Introduction
Wegener's granulomatosis (WG) was first described by
German pathologist Dr Friedrich Wegener as rhinogenic
granulomatosis in 1936 (1) and is an
uncommon vasculitis of small and medium-sized arteries (2). WG, which is an angiogenic and multiple
system necrotizing disease involving the upper and lower
respiratory tract and kidneys (3–6), is affected by a number of
factors, including heredity, infection, the immune system and the
environment, and diagnosis is typically confirmed via clinic and
laboratory examinations (7).
Exposure to environmental agents, including cadmium (8), silica, mercury, sand dust and volatile
hydrocarbons, has been reported to lead to the development of WG;
however, no single causative agent has been identified (9). Modern immunosuppressive treatment
methods greatly improve patient outcomes, with the estimated median
survival time increasing to 21.7 years post-diagnosis (10). Patients may develop WG at any age,
with the mean age at diagnosis being 50 year, and males and females
are equally affected (11). The
majority (~90%) of clinically apparent cases are reported in
Caucasian individuals (11).
The thorax is commonly involved in WG and, as such,
the majority of patients present with pulmonary or upper
respiratory tract involvement (12).
Due to its high sensitivity for detecting pulmonary involvement,
computed tomography (CT) is primarily used to examine the chest in
patients with WG (13–15). However, since certain characteristics of
WG are difficult to detect using clinical, laboratory and imaging
examination it is frequently misdiagnosed.
In the present study, the clinical manifestations,
CT results and diagnosis of 45 patients with diagnosed WG were
retrospectively reviewed. The aim of the present study was to
identify ways of decreasing the rate of misdiagnosis and improving
the diagnostic accuracy of WG involving the chest with CT.
Materials and methods
Patients
A total of 45 patients with clinically confirmed WG
were selected retrospectively using a computerized search of
records from patients who were treated at the Chinese PLA General
Hospital (Beijing, China) between January 2011 and June 2013. The
patients, including 24 men and 21 women, were 19–74 years old
(mean, 46±12.2 years). The clinical symptoms were as follows: Fever
accompanied with or without fatigue (n=26, 26/45; 57.78%), night
sweats (n=2, 2/45; 4.44%), weakness (n=2, 2/45; 4.44%), abnormal
myelogram (n=29, 29/45; 64.44%), cough (n=25, 25/45; 55.56%) and
expectoration (n=15, 15/45; 33.33%). A total of 32 patients
underwent screening examination with antineutrophil cytoplasmic
antibody (ANCA) and a total of 28 patients received biopsy,
including a CT-guided percutaneous transthoracic lung biopsy
(n=10), transbronchoscopic biopy (n=1), paranasal sinuses and nasal
biopsy (n=14), percutanenous renal biopsy (n=1), parotid gland
biopy (n=1) and skin biopsy (n=1). The other 17 patients were
diagnosed based on the 1990 American College of Rheumatology
criteria (Table I) (9), when a minimum of 2 items in Table I were met. All patients received
glucocorticosteroid therapy (predisone) at a dosage of 1.0 mg/kg
per day for 4 weeks and then low dose maintenance. Following
treatment, the symptoms of all patients was relieved to varying
degrees. Written consent was obtained from all participants and the
present study was approved by the Ethics Committee of the Hainan
Branch of the Chinese PLA General Hospital (Hainan, China).
 | Table I.American college of rheumatology
diagnostic criteria for Wegener granulomatosis. |
Table I.
American college of rheumatology
diagnostic criteria for Wegener granulomatosis.
| Criteria | Characteristics |
|---|
| Nasal or oral
inflammation | Painful or painless
oral ulcers, purulent or bloody nasal discharge |
| Abnormal chest
radiograph | Nodules, fixed
infiltrates, cavities |
| Urinary sediment | Microhematuria (>5
red blood cells per high-power field), red cell casts |
| Granulomatous
inflammation at biopsy | Involvement of the
wall of an artery/arteriole, involvement of the perivascular/extra
vascular space |
CT technique
In the present study, 43 patients underwent CT
scanning. CT results were not available for the other 2 patients as
they were transferred to a different hospital. CT scans were
performed using a Siemens Sensation Cardiac 64 detector-row CT
scanner (Siemens AG, Munich, Germany). The scan parameters were the
same for each patient: Tube current, 900 mA; tube voltage, 120 kV;
slice thickness, 5.0 mm; matrix, 512×512. The raw data were
reconstructed using 5 mm thickness standard algorithms combined
with a 2 mm thickness high-resolution construction in the region of
interest. The images were mainly reviewed at the lung window
setting with an about 1600 HU of window width and a 600 HU of
window level. All the CT images were simultaneously reviewed by two
independent and experienced chest radiologists who were blinded to
the study conditions.
Results
Involved location of WG patients
System involvement varied between the 45 patients
assessed in the present study and included thoracic involvement
(n=34), renal involvement (n=10), skin involvement (n=7), larger
joint involvement (n=14) and parotid gland involvement (n=1; data
not shown). A total of 6 patients presented with the classic clinic
triad (upper respiratory tract, lung and renal involvement).
Results of laboratory pathology
investigation
Of the 32 patients who underwent ANCA screening
examination (Table II), 27 (84.38%)
were ANCA-positive, which included cytoplasmic ANCA (n=20) and
perinuclear ANCA (n=7). Among the 28 patients who underwent biopsy,
27 patients received a confirmed diagnosis (96.43%).
 | Table II.Clinical and CT manifestations of 45
patients with Wegener granulomatosis. |
Table II.
