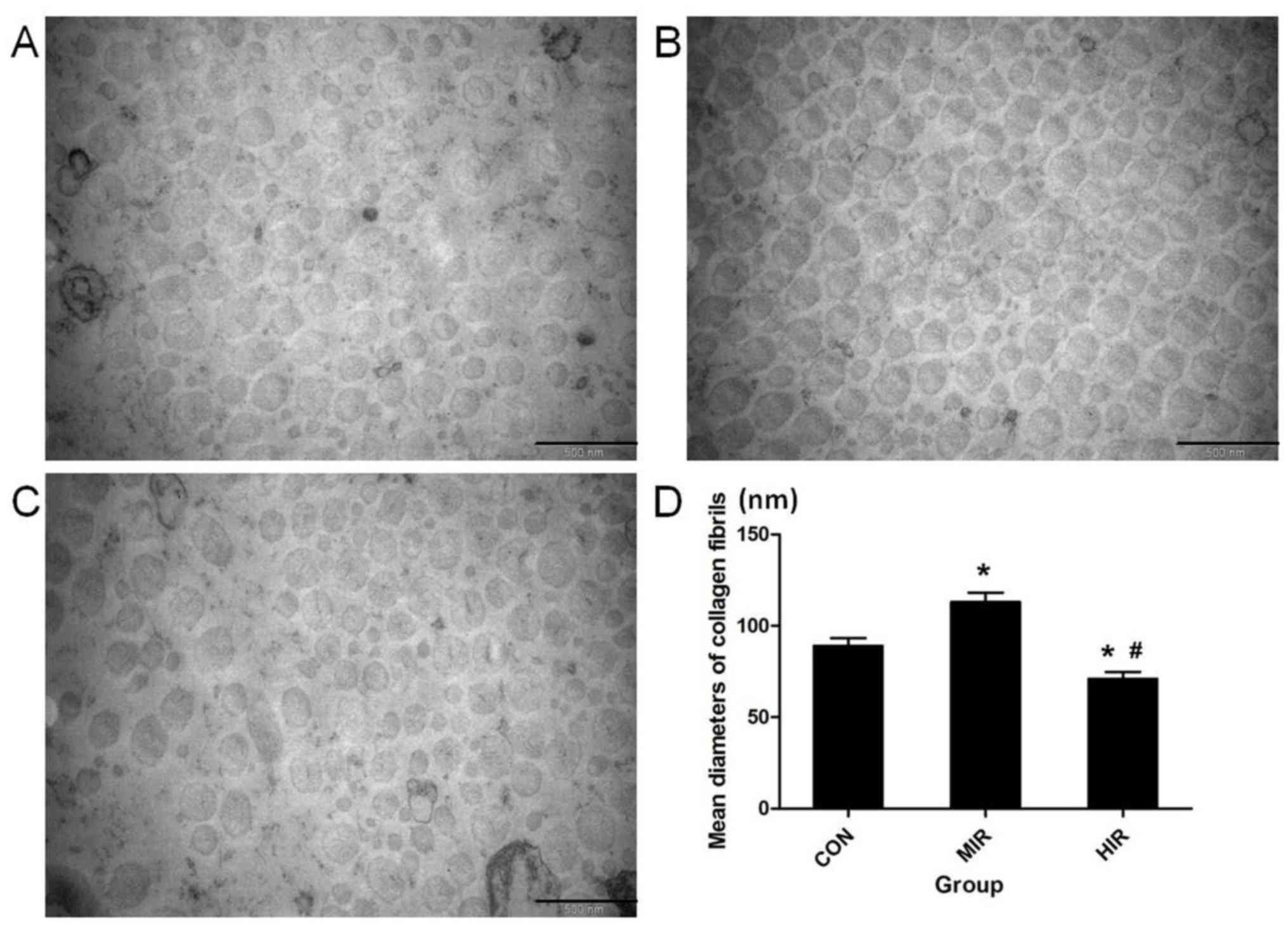
TEM
Representative TEM images of the collagen fibrils in
Achilles tendons in transverse section for each group are presented
in Fig. 2; CON (Fig. 2A), MIR (Fig. 2B) and HIR (Fig. 2C). The diameter of collagen fibrils
was calculated using Scion Image Software (Fig. 2D). Significantly thicker collagen
fibrils were observed in the MIR group (113±5.2 nm) compared with
the CON group (89±4.3 nm; P<0.05). Furthermore, significantly
thinner collagen fibrils were observed in the HIR group (71±3.8 nm)
compared with the CON and MIR groups (P<0.05).
