Introduction
Diffuse large B-cell lymphoma (DLBCL) is an invasive
and malignant tumor derived from mature B lymphocytes. It is the
most common type of non-Hodgkin lymphoma (NHL), accounting for
30–40% of the morbidity of this disease (1). Although a subset of patients may be
successfully treated by chemotherapy, the recurrence rate is high.
Therefore, further study to identify novel therapeutic options to
treat patients with DLBCL is warranted. Oxidative damage induced by
oxidative stress may be associated with the occurrence and
development of lymphoma (2,3). Under physiological conditions, the
Kelch-like ECH associated protein 1 (Keap1)/nuclear factor
erythroid-2-related factor-2 (Nrf2) signaling pathway is a critical
system that responds to oxidative stress in vivo (4). It has been indicated that this
signaling pathway is associated with the development of conditions
including inflammation (5), tumors
(6), nerve injury (7), cardiovascular disease (8) and chronic obstructive pulmonary disease
(9). Most available studies on Keap1
and Nrf2 expression have mainly focused on lymphoma cell lines, and
few have reported on their expression in DLBCL tissues. The present
study was performed to characterize Keap1 and Nrf2 expression in
DLBCL tissues by immunohistochemical staining, and to explore the
role of these proteins in the development and progression of DLBCL.
Specifically, the association between the expression of these
proteins and clinical features was assessed in an attempt to
provide novel approaches for the treatment of DLBCL.
Materials and methods
Patients
The present study included 39 cases of de novo
DLBCL, and paraffin-embedded specimens that were histologically
confirmed by pathologists of Gansu Provincial Hospital (Lanzhou,
China) between October 2012 and November 2016 were utilized.
Samples were derived from 21 males and 18 females, whose age ranged
from 16 to 83 years of age (median age, 56 years). A total of 17
paraffin-embedded specimens comprising cases of reactive lymph node
hyperplasia during the same period, were included in the control
group, and the age of these donors varied from 16–70 years of age
(median age, 41 years). Diagnoses were confirmed by an experienced
pathologist according to the World Health Organization (WHO)
classification of tumors of hematopoietic and lymphoid tissues in
2016 (10). The complete clinical
data were collected for each subject. Patients with the following
diseases were excluded: Other malignant tumors, acute infection,
cardiovascular diseases, chronic obstructive pulmonary disease,
neurodegenerative diseases, liver diseases and ocular diseases,
including age-associated macular degeneration, cataracts, diabetic
retinopathy and glaucoma (5,7–9,11–13). The
present study was approved by the Ethics Committee of Gansu
Provincial Hospital and all patients provided written informed
consent.
Reagents
Rabbit anti-Keap1 antibody (cat. no. bs-4900R;
dilution, 1:400), rabbit anti-Nrf2 antibody (cat. no. bs-1074R;
dilution, 1:400), biotin-conjugated goat anti-rabbit immunoglobulin
(Ig)G/bio antibody (cat. no. bs-0295G-bio; dilution, 1:100),
streptavidin-horseradish peroxidase antibody (cat. no.
bs-0437P-HRP; dilution, 1:500) and diaminobenzidine (DAB) staining
kit (cat. no. C02-04001) were purchased from Beijing Bioss Biotech
Co., Ltd. (Beijing, China).
Immunohistochemical staining
All specimens were fixed in 10% neutral formalin,
embedded in paraffin, sliced to a thickness of 4 µm and stained.
First, the slides were baked at 60°C for 2 h. Subsequently, the
paraffin sections were dewaxed three times using xylene (15 min
each time) and hydrated in a descending series of ethanol. Antigen
retrieval was performed by boiling samples in 0.01 M citrate buffer
(pH 6.0) at 95°C for 15 min, followed by cooling to room
temperature. The paraffin sections were incubated at room
temperature in 3% hydrogen peroxide solution for 20 min to
eliminate endogenous peroxidase activity. Samples were then
incubated in 10% normal goat serum at 37°C for 20 min, and the
primary antibody (anti-Keap1 or anti-Nrf2) was then added, followed
by incubation at 4°C overnight. Following the addition of the
antibody (biotin-conjugated goat anti-rabbit IgG secondary
antibody), sections were incubated at 37°C for 20 min. An antibody
(streptavidin-horseradish peroxidase tertiary antibody) was added
to the sections at 37°C for 20 min. After each completed step, the
slides were washed three times with PBS for 5 min each. DAB
chromogenic reagent was added, and samples were counterstained with
hematoxylin, hydrated in an ascending series of ethanol, cleared in
xylene and mounted. Samples in which the primary antibody
(anti-Keap1 or anti-Nrf2) was replaced with PBS served as a
negative control.