Clinical and CT manifestations of 45
patients with Wegener granulomatosis.
| Item | Value (%) |
|---|
| General
information |
|
|
Male | 24 (53.33) |
|
Female | 21 (46.67) |
| Age
(years) | 46.4±12.2 |
| Clinical
manifestation |
|
| Fever,
combined ear/eye/mouth and nasal symptoms | 22 (48.89) |
| Fever,
chest tightness, cough/cough blood | 6 (13.33) |
|
Cough/cough sputum,
ear/eye/mouth and nose symptoms, no fever | 13 (28.89) |
|
Cough/cough/blood, no
fever | 4 (8.89) |
| Laboratory
examination |
|
|
Positive ANCA | 27 |
|
Negative ANCA | 1 |
| Thoracic CT
findingsa |
|
|
Nodules: Solid, ground glass
opacity, cavity nodules | 11 (25.58) |
| Patchy
shadows: Ground glass opacity, consolidation, combined cavity | 5 (11.63) |
| Lump,
combined cavity | 1 (2.33) |
| Nodules
combined with patch and/or lump | 12 (27.91) |
|
Intrapulmonary lesion combined
with large airway involvement: diffused tube wall thickening or
intratracheal soft tissue | 2 (4.65) |
| Large
airway involvement: Localized thickening of tracheal wall | 1 (2.33) |
