Introduction
Inflammatory bowel disease (IBD), including
ulcerative colitis (UC) and Crohn's disease (CD), are common
diseases in Europe and North America. However, according to a
recent epidemiologic data (1), IBD
is becoming increasingly common in Asia. Therefore, more attention
and health care resources on IBD would be required around the
world.
The mechanism of IBD is considered to be closely
related to genetic, immunological and environmental factors.
Several therapies, including novel medicine, biologic treatment,
stem cell transplantation etc., have treatment effects since they
are relevant to disease initiation, progression or both. Stem cell
transplantation has been considered as an effective therapy of IBD,
especially in refractory patients with CD who have no other
therapeutic options (2).
Mesenchymal stem cells (MSCs) are undifferentiated
cells that have the unique potential to develop into many different
cell types in the body as well as the function of immunomodulation.
Therefore, these cells have emerged as leading candidates for
regenerative treatment and shown great promise in numerous clinical
trials (3,4). The key process of tissue repair is as
follows: Activated by several inflammatory cytokines and
chemokines, MSCs migrate to sites of damaged location (5,6), and
then modulate the immunological function and repair the damaged
part.
However, a significant barrier to the effective
implementation of MSCs therapy is the inability to target these
cells to tissues of interest with high efficiency and engraftment
(7). Therefore, researches on
promoting migration and engraftment of MSCs to target tissue have
been the focus of attention. Several methods, including cell
surface modification, nanoparticles and advanced biomaterials
(8) packing, have been used to
enhance the ability to migrate.
The stromal-derived factor-1 (SDF-1) plays an
essential role in stem cell homing by recruiting the progenitor
cells that express its cognate receptor, CXC chemokine receptor 4
(CXCR-4). This role of SDF-1 has been confirmed in several
researches (9–12). It is the theoretical foundation of
CXCR-4 gene overexpressed MSCs that improve their homing capacity.
As more MSCs migrate to the damaged location, the effect of repair
becomes more remarkable. In this study, we used a lentiviral gene
vector as a carrier to deliver CXCR-4 gene into MSCs to make this
gene overexpress further. We examined the effects of CXCR-4
overexpressed MSCs migration in vitro as well as the
capability of homing and repairing after transplantation of these
cells in vitro.
Materials and methods
Mice
Four- to eight-week-old male BALB/c mice provided by
the Research and Technology Service Center, 302 Hospital of PLA
(Beijing, China) were were group-housed under controlled
temperature (26°C) and a 12-h light/dark cycle, fed standard mouse
chow and clean water. The mice were maintained under specific
pathogen-free conditions and allowed to acclimatize for 1 week
prior to the commencement of experimental work. All animal
experiments were approved by the Animal Ethics Committee of the
Animal Facility of Chinese PLA General Hospital (Beijing,
China).
Isolation and culture of mice bone
marrow mesenchymal stem cells (BMSCs)
Bone marrow (BM) cell suspension was obtained by
flushing marrow cavity of four- to five-week-old male mice with
α-minimum essential medium (α-MEM; HyClone; GE Healthcare Life
Sciences, Logan, UT, USA). Then, the BM cells were cultured with
α-MEM containing 10% heat-inactivated fetal bovine serum (FBS;
HyClone; GE Healthcare Life Sciences) and penicillin-streptomycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Cells were
cultured at 37°C in a 5% CO2 humidified incubator. To acquire
putative BMSCs, non-adherent cells and tissue debris were removed
by phosphate-buffered saline (PBS) washing after 72 h (13). The culture medium was changed every 3
days until the cell density reached about 90%. BMSCs were detached
by 0.25% trypsin containing 0.02% EDTA (HyClone; GE Healthcare Life
Sciences) and expanded. All BMSCs were utilized for subsequent
experiments.
To better observe the BMSCs, cells were labeled by
cell tracker. The methods were as follows: BMSCs were suspended at
a density of 3×106/ml with α-MEM. Every 1×106 cells were incubated
with 3 µl CM-Dil (1 µg/ml) (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C for 5 min then 4°C for 15 min.
Cells were washed twice and suspended by α-MEM.
Transfection of CXCR-4 gene
A lentiviral vector with both Mice CXCR-4 gene and
Luciferase report gene was used. All operation associated with this
lentiviral vector, including the design, construction and
detection, were performed by Shanghai Genechem Co., Ltd. (Shanghai,
China). Virus aliquots were suspended in PBS and stored at −80°C
until needed. Samples were diluted 1:1 before viral titers were
measured.
The transfection processes were modified according
to the Ricks' method (14). BMSCs at
passage 3 were cultured in 12-well plates at a density of 5×103
cells in 2 ml of α-MEM containing 10% FBS per well. After 24 h
plating, transfection was carried out at 20 multiplicity of
infection (MOI) in the presence of 2 µg/ml Polybrene
(Sigma-Aldrich; Merck KGaA). Cells were cultured in incubator for
10 h, then the transfection medium was replaced with fresh α-MEM
containing 10% FBS. The absorbance of CXCR-4 gene transfected BMSCs
(CXCR-BMSCs) was quantified with a luminometer (Lonza, Switzerland)
according to the manual. To detect the viability and the capability
of differentiation about CXCR-BMSCs, cells were sequentially
expanded thereafter and were used for the next experiment.
Analysis of the CXCR-4 gene expression
in CXCR-BMSCs
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was applied to analyze the expression of CXCR-4
gene in CXCR-BMSCs.
RNA extraction and RT-qPCR
Whole RNA was extracted from BMSCs and CXCR-BMSCs by
the utilization of Total RNA Kit (SinoGene Scientific Co., Ltd.,
Beijing, China) according to the manual. The sequences of primers
for PCR were as follows: Mice CXCR-4 gene, 5′-CCTCTACAGCAGCGTTCT-3′
(forward), 3′-GTTTCCTTGGCCTTTGAC-5′ (reverse); β-actin,
5′-CGTTGACATCCGTAAAGACC-3′ (forward), and
3′-CTAGGAGCCAGAGCAGTAATC-5′ (reverse). The conditions and processes
of PCR amplification were as follows: Pre-denature at 95°C for 10
min, then 40 cycles of denature at 95°C for 20 sec, annealing at
60°C for 30 sec, extension at 60°C for 30 sec. qPCR crossing
threshold (Ct) values were obtained during the exponential
amplification phase and Prizm4 was used to analyze data.
Identification of biological
characteristics of BMSCs
Flow cytometry analysis and experiment about
differentiation of BMSCs were applied to identify BMSCs.