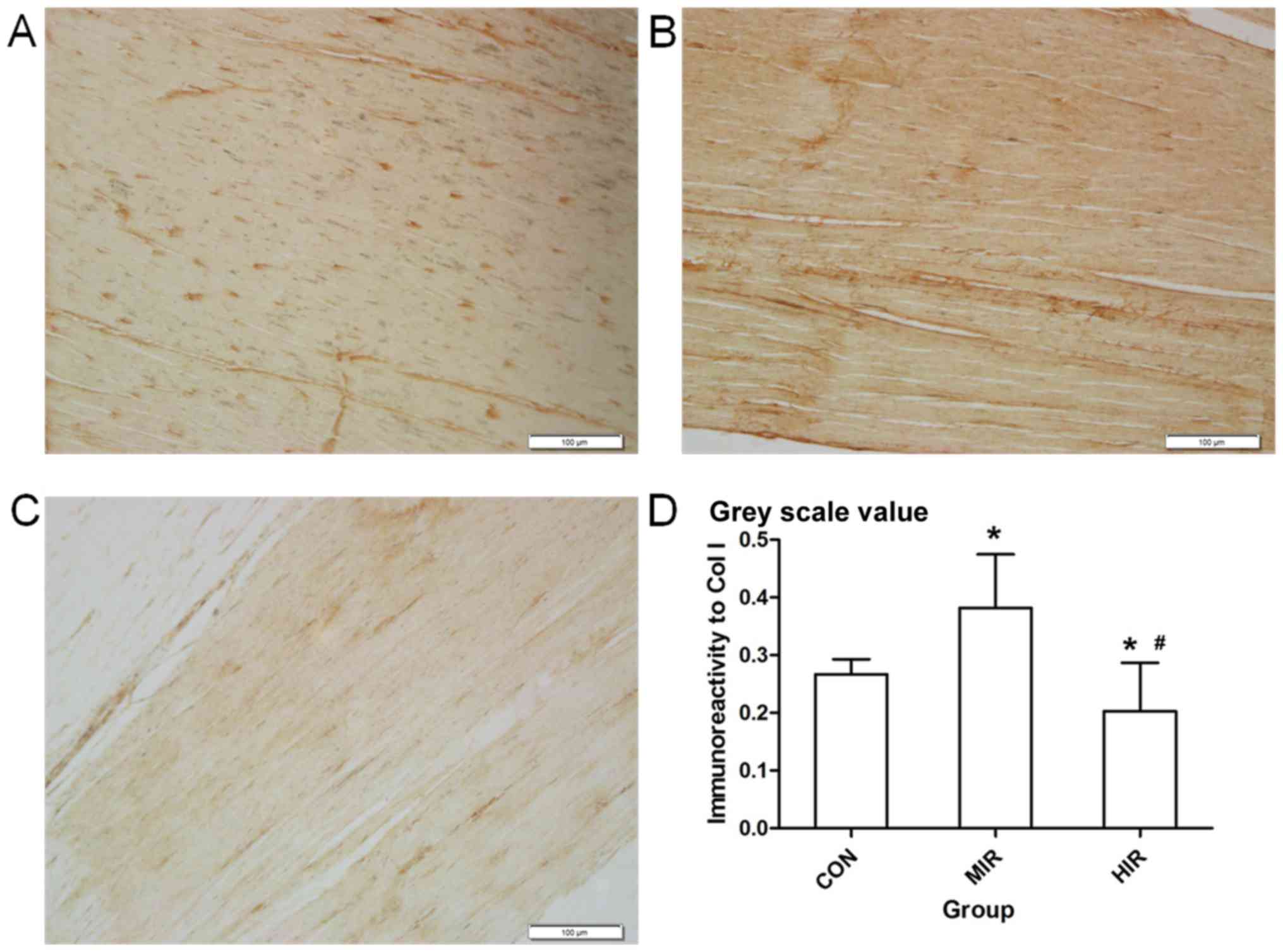
Immunohistochemistry
Fig. 3 presents
representative immunostaining of Col I in Achilles tendon sections
in the CON (Fig. 3A), MIR (Fig. 3B) and HIR (Fig. 3C) groups. Immunohistological analysis
for Col I was performed using Image-Pro Plus 6.0 software and is
presented in Fig. 3D. The Col I
content (image gray value) was significantly higher in the MIR
group (0.382±0.093) compared with the CON group (0.267±0.026;
P<0.05) and was significantly lower in the HIR group
(0.203±0.084) compared with the CON or MIR groups (P<0.05).
