Introduction
Primary hepatocellular carcinoma (HCC) is one of the
most common malignant tumors of the digestive system, with high
intrahepatic recurrence and metastasis rates leading to poor
overall prognosis (1–3). Trousseau (4) reported that patients with cancer
suffered complications due to venous thrombosis; the comorbidity of
venous thrombosis and cancer was thus named Trousseau syndrome.
Venous thrombosis is a common complication that occurs during the
development of malignant tumors and is the second leading cause of
death in patients with Trosseau syndrome after the cancer itself
(5). A previous study suggested that
malignant tumor is a high-risk factor for venous thrombosis, with
~12.3% of patients with cancer developing thrombi within 6 months
of diagnosis (6). However, the
mechanisms underlying the development of tumor thrombosis secondary
to malignant tumors is yet to be elucidated.
The development of HCC is dependent on angiogenesis
(7). Vascular endothelial growth
factor A (VEGFA) serves an important role in the normal physiology
and angiogenesis of various abnormal pathologies (8). The expression of VEGF in HCC cells is
high due to local hypoxia stimulation, particularly near tumor
necrotic areas (9,10). Inhibiting the expression of VEGF or
blocking the corresponding signal transduction pathway and other
pathways has an anti-angiogenic effect (11). Humanized monoclonal antibodies that
bind to VEGFA and block its activity have been approved for the
treatment of neoplastic diseases (12).
Several mRNA and microRNA (miR or miRNA) molecules
are associated with Trousseau syndrome (13), including integrin β3 and the
polyphosphate-factor XII signaling pathway (14). The occurrence of tumor thrombi is
associated with neovascularization (15). VEGF, which is also secreted by tumor
cells, and its signal transduction pathway serve important roles in
the development of tumor thrombosis (16–18). In
tumor angiogenesis, there are interactions between the coagulation
process, inflammatory cytokines and malignant tumor growth and
metastasis (19). The high
permeability of tumor neovascular tissues induces plasma leakage
resulting in increased specific volume, increased blood viscosity
and blood stasis, all risk factors for thrombosis (16).
In the present study, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting were used to analyze the expression of VEGFA in
the tumor thrombi of patients with HCC with portal vein tumor
thrombus (PVTT). miRNAs that regulate the expression of VEGFA were
predicted by bioinformatics methods and validated using a dual
luciferase reporter assay; it was demonstrated that VEGFA is
targeted by miR-381. The association between miR-381 and VEGFA was
investigated and their roles in venous endothelial cell
proliferation were examined using an MTT assay. The results of the
present study may provide a novel insight into the regulatory
mechanisms of PVTT development in HCC.
Materials and methods
Patients
A total of 39 patients with HCC and PVTT were
enrolled in the present study. All patients were diagnosed and
underwent tumor thrombus resection between August 2013 and June
2016 at the Affiliated Tumor Hospital of Guangxi Medical University
(Nanning, China). PVTT samples and paired adjacent tissues were
collected from all patients. The cohort comprised 18 male and 21
female patients with a median age of 46.6 years (range, 26–65
years). All patients were diagnosed by a clinician and diagnoses
were confirmed by histopathology. Prior to surgical resection,
patients did not receive hormone, herbal, radiotherapy or
chemotherapy treatment. PVTT was confirmed by Doppler ultrasound or
based on symptoms and patient history. Written informed consent was
obtained from every patient and the study was approved by the
Ethics Review Board of Guangxi Medical University.
RNA extraction and RT-qPCR
Total RNAs were isolated from thrombi and adjacent
tissues using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. Following quantification using a spectrophotometer at 490
nm, RNA (1 µg) was reverse transcribed into cDNA using a TIANScript
II cDNA 1st strand cDNA Synthesis kit (Tiangen Biotech Co., Ltd.,
Beijing, China). miRNA was reverse transcribed from total RNA using
an miRcute miRNA cDNA Synthesis kit (Tiangen Biotech Co., Ltd.).
qPCR was performed using the SuperReal SYBR Green PreMix (Tiangen
Biotech Co., Ltd.) for mRNA, or the miRcute miRNA qPCR Detection
kit (Tiangen Biotech Co., Ltd.) for miRNA using a Bio-Rad IQ5
thermocycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). For
mRNA, the reaction mixture was incubated at 94°C for 2 min followed
by 35 cycles at 94°C for 30 sec, 55°C for 30 sec and 71°C for 1
min. For miRNA, the reaction mixture was incubated at 95°C for 5
min followed by 40 cycles at 95°C for 15 sec, 60°C for 30 sec, and
72°C for 30 sec. Primers used were as follows: VEGFA. forward
5′-TTGCCTTGCTGCTCTACCTC-3′ and reverse 5′-AAATGCTTTCTCCGCTCTGA-3′;
β-actin, forward 5′-TGACGTGGACATCCGCAAAG-3′ and reverse
5′-CTGGAAGGTGGACAGCGAGG-3′; miR-381, forward
5′-ACACTCCAGCTGGGTATACAAGGGCAAGCT-3′ and reverse
5′-TGGTGTCGTGGAGTCG-3′; U6, forward 5′-CTCGCTTCGGCAGCACA-3′ and
reverse 5′-AACGCTTCACGAATTTGCGT-3′. The relative expression levels
were evaluated using the 2−ΔΔCq method (20). β-actin and small nuclear U6 were used
as internal controls for mRNA and miRNA, respectively.