Evaluation of staining
Keap1 and Nrf2 staining was evaluated and analyzed
by two senior doctors in the pathology department (Dr Yamei Dang
and Dr Fenghui Zhao; Gansu Provincial Hospital, Lanzhou, China)
under low magnification (×100), high magnification (×400) and oil
immersion microscopy (×1,000). If there was a disagreement, the
result of staining was evaluated and analyzed by a third doctor. If
two doctors had the same result, that was considered the final
result. All pathologists were unaware of the clinical data. The
staining results were evaluated as follows (14–16): The
percentage of positively stained cells were as follows: 0, 0–5%; 1,
6–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100% of cells stained.
Sections were also scored on the basis of staining intensity: 0,
negative or no staining; 1, pale yellow, weak staining intensity;
2, yellow, moderate staining intensity; and 3, brown, strong
staining intensity. The semi-quantitative score was based on the
multiplication of positive cell percentage and positive cell
staining intensity score. Staining results were divided into four
grades based on the scores regarding the percentage of positively
stained cells and staining intensity. Results were presented as
follows: 0 (−, negative), 1–4 (+, weakly positive), 5–8 (++,
moderately positive) and 9–12 (+++, strongly positive). Samples
rated as (+) - (+++) were regarded as positive.
Statistical analysis
Data was analyzed using SPSS 22.0 software (IBM
Corp., Armonk, NY, USA). The positive rates between different
