Introduction
Currently, adipose tissue is considered an endocrine
organ which synthesizes a variety of adipokines and chemokines that
are released into the circulation to exert their effects on various
tissues.
Similar to sex-specific differences in body fat
distribution, differential plasma concentrations of various
adipokines have been reported. It is well known that from the
beginning of puberty, sex-specific differences in plasma levels of
leptin, adiponectin and ghrelin prevail, with these values being
higher in women than in men (1,2).
Lipocalin-2 (LCN2), also known as neutrophil
gelatinase-associated LCN, has drawn the attention of numerous
researchers due to its implication in metabolic pathologies
(3). LCN2 is a member of the LCN
superfamily comprised of small secreted proteins, characterized by
the presence of three conserved motifs, constituting a single
eight-stranded anti-parallel beta-barrel similar to a calyx that
has the ability to bind organic ligands, specific cell surface
receptors or to form complexes with soluble macromolecules. These
three specific features confer a vast functional diversity. Thus,
LCNs are involved in different roles, including retinol transport,
cryptic coloration, olfaction, pheromone transport and enzymatic
synthesis of prostaglandins. They are also implicated in the
regulation of the immune response and cell homeostasis (4).
The human LCN2 gene is located on chromosome 9q34
and comprises a 3,696-bp coding region, which contains seven exons
and six introns (5). The
corresponding protein is a 25-kDa secreted glycoprotein, initially
identified in neutrophils covalently linked to matrix
metalloproteinase (MMP)-9. It is also present as a 46-kDa
disulphide-linked homodimer and a 135-kDa disulphide-linked
heterodimer (6). This adipokine
binds to a specific cell surface receptor, the 24p3 receptor
(24p3R), in order to be internalized into the cell to regulate
various physiological processes, including iron delivery or uptake,
and cellular apoptosis (7). The
corresponding rat gene, designated as LCN2, 24p3 or Sip24, is
located on chromosome 3p12, and encodes an mRNA of 596 bp with only
one exon and a 24-kDa protein with 198 amino acids (8).
Clinical studies have reported an association of
high circulating levels of LCN2 with obesity and insulin resistance
(9). Conversely, others have
demonstrated a reduction of LCN2 levels in obese and non-obese
diabetic individuals, or in women with polycystic ovarian syndrome
(10,11); however, in these two studies, cardiac
alterations were present in the patients analyzed. Regarding the
latter, it is worth mentioning that this adipokine is an important
modulator of inflammation (12).
Therefore, it may be suggested that this ambivalence in the
reduction or increment of LCN2 levels within cardiometabolic
alterations depends on the level of inflammation due to the disease
stage and whether or not such disease has been controlled. In this
context, a previous study by our group reported a statistically
significant decrease in plasma levels of LCN2 in Mexican patients
with type 2 diabetes mellitus in comparison with those in control
subjects (13). Another previous
study by our group reported sex-associated differences in LCN2
plasma levels in healthy individuals (14). However, few studies have assessed the
possibility of sex-specific differences in the levels of LCN2,
either in its circulating concentration or its expression in other
organs (13–15). To the best of our knowledge, LCN2 has
not been previously determined within the gonads.
In order to identify differentially expressed genes
in the neonatal murine ovary, a previous study by our group
employed a DNA microarray to interrogate the mouse genome,
identifying the LCN2 gene within a cluster of DNA sequences whose
expression profiles were increased during the first 4 postnatal
days, when folliculogenesis takes place (16). This result, as well as the fact that
the murine LCN2 gene contains estrogen recognition sites within its
promoter region (17), suggested the
presence of LCN2 protein in the gonads and its regulation by
hormones, and therefore its sex-specific differential
expression.
Taking this into account, the present study analyzed
the mRNA expression levels of LCN2 and its receptor 24p3R, as well
as the respective protein profiles in ovaries and testes of
wild-type rats.
Materials and methods
Animals
Animal experiments were performed using Sprague
Dawley rats obtained from an inbred colony at the National Medical
Center, Mexican Social Security Institute (México City, México). A
total of 10 female and 10 male rats (3 months old and 200–250 g)
were housed under a 12-h light/dark cycle, temperature of 21±2°C
and 60% humidity, and were given free access to rodent chow and tap
water (5008 Formulab Diet; PMI Nutrition International, Brentwood,
MO, USA). The experimental protocol was approved by the Research
Committees of the National Medical Center and the National
Autonomous University of Mexico (México City, México; approval no.
UNAM-003-2013), and was performed in accordance with the American
Association for Accreditation of Laboratory Care and National
Institutes of Health guidelines.
Following an adaptation period, each of ten female
rats was mated with a male. The presence of vaginal plugs was
examined the morning after mating. Confirmation of a vaginal plug
was designated as postcoitum day 1 (1 dpc). Likewise, the day of
birth was designated as postnatal day 0 (0 dpn). Following birth,
offspring from the 10 pregnant rats were weighed and, in order to
assure adequate and standardized nutrition until weaning, litter
sizes were normalized to eight pups per litter (4 females and 4
males). The remaining pups of each litter were sacrificed by
decapitation immediately following birth. Maternal animals were fed
ad libitum during lactation. Each litter was weighed
weekly.
A total of 3 pregnant rats were sacrificed at 21
dpc, and the ovaries and testes from the 12 female and 12 male pups
were collected. Ovaries and testes from 4 females and 4 males pups
at each of the following time points: 0, 2, 4, 6, 12, 20 and 30
dpn, were also collected immediately after pups were sacrificed by
decapitation. Upon collection, gonadal tissue was either frozen on
dry ice for RNA isolation or fixed for immunohistochemistry.
RNA isolation
Total RNA was isolated using the RNeasy Mini kit
(Qiagen, Hilden, Germany) following the manufacturer's
instructions. In brief, the tissue was homogenized in TRI reagent
(Molecular Research Center, Cincinnati, OH, USA), and the aqueous
and organic phases were separated by addition of one volume of
bromo-3-chloropropane (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), followed by centrifugation at 13, 800 × g for 15 min at
4°C. Thereafter, 70% ethanol (350 µl) was added to each sample,
which was then applied to an RNeasy minicolumn (Qiagen), followed
by washing by centrifugation at 735 × g for 2 min at room
temperature with buffers containing guanidine and ethanol. To elute
the RNA, 30 µl RNase-free water was added directly onto the
silica-gel membrane of each of the columns, which were then
centrifuged for 1 min at 13, 800 × g at room temperature. The RNA
was quantified by measuring the absorbance at 260 nm and stored at
−85°C until use. The quality of each RNA sample was assessed on a
2% formaldehyde denaturing agarose gel.
Semi-quantitative reverse
transcription polymerase chain reaction (RT-PCR)
To assess the relative expression of LCN2 and 24p3R
mRNA in ovaries and testes collected at the different stages
mentioned above, total RNA from all samples was first
reverse-transcribed using the Superscript First-Strand kit
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
following the manufacturer's instructions. All reactions were
performed in a total volume of 20 µl. Initially, 200 ng total RNA,
isolated from the gonads, was annealed at 65°C for 5 min in the
presence of 0.5 µg oligo (dT) 12–18 primer (0.5 µg/µl) and 1 µl
dinucleoside triphosphate (dNTP) cocktail (10 mM). The annealed
RNA-primer samples were incubated for 1 h at 42°C with RT buffer
(10X), MgCl2 (25 mM), RNaseOUT (40 U/µl) and 50 U
Superscript II reverse transcriptase (50 U/µl). Reactions were
terminated by incubation at 70°C for 15 min, followed by incubation
at 37°C for 20 min with 2 U of Escherichia coli RNase H (2
U/µl).
