Introduction
Diabetes mellitus (DM) is a chronic disease with
high prevalence in Taiwan (1,2). DM is
characterized by a relative or absolute lack of insulin, resulting
in hyperglycaemia (3). Chronic
hyperglycaemia leads to a variety of complications such as
neuropathy, nephropathy and retinopathy and increased risk of
cardiovascular disease (4–7). According to the World Health
Organization Global report, 422 million individuals were diagnosed
with DM in 2016 (8). In Taiwan, DM
was reported to be the fifth leading cause of death in 2015 by the
Ministry of Health and Welfare of Taiwan (9). DM has two subtypes, namely type 1 and
type 2 DM (10,11). Type 1 DM (T1D) is an autoimmune
disease that leads to the destruction of the insulin-producing
pancreatic β cells in the islets of Langerhans (12). In children and young adults, T1D is
the most commonly diagnosed type of DM. T1D is associated with low
endogenous insulin production in affected patients, and insulin
supplementation by subcutaneous injection is required (13). The blood glucose levels must be
frequently monitored to manage the risk of hypoglycaemia (14). Genetic influences and environmental
factors have an important role in disease development (15,16).
However, T2D is the most common type of DM in Taiwan. It is most
commonly diagnosed in middle-aged adults (17). T2D is associated with insulin
resistance, which is a lack of appropriate compensation by the β
cells, leading to a relative insulin deficiency (18,19). In
the early stage, insulin resistance may be improved by weight
reduction and exercise (20). A
variety of drugs are available for treating T2D (21,22).
Treatment with drugs such as sulphonylureas stimulates insulin
production by the β cells (23,24),
while that with drugs such as biguanides or metformin reduces
hepatic glucose production (25,26),
while α-glucosidase inhibitors delay carbohydrate uptake in the gut
(27,28), thiazolidinediones improve insulin
action (29,30) and as glucagon-like peptide 1 (GLP-1)
receptor agonists or dipeptidyl peptidase-4 inhibitors target the
GLP-1 axis (31,32). DM represents is a complex disease
involving various bodily systems. Thus, animal models should be
carefully selected, depending on what aspect of the disease is
being investigated (33).
One of the mouse models that has not been
extensively examined by the National Institutes of Health-sponsored
Animal Models of Diabetic Complications Consortium is the KK
Cg-Ay/J (KK-Ay) strain (34,35). The
KK-Ay mouse is a typical T2D model, and one of the
inbred strains established from Japanese native mice (33,35).
Yellow KK-Ay mice carry the yellow obesity gene
Ay and develop marked adiposity and DM symptoms in
comparison with control black KK-α/α mice (36). This model exhibits marked obesity,
glucose intolerance and insulin resistance of peripheral tissue,
hyperglycemia, dyslipidemia, hypertension and renal glomerular
changes (33,35,36).
Furthermore, the pancreatic islets in yellow KK-Ay mice
are hypertrophic and de-granulated. This mouse strain also presents
with signs of diabetic nephropathy (33,35,36).
T2D is a phenotypically and genetically diverse
disease characterized by insulin resistance (22,28).
Candidate gene mapping and positional cloning have suggested
numerous putative susceptibility variants, but only a few genetic
variants leading to T2D have been clearly identified, including
transcription-factor-7-like 2 (TCF7L2) and hematopoietically
expressed homeobox (HHEX) protein (37–39). The HHEX gene is
located on chromosome 10q23.33 and encodes a 270 amino-acid protein
(40). HHEX contains the
insulin-degrading enzyme (IDE) and kinesin family member 11
(40,41). The HHEX gene encodes a
transcription factor involved in hepatic and pancreatic development
via the Wnt signal pathway, which is fundamental for cell growth
and differentiation (42,43). Numerous studies have identified the
HHEX gene polymorphisms of rs1111875 T>C and rs7923837
A>G in T2D patients (38,44,45). A
genome-wide scan for association provided evidence that HHEX is an
excellent candidate susceptibility gene for T2D, and indicated a
significant association of rs1111875 and rs7923837 with T2D
(38). Although the association
between HHEX polymorphisms and T2D has been well studied in humans,
it has remained elusive in KK-Ay mice. A preliminary
study by our group clearly demonstrated that HHEX mRNA was
downregulated in liver tissues of KK-Ay mice as compared
with that in KK-α/α control mice by complementary DNA microarray
analysis (unpublished data). The present study focused on
investigating the association between HHEX and T2D in
KK-Ay mice and in a Han Chinese Population in
Taiwan.
Materials and methods
KK-α/α and KK-Ay mice
A total of 5 four-week-old male control KK-α/α mice
and 5 four-week-old male KK-Ay mice were obtained from
Jackson Laboratories (Bar Harbor, ME, USA), the mice were divided
into two groups (5 mice/group). The animals were housed in
individual cages and provided lab chow (LabDiet 5k52; St. Louis,
MO, USA) and water ad libitum in a room with a constant
temperature (22–25°C), relative humidity (50–70%) and photoperiod
(12-h light/dark cycle). The study was approved by the
Institutional Animal Care and Use Committee of China Medical
University (IACUC permit no. 102-217) as previously described
(46).
Immunohistochemical (IHC)
analysis
IHC was performed on paraffin-embedded liver
sections as previously described (46,47). IHC
staining for HHEX was performed using a Leica Bond MAX automated
immunostainer (Leica Microsystems Inc., Buffalo Grove, IL, USA).