groups were compared by performing a χ2 test [n≥40;
theoretical frequency (T) ≥5], continuity correction of
χ2 test (n≥40, 1≤T<5) and Fisher's exact test
(n<40 or T<1). Spearman's rank correlation analysis was used
to assess the correlation between Keap1 and Nrf2. P<0.05 was
considered to indicate a statistically significant difference.
Results
Keap1 and Nrf2 expression in reactive
lymph node hyperplasia and DLBCL tissues
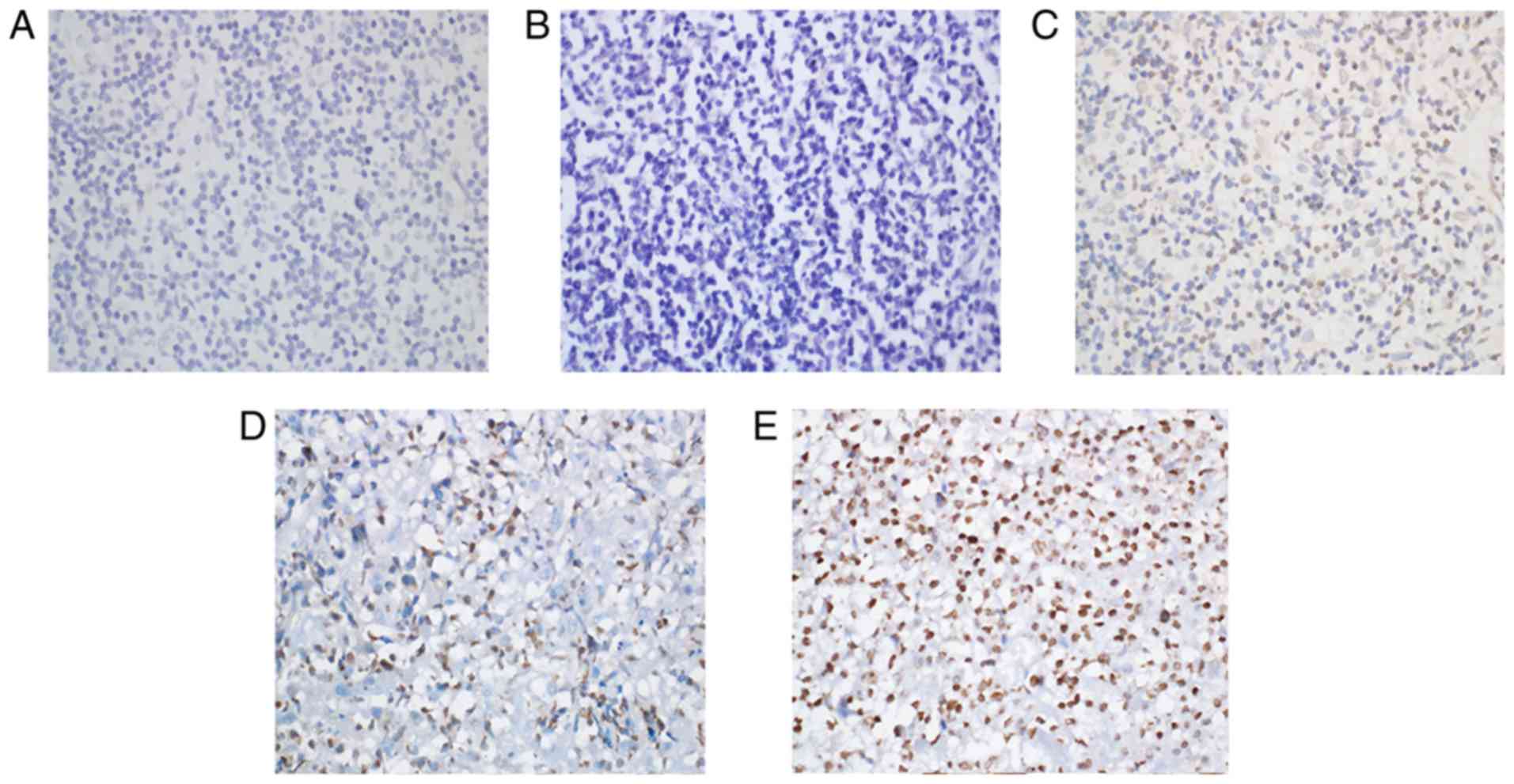
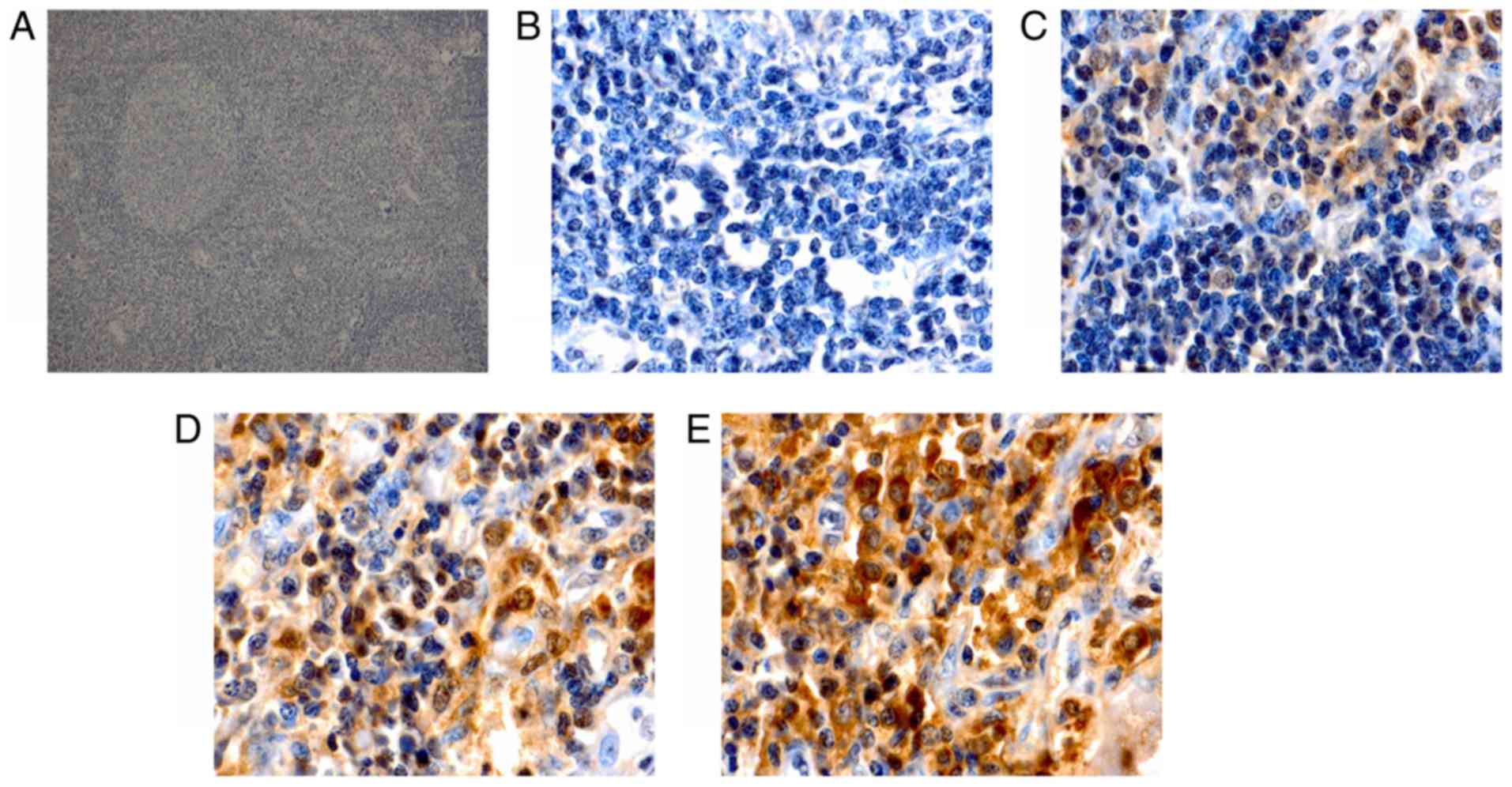
Immunohistochemical staining for Keap1 was observed
as yellow or brown granules that were predominantly located in the
cytoplasm, with a few situated in the nucleus (Fig. 1). However, Nrf2 staining presented as
yellow or brown granules, which were mainly located in the cellular
nuclei, with little staining was detected in the cytoplasm
(Fig. 2). In the reactive lymph node
hyperplasia samples, Nrf2 staining for 1 case was positive in the
nuclei of the follicle cells, while it was negative for the other
cases. In 3 cases, Keap1 staining was scattered in the cytoplasm of
the follicle cells, plasmacytes and histiocytes. In most DLBCL
tissues, a strong expression of Keap1 was observed, with a diffuse
distribution in the cytoplasm of heterotypic malignant lymphocytes
(Fig. 1E). Furthermore, a strong
expression of Nrf2 was mainly observed as a diffuse distribution in
the nuclei of heterotypic malignant lymphocytes (Fig. 2E). In addition, a weak or moderate
expression of Keap1 and Nrf2 was observed in malignant lymphocytes