Flow cytometry analysis
Two groups of cells, BMSCs group and CXCR-BMSCs
group, were trypsinized, inactivated with FBS and washed twice with
PBS. These cells were suspended in 100 µl PBS and incubated at 4°C
for 20 min with PE-conjugated anti-mouse CD90.2 (BD Biosciences,
Franklin Lakes, NJ, USA), Alexa Fluour-conjugated anti-mouse CD105
(BD Biosciences), PE Cy™-conjugated anti-mouse CD11b (eBioScience;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
FITC-conjugated anti-mouse CD45 (BD Biosciences) as described
previously (13).
Experiment on differentiation of
BMSCs
The function of BMSCs differentiation into
osteogenic and adipogenic lineages was examined to illustrate the
biological characteristics of BMSCs (15). Two groups of cells were planted
separately in 24-well plates with the concentration of 2×104
cells/well. All details about experiment and detection of BMSCs
differentiation into osteogenic and adipogenic lineages were as
previously described (13).
Cell viability evaluation
Viability of two groups of cells was confirmed by
Trypan Blue staining exclusion. The numbers of live cells and dead
cells were counted and the rate of living cell calculated. Living
cell rate (%) = (living/total cells) × 100%.
Migration assay in vitro
To investigate the migration ability of these two
groups of cells, a chemotaxis assay was performed by the
utilization of 24-well plate transwell chamber with 8 µm pore
filters (Corning International Co., Tokyo, Japan). Two groups of
cells were plated in the upper chambers with 2×105 cells/ml in 200
µl of FPS free α-MEM containing 0.1% BSA, and the lower chambers
with 600 µl recombinant mouse SDF-1α (50 ng/ml). The whole
transwell chamber was set at 5% CO2, 37°C for 10 h. The migrated
cells on the lower side of the filter was stained by crystal violet
and their number was determined by two independent observers by
counting three random fields per well using an Olympus BH-2
microscope (Olympus, Tokyo, Japan; magnification, ×200).
Mice colitis model and BMSCs
transplantation
Six- to eight-week-old female BABL/C mice were
randomly assigned into four groups: Control group (Ctr group),
colitis model group (T group), BMSCs transplantation group (MT
group) and CXCR-BMSCs transplantation group (CMT group),
n=12/group. Colitis was induced by 2.0 mg TNBS/50% ethanol enema
(Sigma-Aldrich; Merck KGaA), as previously described (13). At 24 h after the colitis model was
established, mice in MT group and CMT group were injected 1×106
cells in 100 µl PBS via tail vein. Mice in Ctr group and T group
were given pure PBS in the volume equivalent to the PBS of MT group
and CMT group. The body weight and Disease Activity Index (DAI) of
mice in each group were recorded and calculated. Mice were
respectively sacrificed at the 3rd, 5th, 9th, 13th day after BMSCs
transplantation. The sections of tissues were H&E-stained to
assess the severity of inflammation in colitis.
Identification of homing of BMSCs
Observation of BMSCs with red fluorescence and
detection of mouse Y-chromosome (Sry) gene in colon mucosa were
applied to identify the homing of BMSCs.
Fluorescence observation
The sections of colonic specimens were observed with
fluorescent microscopy (Olympus).
Sry gene detection
Whole DNA was extracted from colon tissues in each
group by the utilization of Total DNA Kit (SinoGene Scientific Co.,
Ltd., Beijing, China) according to the manual. The sequences of
primers for PCR were as follows: Mouse Sry,
5′-TCGGAGGGCTAAAGTGTC-3′ (forward), and 3′-TCTTGCCTGTATGTGATGG-5′
(reverse). The conditions and processes of PCR amplification were
as follows: Pre-denature at 94°C for 3 min, then 30 cycles of
denature at 94°C for 20 sec, annealing at 58°C for 20 sec, and
extension at 72°C for 30 sec. qPCR crossing threshold (Ct) values
were obtained during the exponential amplification phase and Prizm4
was used to analyze data.
Immunohistochemistry
Immunohistochemistry was performed to detect the
expression of occludin and vascular endothelial growth factor
(VEGF) in colon mucosa. The conditions and processes of
immunohistochemistry were as follows: The slices of 4 µm
formalin-fixed and paraffin-embedded colon sections were
respectively incubated with a rabbit anti-mouse primary antibody to
Occludin (1:200, ab64482; Abcam) and a rabbit anti-VEGF antibody
(1:200, bs-0279R; Bioss, Beijing, China) at 4°C overnight, after
washed with PBS 3 times, the slices were incubated with a goat
anti-rabbit secondary polyclonal antibody (1:1,000, cat. no.
ZDR-5306; ZSGB-Bio, Beijing, China) at 37°C for 30 min, the samples
were finally lightly stained with H&E and examined.
Statistical analysis
Data were represented as mean ± standard deviation.
The t-test was used for comparison between two groups. Statistical
comparisons were performed using one-way ANOVA followed by Tukey's
post hoc test by SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Transfection of CXCR-4 gene in
BMSCs
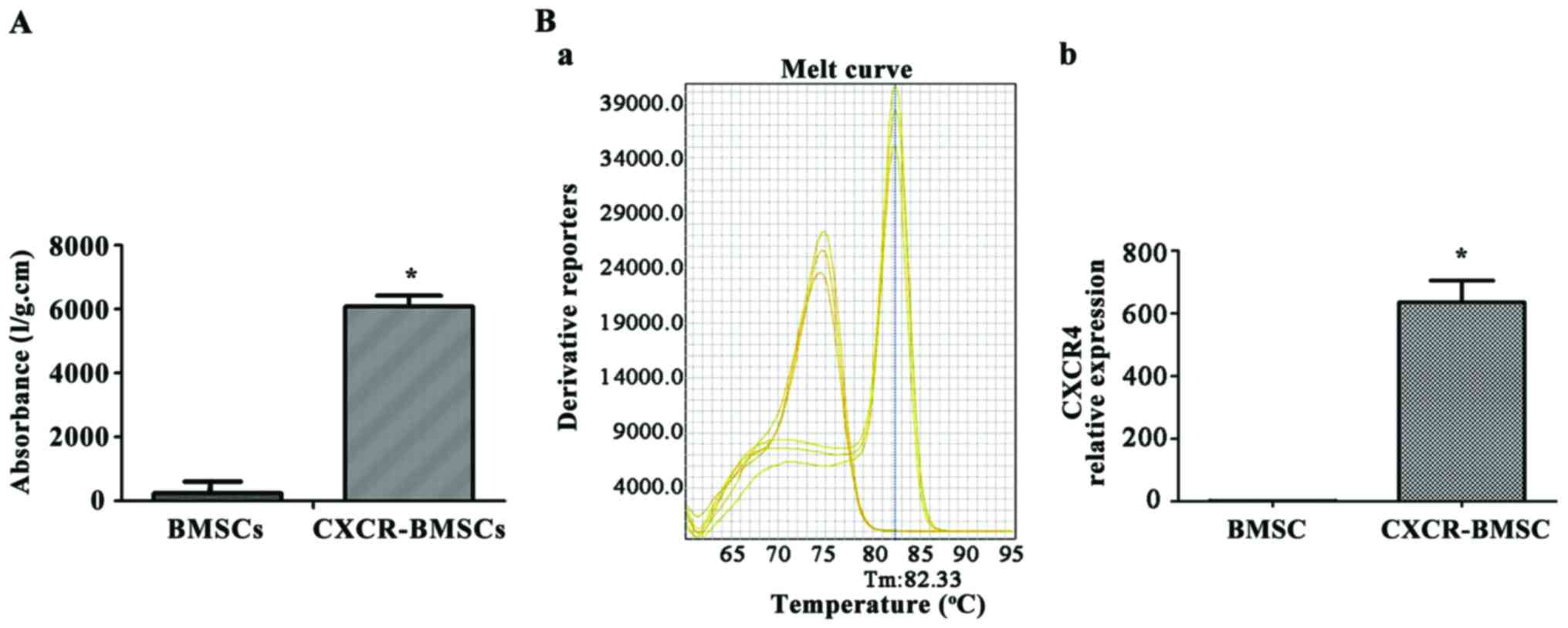
The absorbance of samples from BMSCs and CXCR-BMSCs
was quantified to illustrate the existence of CXCR-4 gene in these
cells (Fig. 1A). The luciferase
report gene absorbance was low in BMSCs, but significantly high in
CXCR-BMSCs (P<0.05).