Tissue sections (5-µm-thick) were de-waxed, treated with Proteinase
K enzyme (cat. no. P2308; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), followed by blocking with 3% hydrogen peroxide (cat. no.
H1009; Sigma-Aldrich; Merck KGaA). The slides were incubated in
anti-HHEX (cat. no. GTX84369; GeneTex, Hsinchu, Taiwan) antibodies
(1:250 dilution) for 30 min at room temperature, followed by
horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin
G secondary antibodies (cat. no. 61-6520; 1:1,000; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 15 min at 37°C. Chromogen
visualization was performed using 3,3′-diaminobenzidine
tetrahydrochloride (DAB; cat. no. D12384, Sigma-Aldrich; Merck
KGaA) for 5 min at room temperature. The sample was washed with
0.05% Tween 20 in Tris-buffered saline (DAKO, Carpinteria, CA, USA)
between all steps.
Patients and sample collection for
genotyping
A total of 570 patients diagnosed with T2D by
endocrinologist at China Medical University Hospital (Taichung,
Taiwan) were recruited between August 2014 and August 2015 for the
present study from China Medical University Hospital. To compare
the prevalence of polymorphisms in patients with that in a healthy
population, the genotype frequency data of 1,700 healthy controls
were downloaded from the Taiwan Biobank (https://taiwanview.twbiobank.org.tw/taiwanview/dl.do).
The single nucleotide polymorphisms (SNPs) in the target gene were
queried from the National Center of Biotechnology Information SNP
database (http://www.ncbi.nlm.nih.gov/snp). The SNPs of the
genes of interest were obtained and compared between the disease
and control groups. Chi-square tests were used to calculate odds
ratios and P-values. The study was approved by the ethics
committee/Institutional Review Board of China Medical University
Hospital (no. CMUH103-REC2-071).
Statistical analysis
The Chi-square test was used to determine
statistically significant differences in allele/genotype
frequencies of HHEX SNP rs61862780 between the case and control
groups. Differences were considered statistically significant when
P<0.05. The odds ratios (ORs) with 95% confidence intervals (95%
CIs) were calculated for the genotypic and allelic frequencies of
the HHEX SNP rs61862780. The statistical analysis was performed
using SPSS version 11 (SPSS, Inc., Chicago, IL, USA).
Results
Diabetes-associated features of yellow
KK-Ay mouse model
The diabetes-associated features of yellow
KK-Ay mice according to the supplier's information and
previous studies are listed in Table
I (33,34). In the late stage (42 weeks),
KK-Ay mice presented with a variety of T2D-like
characteristics, including abnormal lipid homeostasis, increase in
blood glucose levels, hyperglycemia (>400 mg/dl), glucose
intolerance, increased circulating insulin and urine glucose
levels, insulin resistance, adipose tissue weight increases,
