Introduction
Cardiorenal syndrome (CRS) refers to the clinical
syndrome when the patients suffer from cardiac insufficiency and
renal impairment that have a complex interaction (1). CRS includes different
pathophysiological disorders of the heart and the kidney.
Furthermore, acute and chronic dysfunction of any organ would lead
to acute and chronic dysfunction of another, both of which are
clinical risk factors for each other. Their mutual aggravation
leads to rapid deterioration of cardiac and renal functions to
increase mortality. Therefore, it is now necessary for us to expand
our knowledge regarding its pathogenesis, prevention, and potential
treatment, and it is extremely urgent to seek early specific and
sensitive biomarkers to predict cardiac and renal damage (2).
Biomarkers may improve risk predictive models in the
future. Biomarkers pertinent to the cardiovascular (CV) and renal
interface are defined as renocardiovascular biomarkers and
currently the emerging biomarkers such as B-type natriuretic
peptide (BNP), cardiac troponins, copeptin (CPP), the biomarker of
renal injury (neutrophil gelatinase-associated lipocalin) have been
introduced (3). For example, CPP
recently has been demonstrated to be potential suitable for guiding
management of patients with cardiac and renal damage. CPP is part
of the original C-terminal of arginine vasopressin (AVP), which is
stable in vivo and easy to be detected. AVP is an effective
osmoregulator that can increase peripheral vasoconstrictive
activity through interaction with its receptor V1. On the other
hand, binding to the V2 receptor mediates water retention in renal
tubules (4). Unfortunately, the
circulatory half-life of AVP is very short rendering it
inaccessible for clinical routine determination. CPP has been found
to have an important clinical value in the early diagnosis and
prognosis of CV and cerebrovascular diseases such as acute MI
(3,5). In addition, it could also be useful in
the diagnosis of left ventricular dysfunction (LV dysfunction) in
hemodialysis patients (6). However,
few studies are related to the occurrence of CRS as well as its
diagnosis and treatment, leading to the lack of animal model data,
although CPP has been found to be associated with renal function in
chronic kidney disease (CKD) (7).
Therefore, this study used the combination of partial nephrectomy
and myocardial infarction (SNX + MI) to aggravate organ damage for
establishing CRS rat model to observe the dynamic changes in serum
and urine CPP concentrations. Moreover, a comparative analysis was
performed with indicators such as BNP to evaluate the predictive
value of CPP level in CRS rats, which provided a basis for seeking
new markers for the diagnosis and prognosis of CRS.
Materials and methods
Animal modeling and grouping
A total of 60 specific pathogen-free male
Sprague-Dawley (SD) rats weighed 250–280 g were purchased from
Shanghai Laboratory Animal Center Laboratory Animal Co., Ltd. The
license number was SCXK (Shanghai) 2012–0002, and the certificate
number was 0278317. After the experimental animals were adapted in
the animal room for 6 days, they were divided into 4 groups (15
rats in each group) using random selection (the animals were
numbered, and 2 rats were randomly selected from each brood to be
placed in the same group), including CK group (blank control
group), SNX group (renal failure group), MI group (heart failure
group), and CRS group (heart and renal failure group). Modeling was
performed using combined surgery according to the literature
(8).
The blank control group (CK group): The rats in this
group were routinely fed without any other treatment.
The SNX group: The rats in this group were
abdominally anesthetized using 10% chloral hydrate (300 mg/kg,
i.p.; Qingxi Chemical Technology Co., Ltd., Shanghai, China) and
fixed in a supine position. The fur of the abdomen was removed, and
disinfection was performed using iodophor. The abdominal cavity was
opened to ligate renal artery of the left kidney, renal vein, and
ureter, and then the left kidney was excised. The abdominal cavity
was closed, and suturing was performed layer by layer. The animals
were placed back into the cage after they awoke, and 100,000 units
of penicillin sodium were intramuscularly injected for
anti-infection 3 days after the surgery.
The MI group: The rats in this group were
abdominally anesthetized using 10% chloral hydrate (300 mg/kg,
i.p., Qingxi Chemical Technology Co., Ltd.) and fixed in a supine
position. The fur of the chest was removed, and disinfection was
performed using iodophor. Electrocardiogram (ECG) was monitored
using limb II lead. The rats wore breathing masks (made by
researchers) on the mouth and nose, which were connected to the
ventilator to maintain positive end-expiratory pressure (parameters
of the ventilator were set as follows: Respiratory rate of 55–60
times/min, breathing ratio of 1:2, and tidal volume of 20 ml/kg).
The skin of the left chest was cut off, and the muscle was bluntly
separated. The intercostal opener was used to stretch ribs in third
and fourth intercostal spaces, and the cardiac pericardium was torn
open to expose the heart. A needle was injected 2–3 mm below the
lower edge of the left atrial appendage using great cardiac vein
between the left atrial appendage and the pulmonary artery cone as
a marker, and the needle was removed from the pulmonary artery
cone. The depth of the injection was 1.5–2 mm, and the width was
2–3 mm. The left coronary artery was ligated. It was visually
observed that the left ventricular wall was whitened, ECG QRS wave
was heighted and broadened, and the intersection (J point) of the S
wave and T wave elevated significantly, or the R wave disappeared.