RT-qPCR was performed to detect CXCR-4 gene
expression in BMSCs. The mRNA transcription level in BMSCs (P4) was
low, but significantly high in CXCR-BMSCs. The result indicated
that the expression of CXCR-4 gene in CXCR-BMSCs (Fig. 1B) was significantly higher than that
in BMSCs (P<0.05).
Biological characteristics of BMSCs
and CXCR-BMSCs
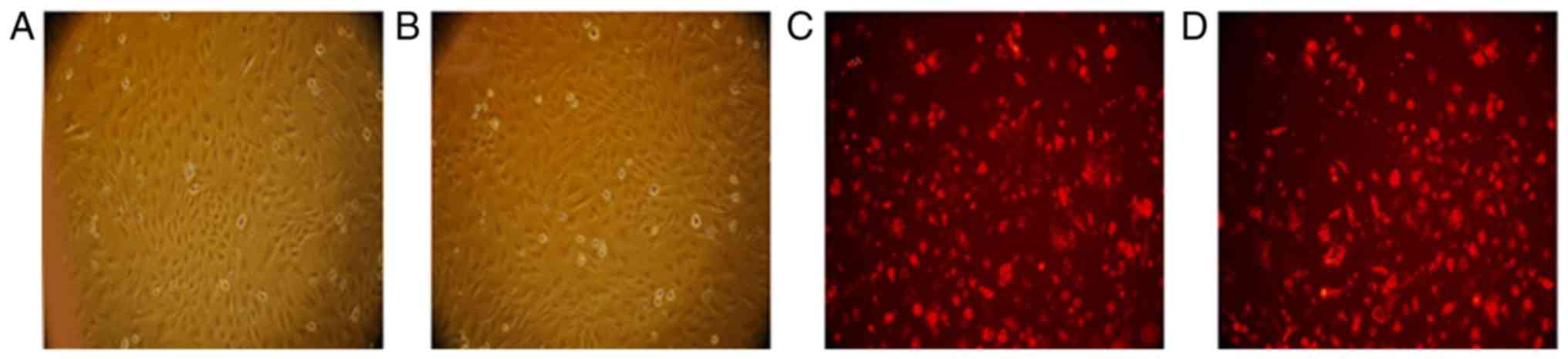
Comparing the morphological characteristics of BMSCs
and CXCR-BMSCs on the same passage, we found that all cells were
long spindle and arranged closely (Fig.
2A and B). The cell membrane of both BMSCs and CXCR-BMSCs
presented red under the green fluorescence observation after being
marked by CM-Dil (Fig. 2C and
D).
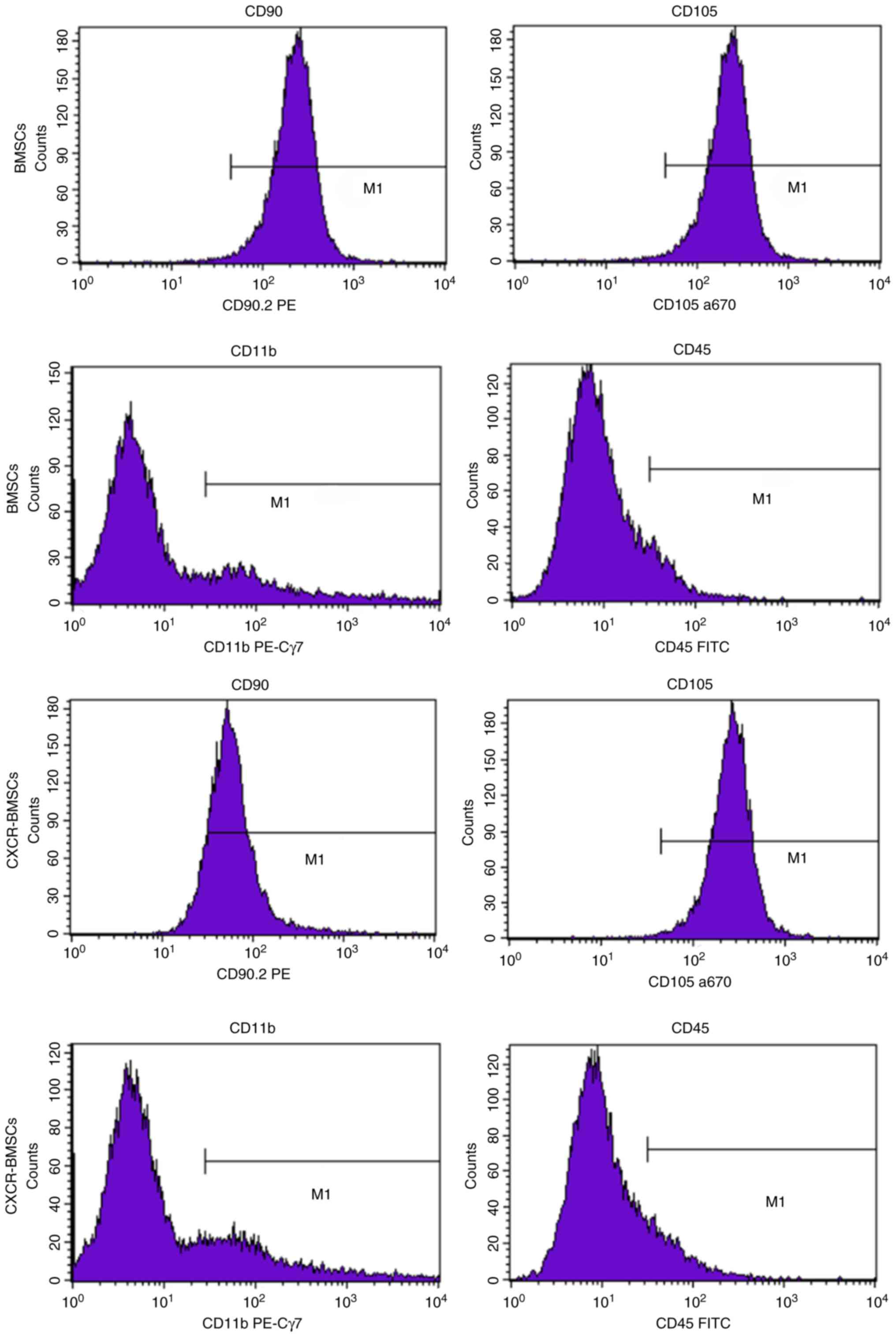
Cell phenotype of the BMSCs and CXCR-BMSCs were
authenticated by flow cytometry. The expression of bone marrow
progenitor cell marker CD90 and CD105 were positive, while the
expression of amonocyte/macrophage marker CD11b and pan leukocyte
marker CD45 were negative. Details were as follows: The expression
of CD90 (92.29%) and CD105 (99.30%), CD11b (19.07%) and CD45
(8.86%) in BMSCs; the expression of CD90 (88.79%) and CD105
(99.68%) b, CD11b (17.87%) and CD45 (10.37%) in CXCR-BMSCs
(Fig. 3). It demonstrated that BMSCs
were cultured successfully and transfection of CXCR-4 gene had no
impact on the cell phenotype.