increased susceptibility to weight gain, abnormal morphology of
pancreatic islets, renal glomerulus and renal tubules,
de-granulated pancreatic β cells, dilated renal tubules, expanded
mesangial matrix, increased renal glomerulus basement membrane
thickness and hyaline cast present in tubules (2,21,31,33).
 | Table I.General characteristics of diabetic
features of yellow KK Cg-Ay/J mice according to the
supplier. |
Table I.
General characteristics of diabetic
features of yellow KK Cg-Ay/J mice according to the
supplier.
| Parameter | 6 weeks | 16 weeks | 42 weeks |
|---|
| Abnormal lipid
homeostasis | − | + | ++ |
| Increase in blood
glucose levels | + | ++ | +++ |
| Marked
hyperglycemia (400–500 mg/dl) | − | − | + |
| Impaired glucose
tolerance | − | + | ++ |
| Increased
circulating insulin level | + | ++ | +++ |
| Increased urine
glucose level | + | ++ | +++ |
| Insulin
resistance | − | + | ++ |
| Adipose tissue
weight increases | − | + | ++ |
| Increased
susceptibility to weight gain | + | ++ | +++ |
| Abnormal pancreatic
islet morphology | − | + | ++ |
| Degranulated
pancreatic beta cells | + | ++ | +++ |
| Abnormal renal
glomerulus morphology | + | ++ | +++ |
| Abnormal renal
tubule morphology | + | ++ | +++ |
| Dilated renal
tubules | + | ++ | +++ |
| Expanded mesangial
matrix | + | ++ | +++ |
| Increased renal
glomerulus basement membrane thickness | + | ++ | +++ |
| Hyaline cast is
present in tubules | + | ++ | +++ |
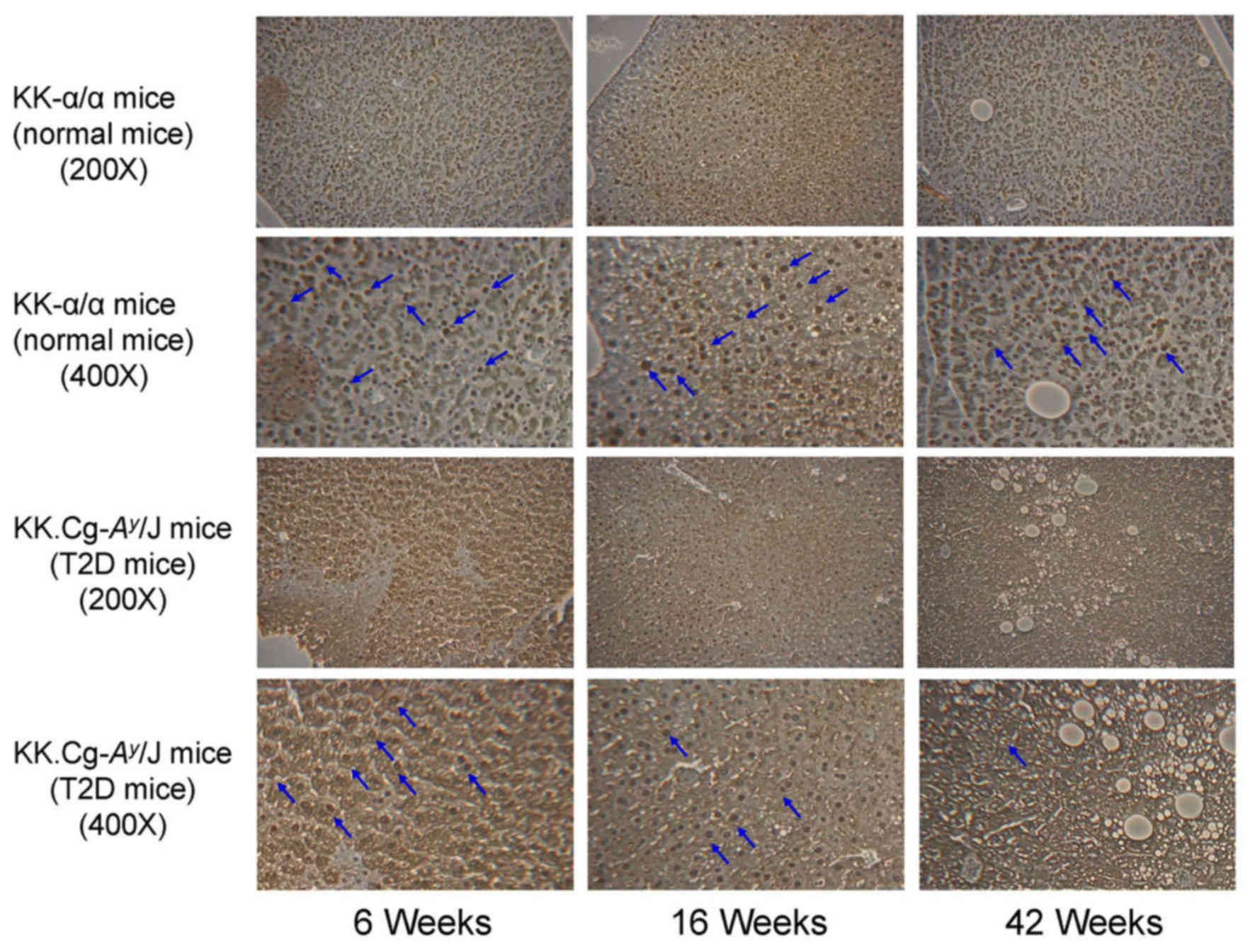
HHEX protein expression in liver
tissues of KK-Ay T2D model mice
KK-α/α mice (control group) and KK-Ay
mice (T2D group) were individually sacrificed at 6, 16 and 42 weeks
of age, and liver tissues were excised, fixed, embedded and
sectioned for IHC staining. As presented in Fig. 1, IHC analysis indicated that the HHEX
protein expression in liver tissue was decreased with time. Based
on these results, HHEX protein expression in liver tissues of
KK-Ay mice (T2D group) was significantly lower than that
in KK-α/α mice (control group). In addition, it was demonstrated
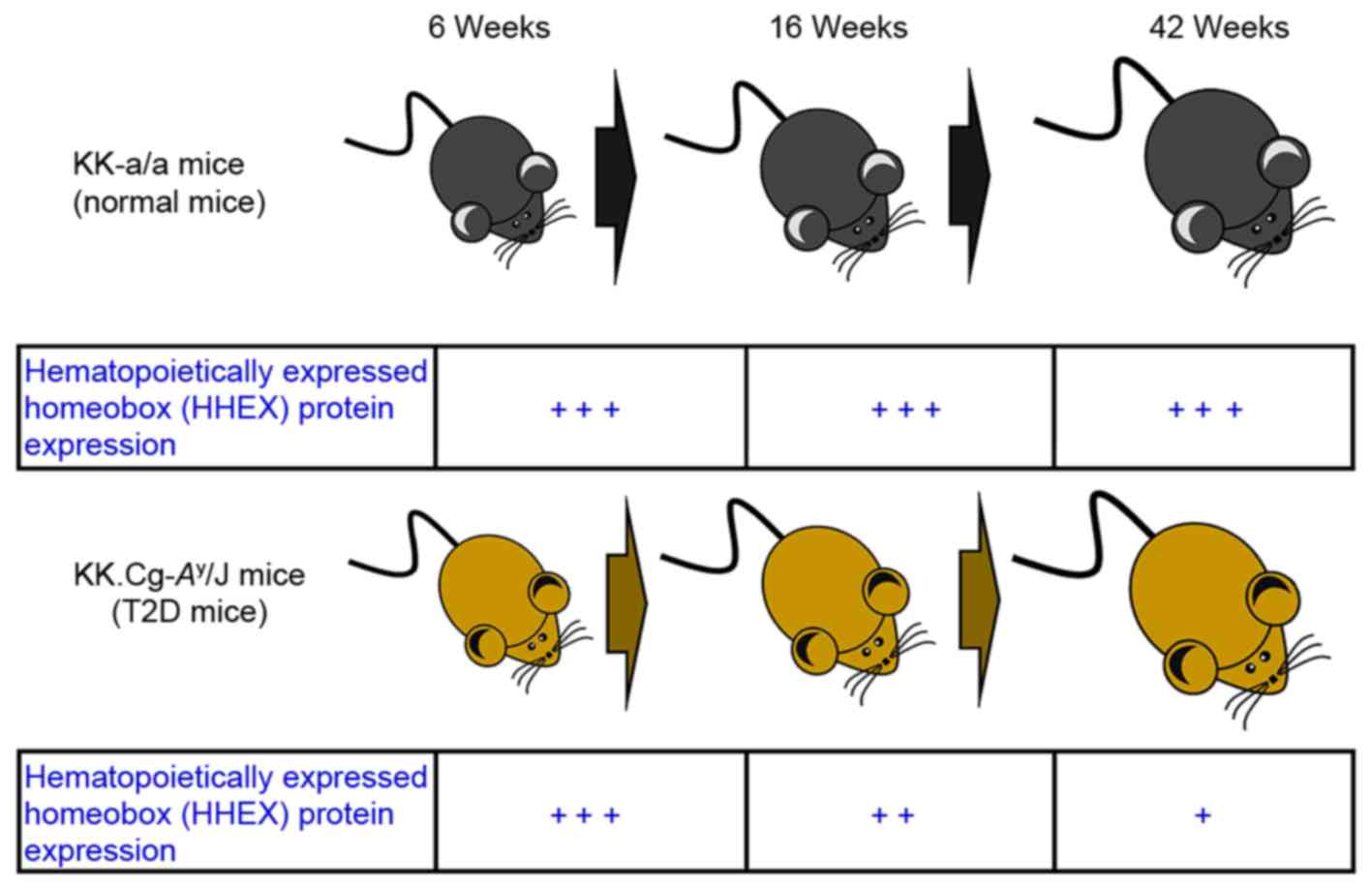
that DM mice (KK-Ay) grew in the period that relative
HHEX protein expression were decreased (Fig. 2). Although KK-α/α mice (control
group) grew in the same period HHEX protein expression did not
change.
Genotypic and allelic frequency
distributions of the rs61862780 SNP in the HHEX gene among Taiwan's
Han Chinese population
The genotypic and allelic frequency distributions of
the rs61862780 SNP in the HHEX gene located within Chromosome
10q23.3 region (92,708,886 bp) are summarized in Table II. The Hardy-Weinberg model was used
to describe and predict genotype and allele frequencies in the
study cohort. It was observed that for the rs61862780 (C/T) SNP in
the 3′-UTR of HHEX, the frequency of the CC genotype was higher in