|
Intrapulmonary lesion combined
with pulmonary artery: Tube wall thickening combined with
embolism | 2 (4.65) |
| Normal
thoracic CT findings | 9 (20.93) |
Thoracic CT manifestations
A total of 34 patients presented with abnormal
pulmonary CT findings (Table II),
including large airway involvement (n=1; 2.94%), abnormal lung
findings (n=31; 91.18%) and combined large airway and lung
involvement (n=2; 5.88%). Bilateral lung abnormalities were
observed in 20 patients (58.82%), unilateral lung abnormalities in
13 patients (13/34, 38.24%), pleural alterations combined with
effusion in 4 patients (11.76%) and pulmonary artery involvement in
2 patients (5.88%).
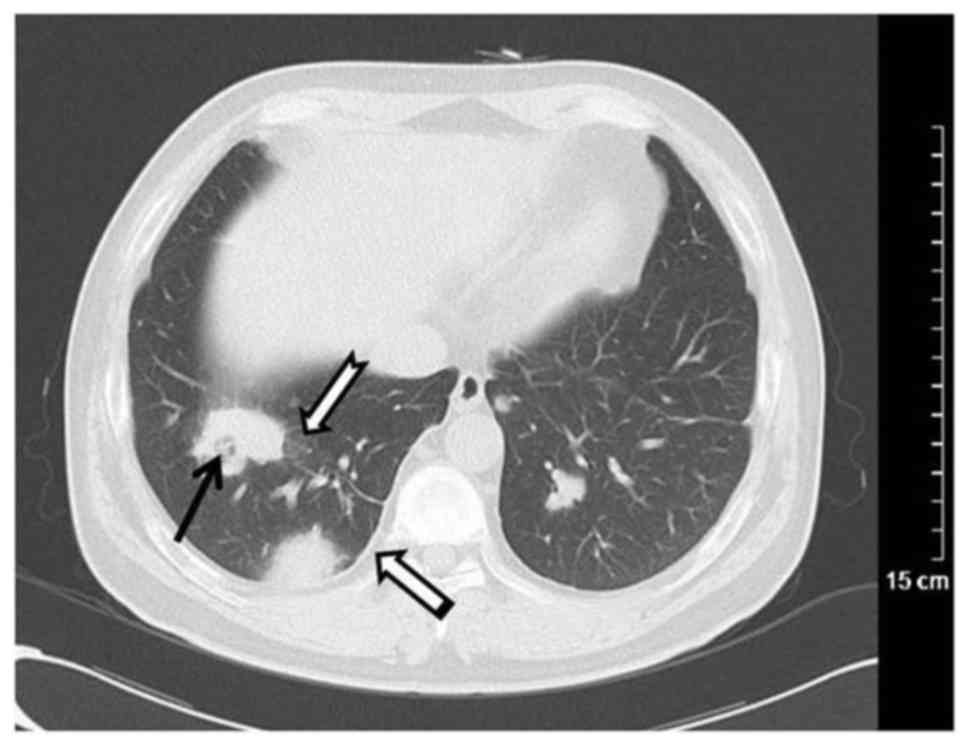
The most common finding was pulmonary nodules
ranging in size from 0.3–3.0 cm, which were detected in 25 patients
(73.53%; Fig. 1), including
unilateral pulmonary nodules (n=7), solitary nodule (n=3),
ground-glass nodules (n=2) and nodules combined with halo sign
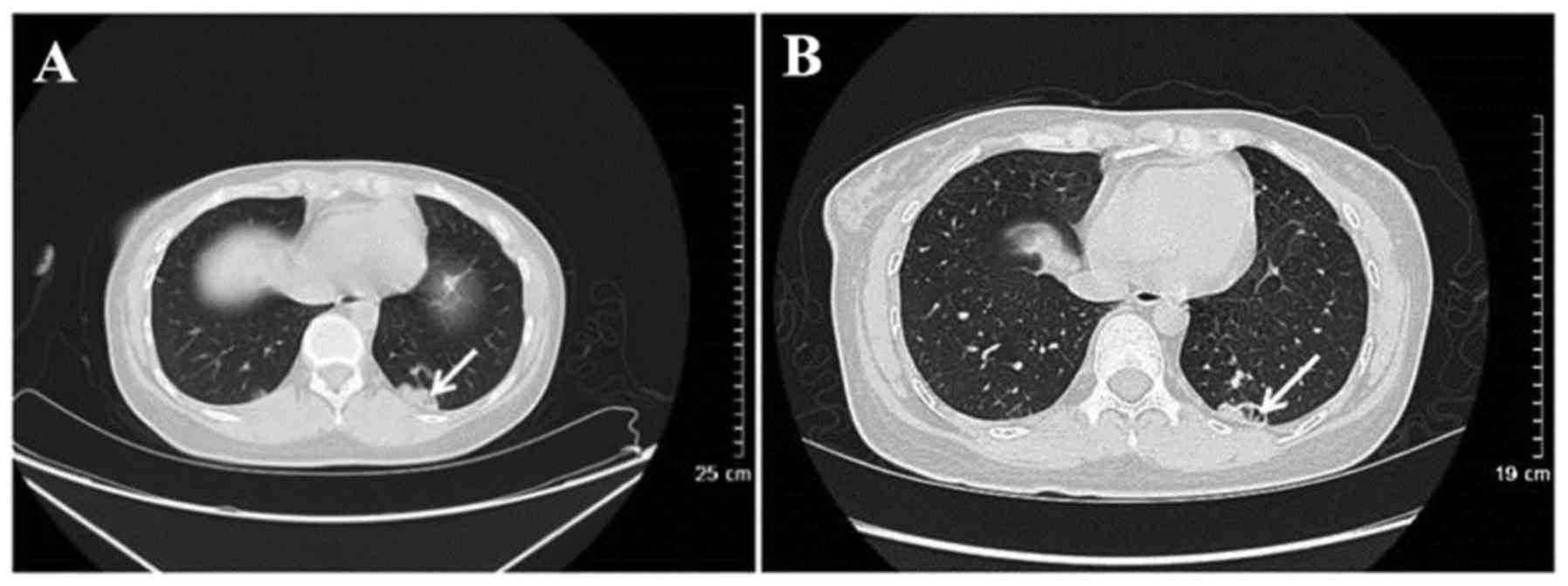