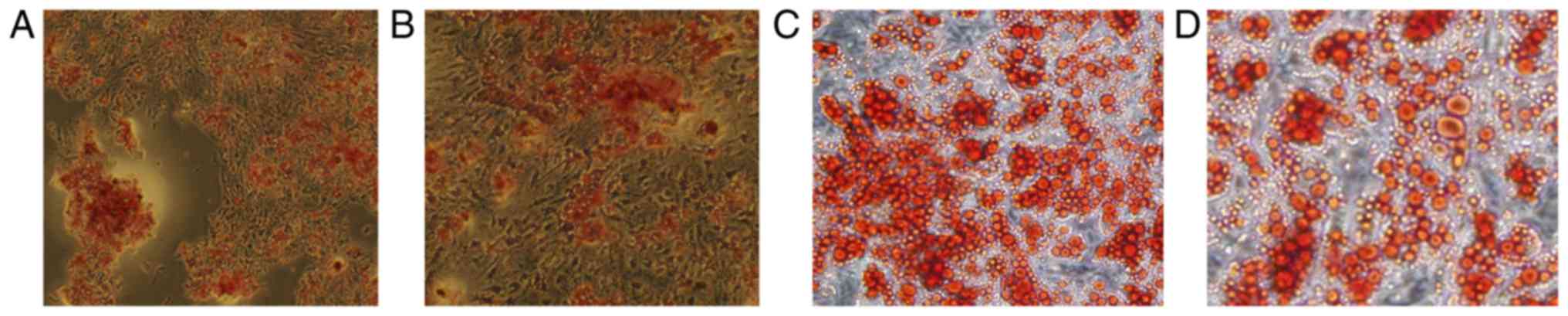
The capability of differentiation into osteoblasts
and adipocytes is a crucial biological feature of MSCs. After the
culturing with osteogenic medium for 3 weeks and alizarin red
staining, mineralized nodules were formed and could be observed in
both two groups of cells (Fig. 4A and
B). Red fat particles could also be observed after cells were
induced with adipogenic medium for 8 days and stained by Oil Red O
(Fig. 4C and D).
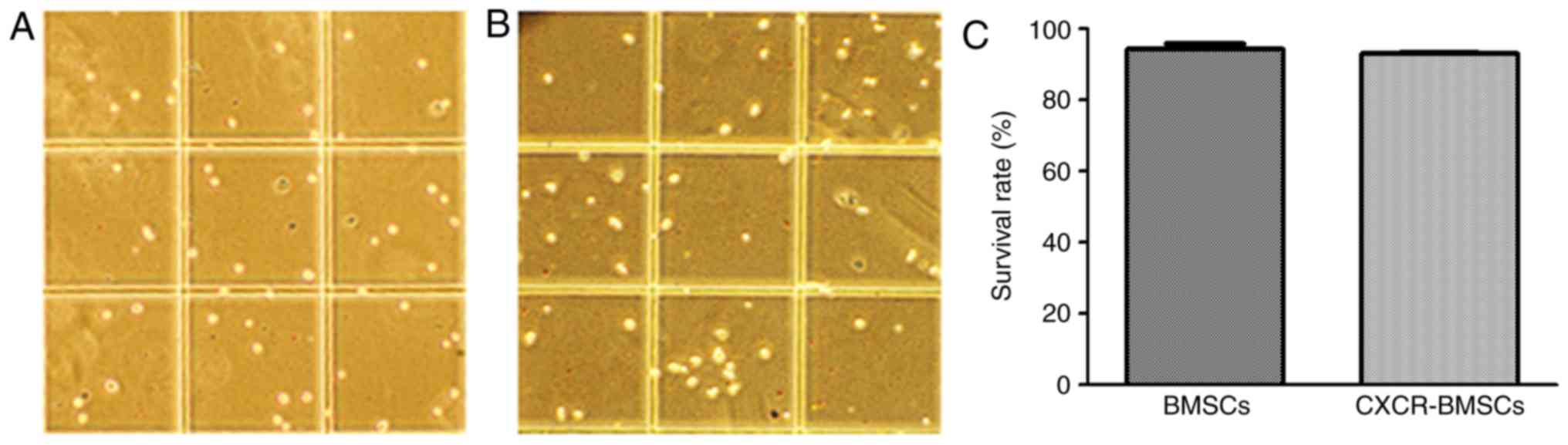
Cell viability of BMSCs and
CXCR-BMSCs
The DNA of inanimate cells can be stained by Trypan
Blue, but living cells with completed membrane can inhibit this
function. By counting the numbers of live cells and dead cells, the
rate of living cell can be calculated. In both groups, rates of
living cell were above 93% and no statistical difference (Fig. 5). It demonstrated that transfection
of CXCR-4 gene had no influence on the viability of BMSCs.