patients (6.0%) than in controls (2.7%). In addition, the frequency
of the CT genotype was higher in patients (30.0%) than in controls
(29.2%). In comparison with the TT genotype, the OR of the CC
genotype was 2.4 (95% CI=1.51–3.8; P<0.001). The allelic
frequency of the C allele was higher in patients (21.0%) than in
controls (17.3%). In comparison with the T allele, the OR for the C
allele was 1.27 (95% CI=1.08–1.5; P<0.005). It was therefore
indicated that the HHEX SNP rs61862780 is associated with the
susceptibility to T2D.
 | Table II.Genotypic and allelic frequencies in
the rs61862780 single nucleotide polymorphism of the 3′
untranslated region of the HHEX gene in patients with T2D (n=570)
and controls (n=1,698). |
Table II.
Genotypic and allelic frequencies in
the rs61862780 single nucleotide polymorphism of the 3′
untranslated region of the HHEX gene in patients with T2D (n=570)
and controls (n=1,698).
| Parameter | Patients with T2D,
n (%) | Control, n (%) | OR (95% CI) | P-value |
|---|
| Genotype |
|
|
|
|
| CC | 34 (6.0) | 45 (2.7) | 2.4
(1.51–3.8)a | <0.001 |
| CT | 171 (30.0) | 496 (29.2) | 1.09
(0.89–1.35) |
|
| TT | 365 (64.0) | 1,157 (68.1) |
|
|
| Allele
frequency |
|
|
|
|
| C | 239 (21) | 586 (17.3) | 1.27
(1.08–1.5)b | <0.005 |
| T | 901 (79) | 2,810 (82.7) |
|
|
Discussion
Numerous animal models of T2D are obese to reflect
the human condition where obesity is closely linked to T2D
development (33). Two genetic mouse
models of T2D exist, namely monogenic models (including
Lepob/ob and Leprdb/db mice)
and polygenic models (including KK Cg-Ay/J mice)
(33). A variety of different
polygenic mouse models of obesity, glucose intolerance and diabetes
are available, and the variety of genotypes, for example KK
Cg-Ay/J mice, which are one of the spontaneous animal
models of T2D, might allow for more better modeling of T2D in
humans compared with other obesity models (33,34,48). In
the present study, KK-Ay mice were used to study the
protein expression of HHEX in the liver. The KK-Ay mouse
strain is glucose-intolerant, insulin-resistant, dyslipidemic and
hypertensive (2,21,31). The
complex pathogenesis of T2D in obese and non-obese patients
involves genetic and environmental factors (49,50).
Genome-wide association studies (GWAS) have indicated an
association of T2D with several newly identified genes (51,52).
Associations of T2D with common variants in HHEX, hepatocyte
nuclear factor 4α, potassium voltage-gated channel subfamily J
member 11 (KCNJ11), peroxisome proliferator-activated receptor γ,
cyclin dependent kinase inhibitor 2A (CDKN2A)/2B, solute carrier
family 30 member 8, cell division cycle 123
(CDC123)/calcium/calmodulin dependent protein kinase ID (CAMK1D),
TCF7L2, ATP binding cassette subfamily A member 1 and
solute-carrier-family-16-member 11 (SLC16A11) genes have been
reported in European, Asian and Latin American populations (53–59).