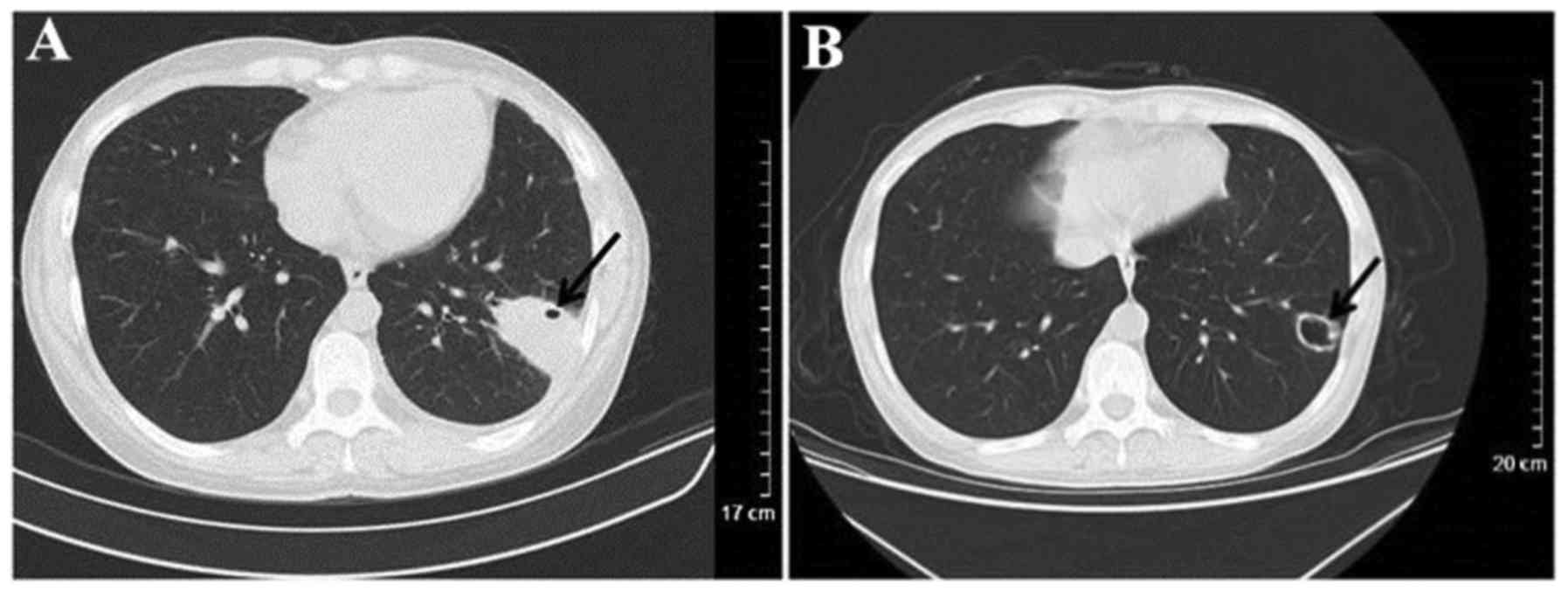
(n=4). CT results for 14 patients revealed cavitary nodules and 5
patients presented with irregular cavity walls combined with
necrotic debris (Fig. 2). Nodules
combined with lobulation, speculation and vacuoles were observed in
5 patients and 1 patient presented with signs of vessel
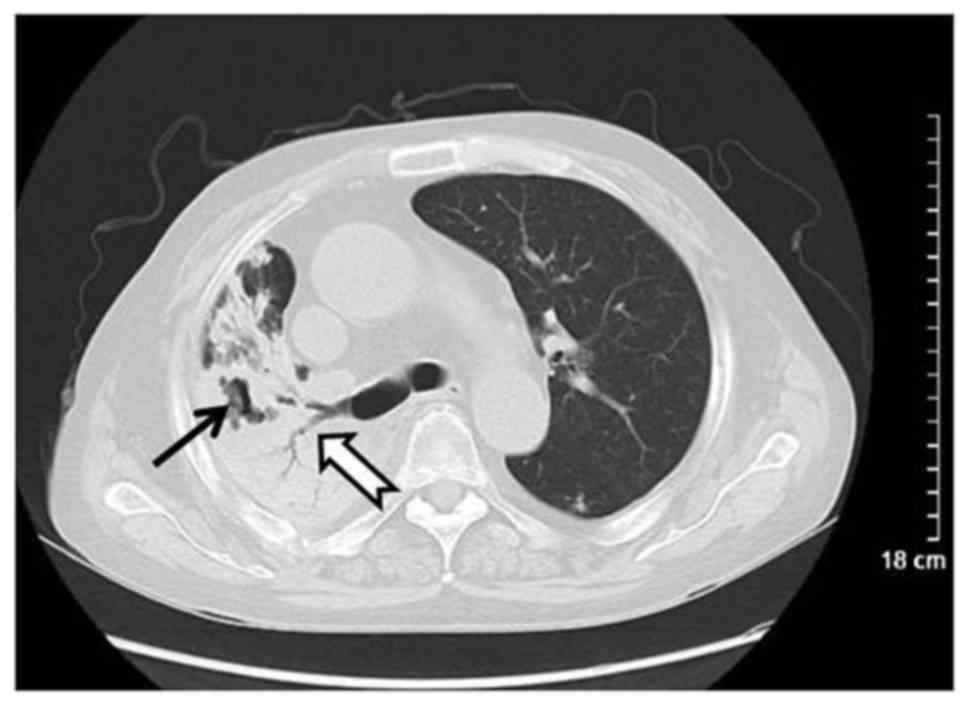
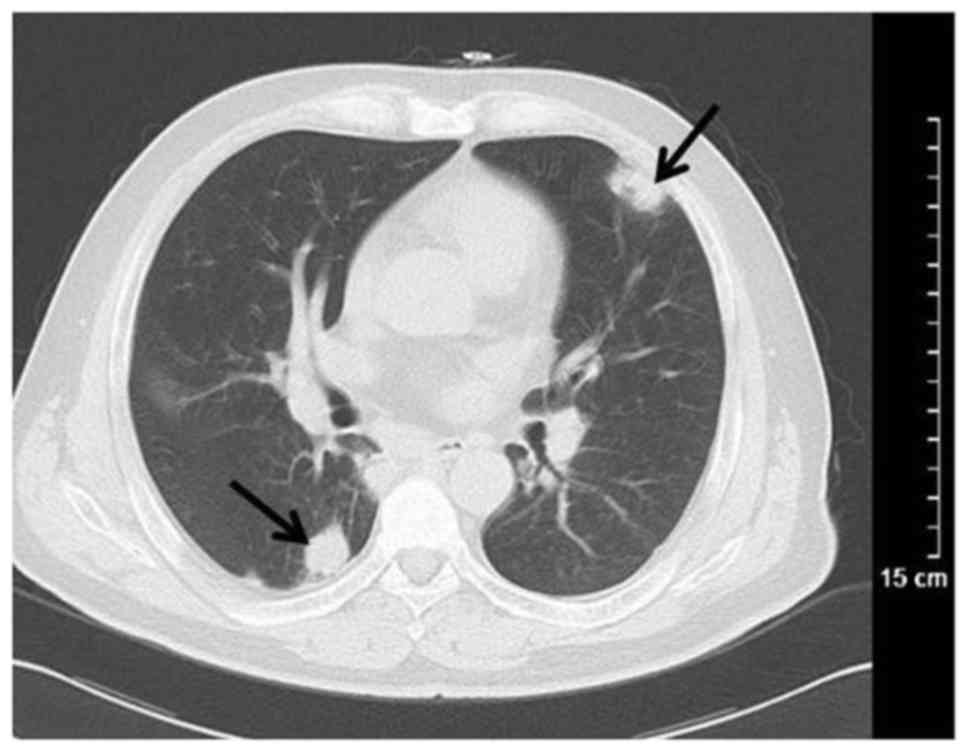
convergence. Additionally, 15 patients exhibited plaques (44.12%)
with ground glass opacity (n=6), pulmonary consolidation (n=6) and
cavitation (n=3; Fig. 3). Masses (≥3
cm) were observed in 6 patients (17.65%) and were combined with
cavitations in 4 patients, had fuzzy limits and irregular shape in
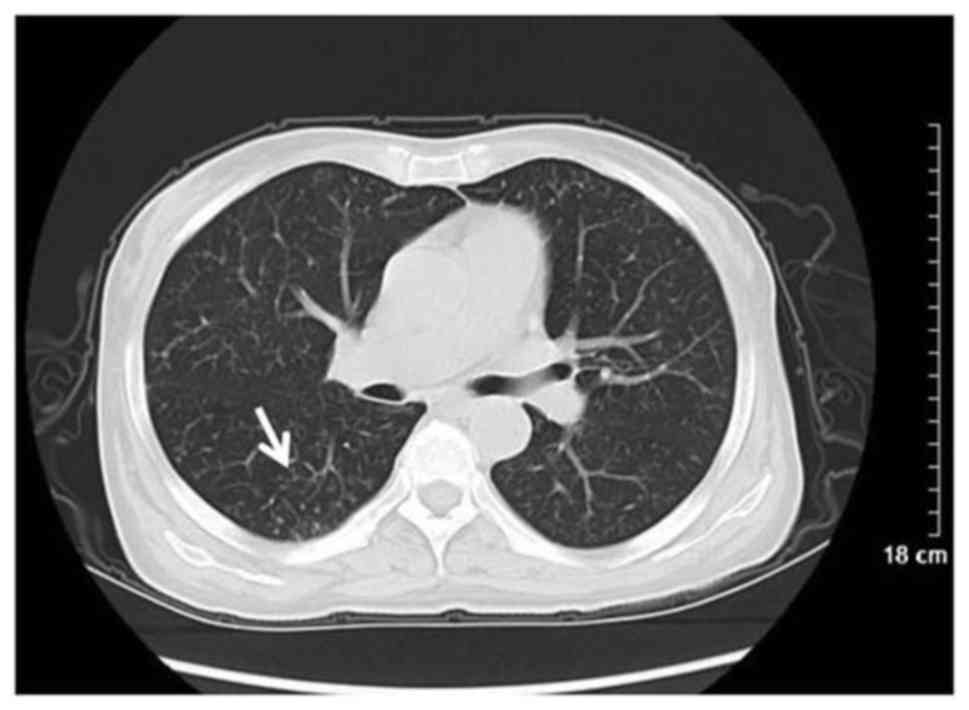
6 patients, and spiculation in 3 patients (Fig. 1). Bilateral pulmonary interstitial
alterations were observed in 1 patient (Fig. 4).
Of the 34 patients who had abnormal pulmonary CT
findings, severe airway alterations were observed in 3 (8.82%).