Western blotting
Proteins were extracted using
radioimmunoprecipitation assay buffer and protease inhibitor
phenylmethane sulfonyl fluoride. The protein concentration was
determined by using a bicinchoninic acid assay kit [RTP7102;
Real-Times (Beijing) Biotechnology Co., Ltd., Beijing, China]. A
total of 20 µg/lane protein was separated by 10% SDS-PAGE and
transferred to polyvinylidene difluoride membranes. After blocking
with 5% skimmed milk at room temperature for 1 h, the membranes
were probed with the rabbit anti-VEGFA (1:1,000; ab46154) or rabbit
anti-β-actin (1:5,000; ab129348; both Abcam, Cambridge, MA, USA) at
4°C overnight. After washing five times with Tris-buffered saline
and Tween-20 (5 min each time), the membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibodies (1:3,000; ab6721; Abcam) at room temperature for 1 h.
Signal detection was performed using an enhanced chemiluminescence
reaction kit (ab65623; Abcam) on a Gel Doc XR+ system (Bio-Rad
Laboratories, Inc.). The acquired images were analyzed using Image
lab 3.0 (Bio-Rad Laboratories, Inc.) and the relative protein
expression was expressed as the densitometric value ratio of VEGFA
to the β-actin band.
Bioinformatics analysis
To further identify the miRNAs that may regulate the
expression of VEGFA the following bioinformatics databases were
used: miRanda (34.236.212.39/microrna/home.do), TargetScan
(targetscan.org), PiTa (genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNAhybrid (bibiserv.techfak.uni-bielefeld.de/rnahybrid/) and
PICTA (pictar.mdc-berlin.de/) were used.
Dual-luciferase reporter gene
assay
Based on the results of bioinformatics prediction,
the conservative miR-381 binding sequence on 3′-untranslated region
(UTR) of wild type (WT) or the mutant VEGFA mRNA was cloned.
The primers to clone the WT miR-381 binding sequence were designed
by CE Design V1.04 (Vazyme, Nanjing, China) ad follows: WT, forward
5′-tga tga aag ctg cgc act agt AAAGAGTAGGGTTTTTTTTCAGTATTCTT-3′ and
reverse 5′-aaaagatcctttattaagcttTGCTGGGGAGCCAGGGGA-3′. Primers for
the mutated sequence were constructed using a Mut Express II Fast
Mutagenesis kit V2 C214-01/02 (Vazyme). Mutant, forward
5′-GTACCGGTTTaacaataATAAAATTCATGTTTCCAATCTCTCTCT-3′ and reverse
5′-tattgttAAACCGGTACAAATAAGAGAGCAAG-3′. The mutant binding sequence
is underlined below:
2821
tgtatcttttgctctctcttgctctcttatttgtaccggtttTTGTATataaaattcatg
2881
tttccaatctctctctccctgatcggtgacagtcactagcttatcttgaacagatattta
Luciferase reporter plasmids were generated by
inserting WT or mutant sequences of VEGFA into the multiple
cloning site (Spe-1 and HindIII) downstream of the luciferase
reporter gene in the pMIR-REPORT™ Luciferase (Thermo Fisher
Scientific, Inc.). The 293T cells (American Type Culture
Collection, Manassas, VA, USA) were transfected with 0.8 µg
constructed luciferase reporters and 100 nM antagomir (agomiR)-381
(Sangon Biotech, Shanghai, China) or negative control RNA (NC)
using ExFect Transfection reagent (cat. no. T101-02; Vazyme,
Piscataway, NJ, USA). A total of 10 ng pMIR-REPORT™ β-gal Control
Plasmid was transfected as an internal control for transfection
efficiency. Luminescence was measured at 24 h after transfection
using a Dual-Luciferase® Reporter Assay System (Promega
Corp., Madison, WI, USA) according to the manufacturer's protocol
Measurements of luminescence were performed using a Glomax 20/20
(Promega Corp.).
Cell culture and transfection
Human venous endothelial cells EAhy926 (American
Type Culture Collection) were cultured in Dulbecco's Modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.) with 10%
heat-inactivated fetal bovine serum (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 1% penicillin and
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2. A total of 3×105 cells were seeded in a
24-well plate and transfected with 25 pmol of agomiR-381 (Sangon
Biotech) using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. As a control, EAhy926
cells were transfected with NC RNA without specifically targeting
any human gene products. To silence the expression of VEGFA, cells
were transfected with 50 nM VEGFA siRNA (siVEGFA) or NC (Sangon
Biotech, Shanghai, China). Cells were collected at 48 h
post-transfection for further experiments.
MTT assay
Cells were seeded into 96-well plates at a density
of 2,000 cell/well. Cells were cultured at in a humidified
atmosphere containing 5% CO2 at 37°C. At 24, 48, and 72
h, 20 µl of MTT reagent (5 mg/ml; JRDUN biotechnology, Shanghai,
China) were added and cells were incubated at 37°C for 4 h until
purple precipitate was visible. On the last day the culture
supernatant was removed and 150 µl dimethyl sulfoxide per well was
added. The 96-well plate was oscillated for 10 min until purple
precipitate was dissolved. The absorbance was measured at 490 nm on
a microplate reader. A cell growth curve was generated based on
these absorbance values.