Kong et al (53,60,61)
demonstrated associations of SNPs in wolframin ER transmembrane
glycoprotein, CDK5 regulatory subunit associated protein 1 like 1,
CDKN2A/2B, CDC123/CAMK1D, HHEX, TCF7L2, KCNQ1 and melatonin
receptor 1B with T2D in a Chinese population. Sladek et al
(62) reported variants of the
TCF7L2, SLC30A8 and HHEX genes as novel loci
associated with T2D by GWAS analysis in a French population. The
present study proved that the frequency of the CC genotype of the
rs61862780 (C/T) SNP in the 3′UTR of HHEX was higher in T2D
patients than in controls compared with that of the TT genotype. In
addition, the allelic frequency of the C allele was higher in
patients than in controls compared with that of the T allele, which
is in agreement with the previous studies (53,60,61). The
HHEX gene regulates cell proliferation and tissue
differentiation underlying vascular, hepatic differentiation and
forebrain neuro-development (63–65). HHEX is essential for heart
(66,67), thyroid (68), hepatic and pancreatic development and
is a target of the Wnt signaling pathway (43,69).
Decreased levels in HHEX-deficient islets cause disrupted paracrine
inhibition of insulin release from β cells (70). HHEX activity has been described in
the mouse lung, thyroid and liver. Numerous studies reported
associations between HHEX/insulin-degrading enzyme variants
(rs1111875) and the insulin secretion response following a glucose
load, suggesting that the HHEX gene may influence the T2D
risk primarily through an effect on β-cell function (47,71,72).
However, to the best of our knowledge, HHEX protein expression has
not been previously reported in KK-Ay T2D mice. The
present study was the first to demonstrate the role of HHEX in
KK-Ay mice. The IHC results were corroborated by results
of a complementary DNA microarray analysis of the liver tissues
(data not shown), which indicated a significant decrease in the
expression of HHEX in KK-Ay mice at 16 and 42 weeks.
High insulin levels (hyperinsulinemia) are a major feature of T2D
in the early stage, and the pancreas fails to produce sufficient
insulin to overcome the insulin resistance; however, failure of
β-cell function in the late stages of T2D causes a reduction of
insulin secretion (73,74). The present results suggested that
downregulation of HHEX expression in the liver may contribute to
disrupted paracrine control of insulin secretion in
KK-Ay T2D mice at 16 and 42 weeks.
Diabetes is a risk factor of various types of
cancer, including colorectal cancer (CRC), as well as breast,
bladder, liver and pancreatic cancer (75–77). Ma et al
(54) demonstrated that
T2D-associated variants of HHEX (rs7923837), TCF7L2 (rs290481) and
CDKAL1 (rs7756992) increased the risk of cancer in patients with
diabetes. Sun et al (78)
also demonstrated that two variants on the T2D susceptibility gene
HHEX are associated with the risk of CRC in a Chinese population.
Therefore, the factors associated with an increased risk for CRC in
T2D patients and the underlying mechanisms still require further
study. The time-dependent changes in the protein levels of HHEX in
KK-Ay T2D mice are summarized in Fig. 2. The present results demonstrated a
downregulation of HHEX protein in the liver of KK-Ay T2D
mice and an association of the HHEX SNP rs61862780 with T2D in
Taiwanese populations, indicating that loss/mutation of HHEX may
have an important role in the pathogenesis of T2D.
Acknowledgements
Not applicable.
Funding
This study was supported by the Biosignature
project, Academia Sinica, Taiwan and the China Medical University
Hospital (Taichung, Taiwan; grant no. DMR-107-123).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CCLu, JSY and FJT conceived and designed the
experiments. JWT and YNJ performed the experiments. YTC, SYC, YMH
and CCLi analyzed the data. CCLu, JSY and FJT wrote and modified
the paper. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the
Institutional Animal Care and Use Committee of China Medical
University (IACUC permit no. 102-217). The human experiments were
approved by the ethics committee/Institutional Review Board of
China Medical University Hospital (no. CMUH103-REC2-071).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hou WH, Li CY, Chen LH, Wang LY, Kuo KN,
Shen HN and Chang MF: Prevalence of hand syndromes among patients
with diabetes mellitus in Taiwan: A population-based study. J
Diabetes. 9:622–627. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen SY, Hsu YM, Lin YJ, Huang YC, Chen
CJ, Lin WD, Liao WL, Chen YT, Lin WY, Liu YH, et al: Current
concepts regarding developmental mechanisms in diabetic retinopathy
in Taiwan. Biomedicine (Taipei). 6:72016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Middleton P, Crowther CA and Simmonds L:
Different intensities of glycaemic control for pregnant women with
pre-existing diabetes. Cochrane Database Syst Rev: CD008540. 2016.
View Article : Google Scholar
|
|
4
|
Adeshara KA, Diwan AG and Tupe RS:
Diabetes and complications: Cellular signaling pathways, current
understanding and targeted therapies. Curr Drug Targets.
17:1309–1328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zatalia SR and Sanusi H: The role of
antioxidants in the pathophysiology, complications, and management
of diabetes mellitus. Acta Med Indones. 45:141–147. 2013.PubMed/NCBI
|
|
6
|
Resl M and Clodi M: Diabetes and
cardiovascular complications. Wien Med Wochenschr. 160:3–7.