This includes 1 patient with changes in the vocal cord and
subglottic region, 1 with intratracheal soft tissue shadow and
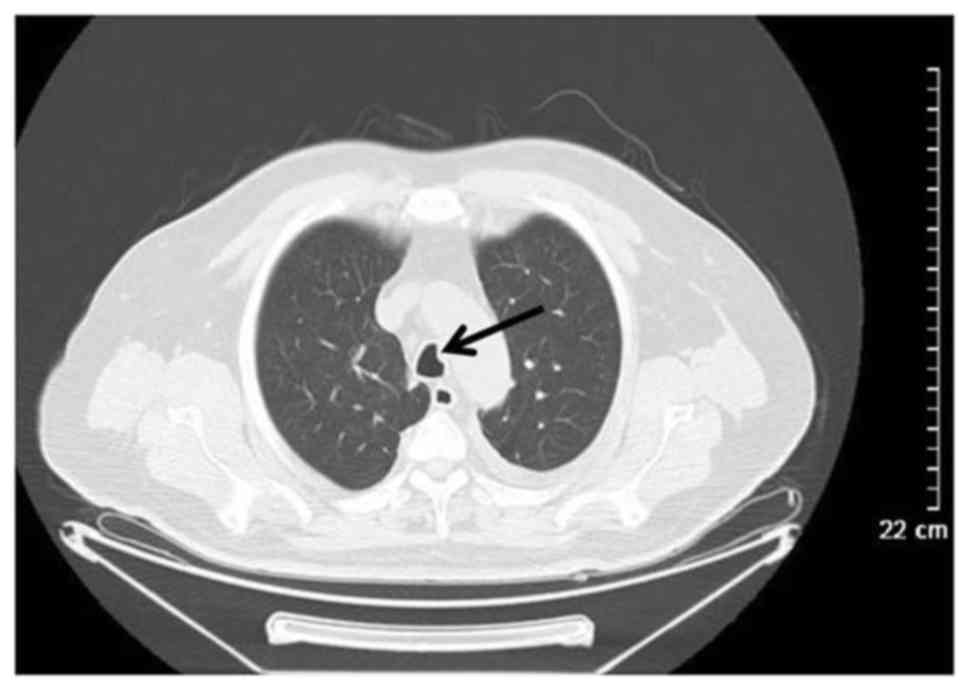
another with bronchial wall thickening (Fig. 5). A total of 2 patients with
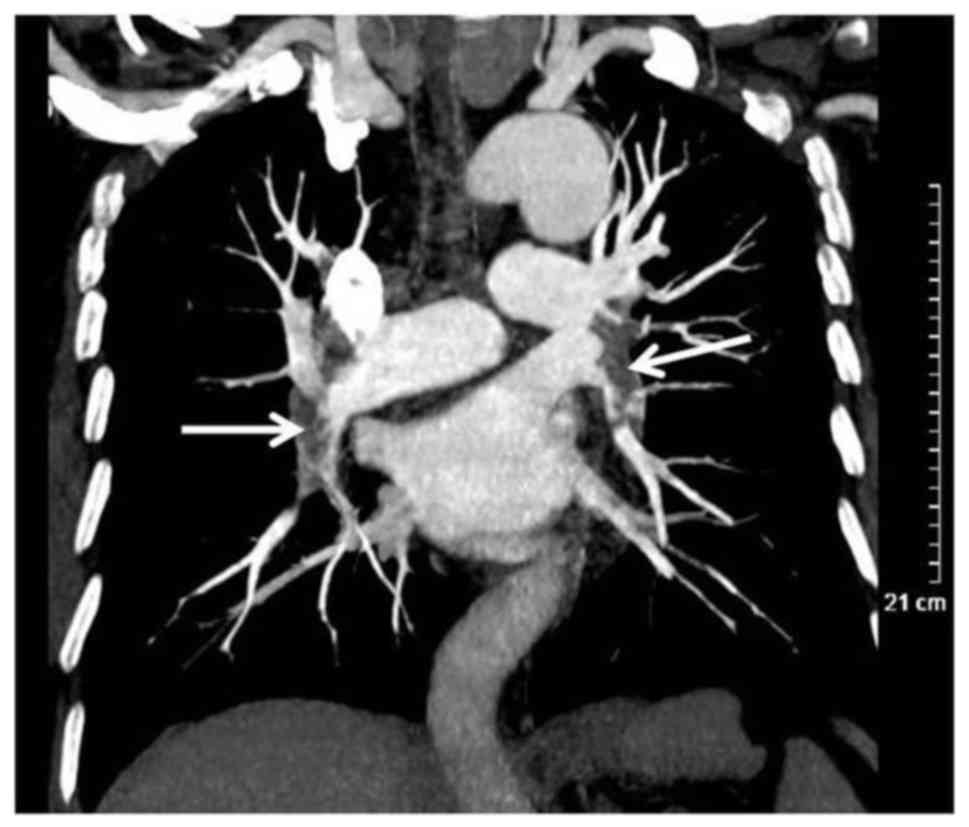
pulmonary artery involvement were demonstrated to have undergone
pulmonary artery alteration, including pulmonary artery wall
thickening, which may lead to pulmonary artery embolism (Fig. 6).
Misdiagnosis based on CT findings
Of the 34 patients with clinically confirmed WG, 12
were diagnosed using CT results (35.29%), 4 patients had
inconclusive diagnoses (11.79%), and 18 patients were misdiagnosed
(52.94%). Patient misdiagnoses were as follows: 12 were diagnosed
with inflammation, including opportunistic infections in 1 patient,
mixed infection in 1 patient, interstitial pneumonia in 1 patient,
pneumonia with lymphocytic infiltrate in1 patient, fungal
infections in 5 patients and viral infection in 3 patients. The CT
images obtained for patients who were misdiagnosed with
inflammation featured ground glass opacity in 3 patients, patchy
shadows in 8 patients, military nodules in 2 patients, pulmonary
interstitial alterations in 1 patient, masses in 2 patients and
pleural effusion in 2 patients. A total of 6 patients were
misdiagnosed with tumors, including combined airway abnormality in
2 patients (airway thickening in 1 patient) and pulmonary
interstitial alterations combined with fungal pneumonia in 1
patient.
In WG with lung involvement there may be various
imaging manifestations. The atypical CT findings of WG were
typically misdiagnosed as infectious lesions or neoplastic lesions.
The irregular cavities were typically observed in patients with WG
(Fig. 7).