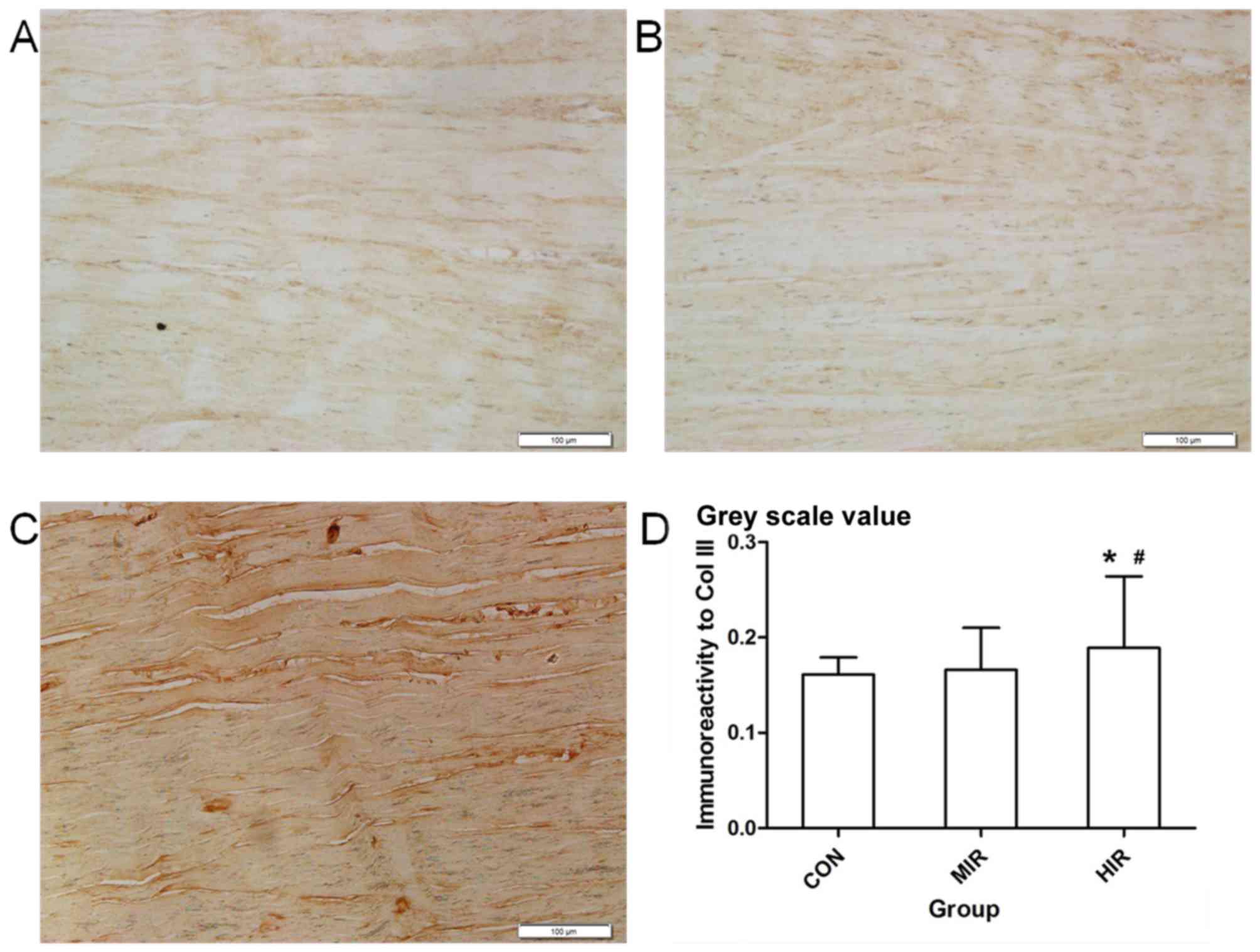
Representative immunostaining of Col III in Achilles
tendon sections is presented for each group in Fig. 4; CON (Fig.