2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hahr AJ and Molitch ME: Diabetes,
cardiovascular risk and nephropathy. Cardiol Clin. 28:467–475.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
World Health Organization, . Global report
on diabetes. WHO; Geneva: 2016, http://www.who.int/diabetes/global-report/en/PubMed/NCBI
|
|
9
|
Ministry of Health and Welfare: 2015
statistics of causes of deathMinistry of Health and Welfare.
Taiwan, R.O.C.: https://www.mohw.gov.tw/cp-3265-30093-2.htmlLast
Updated July 18. 2017
|
|
10
|
Yang X, Xu Z, Zhang C, Cai Z and Zhang J:
Metformin, beyond an insulin sensitizer, targeting heart and
pancreatic β cells. Biochim Biophys Acta. 1863:1984–1990. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murillo-Maldonado JM and Riesgo-Escovar
JR: Development and diabetes on the fly. Mech Dev. 144:150–155.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boldison J and Wong FS: Immune and
pancreatic β cell interactions in type 1 diabetes. Trends
Endocrinol Metab. 27:856–867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kesavadev J: Insulin pump therapy in
pregnancy. J Pak Med Assoc. 66 9 Suppl 1:S39–S44. 2016.PubMed/NCBI
|
|
14
|
Bolinder J, Antuna R, Geelhoed-Duijvestijn
P, Kröger J and Weitgasser R: Novel glucose-sensing technology and
hypoglycaemia in type 1 diabetes: A multicentre, non-masked,
randomised controlled trial. Lancet. 388:2254–2263. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou D, Ye Y, Zou N and Yu J: Analysis of
risk factors and their interactions in type 2 diabetes mellitus: A
cross-sectional survey in Guilin, China. J Diabetes Investig.
8:188–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hotta-Iwamura C and Tarbell KV: Type 1
diabetes genetic susceptibility and dendritic cell function:
Potential targets for treatment. J Leukoc Biol. 100:65–80. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng CA, Chien WC, Hsu CY, Lin HC and
Chiu HW: Risk analysis of carotid stent from a population-based
database in Taiwan. Medicine (Baltimore). 95:e47472016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakai S, Tanimoto K, Imbe A, Inaba Y,
Shishikura K, Tanimoto Y, Ushiroyama T, Terasaki J and Hanafusa T:
Decreased β-cell function is associated with reduced skeletal
muscle mass in japanese subjects without diabetes. PLoS One.
11:e01626032016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rezai S, LoBue S and Henderson CE:
Diabetes prevention: Reproductive age women affected by insulin
resistance. Womens Health (Lond). 12:427–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arias-Loste MT, Ranchal I, Romero-Gómez M
and Crespo J: Irisin, a link among fatty liver disease, physical
inactivity and insulin resistance. Int J Mol Sci. 15:23163–23178.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Skliros NP, Vlachopoulos C and Tousoulis
D: Treatment of diabetes: Crossing to the other side. Hellenic J
Cardiol. 57:304–310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marín-Peñalver JJ, Martín-Timón I,
Sevillano-Collantes C and Del Cañizo-Gómez FJ: Update on the
treatment of type 2 diabetes mellitus. World J Diabetes. 7:354–395.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nenquin M and Henquin JC: Sulphonylurea
receptor-1, sulphonylureas and amplification of insulin secretion
by Epac activation in β cells. Diabetes Obes Metab. 18:698–701.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abdelmoneim AS, Welsh RC, Eurich DT and
Simpson SH: Sulfonylurea use is associated with larger infarct size
in patients with diabetes and ST-elevation myocardial infarction.
Int J Cardiol. 202:126–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Winkler G: Metformin-new data for an
‘old’, but efficient, safe and reliable antidiabetic drug. Orv
Hetil. 157:882–891. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Souza A, Khawaja KI, Masud F and Saif
MW: Metformin and pancreatic cancer: Is there a role? Cancer
Chemother Pharmacol. 77:235–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Chen Q, Li L, Kwong JS, Jia P,
Zhao P, Wang W, Zhou X, Zhang M and Sun X: Alpha-glucosidase
inhibitors and hepatotoxicity in type 2 diabetes: A systematic
review and meta-analysis. Sci Rep. 6:326492016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smith JD, Mills E and Carlisle SE:
Treatment of pediatric type 2 diabetes. Ann Pharmacother.
50:768–777. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahuja V and Chou CH: Novel therapeutics
for diabetes: Uptake, usage trends, and comparative effectiveness.
Curr Diab Rep. 16:472016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salazar JJ, Ennis WJ and Koh TJ: Diabetes
medications: Impact on inflammation and wound healing. J Diabetes
Complications. 30:746–752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bae EJ: DPP-4 inhibitors in diabetic
complications: Role of DPP-4 beyond glucose control. Arch Pharm
Res. 39:1114–1128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bonora E and Cigolini M: DPP-4 inhibitors
and cardiovascular disease in type 2 diabetes mellitus.
Expectations, observations and perspectives. Nutr Metab Cardiovasc
Dis. 26:273–284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
King AJ: The use of animal models in
diabetes research. Br J Pharmacol. 166:877–894. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
O'Brien SP, Smith M, Ling H, Phillips L,
Weber W, Lydon J, Maloney C, Ledbetter S, Arbeeny C and Wawersik S:
Glomerulopathy in the KK. Cg-A(y)/J mouse reflects the pathology of
diabetic nephropathy. J Diabetes Res. 2013:4989252013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ikeda H: KK mouse. Diabetes Res Clin
Pract. 24 Suppl:S313–S316. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iwatsuka H, Shino A and Suzuoki Z: General
survey of diabetic features of yellow KK mice. Endocrinol Jpn.