CT imaging of pulmonary abscesses usually reveals
thick-walled cavitations based on a high consolidation with the
appearance of liquid-gas level in the cavity, which differs from
WG. Fungal infections vary widely; floating bacteria may be
observed in the void, which is not normally present in WG (16). WG may be misdiagnosed as primary
pulmonary or pulmonary metastatic tumors with single and multiple
solid mass or nodules (17). When WG
appears as a single solid mass, it is not easily distinguished from
other lung tumors using CT alone. There are certain differences
between WG and pulmonary metastasis in CT findings of multiple
nodules (18). It was observed in
the present study that the multiple lung nodules of WG are
typically shown as variable irregular shapes and the shadow
connected to the lesion (Fig. 8).
However, multiple pulmonary metastases are generally located in the
peripheral region of the lung, with a variable size and smooth
boundaries.
Discussion
WG is a rare and angiogenic necrotizing vasculitis,
involving the upper and lower respiratory tract, kidney and skin
(19). The sex distribution
(male:female) of WG is 3:2 and the peak incidence occurs in
patients aged between 50 and 60 years (20). The incidence of WG has been
increasing annually, which may be a result of increased
environmental factors including cadmium, silica, mercury, sand dust
and volatile hydrocarbons (9). WG
may be a type of inflammation resulting from a hypersensitive
response to external antigens (21).
Trimethoprim-sulphamethoxazole has been previously used to
successfully control the progression and relapse of WG (22).
Any part of the body may be involved in WG and the
majority of patients present with classic clinic triad of the upper
respiratory tract, lung and renal involvement (23). While the number of patients with
renal involvement was low in the present study, the ratio of
occurrence was lower in those who presented with the classic clinic
triad. With the progression of the disease, 80–90% of patients with
WG generally present with renal involvement (24). The clinical manifestations of WG are
varied, a number of patients with WG present with long-term nasal
congestion, which is often misdiagnosed as chronic sinusitis
(25,26). Furthermore, some patients present
with acute renal and respiratory failure (27,28).
Patients with WG with thoracic involvement were typically present
with cough, dyspnea, fever and chest pain (29).
WG has been reported to be a type of angiitis
associated with ANCA, in particular cytoplasm ANCA (c-ANCA)
(30). It was reported that the
serum c-ANCA levels during the active phase of WG are elevated in
90% of patients (31). In the
present study, 84.38% patients were ANCA-positive and 74.07%
patients were positive for elevated c-ANCA levels (20/27); However
other diseases, including polyarteritis nodosa, systemic lupus
erythematosus, anaphylactoid purpura and Kawasaki disease may also
present as c-ANCA-positive (9).
Hence, biopsy is the gold standard in the diagnosis of WG (32).
As the majority of WG patients present with upper
respiratory tract involvement and 90% of patients exhibit lung
involvement (33), CT scans are
essential for the diagnosis of WG. Multiple pulmonary nodules and
masses are the most common manifestation of pulmonary involvement,
the incidence rate of which was 40–70% in the present study. The
rate of pulmonary masses was 17.65% (6/34) and the rate of nodules
was 73.53% (25/34). Nodules were usually bilateral, numerous and
scattered, ranging from 2–4 cm (15,34,35). The
nodules in pulmonary involvement were similar, with signs of
pulmonary tuberculosis, allergic reaction and acute bacterial,
viral and fungal pneumonia with centrilobular distribution. The
incidence of cavitation was 25% among patients with nodules >2
cm (15,35). The walls of cavitations, which were
eccentric or nodular, had varying thickness and were smooth or
irregular. In the present study, the chest CT images revealed
cavitation lesions with piecemeal necrosis in certain patients.
Cavitations are susceptible to infection, leading to an increase in
air-fluid levels, which is easily misdiagnosed as metastatic tumor,
pulmonary abscess or septic pulmonary embolism (17). However, flocculent piecemeal fragment
in the cavity of metastatic cavity and pulmonary abscess is rare,
which may be a useful CT sign for the differential diagnosis
between metastatic cavity and pulmonary abscess. Hemorrhage
surrounding the nodules manifested as a halo signal on CT images,
which was visible at traumatic hemorrhage or alveolar exudation,
including bronchioloalveolar carcinoma and abundant vascularity
metastasis. In the present study, nodules with cavitation were
observed in 14 patients, as indicated by increased air-fluid levels
and uneven thickness of cavity walls. A total of 5 patients with
nodules with cavitation were misdiagnosed, with a rate of 35.71%; 4
patients were misdiagnosed with infection, and 1 was misdiagnosed
with a tumor. Nodules with halo signals were observed in 4
patients. The lung parenchyma of patients with WG in the active
stage with hemorrhage resulted in ground glass density and
consolidation, which was misdiagnosed as pneumonia. The involvement
of pulmonary arterioles in WG patents presented as mosaic perfusion
and tree-in-bud (36). Ground glass
density in the lungs of patents with WG may occur secondary to
hemorrhages, exudation and mosaic perfusion of pulmonary alveoli in
small vessel vasculitis (37).
Pulmonary consolidation of patients with WG may be caused by
pulmonary infarction or pulmonary infection due to small vessel
vasculitis (38), which is similar
to bacterial, viral and fungal pneumonia, pulmonary tuberculosis,
pulmonary edema, acute respiratory distress syndrome or
bronchioloalveolar carcinoma (39).
A total of 15 patients presented with ground glass opacity and
consolidation; 6 among them were misdiagnosed with different types
of infection including opportunistic infection in one patient,
lymphocytic interstitial pneumonia in another, tumor in 1 patient
and fungal infection in 3 patients. Airway involvement has been
identified as a late complication of WG, the incidence rate of
which has been reported to be 15–25% (40). Airway involvement in WG has been
misdiagnosed as tracheo-bronchial tumor and amyloidosis (39). The involvement of the heart and large
vessels are rare manifestations of thoracic involvement in WG; 2
patients in the present study presented with damage to pulmonary
arteries, which may be caused by the spread of granuloma. However,
due to the incomplete information, there is a lack of sufficient
evidence to support this point of view.
WG is frequently misdiagnosed as tumor-like lesions,
including metastatic tumors and bronchial lung cancer. WG
presenting with multiple solid nodules has been misdiagnosed as
metastatic tumor; however, CT scans differ from those of patients
with metastatic tumors (41).