Statistical analysis
Data analysis was performed using SPSS version 18.0
(SPSS, Inc., Chicago, IL, USA) and expressed as the mean standard
deviation. Differences between groups were evaluated for
significance using one-way analysis of variance with Student Newman
Keuls, Tamhane's T2 or Dunnett's T3 post hoc tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
VEGFA is upregulated in tumor
thrombi
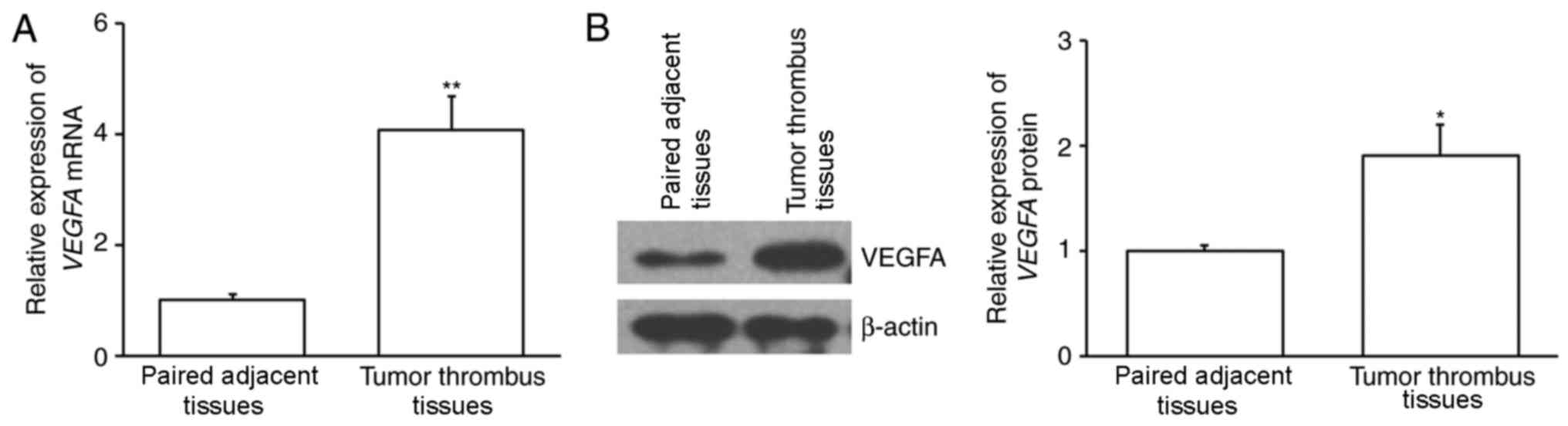
The expression of VEGFA mRNA was assessed
using RT-qPCR. The results demonstrated that VEGFA mRNA
expression was significantly increased in tumor thrombus samples
compared with paired adjacent tissues (P<0.01; Fig. 1A). The expression of VEGFA protein
was also assessed using western blotting and the results revealed
that it was significantly upregulated in tumor thrombi compared
with paired adjacent tissues (P<0.05; Fig. 1B). This suggests that VEGFA may be
associated with the formation of tumor thrombi.
VEGFA is a direct target of
miR-381
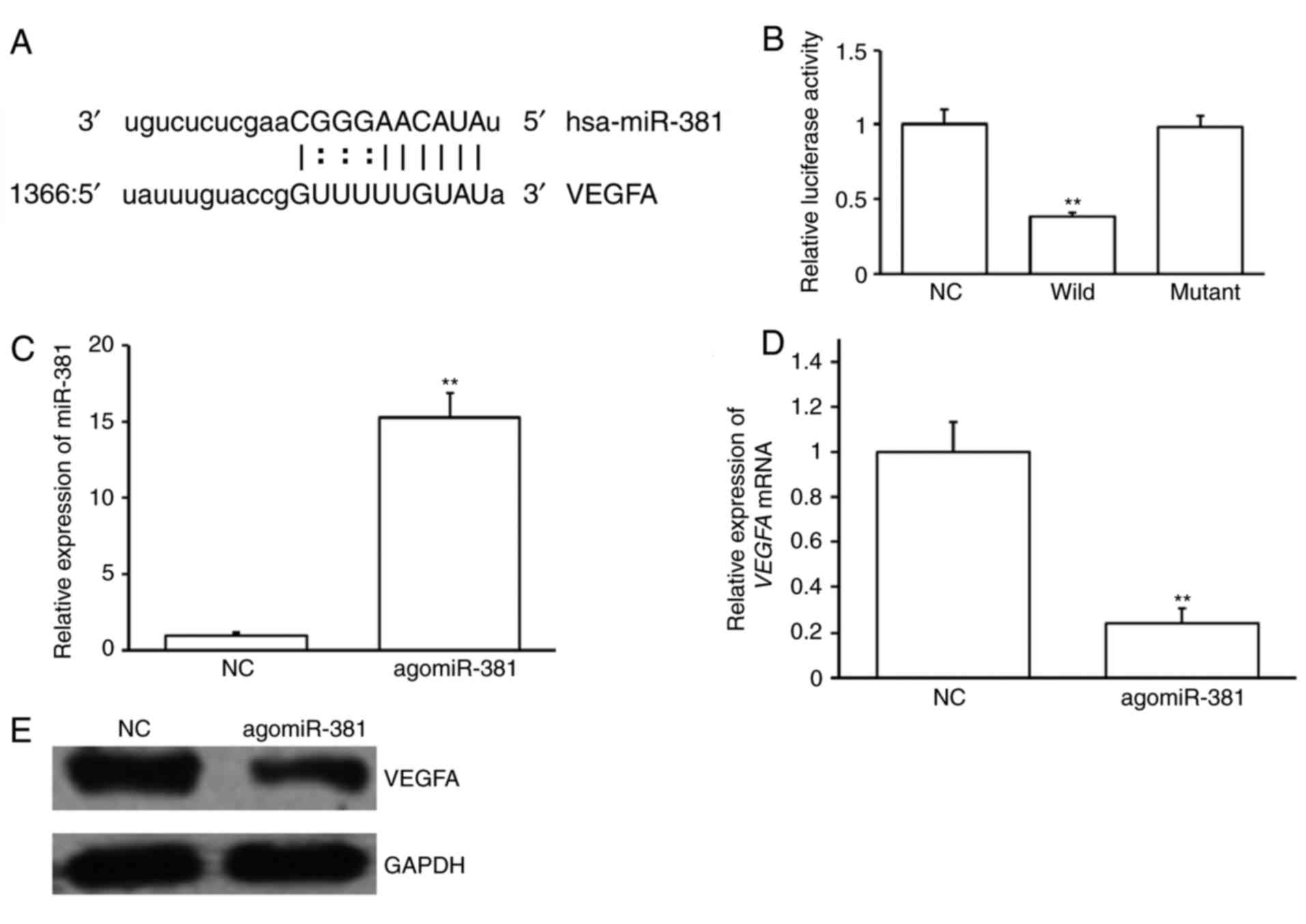
Potential upstream miRNA regulators of VEGFA were
investigated. miRNAs that may bind to the 3′-UTR of VEGFA were
predicted using bioinformatics. An miR-381 binding site was
identified at the 3′-UTR of VEGFA mRNA (Fig. 2A). A dual luciferase reporter assay