17:23–35. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Qiao W, Zhao X and Tao M:
Quantitative assessment of the influence of hematopoietically
expressed homeobox variant (rs1111875) on type 2 diabetes risk. Mol
Genet Metab. 102:194–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van Vliet-Ostaptchouk JV, Onland-Moret NC,
van Haeften TW, Franke L, Elbers CC, Shiri-Sverdlov R, van der
Schouw YT, Hofker MH and Wijmenga C: HHEX gene polymorphisms are
associated with type 2 diabetes in the Dutch Breda cohort. Eur J
Hum Genet. 16:652–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pechlivanis S, Scherag A, Mühleisen TW,
Möhlenkamp S, Horsthemke B, Boes T, Bröcker-Preuss M, Mann K, Erbel
R, Jöckel KH, et al: Coronary artery calcification and its
relationship to validated genetic variants for diabetes mellitus
assessed in the Heinz Nixdorf recall cohort. Arterioscler Thromb
Vasc Biol. 30:1867–1872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao J, Deliard S, Aziz AR and Grant SF:
Expression analyses of the genes harbored by the type 2 diabetes
and pediatric BMI associated locus on 10q23. BMC Med Genet.
13:892012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Feuk L, McCarthy S, Andersson B, Prince JA
and Brookes AJ: Mutation screening of a haplotype block around the
insulin degrading enzyme gene and association with Alzheimer's
disease. Am J Med Genet B Neuropsychiatr Genet. 136B:69–71. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bort R, Signore M, Tremblay K, Barbera
Martinez JP and Zaret KS: Hex homeobox gene controls the transition
of the endoderm to a pseudostratified, cell emergent epithelium for
liver bud development. Dev Biol. 290:44–56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hindy G, Mollet IG, Rukh G, Ericson U and
Orho-Melander M: Several type 2 diabetes-associated variants in
genes annotated to WNT signaling interact with dietary fiber in
relation to incidence of type 2 diabetes. Genes Nutr. 11:62016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kommoju UJ, Samy SK, Maruda J, Irgam K,
Kotla JP, Velaga L and Reddy BM: Association of CDKAL1, CDKN2A/B
& HHEX gene polymorphisms with type 2 diabetes mellitus in the
population of Hyderabad, India. Indian J Med Res. 143:455–463.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qian Y, Lu F, Dong M, Lin Y, Li H, Chen J,
Shen C, Jin G, Hu Z and Shen H: Genetic variants of IDE-KIF11-HHEX
at 10q23.33 associated with type 2 diabetes risk: A fine-mapping
study in Chinese population. PLoS One. 7:e350602012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen YT, Liao JW, Tsai YC and Tsai FJ:
Inhibition of DNA methyltransferase 1 increases nuclear receptor
subfamily 4 group A member 1 expression and decreases blood glucose
in type 2 diabetes. Oncotarget. 7:39162–39170. 2016.PubMed/NCBI
|
|
47
|
Steneberg P, Bernardo L, Edfalk S,
Lundberg L, Backlund F, Ostenson CG and Edlund H: The type 2
diabetes-associated gene ide is required for insulin secretion and
suppression of α-synuclein levels in β-cells. Diabetes.
62:2004–2014. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Asrafuzzaman M, Cao Y, Afroz R, Kamato D,
Gray S and Little PJ: Animal models for assessing the impact of
natural products on the aetiology and metabolic pathophysiology of
Type 2 diabetes. Biomed Pharmacother. 89:1242–1251. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kaul N and Ali S: Genes, genetics, and
environment in Type 2 diabetes: Implication in personalized
medicine. DNA Cell Biol. 35:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cornelis MC, Zaitlen N, Hu FB, Kraft P and
Price AL: Genetic and environmental components of family history in
type 2 diabetes. Hum Genet. 134:259–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Flannick J and Florez JC: Type 2 diabetes:
Genetic data sharing to advance complex disease research. Nat Rev
Genet. 17:535–549. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fuchsberger C, Flannick J, Teslovich TM,
Mahajan A, Agarwala V, Gaulton KJ, Ma C, Fontanillas P, Moutsianas
L, McCarthy DJ, et al: The genetic architecture of type 2 diabetes.
Nature. 536:41–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kong X, Zhang X, Xing X, Zhang B, Hong J
and Yang W: The association of Type 2 diabetes loci identified in
genome-wide association studies with metabolic syndrome and its
components in a chinese population with Type 2 diabetes. PLoS One.
10:e01436072015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ma RC, So WY, Tam CH, Luk AO, Ho JS, Wang
Y, Lam VK, Lee HM, Kong AP, Tong PC, et al: Genetic variants for
type 2 diabetes and new-onset cancer in Chinese with type 2
diabetes. Diabetes Res Clin Pract. 103:328–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Klimentidis YC, Lemas DJ, Wiener HH,
O'Brien DM, Havel PJ, Stanhope KL, Hopkins SE, Tiwari HK and Boyer
BB: CDKAL1 and HHEX are associated with type 2 diabetes-related
traits among Yup'ik people. J Diabetes. 6:251–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sanghera DK, Ortega L, Han S, Singh J,
Ralhan SK, Wander GS, Mehra NK, Mulvihill JJ, Ferrell RE, Nath SK
and Kamboh MI: Impact of nine common type 2 diabetes risk
polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2,
TCF7L2 and FTO variants confer a significant risk. BMC Med Genet.