PCR amplification was performed using the QuantumRNA
18S Internal Standard kit (Ambion; Thermo Fisher Scientific, Inc.)
in a total volume of 25 µl, containing 2.5 µl 10X PCR buffer, dNTPs
(0.1 mM) and 0.15 µl Taq polymerase (5 U/µl; HotStar Taq; Qiagen)
plus 2.5 µl of a mixture of 18S primers/competimers at a ratio of
3:7 and 1 µl complementary (c)DNA template annealed to 10 pmol of
LCN2- and 24p3R-specific primers (Table
I).
 | Table I.Primers complementary to LCN2, 24p3R
and 18s genomic sequences used for complementary DNA
amplification. |
Table I.
Primers complementary to LCN2, 24p3R
and 18s genomic sequences used for complementary DNA
amplification.
| Gene
name/direction | Sequence
(5′-3′) | Product length
(bp) |
|---|
| LCN2 |
|
|
|
Forward |
TCTCGATTCCGTCGGGTGGTGG | 592 |
|
Reverse |
CCTGGGTGTCCTGTGTCTG |
|
| 24p3R |
|
|
|
Forward |
AGGACTGGGACTACAACGGA | 507 |
|
Reverse |
GTGCGGACTCCAGAAACAGA |
|
| 18s |
|
|
|
Forward |
TCTCGATTCCGTCGGGTGGTGG | 360 |
|
Reverse |
CTTATGACCCGCACTTACTCG |
|
The PCR conditions used for LCN2, 24p3R and 18s
amplification were 5 min at 94°C to activate the HotStar Taq
enzyme, followed by 35 cycles of 1 min of denaturation at 94°C, 1
min of annealing at 60°C and 1 min of extension at 72°C, followed
by a 10-min final extension at 72°C. In all PCR experiments, a
reaction with all PCR components with the exception of DNA was used
as a negative control. Equal volumes of PCR products were
electrophoresed on 2% agarose gels stained with ethidium bromide.
Subsequently, the gels were scanned and the images were quantitated
by densitometry using image analysis software (Quantity One;
version 4.6.9; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
In order to verify LCN2 and 24p3R cDNA
amplification, purified samples were sequenced on an Applied
Biosystems DNA Sequencer model 377, using the Big Dye™
Terminator Sequencing Ready Reaction kit version 3.1 (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Sequencing was
performed following the protocol supplied by the manufacturer.
Sequencing results were compared against the GenBank sequence
database by means of the Basic Local Alignment Search Tool
algorithm of the National Center for Biotechnology Information
(https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Western blot analysis
To determine the protein levels of LCN2 and 24p3R in
rat gonadal tissue, total protein extracts were obtained from
ovaries, testes and kidneys of adult wild-type rats by
homogenization using a low-ionic-force buffer containing
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (10 mM),
MgCl2 (1.5 mM) and KCl (10 mM), supplemented with
aprotinin (10 µg/ml), penylmethane sulfonylfluoride (1 mM),
dithiothreitol (0.5 mM) and the protease-inhibitor
1,10-phenantroline (10 mM; Sigma-Aldrich; Merck KGaA). Protein
concentrations were determined using the Bradford assay (Bio-Rad
Laboratories, Inc.) and thereafter, proteins were denatured at 37°C
for 30 min, and 50 µg/well of denatured protein was electrophoresed
on a 10% Tris-Glycine SDS-PAGE gel, transferred onto an Immobilon-P
membrane (EMD Millipore, Billerica, MA, USA) and blocked at 4°C for
1 h, with freshly prepared solution of 5% bovine serum albumin
(BSA; Bio-Rad Laboratories, Inc.) in a buffer containing 10 mM Tris
(pH 8.0), 150 mM NaCl and 0.05% Tween 20. The membrane was
immunoblotted overnight at 4°C with gentle agitation using either a
rat polyclonal antibody against LCN2 or against 24p3R (cat. no.
ab41105 and ab124506, respectively; Abcam, Cambridge, MA, USA)
diluted at 1:200, followed by incubation with an anti-goat
horseradish peroxidase-conjugated immunoglobulin (Ig)G (1:15,000
dilution; cat. no. 305035003; Jackson Immuno-Research, West Grove,
PA, USA) for 1 h at room temperature. After stripping the membrane
for 2 h at 55°C in a 62.5 mM Tris-HCl buffer (pH 6.8) containing
100 mM 2-mercaptoethanol and 2% SDS, the membrane was re-blocked
and re-probed using an anti-GAPDH mouse monoclonal antibody (cat.
no. MAB374; Merck KGaA, Darmstadt, Germany) at 1:10,000 dilution
overnight at room temperature and detected with an anti-mouse
HRP-conjugated IgG (cat. no. 115035003; Jackson Immuno-Research) at
a dilution of 1:15,000 at room temperature for 2 h. The reaction
was visualized after 6 min of exposure with enhanced
chemiluminescence reagents (Perkin Elmer; Thermo-Fisher Scientific,
Inc.). 293 cells (American Type Culture Collection, Manassas, VA,
USA) were used as a negative control for LCN2 signaling. Protein
extracts from 293 cells were obtained following the culturing of
the cells in 75 cm2 culture plates (Corning
Incorporated, Corning, NY, USA) containing high glucose Dulbecco's
modified Eagle medium, 5% fetal bovine serum, 2.0 mM L-glutamine,
50 IU/ml penicillin and 100 µg/ml streptomycin (all Gibco; Thermo
Fisher Scientific, Inc.). The cells were cultured in 5%
CO2 at 37°C for 24 h.
Immunohistochemistry
In order to determine LCN2 and 24p3R protein
signaling within gonads of wild-type rats, 5-µm sections of
formalin-fixed, paraffin-embedded gonadal samples collected from
30-day-old rats were obtained and mounted on glass slides
previously coated with poly-L-lysine, and then deparaffinized and
rehydrated in a graded series of ethanols (100, 90, 70 and 30% and
water). Sections were then microwave-heated with antigen retrieval
solution (Vector Laboratories, Burlingame, CA, USA), rinsed in 1X
PBS (pH 7.4) and incubated for 30 min in 3%
H2O2 in methanol to inactivate endogenous
peroxidase, and subsequently blocked with 10% BSA in 1X PBS for 30
min. Tissues were then incubated with either primary rat anti-LCN2
or rat anti-24p3R antibody (dilution, 1:150; Abcam) at 4°C
overnight. Sections were washed in PBS, incubated at room
temperature for 2 h with the Mouse/Rabbit Immunodetector
HRP/diaminobenzidine (DAB; Bio SB, Inc., Goleta, CA, USA) and
washed with 1X PBS. The peroxidase reaction was developed with DAB
and H2O2 generating a brown precipitate.
Finally, slides were counterstained with hematoxylin, dehydrated
and mounted with synthetic resin. Sections of uterus collected from
the same wild-type female rats were used as a positive control. The
negative control was treated with BSA in PBS instead of primary
antibody.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Comparisons between two groups were made by an unpaired
two-tailed Student's t-test. Comparisons between the mRNA
expression levels in ovaries and testicles were made by two-way
analysis of variance followed by Tukey's post hoc test. Statistical
analyses were performed using GraphPad Prism 4 for Windows
(GraphPad Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference. To obtain
significant results 80 animals were used with 8 per stage.