was performed to confirm this prediction. Cotransfection of
agomiR-381 and pMIR-REPORT-VEGFA WT construct significantly
decreased luciferase activity compared with NC and
pMIR-REPORT-VEGFA WT construct (P<0.05; Fig. 2B). However, cotransfection with
agomiR-381 and pMIR-REPORT-VEGFA mutant construct had no
significant effect on luciferase activity (Fig. 2B). These results suggest that VEGFA
is a possible target of miR-381, with miR-381 binding to its 3′-UTR
sequence.
To confirm the results of the dual luciferase
reporter assay, EAhy926 cells were transfected with agomiR-381 or
NC. AgomiR-381 transfection significantly increased miR-381
expression (P<0.01; Fig. 2C) and
significantly decreased VEGFA mRNA expression in EAhy926
cells (P<0.01; Fig. 2D).
AgomiR-381 transfection also markedly reduced VEGFA protein levels
(Fig. 2E). These results suggest
that the expression of VEGFA is regulated by miR-381.
miR-381 is downregulated in tumor
thrombi
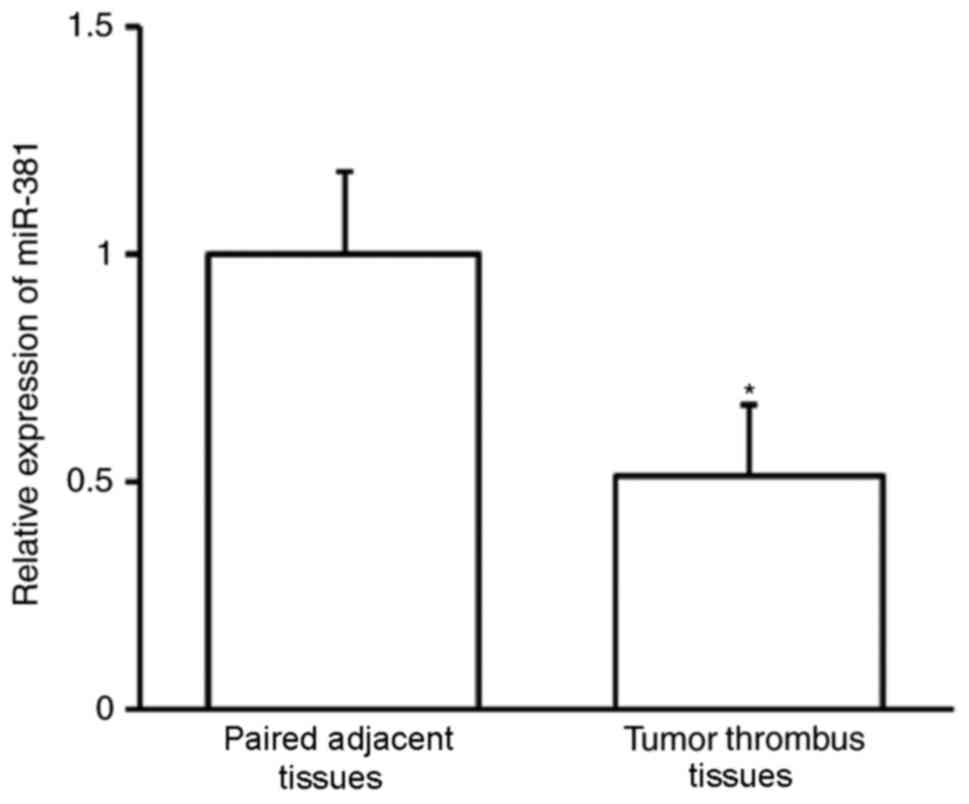
The expression of miR-381 was assessed using
RT-qPCR. miR-30b expression was significantly downregulated in
tumor thrombi compared with adjacent paired tissues (P<0.01;
Fig. 3). This indicates that miR-381
may contribute to the development of PVTT.