4A), MIR (Fig. 4B) and HIR
(Fig. 4C). Immunohistological
analysis for Col III was completed using Image-Pro Plus 6.0
software (Fig. 4D). The Col III
content (image gray value) was significantly higher in the HIR
group (0.189±0.075) compared with the CON (0.161±0.018) or MIR
(0.166±0.044; P<0.05) groups. No significant difference was
observed between the CON and MIR groups.
RT-qPCR
Changes in the mRNA gene expression in rat Achilles
tendons in CON, MIR and HIR groups is presented in Fig. 5. The expression of Col I was
significantly upregulated in the MIR group compared with the CON
group (P=0.024; Fig. 5A). However,
Col I expression was significantly downregulated in the HIR group
compared with the CON or MIR groups (P=0.037 and P=0.019,
respectively; Fig. 5A). The
expression of Col III was significantly upregulated in the HIR
group when compared with the CON or MIR group (P=0.024, P=0.047,
respectively; Fig. 5A). The changes
in Col III mRNA gene expression were less pronounced in the MIR
group in comparison with the CON group (P=0.196; Fig. 5A).
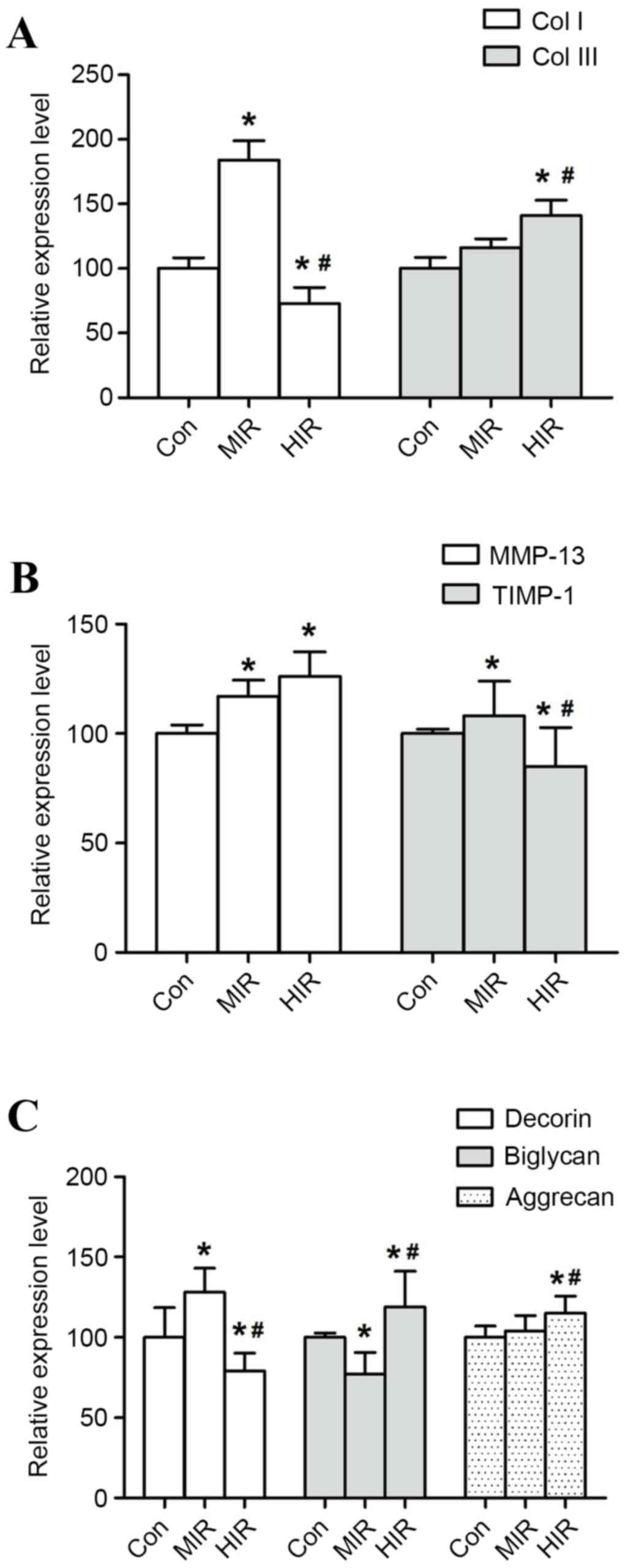 | Figure 5.mRNA expression in rat Achilles
tendons. mRNA expression of (A) Col I and Col III, (B) MMP-13 and
TIMP-1 and (C) decorin, biglycan and aggrecan in rat Achilles
tendons following MIR and HIR were determined by reverse
transcription-quantitative polymerase chain reaction. GAPDH was
used as an endogenous reference. Data are presented as mean ±
standard deviation, n=3, *P<0.05 vs. CON; #P<0.05
vs. MIR group. Col I, collagen type I; Col III, collagen type III;
MMP-13, metalloproteinase-13; TIMP-1, tissue inhibitor of
metalloproteinases-1; MIR, medium-intensity running; HIR,
high-intensity running. |
The expression of MMP-13 was significantly increased
in the MIR and HIR groups in comparison with the CON group
(P=0.028, P=0.013, respectively; Fig.
5B). However, a less pronounced change of MMP-13 mRNA
expression was recorded in the HIR group in comparison with the MIR
group (P=0.075; Fig. 5B). The
expression of TIMP-1 was increased in the MIR group compared with
the CON group (P=0.018; Fig. 5B),
but significantly decreased in the HIR group compared with the CON