Results
LCN2 mRNA expression profiling in rat
gonads
To determine LNC2 mRNA expression profiling during
ovarian and testicular development in wild-type rats, RT-PCR using
a set of primers that amplified a 592-bp fragment of the LCN2
sequence (Table I) was performed. As
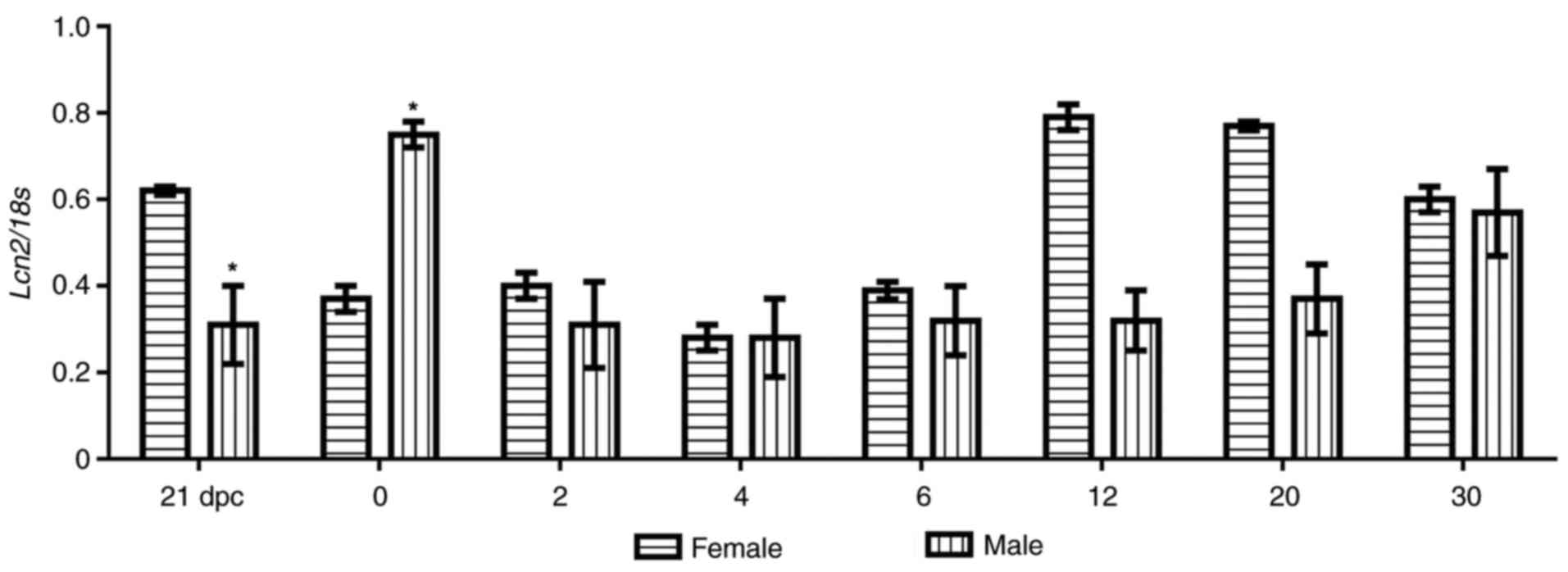
presented in Fig. 1, in rat ovaries,
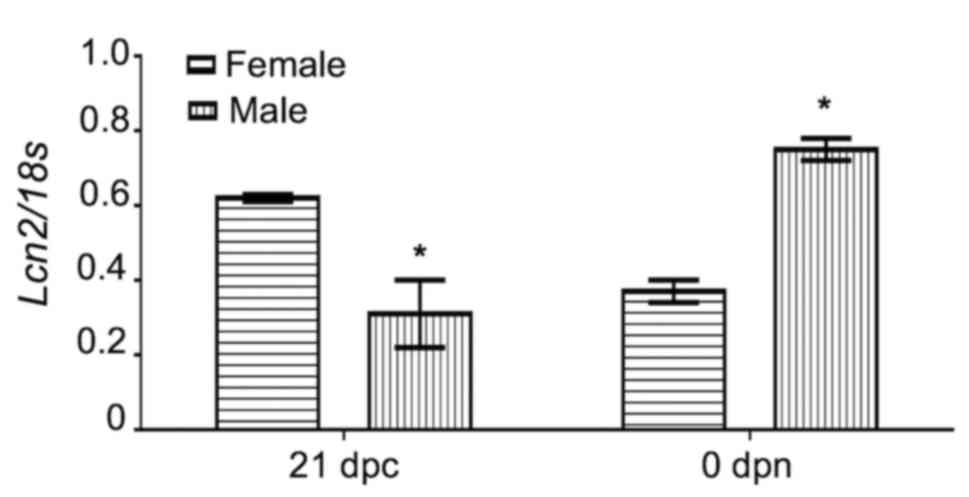
the relative expression of LCN2 mRNA was abundant at 21 dpc, but
significantly decreased by ~50% at <24 h after birth
(P<0.05), increased again at 12 and 20 dpn and stayed at this
relatively high level until 30 dpn, when LCN2 mRNA expression
decreased slightly. Conversely, in testicles, the LCN2 levels were
low at 21 dpc, the mRNA was then abundant at <24 h postpartum
(P<0.05), but from the second postnatal day onwards, its
expression decreased again until 30 dpc, when LCN2 was expressed at
approximately the same rate in the gonads from male and female
animals (Fig. 1). These changes
suggest that LCN2 mRNA expression in perinatal and pre-pubertal
gonads exhibits a sex-specific pattern (Figs. 1 and 2). This also raised the question as to
whether this specific pattern of expression is mediated via 24p3R.