(Fig. 1C and D; Fig. 2C and D). The Keap1 positivity rate in
DLBCL tissues was 46.2%, which was significantly higher than that
of reactive lymph node hyperplasia (17.7%; P<0.05; Table I). Furthermore, statistical analysis
indicated that the positivity rate of Nrf2 was 35.9% in DLBCL
tissues and only 5.9% in reactive lymph node hyperplasia, and this
difference was statistically significant (P<0.05; Table I).
 | Table I.Keap1 and Nrf2 expression in DLBCL
tissues and reactive lymph node hyperplasia. |
Table I.
Keap1 and Nrf2 expression in DLBCL
tissues and reactive lymph node hyperplasia.
|
|
| Keap1 | Nrf2 |
|---|
|
|
|
|
|
|---|
| Group | n | Positives | χ2 | P-value | Positives | χ2 | P-value |
|---|
| DLBCL tissues | 39 | 18 (46.2) | 4.105 | 0.043a | 14 (35.9) | 4.016 | 0.045b |
| Reactive lymph node
hyperplasia | 17 | 3 (17.7) |
|
| 1 (5.9) |
Association between Keap1 or Nrf2
expression and clinicopathological features in DLBCL
In DLBCL tissues, the expression levels of Keap1 and
Nrf2 were associated to the international prognostic index (IPI)
and Ann-Arbor clinical stage (P<0.05), and Keap1 and Nrf2
expression was higher in DLBCL patients with stage III–IV (68.4 and
52.6%, respectively) compared with those with stage I–II (25.0 and
20.0%, respectively). However, Keap1 or Nrf2 expression was not
associated with patient age, sex and lactate dehydrogenase levels
(P>0.05; Table II).
 | Table II.Association between Keap1 or Nrf2
expression and clinicopathological features of patients with
diffuse large B-cell lymphoma. |
Table II.