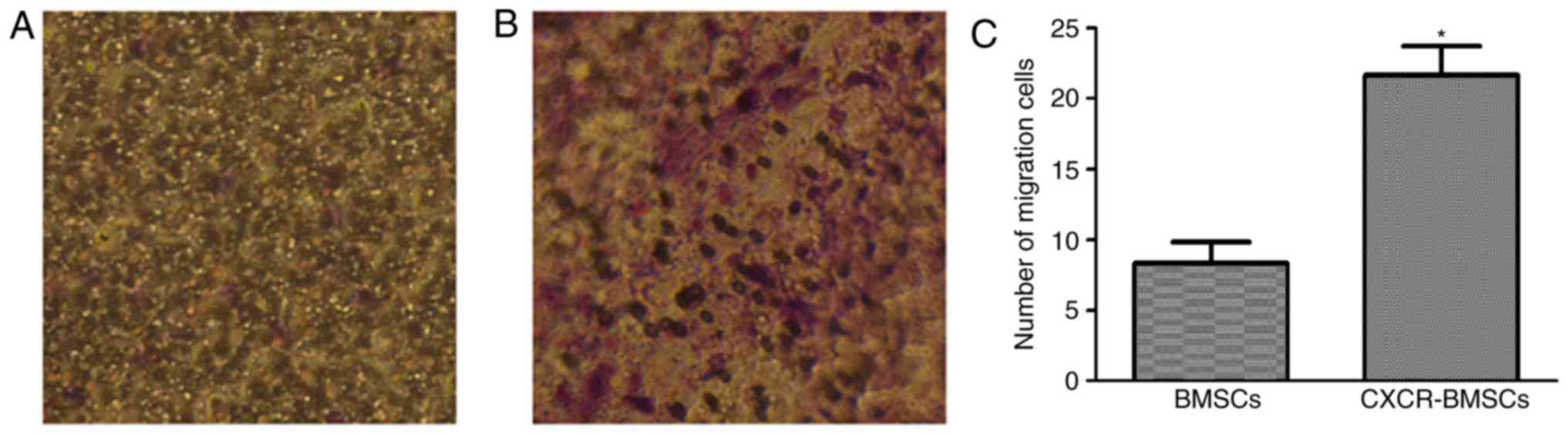
CXCR-4 promotes the migration of BMSCs
to SDF-1 in vitro
The cells stained by crystal violet on the lower
side of the filter in both groups (Fig.
6) were counted and compared. The results showed that the
number of CXCR-BMSCs migrating to SDF-1 was more than that of BMSCs
(P<0.05).
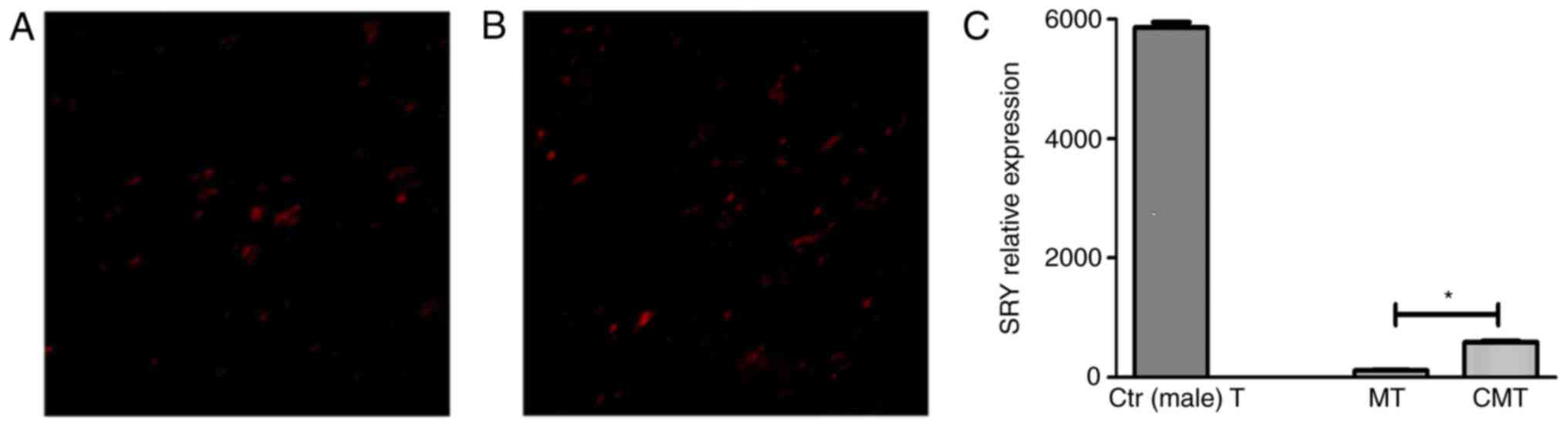
CXCR-4 promotes the homing of BMSCs to
damaged intestinal mucosa
Red-labeled BMSCs on the injured colon were observed
in both MT and CMT group (Fig. 7A and
B). No red fluorescence was seen in T group.
Sry gene was related to Y-chromosome in male.
Through the detection of Sry gene in colon tissue, the situation of
BMSCs homing to damaged intestinal mucosa can be reflected. The
results showed the existence of Sry gene in both MT and CMT group,
and the relative expression of sry gene in CMT group was more than
that in MT group (P<0.05). Besides, there was no expression of
Sry gene in T group, in which the female mice were not injected the
BMSCs isolated from the male mice (Fig.
7C).