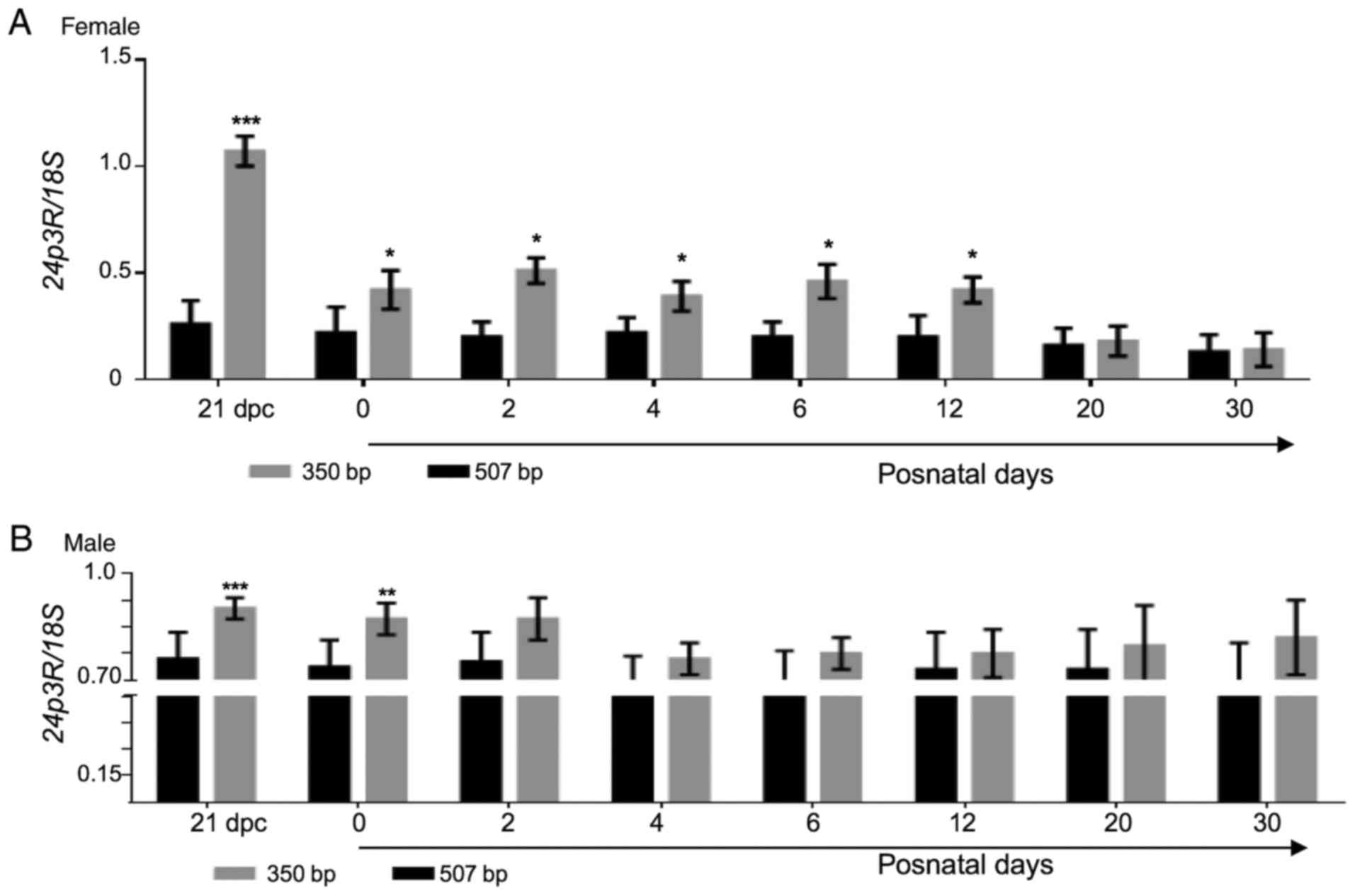
To address this, another RT-PCR assay was performed to amplify the
507-bp fragment of the 24p3R mRNA sequence, employing a specific
set of primers (Table I).
Electrophoresis of the PCR products revealed the presence of the
intended 507-bp band, as well as a lower-size band (350 bp). The
507-bp fragment exhibited a constant relative expression throughout
the stages analyzed, being much lower in the female samples than in
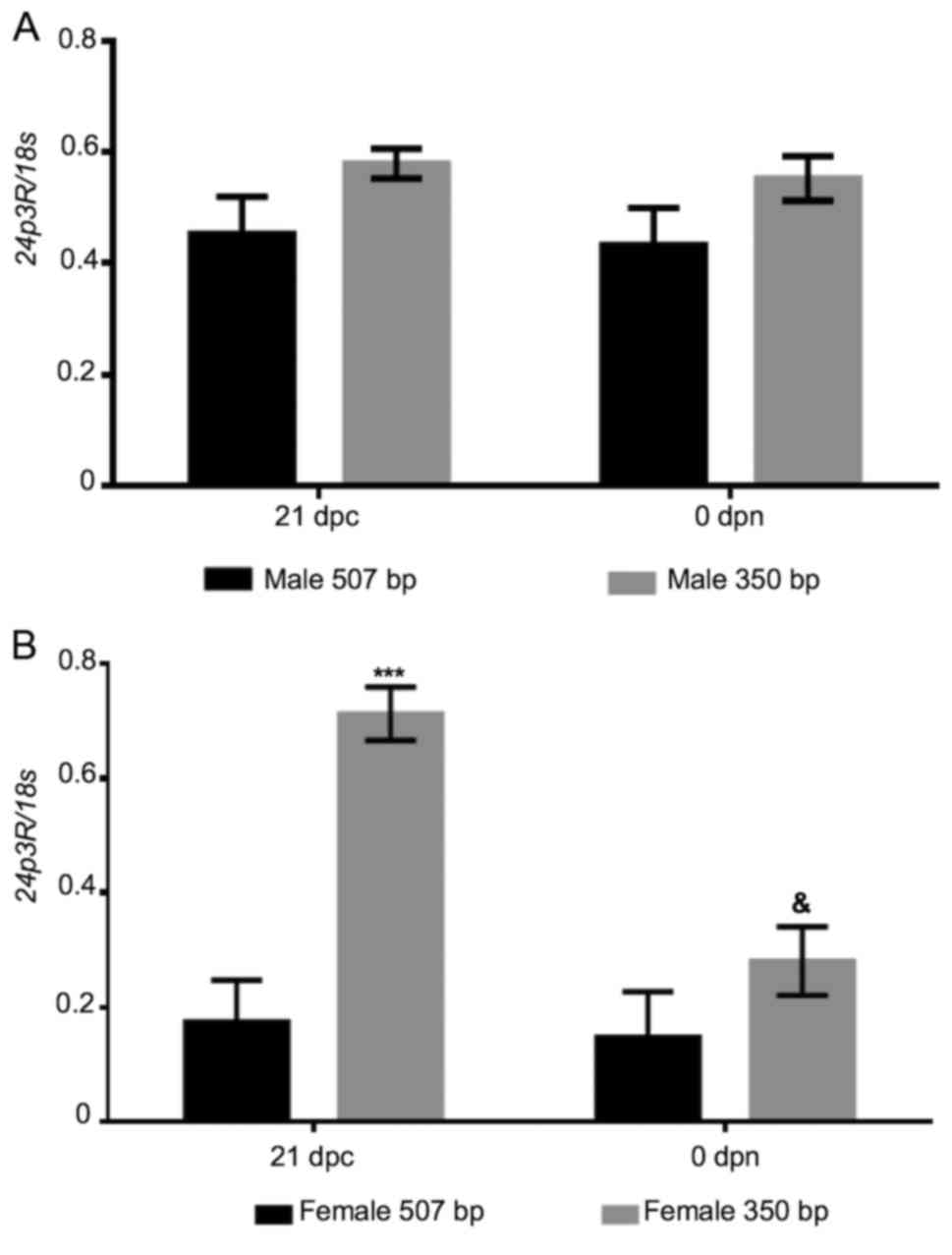
the male ones (Fig. 3). Of note,
only in perinatal ovarian samples, the relative expression of the
small fragment of 24p3R cDNA (350 bp) was similar to that of LCN2
at the same perinatal stage. In other words, as that of LCN2, the
relative expression of 24p3R was high at 21 dpc and decreased hours
after birth (P≤0.05; Fig. 4). As a
positive control, a portion of the ubiquitous 18s gene (360 bp)
obtained from the same cDNA samples was also amplified.