Association between Keap1 or Nrf2
expression and clinicopathological features of patients with
diffuse large B-cell lymphoma.
|
|
| Keap1
expression | Nrf2
expression |
|---|
|
|
|
|
|
|---|
| Variable | n | Positives |
P-valuea | Positives |
P-valuea |
|---|
| Age (years) |
|
| 1.000 |
| 0.740 |
|
≤50 | 15 | 7 (46.7) |
| 6 (40.0) |
|
|
>50 | 24 | 11 (45.8) |
| 8 (33.3) |
|
| Sex |
|
| 0.752 |
| 0.180 |
|
Male | 21 | 9 (42.9) |
| 10 (47.6) |
|
|
Female | 18 | 9 (50.0) |
| 4 (22.2) |
|
| LDH (U/l) |
|
| 0.192 |
| 0.740 |
|
≤250 | 16 | 5 (31.3) |
| 5 (31.3) |
|
|
>250 | 23 | 13 (56.5) |
| 9 (39.1) |
|
| Clinical stage |
|
| 0.010 |
| 0.048 |
|
I–II | 20 | 5 (25.0) |
| 4 (20.0) |
|
|
III–IV | 19 | 13 (68.4) |
| 10 (52.6) |
|
| IPI |
|
| 0.025 |
| 0.043 |
|
0–2 | 23 | 7 (30.4) |
| 5 (21.7) |
|
|
3–5 | 16 | 11 (68.8) |
| 9 (56.3) |
|
Correlation between Keap1 and Nrf2
expression in DLBCL tissues
Out of 14 cases of DLBCL with expression of Nrf2,
expression of Keap1 was detected in 9 cases. Statistical analysis
indicated that Nrf2 expression was not correlated with Keap1
expression in DLBCL tissues (r=0.272, P=0.094; Table III).
 | Table III.Correlation between Keap1 and Nrf2
expression in diffuse large B-cell lymphoma tissues. |
Table III.
Correlation between Keap1 and Nrf2
expression in diffuse large B-cell lymphoma tissues.
|
| Nrf2 expression
(n) |
|
|
|---|
|
|
|
|
|
|---|
| Keap1 expression
(n) | Positives (%) | Negatives (%) | r-value | P-value |
|---|
| Positives | 9 (23.1) | 9 (23.1) | 0.272 | 0.094 |
| Negatives | 5 (12.8) | 16 (41.0) |
|
|
Discussion
The present study provided the first evidence of
high expression of Keap1 and Nrf2 proteins in DLBCL tissues, to the
best of our knowledge. The Keap1-Nrf2/antioxidant response element
(ARE) signaling pathway is a key endogenous antioxidant stress
response pathway and induces endogenous antioxidant reactions. This
pathway mainly comprises three parts, namely Keap1, Nrf2 and ARE
(17). Keap1 is aptly named because
it is similar to the Drosophila actin binding protein Kelch,
which is a polypeptide containing 624 amino acids (18). Nrf2 is a basic leucine zipper
transcription factor and belongs to the Cap'n'Collar subfamily,
which was first reported by Moi et al (19) in 1994. Keap1 is a large cytoplasmic
chaperone of Nrf2 that has a negative role in the regulation of
Nrf2 transcriptional activity. Under oxidative stress from
endogenous and exogenous sources, Nrf2 dissociates from Keap1,
translocates to the nucleus, and transactivates a series of
downstream antioxidant enzymes and phase-II detoxification enzymes
to combat oxidative stress (11).
Therefore, the expression of Nrf2 and Keap1 indicates that Nrf2
dissociates from Keap1 and in turn has been activated. Relevant
studies report that Nrf2 has opposing actions in humans (5,20).
Furthermore, the present study suggests that Nrf2 does not only
protect normal cells, but also cancer cells from oxidative damage,
thereby promoting their growth and survival (21).
Previous studies have demonstrated that Keap1 and
Nrf2 are aberrantly expressed in solid tumors. Kawasaki et
al (22) reported that abnormal
expression of Nrf2 is closely associated with clinical
characteristics of gastric cancer, including lymph node metastasis
and clinical stage. Nrf2 expression may also be associated with
resistance to chemotherapy. In addition, Isohookana et al
(23) suggested that Keap1
overexpression may be a promising prognostic biomarker for
pancreatic cancer. Shibata et al (24) utilized resequencing analysis to
demonstrate that esophageal squamous cancer is associated with an
Nrf2 gene mutation, which is linked with poor prognosis and
recurrence. Furthermore, Nrf2 mutations were identified to be
mainly located in the Keap1 binding domain. Another study by
Shibata et al (25) indicated
that Keap1 gene mutations in gallbladder cancer may result in Nrf2
overexpression. These authors speculated that this may be the
molecular mechanism of chemotherapy resistance.