Multiple solid nodules of metastatic tumors are always variable in
size and are mainly distributed in the peripheral lung, whereas
multiple solid nodules of WG typically present with irregular
outlines combined with smooth edges, as well as irregular nodules.
CT scans of single nodules or masses in WG are also different in
appearance to scans from patients with bronchial lung cancer
(42). Additionally, the clinical
manifestation and typical laboratory results for WG differ from
tumor-like lesions (41). Hence, CT
scans combined with clinical and laboratory analysis may reduce the
rate of misdiagnosis.
Thorax involvement in WG is common and the CT
manifestations are varied. The present study had several
limitations, including the relatively small sample size. To improve
the accuracy of the diagnosis of WG, it is necessary to perform a
large number of case studies. Another shortcoming of the present
study was the inconsistent diagnostic criteria. Of the 45 patients,
only 28 cases were confirmed histologically, the other 17 cases
were diagnosed clinically based on the American College of
Rheumatology diagnostic criteria for Wegener granulomatosis.
Additionally, another disadvantage was that the present study was
retrospective and selection bias may affect the results.
In summary, WG is a rare disease with unknown
etiology and complex clinical manifestation. Furthermore, due to
difficulties with diagnosis and unfavorable prognosis, early
diagnosis of WG is important to manage the symptoms of WG, prevent
recurrence and improve the survival rate of patients. Chest CT
scans may be used as a basis for the primary diagnosis of WG. The
CT manifestation of pulmonary involvement in WG was multitudinous,
including multiple pulmonary nodules, ground glass opacity and
consolidation, which were misdiagnosed as pneumonia, tumor and
other diseases. The results of the present study suggest that
multiple nodules and cavitations, irregular cavity walls and
piecemeal necrosis in cavitations may be regarded as signs
exclusive to WG. Hence, patients with the multiple solid nodules,
ground glass opacity and consolidation may be diagnosed with WG.
The exclusive signs combined with clinical and laboratory analysis,
particularly negative ANCA, may be used as a basis for the
diagnosis of WG. CT scans combined with clinical and laboratory
analysis and biopsy may be required for the definite diagnosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JKL designed the study, CGL performed the
experiments and JJL analysed the data.
Ethics approval and consent to
participate
Written consent was obtained from all participants
and the present study was approved by the Ethics Committee of the
Chinese PLA General Hospital (Hainan, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wegener F: Über eine eigenartige rhinogene
Granulomatose mit besonderer Beteiligung des Arterien systems und
der Nieren. Beiträge Zur Pathologie. 158:127–143. 1976. View Article : Google Scholar
|
|
2
|
Orden AO, Muñoz SA, Basta MC and Allievi
A: Clinical features and outcomes of 37 Argentinean patients with
severe granulomatosis with polyangiitis (Wegener Granulomatosis). J
Clin Rheumatol. 19:62–66. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bişkin S, Yazici ZM, Kayhan FT and Erdur
Ö: Wegener Granülomatoziste KBB Tutulumu. KBB ve BBC Dergisi.
17:62–65. 2009.
|
|
4
|
Rossini BA, Bogaz EA, Yonamine FK, Testa
JR and Penido Nde O: Refractory otitis media as the first
manifestation of Wegener's granulomatosis. Braz J Otorhinolaryngol.
76:541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scalcon MRR, Pereira IA, Filho AR and
Paiva EDS: Manifestação otológica localizada em paciente com
granulomatose de Wegener Localized otologic manifestation in a
patient with Wegener's granulomatosis. Rev Bras Reumatol.
48:253–255. 2008. View Article : Google Scholar
|
|
6
|
Fonseca FP, Benites BM, Ferrari ALV,
Sachetto Z, de Campos GV, de Almeida OP and Fregnani ER: Gingival
granulomatosis with polyangiitis (Wegener's granulomatosis) as a
primary manifestation of the disease. Aust Dent J. 62:102–106.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bîrluţiu V, Rezi EC, Bîrluţiu RM and
Zaharie IS: A rare association of chronic lymphocytic leukemia with
c-ANCA-positive Wegener's granulomatosis: A case report. World J
Surg Oncol. 14:1452016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gambini G and Leurini D: Cadmium exposure
and Wegener's granulomatosis: Case report. Med Lav. 83:349–351.
1992.PubMed/NCBI
|
|
9
|
Martinez F, Chung JH, Digumarthy SR, Kanne
JP, Abbott GF, Shepard JA, Mark EJ and Sharma A: Common and
uncommon manifestations of Wegener granulomatosis at chest CT:
Radiologic-pathologic correlation. Radiographics. 32:51–69. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wojciechowska J, Krajewski W, Krajewski P
and Kręcicki T: Granulomatosis with polyangiitis in
otolaryngologist practice: A review of current knowledge. Clin Exp
Otorhinolaryngol. 9:8–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olivencia-Simmons I: Wegener's
granulomatosis: Symptoms, diagnosis, and treatment. J Am Acad Nurse
Pract. 19:315–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eustaquio ME, Chan KH, Deterding RR and
Hollister RJ: Multilevel airway involvement in children with
Wegener's granulomatosis: Clinical course and the utility of a
multidisciplinary approach. Arch Otolaryngol Head Neck Surg.
137:480–485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jbara MH, Vanlandingham A, Tawadros F and
Moorman J: Cavitary lung lesion with pulmonary embolism: Case of
active granulomatosis with polyangitis (Wegener's Granulomatosis).
Poster Presentation, no.174Appalachian Student Research Forum. D.P.
Culp Centre at ETSU; Johnson City, TN, USA: April 6–7–2016
|
|
14
|
Xia LL, Radiology DO and Hospital XR:
Clinical evaluation onthe diagnostic value of multi-slice CT in the
diagnosis of pulmonary Wegener's granulomatosis. Qiqihar Da Xue Xue
Bao Yi Xue Ban. 31:192015.
|
|
15
|
Guneyli S, Ceylan N, Bayraktaroglu S,
Gucenmez S, Aksu K, Kocacelebi K, Acar T, Savas R and Alper H:
Imaging findings of pulmonary granulomatosis with polyangiitis
(Wegener's granulomatosis): Lesions invading the pulmonary fissure,
pleura or diaphragm mimicking malignancy. Wien Klin Wochenschr.