or MIR groups (P=0.033, P=0.027, respectively).
Additionally, decorin expression was significantly
higher in MIR group than that in CON group (P=0.034), whereas it
was significantly lower in HIR group compared with the CON or MIR
groups (P=0.039, P=0.032, respectively; Fig. 5C). Conversely, biglycan expression
was significantly lower in MIR group than in the CON group
(P=0.022), while significantly higher in the HIR group compared
with the CON or MIR groups (P=0.038, P=0.027, respectively;
Fig. 5C). Aggrecan expression was
significantly higher in the HIR group compared with the CON or MIR
groups (P=0.017, P=0.013, respectively). The change in mRNA gene
expression of aggrecan was less significant in the MIR group in
comparison with the CON group (P=0.073; Fig. 5C).
Discussion
In the present study, a running treadmill model at
different speeds and inclinations was used to represent distinct
exercise intensity and differentiate moderate and strenuous
exercise. The results of the current study indicate that the
crucial component, Col I was significantly increased in the MIR
group compared with that in CON group (P<0.05). However, Col I
was significantly decreased in the HIR group compared with the CON
or MIR groups (P<0.05). Therefore demonstrating that synthesis
or degradation of Col I occurred following 8 weeks of treadmill
running. To evaluate the metabolism of collagen, the expression of
MMP-13 and TIMP-1 was examined. Data from the present study
indicated that the expression of MMP-13 and TIMP-1 was
significantly increased in the MIR group (P<0.05). However, the
expression of MMP-13 increased and the expression of TIMP-1
decreased in the HIR group. The aforementioned results suggested
that MMP-13 and TIMP-1 were balanced and collagen synthesis
occurred following MIR, but were imbalanced following HIR, which
may lead to collagen degradation. These findings suggest that
moderate exercise induced a balanced expression of MMPs and TIMPs,
allowing collagen synthesis to improve mechanical strength for
transmission. However, strenuous exercise induced an imbalance,
leading to collagen degradation which may weaken mechanical
strength and increase the risk of damage (24).