At present, little is known regarding Keap1 and Nrf2
expression in lymphoma tissues. In the present study, Keap1 and
Nrf2 expression was detected in DLBCL tissues by
immunohistochemistry. The results indicated that Keap1 and Nrf2
expression was higher in DLBCL tissues than that in reactive lymph
node hyperplasia tissues, and their expression was correlated with
the IPI and Ann-Arbor clinical stage (P<0.05). In addition, and
Keap1 and Nrf2 expression was higher in patients with stage III/IV
DLBCL. In addition, no correlation was identified between Nrf2 and
Keap1 expression in DLBCL tissues. The reason for this remains
elusive but it may be due to Keap1 mutations, which alter the
structure of Keap1 such that the degradation of Nrf2 is impaired
(26). The present results suggest
that Keap1 and Nrf2 may have crucial roles in disease development,
and hence may be used as prognostic indicators.
Studies have been found that, in many other cancers,
Nrf2 may be closely linked to chemotherapy resistance, promote
tumor growth and lead to poor prognosis, including lung cancer
(27), pancreatic carcinoma
(23,28), breast cancer (29,30),
head and neck cancer (31), prostate
cancer (32), ovarian carcinoma
(33,34) and cervical cancer (35). In addition, in hematopoietic
malignancies, only few studies have reported on Nrf2 expression in
lymphoma cell lines. Zha et al (36) reported that in the Raji cell line,
treatment with disulfiram (DS) and DS/Cu causes excessive
production of reactive oxygen species such that Nrf2 expression is
inhibited, which subsequently promotes apoptosis in transplanted
tumors in nude mice. It has also been indicated that inhibition of
Nrf2 expression may promote apoptosis of lymphoma cells. A study by
Chen et al (37) on the
relapse of mantle cell lymphoma compared Nrf2 expression between
bortezomib-sensitive cell lines (Jeko and SP53) and resistant cell
lines (Mino and Rec-1) after bortezomib treatment. The results
indicated that Nrf2 expression was upregulated in
bortezomib-resistant cell lines, whereas it was decreased or not
significantly changed in sensitive cell lines. This suggested that
elevated Nrf2 expression may be associated with bortezomib
resistance in mantle cell lymphoma. These studies indicated that
elevated Keap1 and Nrf2 expression may be associated with
chemotherapy resistance and replace of lymphoma, but the underlying
mechanisms remain to be elucidated.
According to the results of the present study, Keap1
and Nrf2 may have crucial roles in the development of DLBCL and are
associated with patient prognosis. However, the present study has
certain limitations: According to the 2016 revision of the WHO
classification of lymphoid neoplasms, DLBCL comprises numerous
subtypes. Therefore, in the present study, different subtypes of
DLBCL cases were collected to increase the sample size and minimize
the error of the final statistical results. In addition, the
present study examined a relatively small number of cases, none of
the patients were followed up, and the median survival was unknown.
Therefore, further experiments are required that include the
examination of more samples and follow-up analyses for the same or
even different subtypes of DLBCL patients. Furthermore, cell and
animal experiments will be performed in the future to explore the
specific molecular mechanisms through which Keap1 and Nrf2 regulate
DLBCL and further determine whether Keap1 and Nrf2 expression is
associated with chemotherapy resistance in DLBCL.
Acknowledgements
The authors would like to thank two pathologists, Dr
Yamei Dang and Dr Fenghui Zhao in the Department of Pathology of
Gansu Provincial Hospital (Lanzhou, China), for their guidance
during the evaluation of staining.
Funding
This study was supported by National Natural Science
Foundation of China (grant nos. 81560498, 81260342 and
30960438).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL conceived and designed the experiments. XY, YZ,
LX, JZ, YQ and QJ performed the experiments. XY, LX and JZ analyzed
the data. XY drafted the manuscript, and HL and XY revised the
manuscript. The final version of the manuscript has been read and
approved by all authors, and each author believes that the
manuscript represents honest work.
Ethical approval and consent to
participate
The present study was approved by the Ethics
Committee of Gansu Provincial Hospital (Lanzhou, China) and all
patients provided written informed consent.
Consent for publication
The patient provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests regarding this study.
Glossary
Abbreviations
Abbreviations:
|
Keap1
|
kelch-like ECH-associated protein
1
|
|
Nrf2
|
nuclear factor erythroid-2-related
factor-2
|
|
DLBCL
|
diffuse large B-cell lymphoma
|
|
IPI
|
international prognostic index
|
|
ARE
|
antioxidant response element
|
References
|
1
|
Menon MP, Pittaluga S and Jaffe ES: The
histological and biological spectrum of diffuse large B-cell
lymphoma in the World Health Organization classification. Cancer J.