128:809–815. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bülbül Y, Ozlü T and Oztuna F: Wegener's
granulomatosis with parotid gland involvement and pneumothorax. Med
Princ Pract. 12:133–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang XL, Tan RS and Liu X: The CT
manifestations analysis of pulmonary Wegener's granulomatosis with
literature review. JPMI. 16:494–497. 2015.
|
|
18
|
Weir IH, Müller NL, Chiles C, Godwin JD,
Lee SH and Kullnig P: Wegener's granulomatosis: Findings from
computed tomography of the chest in 10 patients. Can Assoc Radiol
J. 43:31–34. 1992.PubMed/NCBI
|
|
19
|
Hanisch M, Fröhlich LF and Kleinheinz J:
Gingival hyperplasia as first sign of recurrence of granulomatosis
with polyangiitis (Wegener's granulomatosis): Case report and
review of the literature. BMC Oral Health. 17:332016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wallace ZS, Lu N, Miloslavsky E, Unizony
S, Stone JH and Choi HK: Nationwide trends in hospitalizations and
in-hospital mortality of granulomatosis with polyangiitis
(Wegener's). Arthritis Care Res (Hoboken). 69:915–92. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gerhild TL, PMID and Wegener: Wegener'S
Granulomatosis. Polic. 2012.
|
|
22
|
Kronbichler A, Jayne DR and Mayer G:
Frequency, risk factors and prophylaxis of infection in
ANCA-associated vasculitis. Eur J Clin Invest. 45:346–368. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
White ES and Lynch JP: Pharmacological
therapy for Wegener's granulomatosis. Drugs. 66:1209–1228. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Ding Y, Liu Z, Zhang H, Liu Z and
Hu W: Long-term outcomes in antineutrophil cytoplasmic
autoantibody-positive eosinophilic granulomatosis with polyangiitis
patients with renal involvement: A retrospective study of 14
Chinese patients. BMC Nephrol. 17:1012016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ross JA, Mccune WJ and Sanders G: A
50-year old woman with nasal congestion, cough, and dyspnea.
Allergy Asthma Proc. 34:188–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zycinska K, Straburzynski M, Nitsch-Osuch
A, Krupa R, Hadzik-Błaszczyk M, Cieplak M, Zielonka TM and Wardyn
K: Lund-mackay system for computed tomography evaluation of
paranasal sinuses in patients with granulomatosis and polyangiitis.
Adv Exp Med Biol. 884:13–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaya H, Yilmaz S, Sezgi C, Abakay O,
Taylan M, Sen H, Demir M, Akkurt ZM and Senyigit A: Two cases of
extrapulmonary onset granulomatosis with polyangiitis which caused
diffuse alveolar haemorrhage. Respir Med Case Rep. 13:32–36.
2014.PubMed/NCBI
|
|
28
|
Malavieille F, Page M, Ber CE, Christin F,
Bonnet A and Rimmele T: The acute pulmonary renal syndrome: An
unusual presentation of granulomatosis with polyangiitis. Rev Mal
Respir. 31:636–640. 2014.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang CY, Liu CT, Wang YJ and Li TZ:
Clinical data analysis and chest radiographic features of Wegener's
granulomatosis with pulmonary involvement. Nan Fang Yi Ke Da Xue
Xue Bao. 30:786–788. 2010.(In Chinese). PubMed/NCBI
|
|
30
|
Gaffo AL: Diagnostic approach to
ANCA-associated Vasculitides. Rheum Dis Clin North Am. 36:491–506.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vega LE and Espinoza LR: Predictors of
poor outcome in ANCA-associated vasculitis (AAV). Curr Rheumatol
Rep. 18:702016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawakami T, Shimosaka R, Takeuchi S and
Soma Y: Importance of appropriate location and frequency of biopsy
for cutaneous manifestations in eosinophilic granulomatosis with
polyangiitis. Int J Dermatol. 55:1388–1390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu XL, Song W, Sui X, Song L, Qn DU and
Wang X: Radiological and clinical features of eosinophilic
granulomatosis with polyangiitis. Zhongguo Yi Xue Ke Xue Yuan Xue
Ba. 38:617–620. 2016.
|
|
34
|
De Geeter F and Gykiere P: (18)F-FDG
PET/CT imaging in granulomatosis with polyangiitis. Hell J Nucl
Med. 19:5–6. 2016.PubMed/NCBI
|
|
35
|
Feragalli B, Mantini C, Sperandeo M,
Galluzzo M, Belcaro G, Tartaro A and Cotroneo AR: The lung in
systemic vasculitis: Radiological patterns and differential
diagnosis. Br J Radiol. 89:201509922016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Castañer E, Alguersuari A, Andreu M,
Gallardo X, Spinu C and Mata JM: Imaging findings in pulmonary
vasculitis. Semin Ultrasound CT MR. 33:567–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mahajan V, Whig J, Kashyap A and Gupta S:
Diffuse alveolar hemorrhage in Wegener's granulomatosis. Lung Indi.
28:52–55. 2011. View Article : Google Scholar
|
|
38
|
Lohrmann C, Uhl M, Kotter E, Burger D,
Ghanem N and Langer M: Pulmonary manifestations of wegener
granulomatosis: CT findings in 57 patients and a review of the
literature. Eur J Radiol. 53:471–477. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ananthakrishnan L, Sharma N and Kanne JP:
Wegener's granulomatosis in the chest: High-resolution CT findings.
AJR Am J Roentgenol. 192:676–682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rodrigues AJ, Jacomelli M, Baldow RX,
Barbas CV and Figueiredo VR: Laryngeal and tracheobronchial
involvement in Wegener's granulomatosis. Rev Bras Reumatol.
52:231–235. 2012.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Uppal S, Saravanappa N, Davis JP, Farmer
CK and Goldsmith DJ: Pulmonary Wegener's granulomatosis
misdiagnosed as malignancy. BMJ. 322:89–90. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lynch JP III and Tazelaar H: Wegener
granulomatosis (granulomatosis with polyangiitis): Evolving
concepts in treatment. Semin Respir Crit Care Med. 32:274–297.
2011. View Article : Google Scholar : PubMed/NCBI
|