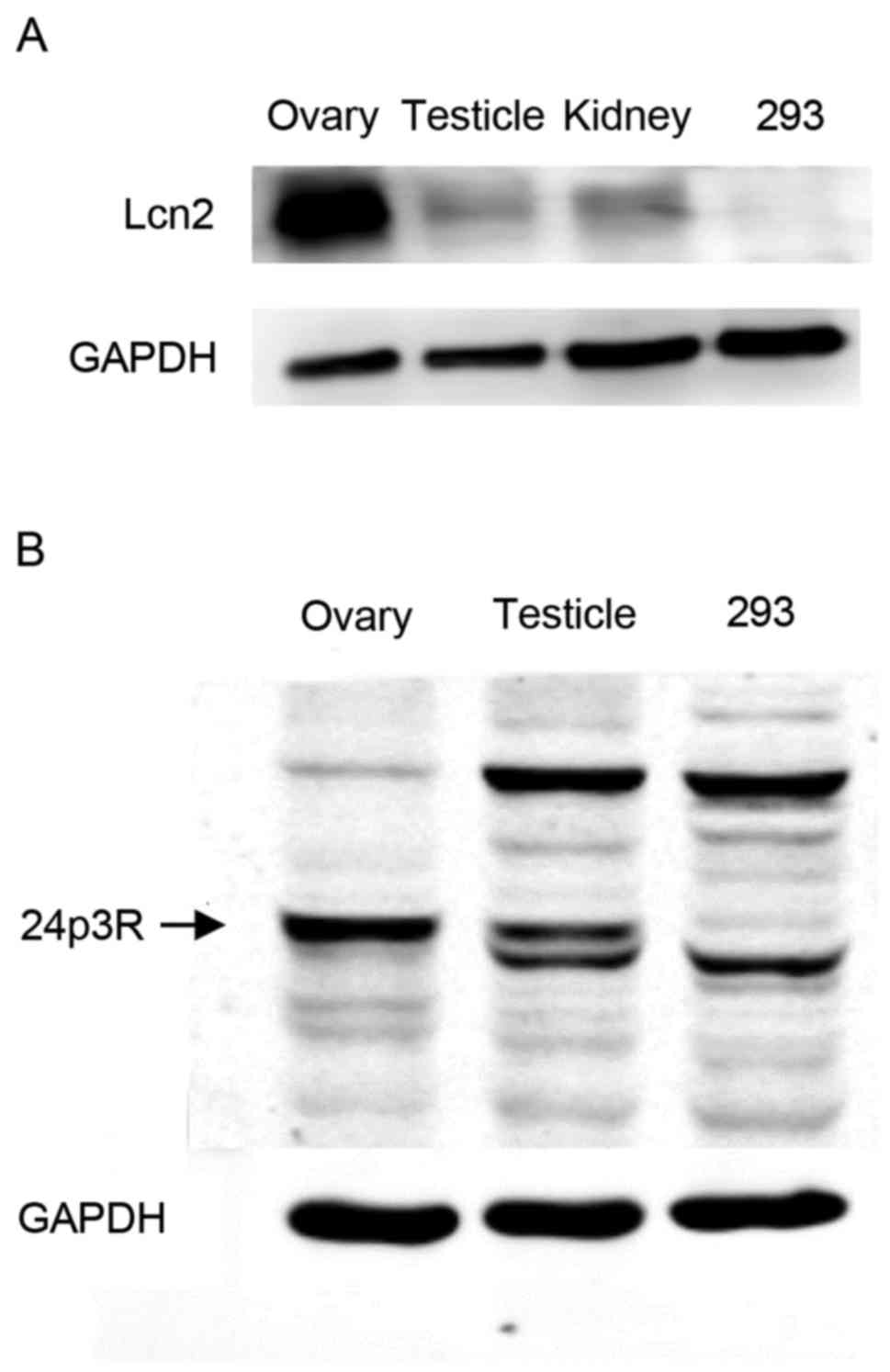
LCN2 protein identification by western
blot analysis
In order to assess the presence of LCN2 and 24p3R
proteins within gonadal tissue, protein extracts from ovaries and
testes of wild-type adult rats were employed to perform a western
blot assay using polyclonal antibodies against LCN2 and 24p3R,
respectively. As presented in Fig.
5A, a 24-kDa band, which corresponds to the molecular weight of
LCN2, was observed in each of the two protein samples. This signal
was also identified in protein extracts isolated from kidneys of
the same animals, where LCN2 synthesis has been demonstrated. In
fact, high concentrations of LCN2 in urine and plasma are now
considered as biological markers for acute kidney injury (18). Furthermore, the 24p3 receptor was
detected within the male and female gonads. As displayed in
Fig. 5B, western blot analysis of
ovarian protein extracts revealed a well-defined 57-kDa band, which
corresponds to the molecular weight of the 24p3 receptor, which was
also present at a lower intensity in the testicular and 293 protein
samples. GAPDH was detected in the protein extracts of the three
organs as a reference (Fig. 5A and
B).
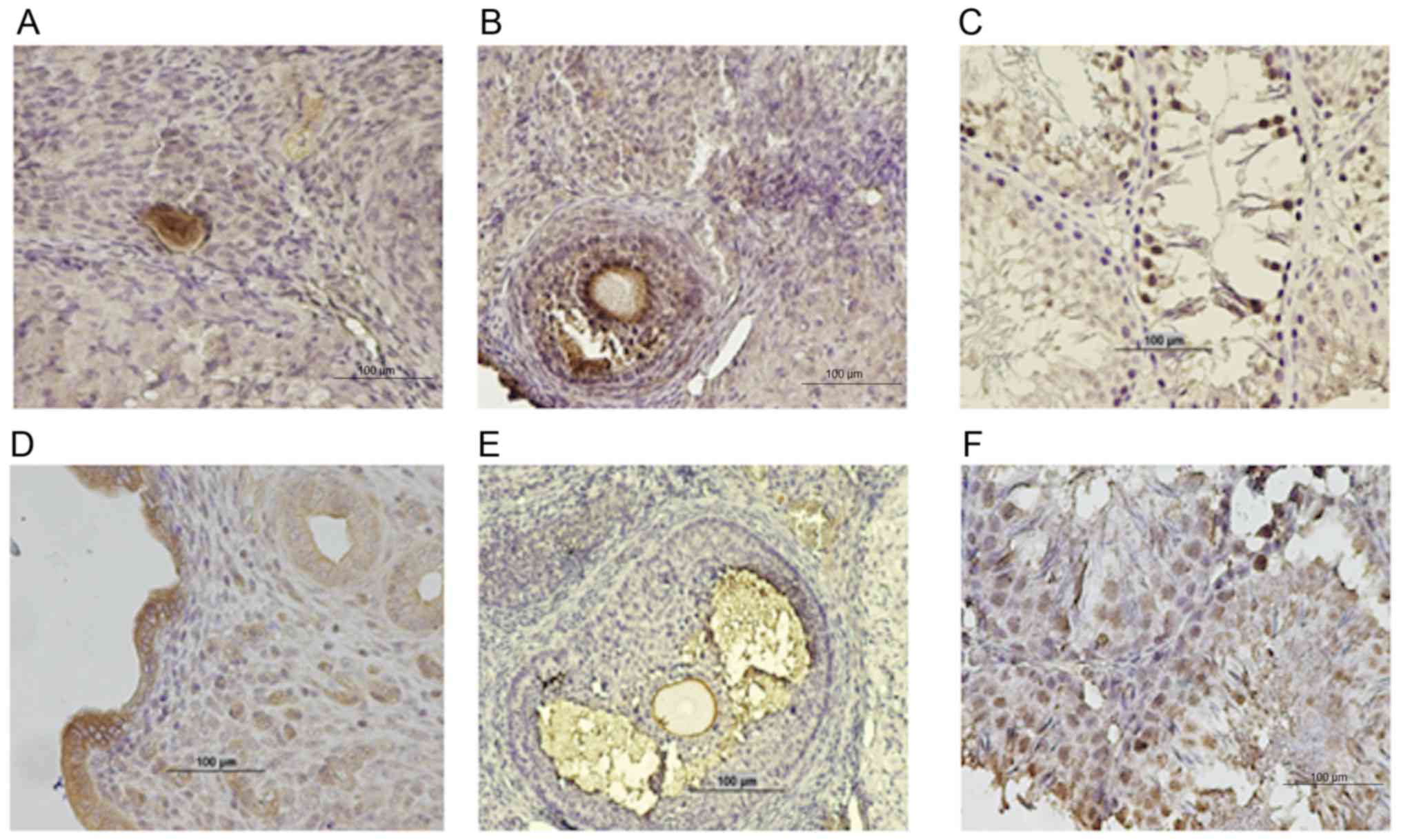
Cellular localization of LCN2 within
gonadal tissue
The cellular localization of LCN2 and 24p3R within
gonads from wild-type rats was determined by means of
immunohistochemistry (Fig. 6). In
sections of paraffin-embedded ovaries, LCN2 immunostaining of
oocytes and granulosa cells of primordial and growing follicles, as
well as in the zona pellucida and antrum of developed follicles,
was observed (Fig. 6A and B).