18:411–420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gustafson HL, Yao S, Goldman BH, Lee K,
Spier CM, LeBlanc ML, Rimsza LM, Cerhan JR, Habermann TM, Link BK,
et al: Genetic polymorphisms in oxidative stress-related genes are
associated with outcomes following treatment for aggressive B-cell
non-Hodgkin lymphoma. Am J Hematol. 89:639–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peroja P, Pasanen AK, Haapasaari KM,
Jantunen E, Soini Y, Turpeenniemi-Hujanen T, Bloigu R, Lilja L,
Kuittinen O and Karihtala P: Oxidative stress and redox
state-regulating enzymes have prognostic relevance in diffuse large
B-cell lymphoma. Exp Hematol Oncol. 1:22012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uruno A and Motohashi H: The Keap1-Nrf2
system as an in vivo sensor for electrophiles. Nitric Oxide.
25:153–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahmed SM, Luo L, Namani A, Wang XJ and
Tang X: Nrf2 signaling pathway: Pivotal roles in inflammation.
Biochim Biophys Acta. 1863:585–597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang DD: The Nrf2-Keap1-ARE signaling
pathway: The regulation and dual function of Nrf2 in cancer.
Antioxid Redox Signal. 13:1623–1626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamazaki H, Tanji K, Wakabayashi K,
Matsuura S and Itoh K: Role of the Keap1/Nrf2 pathway in
neurodegenerative diseases. Pathol Int. 65:210–219. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barančík M, Grešová L, Barteková M and
Dovinová I: Nrf2 as a key player of redox regulation in
cardiovascular diseases. Physiol Res. 65(Suppl 1): S1–S10.
2016.PubMed/NCBI
|
|
9
|
Sandford AJ, Malhotra D, Boezen HM,
Siedlinski M, Postma DS, Wong V, Akhabir L, He JQ, Connett JE,
Anthonisen NR, et al: NFE2L2 pathway polymorphisms and lung
function decline in chronic obstructive pulmonary disease. Physiol
Genomics. 44:754–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taguchi K and Yamamoto M: The KEAP1-NRF2
system in cancer. Front Oncol. 7:852017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng ML, Lu YF, Chen H, Shen ZY and Liu
J: Liver expression of Nrf2-related genes in different liver
diseases. Hepatobiliary Pancreat Dis Int. 14:485–491. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Batliwala S, Xavier C, Liu Y, Wu H and
Pang IH: Involvement of Nrf2 in ocular diseases. Oxid Med Cell
Longev. 2017:17038102017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pasanen AK, Kuitunen H, Haapasaari KM,
Karihtala P, Kyllönen H, Soini Y, Turpeenniemi-Hujanen T and
Kuittinen O: Expression and prognostic evaluation of oxidative
stress markers in an immunohistochemical study of B-cell derived
lymphomas. Leuk Lymphoma. 53:624–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Wang X, Wu W, Dang H and Wang B:
Expression of the Nrf2 and Keap1 proteins and their clinical
significance in osteosarcoma. Biochem Biophys Res Commun.
473:42–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soini Y, Eskelinen M, Juvonen P, Kärjä V,
Haapasaari KM, Saarela A and Karihtala P: Nuclear Nrf2 expression
is related to a poor survival in pancreatic adenocarcinoma. Pathol
Res Pract. 210:35–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu
Y and Dong W: ROS and ROS-mediated cellular signaling. Oxid Med
Cell Longev. 2016:43509652016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
Igarashi K, Engel JD and Yamamoto M: Keap1 represses nuclear
activation of antioxidant responsive elements by Nrf2 through
binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moi P, Chan K, Asunis I, Cao A and Kan YW:
Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic
leucine zipper transcriptional activator that binds to the tandem
NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl
Acad Sci USA. 91:9926–9930. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Villeneuve NF, Lau A and Zhang DD:
Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin
proteasome system: An insight into cullin-ring ubiquitin ligases.