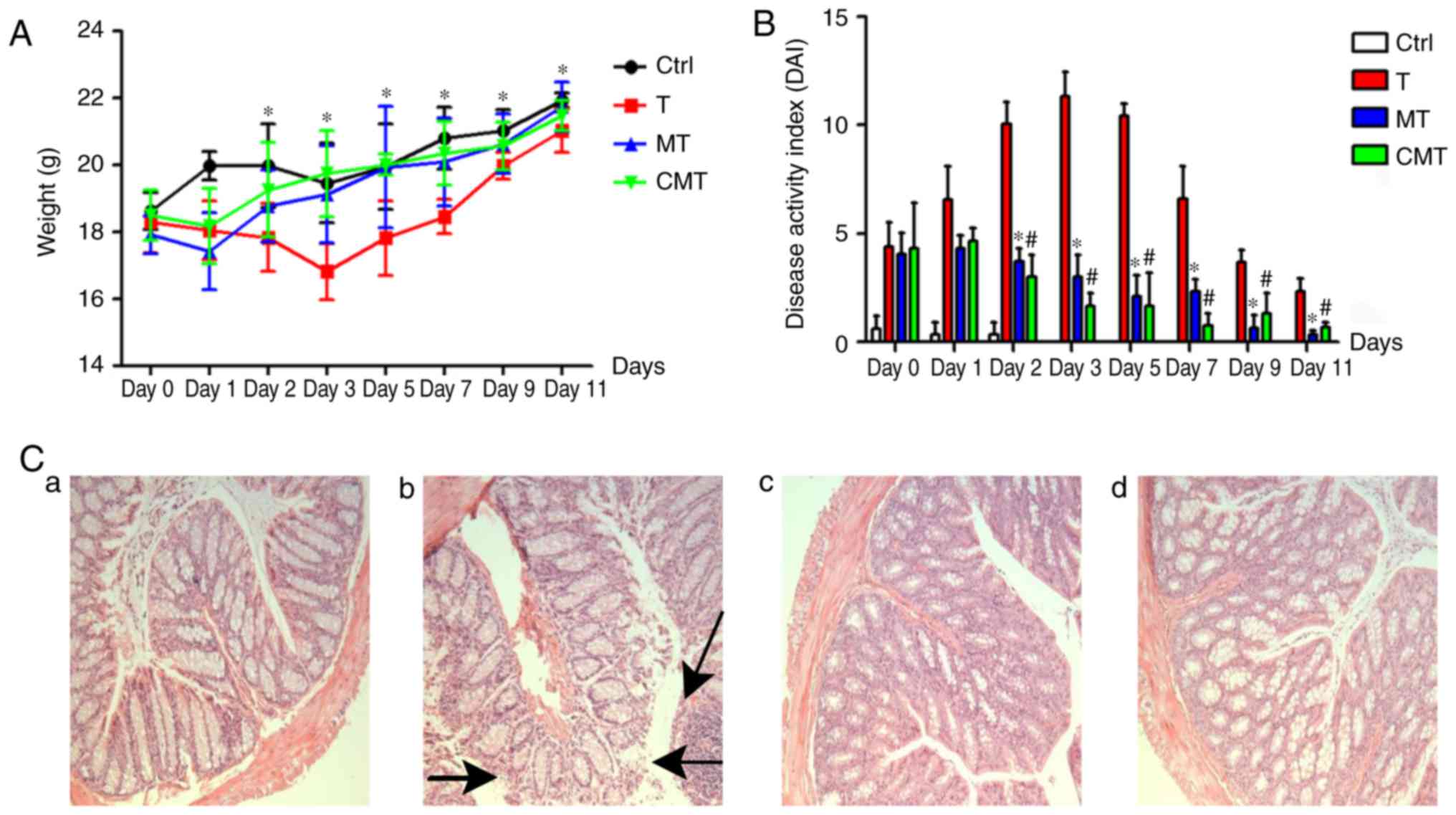
CXCR-4 overexpression BMSCs cure
TNBS-induced colitis better
Clinical manifestation associated with colitis, such
as weight loss, diarrhea, loose stools, even gastrointestinal
bleeding, were observed in groups in which the mice accepted enema
with TNBS. The DAI was calculated according to the Clinical scoring
standard (Table I), which was
modified from the Cooper's scoring method. All manifestations were
improved after injection with two kinds of BMSCs after 2 days
(P<0.05), but the difference in DAI between MT and CMT group was
not significant (P>0.05; Fig. 8A and
B).
 | Table I.Clinical scoring standard. |
Table I.
Clinical scoring standard.
| Stool
consistency | Fecal blood | Body weight loss |
|---|
| 0: Normal | 0: No bleeding | 0: None |
| 1: Wet stools |
| 1: 0–5% weight
loss |
| 2: Soft stools with
sticking surface | 2: Slight
bleeding | 2: 5–10% weight
loss |
| 3: Loose stools | 3: Moderate
bleeding | 3: 10–15% weight
loss |
| 4: Watery
diarrhea | 4: Severe
bleeding | 4: >15% weight
loss |
Compared with the normal colon mucosa in Ctr group,
more serious inflammation and mucosa damage were observed in T
group (Fig. 8C-a and -b). Damaged
mucosa rehabilitated predominantly after BMSCs and CXCR-4 gene
overexpressed BMSCs transplantation, especially in the latter one
(Fig. 8C-c and -d).
CXCR-4 gene overexpressed BMSCs
promotes the repair of damaged intestinal mucosa
Occludin, one of the tight junction proteins, is
critical to protecting the integrity of intestinal mucosa. There
were different amounts of Occludin expression in each group
(Fig. 9A-a-d), with the least
expression in T group, the most expression in CMT group, and the
expression in MT group less than that in CMT group.
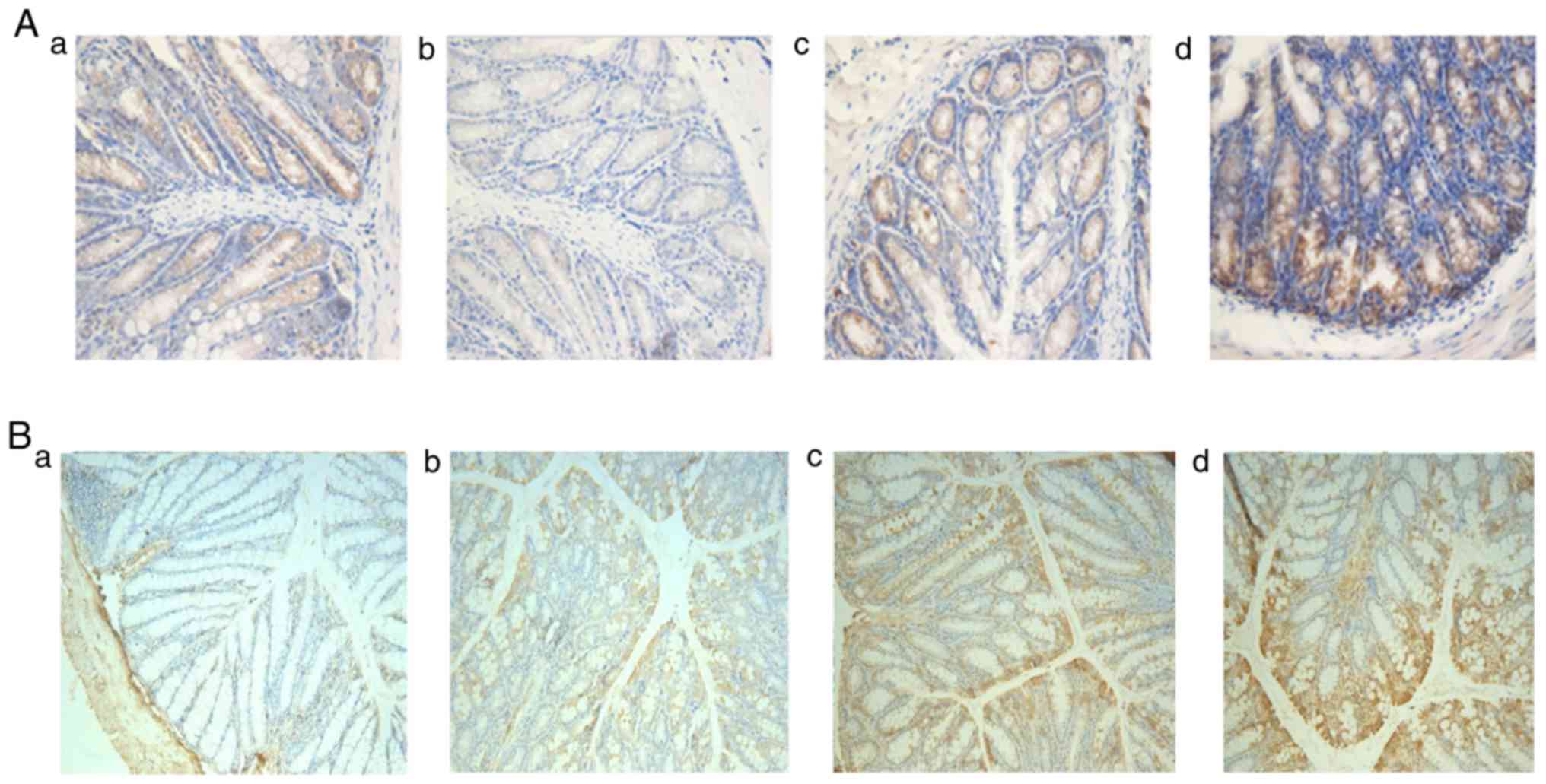 | Figure 9.Immunohistochemistry of occludin and
VEGF in colonic tissues in all groups. (A) Immunohistochemistry of
Occludin in colonic tissues in all groups (a, control group; b, T
group; c, MT group; and d, CMT group) magnification, ×200. (B)
Immunohistochemistry of VEGF in colonic tissues in all groups (a,
control group; b, T group; c, MT group; and d, CMT group)
magnification, ×200. VEGF, vascular endothelial growth factor. |
VEGF promotes the repair of damaged intestinal
mucosa by accelerating the growth of vessels. There were different
amounts of VEGF expression in each group (Fig. 9B-a-d), with the least expression in T
group, the most expression in CMT group, and the expression in MT
group less than that in CMT group.