Regarding the 24p3 receptor, intense staining was observed in the
zona pellucida of oocytes and in the antrum of the fully developed
follicles (Fig. 6E).
In sections of paraffin-embedded testicles, LCN2 and
24p3R are present in germinal cells at different developmental
stages, rather than in cells of epithelial origin (Fig. 6C and F).
Discussion
Adipokines comprise a vast number of molecules
synthetized mainly in adipose tissue but present in other organs,
which are involved in the regulation of numerous physiological
processes, including immunity, appetite control and metabolism, as
well as cardiovascular and reproductive function. Studies performed
in knockout mice have provided evidence for the role of various
adipokines in the regulation of the HPG axis (19). For instance, the participation of
leptin in regulating the HPG axis is evidenced by the fact that
leptin-deficient mice are infertile (20). Leptin is localized to the pituitary
gland, where it stimulates the production of
gonadotrophin-releasing hormone (GnRH) through neurons possessing
leptin receptors. In turn, GnRH causes the release of both the
luteinizing hormone (LH) and the follicle stimulating hormone (FSH)
that subsequently act on male and female gonads (21). In the same manner, adiponectin
regulates reproductive function through the HPG axis. Its
circulating concentration depends on GnRH and gonadotropins levels,
which also vary according to the estrous cycle phase (22). Furthermore, LH and FSH modulate the
expression of the adiponectin receptor 2 in ovarian granulosa cells
in order to increase progesterone secretion (23).
To the best of our knowledge, the present study was
the first to determine the expression profile of LCN2 or 24p3 and
its receptor, 24p3R, in rat ovaries and testicles collected at
different stages of gonadal development. LCN2 and 24p3R mRNA
expression was observed in male and female gonads from 21 dpc
onwards. In this context, the mRNA and protein expression of
adiponectin, visfatin, resistin, chemerin and apelin have been
identified in gonads of several species, leading to the conclusion
that these adipokines are involved, through their specific
receptors, in gonadal functions that are mostly
gonadotropin-dependent, including germinal cell maturation,
steroidogenesis or estradiol secretion (24–30).
Nevertheless, none of these previous studies reported on the
expression of any of the aforementioned adipokines during perinatal
stages when gonadotropin-independent molecular mechanisms are
taking place. In the present study, LCN2 and 24p3R mRNA expression
was observed distinctively at these stages. Taking into account
that LCN2 covalently binds to MMP-9 in order to prevent its
degradation to allow for the modulation of cellular matrix
remodeling (31), the mRNA
expression profile of this metalloproteinase in male and female
gonads was assessed, and a low and constitutive expression was
observed in perinatal stages (data not shown). This is in agreement
with a study by Light and Hammes (32), in which a detectable but extremely
low MMP-9 mRNA expression was identified in primary granulosa cells
of murine ovaries. Therefore, the present study focused on the 24p3
receptor, which, as mentioned above, exhibited a distinct pattern
of expression in the perinatal period. LCN2 in conjunction with
this receptor exerts or triggers different signaling pathways,
including iron transport and regulation of various cellular
processes, including cell differentiation and apoptosis (33,34). It
is known that within male and female murine gonads, mitotic
proliferation of germ cells is arrested by embryonic day 13.5, and
in the case of the ovary, this is followed by progression through
the prophase stage of the first meiotic division until around the
time of birth, or in the case of the testis, a re-entry into the
cell cycle of germ cells arrested in mitosis, in order to start
spermatogenesis (35,36). In this regard, the present study also
indicated that during these perinatal stages, the relative mRNA
expression of LCN2 exhibits sex-specific differences and that at
least in perinatal ovaries, the relative mRNA expression of the
short isoform of 24p3R is identical to that of LCN2. The latter,
may be attributed to the fact that in the female gonad, the
physiological processes performed at this stage are different from
those that occur in the testicle. Therefore, LCN2/24p3R signaling
may have different purposes within ovaries and testes. For
instance, it is well known that during follicular assembly, which
in murine gonads starts at perinatal stages, numerous oocytes are
lost through apoptosis (37). The
fact that binding of apo-LCN2 (iron-free LCN2) to the 24p3 receptor
mediates intracellular iron depletion and subsequently leads to
apoptosis (7), may indicate a
specific role for LCN2/24p3R in apoptotic signaling in the
perinatal ovary. Alternatively, iron-loaded LCN2 may be
internalized through this receptor in order to increase
intracellular iron levels and subsequently promote cell
proliferation (38). Recent in
vitro studies have demonstrated that another adipokine, apelin
13, promotes granulosa cell proliferation and apoptosis inhibition
through the phosphoinositide-3 kinase/Akt signaling pathway
(39). In addition, a protective
role for apelin 13 against apoptosis has been demonstrated in brain
and cardiac tissue (40,41); the latter scenario may also be
alternatively considered for LCN2/24p3 in ovarian physiology. By
contrast, in the male gonad, LCN2 mRNA increases hours after birth,
but two days later, its expression diminishes by half. It is well
established that apoptosis within the testis occurs also at a high
rate, when the first spermatogenic cycle takes place at 10–30 days
after birth. This cell death process is orchestrated primarily by a
balance between pro-apoptotic proteins, including B-cell lymphoma 2
(Bcl-2)-associated X protein and the anti-apoptotic Bcl-2 protein
family (42). Therefore, it is
difficult to associate an apoptotic process within testicles driven
by LCN2 and 24p3 receptor occurring hours after birth with an
increment of LCN2 mRNA expression. Thus, at this stage, the role of
LCN2/24p3R in the procurement of male gonadal cell survival may
also be considered.
Similarly, a distinct difference between the LCN2
mRNA profile of ovaries and testes was observed at postnatal days
12 and 20. The latter coincides with the expression of gonadotropin
receptors and the onset of gonadotropin-dependent mechanisms.
Various studies have localized chemerin, resistin and visfatin
within somatic and germinal cells of the ovary. The first two
adipokines are involved in the downregulation of ovarian
steroidogenesis, mostly by inhibiting aromatase expression in
granulosa cells (19,43). Visfatin appears to be involved in
oocyte maturation, as its concentration in the follicular fluid has
been associated with the number of mature oocytes (25).