Antioxid Redox Signal. 13:1699–1712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang M, Zhang C, Zhang L, Yang Q, Zhou S,
Wen Q and Wang J: Nrf2 is a potential prognostic marker and
promotes proliferation and invasion in human hepatocellular
carcinoma. BMC Cancer. 15:5312015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawasaki Y, Ishigami S, Arigami T,
Uenosono Y, Yanagita S, Uchikado Y, Kita Y, Nishizono Y, Okumura H,
Nakajo A, et al: Clinicopathological significance of nuclear factor
(erythroid-2)- related factor 2 (Nrf2) expression in gastric
cancer. BMC Cancer. 15:52015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Isohookana J, Haapasaari KM, Soini Y and
Karihtala P: Keap1 expression has independent prognostic value in
pancreatic adenocarcinomas. Diagn Pathol. 10:282015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shibata T, Kokubu A, Saito S,
Narisawa-Saito M, Sasaki H, Aoyagi K, Yoshimatsu Y, Tachimori Y,
Kushima R, Kiyono T and Yamamoto M: NRF2 mutation confers malignant
potential and resistance to chemoradiation therapy in advanced
esophageal squamous cancer. Neoplasia. 13:864–873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shibata T, Kokubu A, Gotoh M, Ojima H,
Ohta T, Yamamoto M and Hirohashi S: Genetic alteration of Keap1
confers constitutive Nrf2 activation and resistance to chemotherapy
in gallbladder cancer. Gastroenterology. 135:1358–1368, 1368. e1-4.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayes JD and McMahon M: NRF2 and KEAP1
mutations: Permanent activation of an adaptive response in cancer.
Trends Biochem Sci. 34:176–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh A, Boldin-Adamsky S, Thimmulappa RK,
Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F,
Watson WH, et al: RNAi-mediated silencing of nuclear factor
erythroid-2-related factor 2 gene expression in non-small cell lung
cancer inhibits tumor growth and increases efficacy of
chemotherapy. Cancer Res. 68:7975–7984. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Genrich G, Kruppa M, Lenk L, Helm O,
Broich A, Freitag-Wolf S, Rocken C, Sipos B, Schäfer H and Sebens
S: The anti-oxidative transcription factor Nuclear factor E2
related factor-2 (Nrf2) counteracts TGF-β1 mediated growth
inhibition of pancreatic ductal epithelial cells -Nrf2 as
determinant of pro-tumorigenic functions of TGF-β1. BMC Cancer.
16:1552016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nioi P and Nguyen T: A mutation of Keap1
found in breast cancer impairs its ability to repress Nrf2
activity. Biochem Biophys Res Commun. 362:816–821. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khatri R, Shah P, Guha R, Rassool FV,
Tomkinson AE, Brodie A and Jaiswal AK: Aromatase inhibitor-mediated
downregulation of INrf2 (Keap1) leads to increased Nrf2 and
resistance in breast cancer. Mol Cancer Ther. 14:1728–1737. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stacy DR, Ely K, Massion PP, Yarbrough WG,
Hallahan DE, Sekhar KR and Freeman ML: Increased expression of
nuclear factor E2 p45-related factor 2 (NRF2) in head and neck
squamous cell carcinomas. Head Neck. 28:813–818. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Frohlich DA, McCabe MT, Arnold RS and Day
ML: The role of Nrf2 in increased reactive oxygen species and DNA
damage in prostate tumorigenesis. Oncogene. 27:4353–4362. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zembutsu H: Keap1-Nrf2 pathway and drug
resistance in epithelial ovarian cancer. Pharmacogenomics.
12:1516–1517. 2011.PubMed/NCBI
|
|
34
|
Shim GS, Manandhar S, Shin DH, Kim TH and
Kwak MK: Acquisition of doxorubicin resistance in ovarian carcinoma
cells accompanies activation of the NRF2 pathway. Free Radic Biol
Med. 47:1619–1631. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma JQ, Tuersun H, Jiao SJ, Zheng JH, Xiao
JB and Hasim A: Functional role of NRF2 in cervical carcinogenesis.
PLoS One. 10:e01338762015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zha J, Chen F, Dong H, Shi P, Yao Y, Zhang
Y, Li R, Wang S, Li P, Wang W and Xu B: Disulfiram targeting
lymphoid malignant cell lines via ROS-JNK activation as well as
Nrf2 and NF-kB pathway inhibition. J Transl Med. 12:1632014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen Z, Pittman EF, Romaguera J, Fayad L,
Wang M, Neelapu SS, McLaughlin P, Kwak L and McCarty N: Nuclear
translocation of B-cell-specific transcription factor, BACH2,
modulates ROS mediated cytotoxic responses in mantle cell lymphoma.
PLoS One. 8:e691262013. View Article : Google Scholar : PubMed/NCBI
|