Overexpression of miR-381 inhibits
EAhy926 cell proliferation
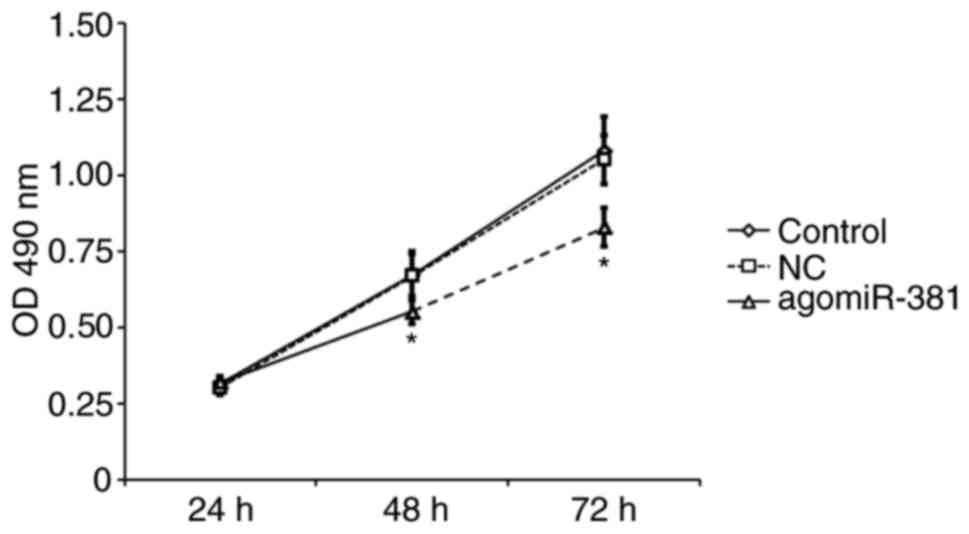
To investigate whether miR-381 regulates cell
proliferation, EAhy926 cells were transiently transfected with
agomiR-381 or NC. An MTT proliferation assay revealed that EAhy926
cells transfected with miR-381 exhibited significantly decreased
cell proliferation compared with untreated cells or those
transfected with NC (P<0.05; Fig.
4). This result suggests that miR-381 is able to inhibit the
proliferation of venous endothelial cells.
Effect of VEGFA on cell
proliferation
Eahy926 cell proliferation was examined following
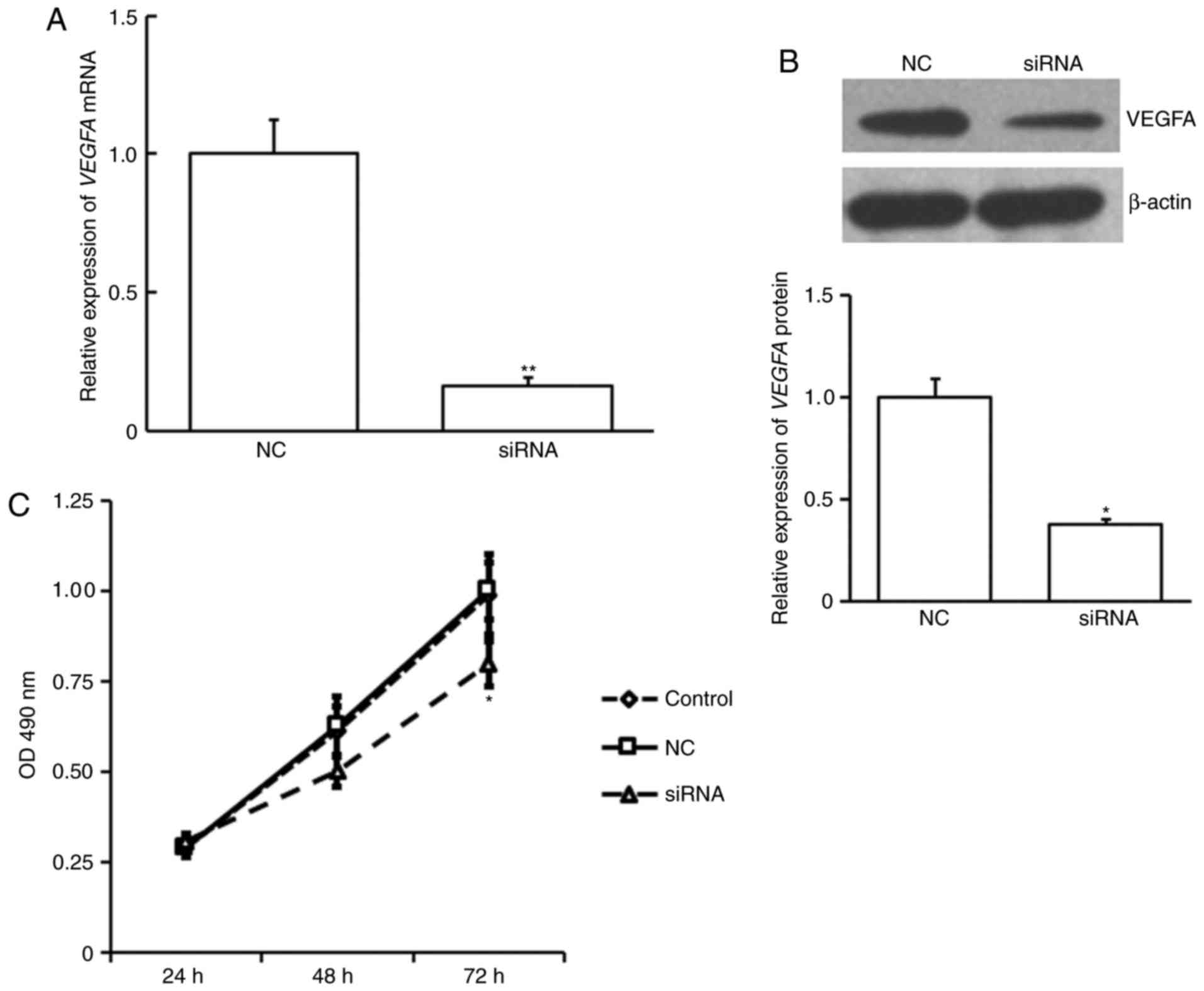
siVEGFA transfection. siVEGFA was demonstrated to significantly
decrease VEGFA expression at the mRNA (P<0.01; Fig. 5A) and protein level (P<0.05;
Fig. 5B). siVEGFA significantly
inhibited cell proliferation at 72 h post transfection compared
with NC or untransfected control cells (P<0.05; Fig. 5C). This suggests that VEGFA also
serves a role in venous endothelial cell proliferation.