In addition, it should be noted that the level of
Col III was increased in the HIR group. Col III has been reported
to intercalate into the Col I fibrils and produce smaller, less
organized fibrils (24). It was also
regarded as an immature collagenous matrix (25). This may in accordance with the
results of TEM in the present study, in which more mature and large
diameter fibrils were observed in the MIR group but in the HIR
group more immature and small diameter fibrils were observed
(Fig. 2).
It has been indicated that PGs serve a vital role in
the self-assembly process of collagen fibrils (26). The results of the present study
revealed that decorin expression was higher in the MIR group and
lower in the HIR group. This supports the observations made
following Picrosirius red staining, in which regular collagen
fibril arrangement in the MIR group was observed, whereas irregular
arrangement was observed in the HIR group. Decorin has been
reported to regulate the fibril diameter and help organize and
orientate the collagen fibrils in tendons (27). However, a converse pattern of changes
was identified in biglycan expression among the three groups. This
is potentially due to high homology and co-expression of biglycan
and decorin (28). Therefore, they
may share common functions and partially compensate for each
other's functions. Additionally, the findings of the current study
indicated that aggrecan expression was higher in the HIR group.
Aggrecan is located between adjacent collagen fiber bundles and
increases the tendon hydration and fibril separation (29). This may explain the presence of more
spaces between and in collagen fibril bundles observed in the HIR
group.
However, the present study was limited by the use of
only one time point (8 weeks) and the difference between rodent and
human MMP expression. In addition, the alterations of a number of
major molecules in ECM were observed, while other molecules may
change and serve important roles in tendon patho-physiology.
In conclusion, the current study demonstrated a
significant intensity-specific effect following treadmill running
on the rat Achilles tendon. These results suggest that moderate
exercise may induce increased collagen synthesis and organize
regular and large collagen fibers, thus benefiting the Achilles
tendon. Nevertheless, overuse may result in collagen degradation
and disturbance, which is predisposed to injury. However, further
studies with more time points and time frames should be completed
to validate the findings.
Acknowledgements
The authors of the present study gratefully
acknowledge Mr PR Zhao for technical assistance. The current study
was supported by Natural Science Foundation of China (grant no.
81371686 and 81572219) and Guangdong Natural Science Foundation
(grant no. S20140006946).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin TW, Cardenas L and Soslowsky LJ:
Biomechanics of tendon injury and repair. J Biomech. 37:865–877.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heinemeier KM and Kjaer M: In vivo
investigation of tendon responses to mechanical loading. J
Musculoskelet Neuronal Interact. 11:115–123. 2011.PubMed/NCBI
|
|
3
|
Juneja SC and Veillette C: Defects in
tendon, ligament, and enthesis in response to genetic alterations
in key proteoglycans and glycoproteins: A review. Arthritis.
2013:1548122013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amiel D, Frank C, Harwood F, Fronek J and
Akeson W: Tendons and ligaments: A morphological and biochemical
comparison. J Orthop Res. 1:257–265. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Franchi M, Torricelli P, Giavaresi G and
Fini M: Role of moderate exercising on Achilles tendon collagen
crimping patterns and proteoglycans. Connect Tissue Res.
54:267–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lui PP, Chan LS, Lee YW, Fu SC and Chan
KM: Sustained expression of proteoglycans and collagen type
III/type I ratio in a calcified tendinopathy model. Rheumatology
(Oxford). 49:231–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reese SP, Underwood CJ and Weiss JA:
Effects of decorin proteoglycan on fibrillogenesis, ultrastructure,
and mechanics of type I collagen gels. Matrix Biol. 32:414–423.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rees SG, Flannery CR, Little CB, Hughes
CE, Caterson B and Dent CM: Catabolism of aggrecan, decorin and
biglycan in tendon. Biochem J. 350:181–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang JH: Mechanobiology of tendon. J
Biomech. 39:1563–1582. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang JH, Thampatty BP, Lin JS and Im HJ:
Mechanoregulation of gene expression in fibroblasts. Gene.