In the present study, even though the relative
expression of 24p3R in male and female gonads remained at a
constant level at all stages, the mRNA abundance of the two
isoforms observed in the ovary was not as much as that identified
in the testis. A study published in 2003 by Burns et al
(44), in which 24p3 is upregulated
by 60-fold in FSH-null mice, suggested an inhibitory effect of FSH
on the expression of this adipokine; this in turn may lead to the
downregulation of 24p3R expression. In fact, a slight decrease in
LCN2 and 24p3R mRNA levels at postnatal day 30 was observed,
coinciding with the decrease of FSH. Regarding the male gonad, FSH
acts in concert with sex hormones (testosterone and estradiol) to
support male germ survival (42).
Thus, the participation of LCN2/24p3R in different mechanisms
within male and female gonads at this time of development should be
considered. This sexually dimorphic expression of LCN2 was also
reported by Guo et al (17)
in 2012, who observed higher levels of LCN2 in inguinal fat depots
of female mice in comparison with the levels in males. Furthermore,
they demonstrated an association between LCN2 and estrogen
production and action in adipose tissue, which provides preliminary
data on the association that may exist between this adipokine and
the corresponding mechanisms, which are dependent on gonadotropic
stimulus.
The localization of LCN2 and 24p3R to somatic and
germ cells within the murine ovary observed in the present study is
in accordance with the observations of studies performed by two
other groups (19,45), which demonstrated that various
adipokines and their corresponding receptors are involved in a
coordinated crosstalk, which ensures proper follicular development.
The latter study also suggests that in the ovary, LCN2/24p3R
signaling may act in a paracrine and/or an autocrine manner. Of
note, in the male gonad, LCN2 and its receptor are localized
exclusively in germ cells of different maturational stages,
indicating that as in the ovary, a coordinated communication
between this adipokine and its receptor may also occur in the
testes in order to achieve adequate germ cell development, but this
communication only occurs in an autocrine way. At present, it is
challenging to explain differences in the pattern of LCN2/24p3R
localization between ovaries and testes due to the limited
information available. In fact, studies focusing on the
participation of adipokines in male gonadal function are scarce and
the majority of them specifically address the association between
metabolic diseases and poor quality, as well as low count or
motility of sperm (19).
The present results may indicate that LCN2/24p3R
signaling is involved in cell proliferation or apoptotic mechanisms
within rat gonads and that such signaling is exerted in a sexually
dimorphic pattern at different stages of gonadal development. Even
though the present study demonstrates the presence of LCN2 and
24p3R in rat gonads, it is limited in terms of not experimentally
demonstrating the participation of LCN2/24p3R signaling in cell
proliferation or apoptotic mechanisms, nor the gonadotropic
stimulus regulation of such signaling.
Acknowledgements
The authors would like to thank Mr Liborio Morán and
Mrs Noemí Castillo (Cardiovascular and Metabolic Diseases Research
Unit, National Medical Center, Mexican Social Security Institute,
México City, México) for their kind assistance with the
histological procedures.
Funding
This study was financially supported by the Mexican
Social Security Institute Foundation (grant no.
FIS/IMSS/PROT/014).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EDLC participated in the conception and design of
the study, performed the immunohistochemistry experiments, analysis
and interpretation of data, and prepared the manuscript. LMA, LD,
AO, MAP and IS collected the biological samples, performed the
semi-quantitative reverse transcription polymerase chain reaction
and western blot analyses, and analysed and interpreted data. JPM
participated in the design of the study, prepared the manuscript
and revised the manuscript for its intellectual content. Also, the
final version of the manuscript has been read and approved by all
authors and each author believes the manuscript represents honest
work.
Ethical approval and consent to
participate
The experimental protocol was approved by the
Research Committees of the National Medical Center and the National
Autonomous University of Mexico (México City, México; approval no.
UNAM-003-2013).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Luque-Ramírez M, Martínez-García MA,
Montes-Nieto R, Fernández-Durán E, Insenser M, Alpañés M and
Escobar-Morreale HF: Sexual dimorphism in adipose tissue function
as evidenced by circulating adipokine concentrations in the fasting
state and after an oral glucose challenge. Hum Reprod.
28:1908–1918. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Makovey J, Naganathan V, Seibel M and
Sambrook P: Gender differences in plasma ghrelin and its relations
to body composition and bone-an opposite sex twin study. Clin
Endocrinol (Oxf). 66:530–537. 2007.PubMed/NCBI
|
|
3
|
Van Dam RM and Hu FB: Lipocalins and
insulin resistance: etiological role of retinol-binding protein 4
and lipocalin-2? Clin Chem. 53:5–7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Flower DR: The lipocalin protein family:
Structure and function. Biochem J. 318:1–14. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kjeldsen L, Cowland JB and Borregaard N:
Human neutrophil-associated lipocalin and homologous proteins in
rat and mouse. Biochim Biophys Acta. 1482:272–283. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kjeldsen L, Johnsen AH, Sengelov H and
Borregaard N: Isolation an primary structure of NGAL, a novel
protein associated with human neutrophil gelatinase. J Biol Chem.
268:10425–10432. 1993.PubMed/NCBI
|
|
7
|
Devireddy LR, Gazin C, Zhu X and Green MR:
A cell-surface receptor for lipocalin-24p3 selectively mediates
apoptosis and iron uptake. Cell. 123:1293–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan YL, Paz V and Wool IG: The primary
structure of rat alpha 2 microglobulin-related protein. Nucleic
Acids Res. 16:113681988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Lam KS, Kraegen EW, Sweeney G,
Zhang J, Tso AW, Chow WS, Wat NM, Xu JY, Hoo RL and Xu A:
Lipocalin-2 is an inflammatory marker closely associated with
obesity, insulin resistance, and hyperglycemia in humans. Clin
Chem. 53:34–41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alkharfy KM, Al-Daghri NM, Vanhoutte PM,
Krishnaswamy S and Xu A: Serum retinol-binding protein 4 as a
marker for cardiovascular disease in women. PLoS One. 7:e486122012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gencer M, Gazi E, Hacivelioglu S,
Binnetoğlu E, Barutçu A, Türkön H, Temiz A, Altun B, Vural A,
Cevizci S, et al: The relationship between subclinical
cardiovascular disease and lipocalin-2 levels in women with PCOS.
Eur J Obstet Gynecol Reprod Biol. 181:99–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cowland JB and Borregaard N: Molecular
characterization and pattern of tissue expression of the gene for
neutrophil gelatinase-associated lipocalin from humans. Genomics.
45:17–23. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De la Chesnaye E, Manuel-Apolinar L,
Zarate A, Damasio L, Espino N, Revilla-Monsalve MC and
Islas-Andrade S: Lipocalin-2 plasmatic levels are reduced in
patients with long-term type 2 diabetes mellitus. Int J Clin Exp
Med. 8:2853–2859. 2015.PubMed/NCBI
|
|
14
|
De la Chesnaye E, Manuel-Apolinar L,
Oviedo-de Anda N, Revilla-Monsalve MC and Islas-Andrade S: Gender
differences in lipocalin-2 plasmatic levels are correlated with age
and the triglyceride/HDL ratio in healthy individuals. Gac Med Mex.