Discussion
In the present study, the expression of VEGFA and
its upstream regulator, miR-381, was assessed in tumor thrombus and
paired adjacent tissues from patients with HCC with PVTT. The
association between miR-381 and VEGFA was also analyzed. The
effects of miR-381 and VEGFA on venous endothelial cell
proliferation were investigated and the mechanism of secondary
development of PVTT in cases of HCC was discussed. To the best of
our knowledge, the present study is the first to investigate the
role of miR-381 and VEGF in tumor thrombosis in patients with
HCC.
The 5-year survival rate of patients with HCC is
<12% in the USA, ~32.5% overall and 59.1% in patients in the
early stages in China (21,22). A number of studies have investigated
the association between venous thromboembolism and malignant
tumors; for example, it has been reported that almost 10% of
patients with renal cell carcinoma develop venous thrombosis
(23,24). In addition, 60% of patients with
tumor thrombus have tumor metastasis (25). For patients with tumors and venous
thrombosis, complete resection of the tumor tissues is currently
the only effective treatment option (24). Venous thrombosis causes complications
in patients with tumors; tumor cells are able to flow through the
blood vessels to the portal vein and gradually grow to form a tumor
thrombus, while the tumor cells can also spread along the vein in
the liver (26,27).
Blocking cell signaling pathways or inhibiting tumor
neovascularization to slow tumor growth is a major focus of tumor
targeted therapy and the use of such neoadjuvant therapies have
been described in several case studies (28–32). In
humans, VEGFA is one of the most effective angiogenic factors as it
is able to promote angiogenesis and increase blood supply (33). In the present study, it was
demonstrated that VEGFA was significantly upregulated in tumor
thrombi compared with normal tissues. Furthermore, silencing VEGFA
inhibited EAhy926 cell proliferation. VEGF is also an important
factor in HCC (34) and the data
herein further suggest that VEGFA serves a crucial role in the
development of PVTT.
miRNAs participate in the development of tumors by
promoting the downregulation of mRNA expression and regulating
protein-coding gene activity (35–37). In
the present study, miR-381 was predicted to be an upstream
regulator of VEGFA, which is consistent with a previous report in
MG-63 cells (38). It has been
reported that miR-381 is significantly downregulated in colon
cancer tissues, and this induces the proliferation and invasion of
colon cancer cells by increasing liver receptor homolog-1
upregulation (39). miRNA-381 in the
p53/pituitary tumor-transforming 1 negative feedback loop inhibits
the growth of pituitary tumors (40). miR-381 is also associated with
multidrug resistance gene 1 and serves a role in multiple drug
resistance (41). The combination of
miR-381 and miR-424 inhibits the activity of cluster of
differentiation 2 in renal cells by targeting WEE1 (42). Furthermore, it has also been
demonstrated that miR-381 is associated with lung adenocarcinoma
(43). Together, these studies
demonstrate that miR-381 is a critical regulator in the development
of various tumors. To further investigate the regulatory mechanism
of miR-381 on VEGFA, miR-381 was overexpressed in EAhy926 cells via
agomiR-381 transfection and cell proliferation was suppressed.
Previous clinical studies and reports have indicated an association
between tumor cell proliferation and PVTT (44–46).
Additionally, miR-381 overexpression resulted in decreased VEGFA
mRNA expression. The results of a dual-luciferase reporter assay
demonstrated that miR-381 is able to directly target VEGFA.
Collectively, the results of the present study indicate that
miR-381 is able to directly regulate VEGFA expression.
In summary, VEGFA was upregulated in tumor thrombi
from patients with HCC and PVTT, whereas miR-381 was downregulated.
miR-381 is able to regulate VEGFA. Furthermore, miR-381 and VEGFA
may regulate the proliferation of venous endothelial cells.
Therefore, the findings of the present study suggest that miR-381
and VEGFA may be associated with the development of PVTT. However,
a limitation of the present study is that the sample size was
relatively small and primarily made up of individuals from the
Chinese population. In order to further investigate the clinical
applications of miR-381 and VEGF in tumor thrombosis, transgenic
mice with miR-381 or VEGF knockout should be used in future
studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JW and SZW performed the experiments, analyzed the
data and drafted the manuscript. TRH conceived and designed the
study and wrote and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from every
patient and the study was approved by the Ethics Review Board of
Guangxi Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wedd JP, Nordstrom E, Nydam T, Durham J,
Zimmerman M, Johnson T, Purcell Thomas W and Biggins SW:
Hepatocellular carcinoma in patients listed for liver
transplantation: Current and future allocation policy and
management strategies for the individual patient. Liver Transpl.