391:1–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dalton S, Cawston TE, Riley GP, Bayley IJ
and Hazleman BL: Human shoulder tendon biopsy samples in organ
culture produce procollagenase and tissue inhibitor of
metalloproteinases. Ann Rheum Dis. 54:571–577. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thampatty BP, Li H, Im HJ and Wang JH: EP4
receptor regulates collagen type-I, MMP-1, and MMP-3 gene
expression in human tendon fibroblasts in response to IL-1 beta
treatment. Gene. 386:154–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsai WC, Hsu CC, Chang HN, Lin YC, Lin MS
and Pang JH: Ibuprofen upregulates expressions of matrix
metalloproteinase-1, −8, −9, and −13 without affecting expressions
of types I and III collagen in tendon cells. J Orthop Res.
28:487–491. 2010.PubMed/NCBI
|
|
14
|
Wisløff U, Helgerud J, Kemi OJ and
Ellingsen O: Intensity-controlled treadmill running in rats: VO(2
max) and cardiac hypertrophy. Am J Physiol Heart Circ Physiol.
280:H1301–H1310. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brew K and Nagase H: The tissue inhibitors
of metalloproteinases (TIMPs): An ancient family with structural
and functional diversity. Biochim Biophys Acta. 1803:55–71. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Firth EC: The response of bone, articular
cartilage and tendon to exercise in the horse. J Anat. 208:513–526.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Magnusson SP, Hansen P and Kjaer M: Tendon
properties in relation to muscular activity and physical training.
Scand J Med Sci Sports. 13:211–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jhingan S, Perry M, O'Driscoll G, Lewin C,
Teatino R, Malliaras P, Maffulli N and Morrissey D: Thicker
Achilles tendons are a risk factor to develop Achilles tendinopathy
in elite professional soccer players. Muscles Ligaments Tendons J.
1:51–56. 2011.PubMed/NCBI
|
|
19
|
Zhang J and Wang JH: The effects of
mechanical loading on tendons-an in vivo and in vitro model study.
PLoS One. 8:e717402013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ni GX, Liu SY, Lei L, Li Z, Zhou YZ and
Zhan LQ: Intensity-dependent effect of treadmill running on knee
articular cartilage in a rat model. Biomed Res Int.
2013:1723922013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Norton AJ, Jordan S and Yeomans P: Brief,
high-temperature heat denaturation (pressure cooking): A simple and
effective method of antigen retrieval for routinely processed
tissues. J pathol. 173:371–379. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dunkman AA, Buckley MR, Mienaltowski MJ,
Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason
DP, Iozzo RV, et al: The tendon injury response is influenced by
decorin and biglycan. Ann Biomed Eng. 42:619–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan SC and Chan O: Achilles and patellar
tendinopathy: Current understanding of pathophysiology and
management. Disabil Rehabil. 30:1608–1615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu SC, Wong YP, Cheuk YC, Lee KM and Chan
KM: TGF-beta1 reverses the effects of matrix anchorage on the gene
expression of decorin and procollagen type I in tendon fibroblasts.
Clin Orthop Relat Res. 226–232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kalamajski S and Oldberg A: The role of
small leucine-rich proteoglycans in collagen fibrillogenesis.
Matrix Biol. 29:248–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scott JE: Elasticity in extracellular
matrix ‘shape modules’ of tendon, cartilage, etc. A sliding
proteoglycan-filament model. J Physiol. 553:335–343. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Säämänen AM, Salminen HJ, Rantakokko AJ,
Heinegård D and Vuorio EI: Murine fibromodulin: cDNA and genomic
structure, and age-related expression and distribution in the knee
joint. Biochem J. 355:577–585. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smith MM, Sakurai G, Smith SM, Young AA,
Melrose J, Stewart CM, Appleyard RC, Peterson JL, Gillies RM, Dart
AJ, et al: Modulation of aggrecan and ADAMTS expression in ovine
tendinopathy induced by altered strain. Arthritis Rheum.
58:1055–1066. 2008. View Article : Google Scholar : PubMed/NCBI
|