Discussion
Several studies and clinical trials have been
performed worldwide to present the advantages of stem cell therapy
in treating a variety of diseases. MSCs transplantation has also
been proposed as a promising strategy for IBD treatment (16,17) and
BMSCs have become an ideal candidate for cell transplantation owing
to their biological characteristics (18). However, the therapeutic effect of
BMSCs transplantation is limited because the efficiency of cell
homing to the damaged tissues is low (19). This low efficiency has been found in
the previous study (13). Thus,
finding a proper method for BMSCs to improve the capacity of homing
is crucial to a successful cell therapy.
Several studies have confirmed that SDF-1 can be
expressed/secreted by several tissues/organs in the body (9) and up-regulated in stressed or injured
tissues (20,21) and the CXCR4 is the specific receptor
of SDF-1. Thus, SDF-1/CXCR4 axis plays a key role in promoting the
migration and homing of stem cells to target site. In this study,
CXCR-4 gene was carried by lentiviral vector and transfected to
BMSCs in order to make it overexpress. The CXCR-4 gene
overexpression in CXCR-BMSCs was confirmed by qPCR technology.
The function of BMSCs in promoting damaged tissues
regeneration is the basis of repairing injured mucosa, which is
intrinsically related to the biological characteristics of BMSCs.
Therefore, the features of both BMSCs and CXCR-BMSCs were detected
by morphology, cell phenotype and capability of differentiation. A
series of related experiments have proved that the overexpression
of CXCR-4 on BMSCs has no influence on original biological features
and viability of BMSCs themselves.
Migration and homing of CXCR-4 gene overexpressed
BMSCs were detected and compared with those of common BMSCs in
vitro and vivo. Migration assay was performed and the
results demonstrated that CXCR-4 gene overexpressed BMSCs were
easier to migrate. In vivo, two groups of cells were
transplanted into the TNBS-induced colitis model. The number of
CXCR-BMSCs homing to the damaged intestinal mucosa was much more
than that of BMSCs, which was confirmed by observation of red
fluorescence and detection of Sry gene. Besides, clinical
manifestation, including the variation of body weight and DAI, also
proved that the curative effect of CXCR-4 gene overexpressed BMSCs
on colitis was better than that of common BMSCs. At present, there
are two methods for colitis animal models construction: One is DSS
oral method, and the other is TNBS enema method. Because we used
enema method in the experiment, and feces of mice will lead to a
greater impact on enema effect. Therefore, mice underwent fasting
for 36 h before the enemata and back to eat after enamata. The
enama was not conducted every day for 12 days (day 0–12). It was
only done once at day 0. For small mammals, eating or not in a
short period of time has great impact on the weight, so after enema
(back to eating) the weight would rise, which apparently is related
to the resumption of eating. In addition, because ‘weight’ itself
is a dynamic index, lack of convincing. In order to better explain
the occurrence of symptoms and remission of symptoms after
treatment, so we use the disease activity score (DAI). The DAI
score prove the successful construction of the model. And we know
that tissue damage is able to self-repair. With the body
self-healing, even the module DAI score is also showing a downward
trend, this is the objective reality.
Available evidence has indicated that BMSCs
accumulate in injured colon and there are several potential
mechanisms for BMSCs to have the therapeutic effect on colitis,
including mucosa repair, immunomodulation and so on. In this study,
the expression of Occludin was detected to reflect the tight
junctional protein production after BMSCs transplantation, and
tight junctional proteins are associated with maintaining the
integrity of epithelium (22), which
is essential for the physiologic function of intestinal mucosa.
Besides, BMSCs can secrete angiogenic cytokines including bFGF and
VEGF (23), which are beneficial to
the growth of vessels to promote the repair of damaged mucosa.
In conclusion, the number of BMSCs located at the
target site has great influence on the therapeutic effect.
Therefore, improving the homing of BMSCs is crucial. SDF-1, a
common cytokine presented on the inflamed colonic tissues, can
specifically combine with CXCR-4. This provides a theoretical
foundation for improving the curative effect by transplantation of
CXCR-4 gene overexpressed BMSCs. In this study, we used lentiviral
vector to transfect the CXCR-4 gene into BMSCs and made it
overexpress. Our study demonstrated that CXCR-4 gene overexpressed
BMSCs could enhance BMSCs homing to damaged intestinal mucosa and
have better effect on treating colitis. In addition, some studies
have used selectin, cell adhesion molecules or peptide ligands to
modify stem cells, so as to increase homing ability (24–26).
Compared with other methods, CXCR-4 gene overexpressed BMSCs method
mediated repair procedure based on BMSCs, which has good
operability and repeatability (27).