9:592008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Scott LJ, Mohlke KL, Bonnycastle LL,
Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS,
Jackson AU, et al: A genome-wide association study of type 2
diabetes in Finns detects multiple susceptibility variants.
Science. 316:1341–1345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Diabetes Genetics Initiative of Broad
Institute of Harvard and MIT, Lund University, and Novartis
Institutes of BioMedical Research, . Saxena R, Voight BF, Lyssenko
V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S,
Hirschhorn JN, et al: Genome-wide association analysis identifies
loci for type 2 diabetes and triglyceride levels. Science.
316:1331–1336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Malhotra A, Coon H, Feitosa MF, Li WD,
North KE, Price RA, Bouchard C, Hunt SC and Wolford JK: American
Diabetes Association GENNID Study Group: Meta-analysis of
genome-wide linkage studies for quantitative lipid traits in
African Americans. Hum Mol Genet. 14:3955–3962. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kong X, Xing X, Hong J, Zhang X and Yang
W: Genetic variants associated with lean and obese type 2 diabetes
in a Han Chinese population: A case-control study. Medicine
(Baltimore). 95:e38412016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kong X, Xing X, Hong J, Zhang X and Yang
W: Association of a type 2 diabetes genetic risk score with insulin
secretion modulated by insulin sensitivity among Chinese Hans. Clin
Genet. 91:832–842. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sladek R, Rocheleau G, Rung J, Dina C,
Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, et al:
A genome-wide association study identifies novel risk loci for type
2 diabetes. Nature. 445:881–885. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Arterbery AS and Bogue CW: Hhex is
necessary for the hepatic differentiation of mouse ES cells and
Acts via Vegf signaling. PLoS One. 11:e01468062016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Watanabe H, Takayama K, Inamura M,
Tachibana M, Mimura N, Katayama K, Tashiro K, Nagamoto Y, Sakurai
F, Kawabata K, et al: HHEX promotes hepatic-lineage specification
through the negative regulation of eomesodermin. PLoS One.
9:e907912014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hunter MP, Wilson CM, Jiang X, Cong R,
Vasavada H, Kaestner KH and Bogue CW: The homeobox gene Hhex is
essential for proper hepatoblast differentiation and bile duct
morphogenesis. Dev Biol. 308:355–367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Deng XP, Zhao LX, Wang BB, Wang J, Cheng
LF, Cheng Z, Suo PS, Li H and Ma X: The HHEX gene is not related to
congenital heart disease in 296 Chinese patients. World J Pediatr.
9:278–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Foley AC and Mercola M: Heart induction by
Wnt antagonists depends on the homeodomain transcription factor
Hex. Genes Dev. 19:387–396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liu S, Chai J, Zheng G, Li H, Lu D and Ge
Y: Screening of HHEX mutations in chinese children with thyroid
dysgenesis. J Clin Res Pediatr Endocrinol. 8:21–25. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
McLin VA, Rankin SA and Zorn AM:
Repression of Wnt/beta-catenin signaling in the anterior endoderm
is essential for liver and pancreas development. Development.
134:2207–2217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang J, McKenna LB, Bogue CW and Kaestner
KH: The diabetes gene Hhex maintains δ-cell differentiation and
islet function. Genes Dev. 28:829–834. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Rong R, Hanson RL, Ortiz D, Wiedrich C,
Kobes S, Knowler WC, Bogardus C and Baier LJ: Association analysis
of variation in/near FTO, CDKAL1, SLC30A8, HHEX, EXT2, IGF2BP2,
LOC387761, and CDKN2B with type 2 diabetes and related quantitative
traits in Pima Indians. Diabetes. 58:478–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Moore AF, Jablonski KA, McAteer JB, Saxena
R, Pollin TI, Franks PW, Hanson RL, Shuldiner AR, Knowler WC,
Altshuler D, et al: Extension of type 2 diabetes genome-wide
association scan results in the diabetes prevention program.
Diabetes. 57:2503–2510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chiasson JL: Early insulin use in type 2
diabetes: What are the cons? Diabetes Care. 32 Suppl 2:S270–S274.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fonseca VA: Defining and characterizing
the progression of type 2 diabetes. Diabetes Care. 32 Suppl
2:S151–S156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Vigneri R, Goldfine ID and Frittitta L:
Insulin, insulin receptors, and cancer. J Endocrinol Invest.
39:1365–1376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
de Miguel-Díez J, Muñoz-Rivas N,
Jiménez-García R, Hernández-Barrera V, Carrasco-Garrido P, Monreal
M, Jiménez D, Guijarro R and de Andrés López A: Type 2 diabetes is
associated with a higher incidence of hospitalization for pulmonary
embolism in Spain: Analysis of hospital discharge data during
2004–2013. Respirology. 21:1277–1284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yu F, Guo Y, Wang H, Feng J, Jin Z, Chen
Q, Liu Y and He J: Type 2 diabetes mellitus and risk of colorectal
adenoma: A meta-analysis of observational studies. BMC Cancer.
16:6422016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sun R, Liu JP, Gao C, Xiong YY, Li M, Wang
YP, Su YW, Lin M, Jiang AL, Xiong LF, et al: Two variants on T2DM
susceptible gene HHEX are associated with CRC risk in a Chinese
population. Oncotarget. 7:29770–29779. 2016.PubMed/NCBI
|