152:612–617. 2016.(In Spanish). PubMed/NCBI
|
|
15
|
Thraikill KM, Moreau CS, Cockrell GE, Jo
CH, Bunn RC, Morales-Pozzo AE, Lumpkin CK and Fowlkes JL: Disease
and gender-specific dysregulation of NGAL and MMP-9 in type 1
diabetes mellitus. Endocrine. 37:336–343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De la Chesnaye E, Kerr B, Paredes A,
Merchant-Larios H, Méndez JP and Ojeda SR: Fbxw15/Fbxo12J is an F
Box protein-encoding gene selectively expressed in oocytes of the
murine ovary. Biol Reprod. 78:714–725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo H, Zhang Y, Brockman DA, Hahn W,
Bernlohr DA and Chen X: Lipocalin-2 deficiency alters estradiol
production and estrogen receptor signaling in female mice.
Endocrinology. 153:1183–1193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moyake N, Buchmann E and Crowther NJ:
Neutrophil gelatinase-associated lipocalin as a diagnostic marker
of acute kidney injury in preclampsia. J Obstet Gynaecol Res.
42:1483–1488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsatsanis C, Dermitzaki E, Avgoustinaki P,
Malliaraki N, Mytaras V and Margioris AN: The impact of adipose
tissue-derived factors on the hypothalamic-pituitary-gonadal (HPG)
axis. Hormones (Athens). 14:549–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chehab FF, Lim ME and Lu R: Correction of
the sterility defect in homozygous obese female mice by treatment
with the human recombinant leptin. Nat Genet. 12:318–320. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Quennell JH, Mulligan AC, Tups A, Liu X,
Phipps SJ, Kemp CJ, Herbison AE, Grattan DR and Anderson GM: Leptin
indirectly regulates gonadotropin-releasing hormone neuronal
function. Endocrinology. 150:2805–2812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kiezun M, Smolinska N, Maleszka A, Dobrzyn
K, Szeszko K and Kaminski T: Adiponectin expression in the porcine
pituitary during the estrous cycle and its effect on LH and FSH
secretion. Am J Physiol Endocrinol Metab. 307:E1038–E1046. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wickham EP III, Tao T, Nestler JE and
McGee EA: Activation of the LH receptor up regulates the type 2
adiponectin receptor in human granulosa cells. J Assist Reprod
Genet. 30:963–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gutman G, Barak V, Maslovitz S, Amit A,
Lessing JB and Geva E: Recombinant luteinizing hormone induces
increased production of ovarian follicular adiponectin in vivo:
Implications for enhanced insulin sensitivity. Fertil Steril.
91:1837–1841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen CJ, Tsai EM, Lee JN, Chen YL, Lee CH
and Chan TF: The concentrations of visfatin in the follicular
fluids of women undergoing controlled ovarian stimulation are
correlated to the number of oocytes retrieved. Fertil Steril.
93:1844–1850. 2013. View Article : Google Scholar
|
|
26
|
Rak A, Drwal E, Karpeta A and
Gregoraszczuk EL: Regulatory role of gonadotropins and local
factors produced by ovarian follicles on in vitro resistin
expression and action on porcine folicular steroidogenesis. Biol
Reprod. 92:1422015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reverchon M, Bertoldo MJ, Rame C, Froment
P and Dupont J: Chemerin (RARRES2) decreases in vitro granulosa
cell steroidogenesis and blocks oocyte meiotic progression in
bovine species. Biol Reprod. 90:1022014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Q, Kim JY, Xue K, Liu JY, Leader A
and Tsang BK: Chemerin a novel regulator of follicular
steroidogenesis and its potential involvement in polycystic ovarian
syndrome. Endocrinology. 153:5600–5611. 2015. View Article : Google Scholar
|
|
29
|
Reverchon M, Cornuau M, Rame C, Guerif F,
Royère D and Dupont J: Chemerin inhibits IGF-1-induced progesterone
and estradiol secretion in human granulosa cells. Hum Reprod.
27:1790–1800. 2015. View Article : Google Scholar
|
|
30
|
Roche J, Ramé C, Reverchon M, Mellouk N1,
Cornuau M, Guerif F, Froment P and Dupont J: Apelin (APLN) and
Apelin receptor (APLNR) in human ovary: Expression, signaling and
regulation of steroidogenesis in primary human luteinized granulosa
cells. Biol Reprod. 95:1042016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan L, Borregaard N, Kjeldsen L and Moses
MA: The high molecular weight urinary matrix metalloproteinase
(MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil
gelatinase-associated lipocalin (NGAL). Modulation of MMP-9
activity by NGAL. J Biol Chem. 276:37258–37265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Light A and Hammes SR: LH-Induced
steroidogenesis in the mouse ovary, but not testis, requires matrix
metalloproteinase 2- and 9-mediated cleavage of upregulated EGF
receptor ligands. Biol Reprod. 93:652015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Langelueddecke C, Roussa E, Fenton RA and
Thévenod F: Expression and function of the lipocalin-2 (24p3/NGAL)
receptor in rodent and human intestinal epithelia. PLoS One.
8:e715862013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Richardson DR: 24p3 and its receptor: Dawn
of a new iron age? Cell. 123:1175–1177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cohen PE and Pollard JW: Regulation of
meiotic recombination and prophase I progression in mammals.
Bioessays. 23:996–1009. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Western PS, Miles DC, van den Bergen JA,
Burton M and Sinclair AH: Dynamic regulation of mitotic arrest in
fetal male germ cells. Stem Cells. 26:339–347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McClellan KA, Gosden R and Taketo T:
Continuous loss of oocytes throughout meiotic prophase in the
normal mouse ovary. Dev Biol. 258:334–348. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schmidt-Ott KM, Mori K, Li JY, Kalandadze
A, Cohen DJ, Devarajan P and Barasch J: Dual Action of neutrophil
gelatinase-associated lipocalin. J Am Soc Nephrol. 18:407–413.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shuang L, Jidong W, Hongjuan P and Zhenwei
Y: Effects of apelin on proliferation and apoptosis in rat ovarian
granulosa cells. Clin Exp Obstet Gynecol. 43:409–413.
2016.PubMed/NCBI
|
|
40
|
Yang Y, Zhang XJ, Li LT, Cui HY, Zhang C,
Zhu CH and Miao JY: Apelin-13 protects against apoptosis by
activating AMP-activated protein kinase pathway in ischemia stroke.
Peptides. 75:96–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boal F, Timotin A, Roumegoux J, Alfarano
C, Calise D, Anesia R, Parini A, Valet P, Tronchere H and Kunduzova
O: Apelin 13 administration protects against
ischemia/reperfusion-mediated apoptosis through FoxO1 pathway in
high-fat diet-induced obesity. Br J Pharmacol. 173:1850–1863. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shaha C, Tripathi R and Mishra DP: Male
germ cell apoptosis: Regulation and biology. Philos Trans R Soc
Lond B Biol Sci. 365:1501–1515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ballinger AB, Savage MO and Sanderson IR:
Delayed puberty associated with inflammatory bowel disease. Pediatr
Res. 53:205–210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Burns KH, Owens GE, Oqbonna SC, Nilson JH
and Matzuk MM: Expression profiling analyses of gonadotropin
responses and tumor development in the absence of inhibins.
Endocrinology. 144:4492–4507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Artimani T, Saidijam M, Aflatoonian R,
Ashrafi M, Amiri I, Yavangi M, SoleimaniAsl S, Shabab N, Karimi J
and Mehdizadeh M: Downregulation of adiponectin system in granulosa
cells and low levels of HMW adiponectin in PCOS. J Assist Reprod
Genet. 33:101–110. 2016. View Article : Google Scholar : PubMed/NCBI
|