We consider that this decoration to BMSCs might be a potentially
novel method to improve the outcomes of BMSCs treatments on
IBD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZC was a major contributor in writing the manuscript
and was responsible for isolation and culture of mice BMSCs. QC
contributed to manuscript preparation and the conception of the
study. HD was a major contributor in preforming the migration
assay. LX performed the qPCR. JW contributed in the data analysis
and manuscript revision. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics Committee of the Animal Facility of Chinese PLA General
Hospital (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ng SC, Tang W, Ching JY, Wong M, Chow CM,
Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, et al: Incidence and
phenotype of inflammatory bowel disease based on results from the
Asia-pacific Crohn's and colitis epidemiology study.
Gastroenterology. 145:158–165.e2. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rogler G: Where are we heading to in
pharmacological IBD therapy? Pharmacol Res. 100:220–227. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Makridakis M, Roubelakis MG and Vlahou A:
Stem cells: Insights into the secretome. Biochim Biophys Acta.
1834:2380–2384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wagner J, Kean T, Young R, Dennis JE and
Caplan AI: Optimizing mesenchymal stem cell-based therapeutics.
Curr Opin Biotechnol. 20:531–536. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawada H, Fujita J, Kinjo K, Matsuzaki Y,
Tsuma M, Miyatake H, Muguruma Y, Tsuboi K, Itabashi Y, Ikeda Y, et
al: Nonhematopoietic mesenchymal stem cells can be mobilized and
differentiate into cardiomyocytes after myocardial infarction.
Blood. 104:3581–3587. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang PY, Zhang BY, Cui S, Qu C, Shao LH,
Xu TK, Qu YQ, Dong LH and Wang J: MSC-derived cytokines repair
radiation-induced intra-villi microvascular injury. Oncotarget.
8:87821–87836. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan D, Xie X, Qi P, Yang X and Jin X:
Human mesenchymal stem cell homing induced by SKOV3 cells. Am J
Transl Res. 9:230–246. 2017.PubMed/NCBI
|
|
8
|
Xinaris C, Morigi M, Benedetti V, Imberti
B, Fabricio AS, Squarcina E, Benigni A, Gagliardini E and Remuzzi
G: A novel strategy to enhance mesenchymal stem cell migration
capacity and promote tissue repair in an injury specific fashion.
Cell Transplant. 22:423–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bobis-Wozowicz S, Miekus K, Wybieralska E,
Jarocha D, Zawisz A, Madeja Z and Majka M: Genetically modified
adipose tissue-derived mesenchymal stem cells overexpressing CXCR4
display increased motility, invasiveness, and homing to bone marrow
of NOD/SCID mice. Exp Hematol. 39:686–696.e4. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trotta T, Di Gioia S, Piro D, Lepore S,
Cantatore S, Porro C, Castellani S, Petrella A, Fortunato F,
Maffione AB and Conese M: Effect of acute lung injury on VLA-4 and
CXCR4 expression in resident and circulating hematopoietic
stem/progenitor cells. Respiration. 85:252–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ratajczak MZ, Kim CH, Abdel-Latif A,
Schneider G, Kucia M, Morris AJ, Laughlin MJ and Ratajczak J: A
novel perspective on stem cell homing and mobilization: review on
bioactive lipids as potent chemoattractants and cationic peptides
as underappreciated modulators of responsiveness to SDF-1
gradients. Leukemia. 26:63–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang SK, Shin IS, Ko MS, Jo JY and Ra JC:
Journey of mesenchymal stem cells for homing: strategies to enhance
efficacy and safety of stem cell therapy. Stem Cells Int.
2012:3429682012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen QQ, Yan L, Wang CZ, Wang WH, Shi H,
Su BB, Zeng QH, Du HT and Wan J: Mesenchymal stem cells alleviate
TNBS-induced colitis by modulating inflammatory and autoimmune
responses. World J Gastroenterol. 19:4702–4717. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pisano F, Mura M, Ciuffreda MC, Calabrò F,
Lanzo N and Gnecchi M: Optimized lentiviral transduction of human
amniotic mesenchymal stromal cells. Pharmacol Res. 127:49–57. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gnecchi M and Melo LG: Bone marrow-derived
mesenchymal stem cells: Isolation, expansion, characterization,
viral transduction, and production of conditioned medium. Methods
Mol Biol. 482:281–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
17
|
Scharl M, McCole DF, Weber A, Vavricka SR,
Frei P, Kellermeier S, Pesch T, Fried M and Rogler G: Protein
tyrosine phosphatase N2 regulates TNFα-induced signalling and
cytokine secretion in human intestinal epithelial cells. Gut.
60:189–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boyd AS and Fairchild PJ: Approaches for
immunological tolerance induction to stem cell-derived cell
replacement therapies. Expert Rev Clin Immunol. 6:435–448. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Castelo-Branco MT, Soares ID, Lopes DV,
Buongusto F, Martinusso CA, do Rosario A Jr, Souza SA, Gutfilen B,
Fonseca LM, Elia C, et al: Intraperitoneal but not intravenous
cryopreserved mesenchymal stromal cells home to the inflamed colon
and ameliorate experimental colitis. PLoS One. 7:e333602012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ponomaryov T, Peled A, Petit I, Taichman
RS, Habler L, Sandbank J, Arenzana-Seisdedos F, Magerus A, Caruz A,
Fujii N, et al: Induction of the chemokine stromal-derived factor-1
following DNA damage improves human stem cell function. J Clin
Invest. 106:1331–1339. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu H, Liu S, Li Y, Wang X, Xue W, Ge G
and Luo X: The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic
effects of hypoxia-preconditioned mesenchymal stem cells for renal
ischemia/reperfusion injury. PLoS One. 7:e346082012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goto Y and Ivanov II: Intestinal
epithelial cells as mediators of the commensal-host immune
crosstalk. Immunol Cell Biol. 91:204–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kinnaird T, Stabile E, Burnett MS, Shou M,
Lee CW, Barr S, Fuchs S and Epstein SE: Local delivery of
marrow-derived stromal cells augments collateral perfusion through
paracrine mechanisms. Circulation. 109:1543–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sackstein R, Merzaban JS, Cain DW, Dagia
NM, Spencer JA, Lin CP and Wohlgemuth R: Ex vivo glycan engineering
of CD44 programs human multipotent mesenchymal stromal cell
trafficking to bone. Nat Med. 14:181–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ko IK, Kim BG, Awadallah A, Mikulan J, Lin
P, Letterio JJ and Dennis JE: Targeting improves MSC treatment of
inflammatory bowel disease. Mol Ther. 18:1365–1372. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kean TJ, Duesler L, Young RG, Dadabayev A,
Olenyik A, Penn M, Wagner J, Fink DJ, Caplan AI and Dennis JE:
Development of a peptide-targeted, myocardial ischemia-homing,
mesenchymal stem cell. J Drug Target. 20:23–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakase H, Matsuura M, Mikami S, Uza N and
Chiba T: Role of the CXC12-CXCR4 axis and CXCL16 in inflammatory
bowel disease. Intest Res. 10:125–133. 2012. View Article : Google Scholar
|