21:1543–1552. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuszyk BS, Beauchamp NJ Jr and Fishman EK:
Neurovascular applications of CT angiography. Semin Ultrasound CT
MR. 19:394–404. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanda M, Sugimoto H and Kodera Y: Genetic
and epigenetic aspects of initiation and progression of
hepatocellular carcinoma. World J Gastroenterol. 21:10584–10597.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trousseau Armand: Lectures on clinical
medicine, delivered at the Hotel-Dieu, Paris. Translated and edited
with notes and appendices by P. Victor Bazire. Med Clin.
1:1801–1867. 1872.
|
|
5
|
Thodiyil PA and Kakkar AK: Variation in
relative risk of venous thromboembolism in different cancers.
Thromb Haemost. 87:1076–1077. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blom JW, Vanderschoot JP, Oostindier MJ,
Osanto S, van der Meer FJ and Rosendaal FR: Incidence of venous
thrombosis in a large cohort of 66,329 cancer patients: Results of
a record linkage study. J Thromb Haemost. 4:529–535. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J National Cancer Institute.
82:4–7. 1990. View Article : Google Scholar
|
|
8
|
Cosmai L, Gallieni M, Liguigli W and Porta
C: Renal toxicity of anticancer agents targeting vascular
endothelial growth factor (VEGF) and its receptors (VEGFRs). J
Nephroll. 30:171–180. 2017. View Article : Google Scholar
|
|
9
|
Wu L, Zhang YS, Ye ML, Shen F, Liu W, Hu
HS, Li SW, Wu HW, Chen QH and Zhou WB: Overexpression and
correlation of HIF-2α, VEGFA and EphA2 in residual hepatocellular
carcinoma following high-intensity focused ultrasound treatment:
Implications for tumor recurrence and progression. Exp Ther Med.
13:3529–3534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rong W, Yang L, Yin L, Gao Y, Xiao T and
Cheng S: PSG9 promotes angiogenesis by stimulating VEGFA production
and is associated with poor prognosis in hepatocellular carcinoma.
Sci China Life Sci. 60:528–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghosh A, Dasgupta D, Ghosh A, Roychoudhury
S, Kumar D, Gorain M, Butti R, Datta S, Agarwal S, Gupta S, et al:
miRNA199a-3p suppresses tumor growth, migration, invasion and
angiogenesis in hepatocellular carcinoma by targeting VEGFA,
VEGFR1, VEGFR2, HGF and MMP2. Cell Death Dis. 8:e27062017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu CH, Kang YK, Yang TS, Shun CT, Shao
YY, Su WC, Su WC, Sandoval-Tan J, Chiou TJ, Jin K, et al:
Bevacizumab with erlotinib as first-line therapy in Asian patients
with advanced hepatocellular carcinoma: A multicenter phase II
study. Oncology. 85:44–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meyer G: Venous thromboembolism and
cancer. Rev Prat. 65:216–219. 2015.(In French). PubMed/NCBI
|
|
14
|
Bianconi D, Schuler A, Pausz C,
Geroldinger A, Kaider A, Lenz HJ, Kornek G, Scheithauer W,
Zielinski CC, Pabinger I, et al: Integrin beta-3 genetic variants
and risk of venous thromboembolism in colorectal cancer patients.
Thromb Res. 136:865–869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kokudo T, Hasegawa K and Kokudo N: Liver,
pancreas, biliary tract cancer. I. Surgical treatment of
hepatocellular carcinoma associated with vascular tumor thrombosis.
Gan To Kagaku Ryoho. 41:1209–1211. 2014.(In Japanese). PubMed/NCBI
|
|
16
|
Posch F, Thaler J, Zlabinger GJ,
Königsbrügge O, Koder S, Zielinski C, Pabinger I and Ay C: Soluble
vascular endothelial growth factor (sVEGF) and the risk of venous
thromboembolism in patients with cancer: Results from the Vienna
cancer and thrombosis study (CATS). Clin Cancer Res. 22:200–206.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Babu Govind K and Bhat GR:
Cancer-associated thrombotic microangiopathy.
Ecancermedicalscience. 10:6492016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Evans CE, Grover SP, Saha P, Humphries J,
Kim JW, Modarai B and Smith A: Suppression of angiogenic response
in local vein wall is associated with reduced thrombus resolution.
Thromb Res. 134:682–685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Di Vito C, Navone SE, Marfia G, Hadi Abdel
L, Mancuso ME, Pecci A, Crisà FM, Berno V, Rampini P, Campanella R
and Riboni L: Platelets from glioblastoma patients promote
angiogenesis of tumor endothelial cells and exhibit increased VEGF
content and release. Platelets. 28:585–594. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang BH, Xia JL, Huang LW, Tang ZY, Chen
MS, Li JQ, Liang AM, Mo QG, Lu HS, Dai CL, et al: Changes of
clinical aspect of primary liver cancer in China during the past 30
years-control study for 3,250 cases with primary liver cancer.
Zhonghua Yi Xue Za Zhi. 83:1053–1057. 2003.(In Chinese). PubMed/NCBI
|
|
23
|
Karnes RJ and Blute ML: Surgery insight:
Management of renal cell carcinoma with associated inferior vena
cava thrombus. Nat Clin Pract Urol. 5:329–339. 2008.PubMed/NCBI
|
|
24
|
Lambert EH, Pierorazio PM, Shabsigh A,
Olsson CA, Benson MC and McKiernan JM: Prognostic risk
stratification and clinical outcomes in patients undergoing
surgical treatment for renal cell carcinoma with vascular tumor
thrombus. Urology. 69:1054–1058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lam JS, Klatte T, Kim HL, Patard JJ, Breda
A, Zisman A, Pantuck AJ and Figlin RA: Prognostic factors and
selection for clinical studies of patients with kidney cancer. Crit
Rev Oncol Hematol. 65:235–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Squillaci E, Fanucci E, Sciuto F, Masala
S, Sodani G, Carlani M and Simonetti G: Vascular involvement in
pancreatic neoplasm: A comparison between spiral CT and DSA. Dig
Dis Sci. 48:449–458. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chow LC and Rubin GD: CT angiography of
the arterial system. Radiol Clin North Am. 40:729–749. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hakenberg OW: Comment on Di Silverio et
al: Neodajuvant therapy with sorafenib in advanced renal cell
carcinoma with vena cava extension submitted to radical
nephrectomy. Urol Int. 80:4542008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shuch B, Riggs SB, LaRochelle JC,
Kabbinavar FF, Avakian R, Pantuck AJ, Patard JJ and Belldegrun AS:
Neoadjuvant targeted therapy and advanced kidney cancer:
Observations and implications for a new treatment paradigm. BJU
Int. 102:692–696. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karakiewicz PI, Suardi N, Jeldres C, Audet
P, Ghosn P, Patard JJ and Perrotte P: Neoadjuvant sutent induction
therapy may effectively down-stage renal cell carcinoma atrial
thrombi. Eur Urol. 53:845–848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harshman LC, Srinivas S, Kamaya A and
Chung BI: Laparoscopic radical nephrectomy after shrinkage of a
caval tumor thrombus with sunitinib. Nat Rev Urol. 6:338–343. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bex A, Van der Veldt AA, Blank C,
Meijerink MR, Boven E and Haanen JB: Progression of a caval vein
thrombus in two patients with primary renal cell carcinoma on
pretreatment with sunitinib. Acta Oncol. 49:520–523. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roberts E, Cossigny DA and Quan GM: The
role of vascular endothelial growth factor in metastatic prostate
cancer to the skeleton. Prostate Cancer. 2013:4183402013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan JJ, Zhang YN, Liao JZ, Ke KP, Chang Y,
Li PY, Wang M, Lin JS and He XX: miR-497 suppresses angiogenesis
and metastasis of hepatocellular carcinoma by inhibiting VEGFA and
AEG-1. Oncotarget. 6:29527–29542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao X, Mohan R, Özcan S and Tang X:
MicroRNA-30d induces insulin transcription factor MafA and insulin
production by targeting mitogen-activated protein 4 kinase 4
(MAP4K4) in pancreatic β-cells. J Biol Chem. 287:31155–31164. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsai HC, Tzeng HE, Huang CY, Huang YL,
Tsai CH, Wang SW, Wang PC, Chang AC, Fong YC and Tang CH: WISP-1
positively regulates angiogenesis by controlling VEGF-A expression
in human osteosarcoma. Cell Death Dis. 8:e27502017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liang Y, Zhao Q, Fan L, Zhang Z, Tan B,
Liu Y and Li Y: Down-regulation of MicroRNA-381 promotes cell
proliferation and invasion in colon cancer through up-regulation of
LRH-1. Biomed Pharmacother. 75:137–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liang HQ, Wang RJ, Diao CF, Li JW, Su JL
and Zhang S: The PTTG1-targeting miRNAs miR-329, miR-300, miR-381,
and miR-655 inhibit pituitary tumor cell tumorigenesis and are
involved in a p53/PTTG1 regulation feedback loop. Oncotarget.
6:29413–29427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu Y, Ohms SJ, Li Z, Wang Q, Gong G, Hu Y,
Mao Z, Shannon MF and Fan JY: Changes in the expression of miR-381
and miR-495 are inversely associated with the expression of the
MDR1 gene and development of multi-drug resistance. PLoS One.
8:e820622013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen B, Duan L, Yin G, Tan J and Jiang X:
Simultaneously expressed miR-424 and miR-381 synergistically
suppress the proliferation and survival of renal cancer cells-Cdc2
activity is up-regulated by targeting WEE1. Clinics (Sao Paulo).
68:825–833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rothschild SI, Tschan MP, Jaggi R, Fey MF,
Gugger M and Gautschi O: MicroRNA-381 represses ID1 and is
deregulated in lung adenocarcinoma. J Thoracic Oncol. 7:1069–1077.
2012. View Article : Google Scholar
|
|
44
|
Wu L, Zheng J, Chen P, Liu Q and Yuan Y:
Small nucleolar RNA ACA11 promotes proliferation, migration and
invasion in hepatocellular carcinoma by targeting the PI3K/AKT
signaling pathway. Biomed Pharmacother. 90:705–712. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hou G, Liu G, Yang Y, Li Y, Yuan S, Zhao
L, Wu M, Liu L and Zhou W: Neuraminidase 1 (NEU1) promotes
proliferation and migration as a diagnostic and prognostic
biomarker of hepatocellular carcinoma. Oncotarget. 7:64957–64966.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang Y, Yu H, Zhang L, Wang K, Guo W, Shi
J, Liu S, Wu M, Wang H and Cheng S: Experimental study on
enhancement of the metastatic potential of portal vein tumor
thrombus-originated hepatocellular carcinoma cells using portal
vein serum. Chin J Cancer Res. 26:588–595. 2014.PubMed/NCBI
|