The ligation was successful when a typical QS wave occurred. Then,
the chest was closed, and the muscle and skin were sutured layer by
layer. An intravenous catheter system was indwelled before closing
the chest to extract air from the chest cavity to restore negative
pressure. The animals were placed back into the cage to be fed
after they awoke. Next, 100,000 units of penicillin sodium was
intramuscularly injected for anti-infection 3 days after the
surgery.
The CRS group: The left kidney of rats in this group
was excised, and the coronary artery was ligated to perform
modeling.
This study was approved by the Animal Ethics
Committee of the JinHua Center of Laboratory Animals. All
experimental procedures were carried out in accordance with the
approval agreement of experimental animals.
Evaluation of cardiac function and
blood pressure (BP)
According to the literature, cardiac function and BP
were detected (9). At week 5 before
sacrifice, the rats in each group were abdominally anesthetized
using 10% chloral hydrate (300 mg/kg, i.p.; Qingxi Chemical
Technology Co., Ltd.). Then, a 2-cm incision was made
longitudinally from the middle of the neck to expose the right
common carotid artery. After that, a PE50 catheter was inserted
into the left ventricle and cardiac function parameters were
analyzed using a biological signal processing system (Techman Soft,
Chengdu, China; model no. BL-420S), including changes in heart rate
(HR), left ventricular systolic blood pressure (LVSP), left
ventricular end diastolic pressure (LVEDP), and ± dp/dt max (change
rate of left ventricular pressure). BP changes of rats in each
group were measured using a tail vein noninvasive BP measurement
system (Techman Soft; model no. BP-300). The measurement was
performed once every other week for a total of five times.
Evaluation of renal function
After successful modeling, the animals were
anesthetized with 10% chloral hydrate (300 mg/kg, i.p., Qingxi
Chemical Technology Co., Ltd.) prior to eye blood collection, then
blood was extracted from the orbital cavity and urine was
collected, once every week for a total of five times, and then
renal function was tested. The serum BUN level in each group was
measured by a biochemical method using a BUN test box (Nanjing
Jiancheng Bioengineering Institute; item no. C013-2; batch no.
20161228), according to the manufacturer's instructions. Serum and
urine Cr levels of each group were measured by the sarcosine
oxidase enzymatic method using a creatine kinase assay kit (Reebio,
Ningbo, China; item no. 2400746; batch no. 2017020802), according
to the manufacturer's instructions also (10).
Measurement of CPP and BNP levels
using enzyme-linked immunosorbent assay
CPP and BNP levels were assayed using enzyme-linked
immunosorbent assay (ELISA) in accordance with the instructions
from the kits provided (11). After
successful modeling, blood was extracted from the orbital cavity
and urine was collected once every week for a total of five times.
ELISA kits for CPP and BNP [ELISA kit for CPP was purchased from
USCN Life Science, Inc., (Wuhan, China), item no. SEA365Ra and
batch no. 161101216; ELISA kit for BNP with the item no. CEA541Ra
and batch no. 161101217] were used to measure serum and urine CPP
together with BNP levels strictly based on the kit instruction.
Histopathological observation of heart
and kidney
The rats were sacrificed at day 9 and week 5 after
modeling. Then, conventional hematoxylin and eosin (H&E)
staining was performed for cardiac and renal tissues, and the
tissue slices were examined under a light microscope to determine
pathological changes in cardiac and renal tissues in CRS rats.
Statistical analysis
All data were analyzed using the SPSS22.0
statistical software package (SPSS, Inc., Chicago, IL, USA).
Continuous data were represented by mean ± standard deviation. The
intragroup comparison was done using the independent-sample t-test
or one-way analysis of variance, further comparison between the
groups was performed using the least significant difference
t-test, and non-normal distribution data was analyzed using
the nonparametric test. The correlation analysis of normal
distribution data was performed using Pearson linear correction,
whereas non-normal distribution data was analyzed using Spearman
rank correlation. Receiver operating characteristic (ROC) curve was
used to determine the early diagnostic value of CPP, BNP, and BUN
on CRS. P<0.05 was considered to indicate a statistically
significant difference.
Results
General condition and survival
rate
After 5 weeks, the rats in each modeling group
showed deeper color of fur, less appetite, no increase in weight
and exercise, and mouth and nose in cyan color compared with the
control group. Asthma and edema in the feet occurred. Moreover,
prehension response was weakened. In the MI group, one rat died
within 5 weeks, and the survival rate was 85.7%. In the CRS group,
two rats died within 5 weeks, and the survival rate was 71.4%. No
death occurred in the CK and SNX groups, and the survival rate was
100%.
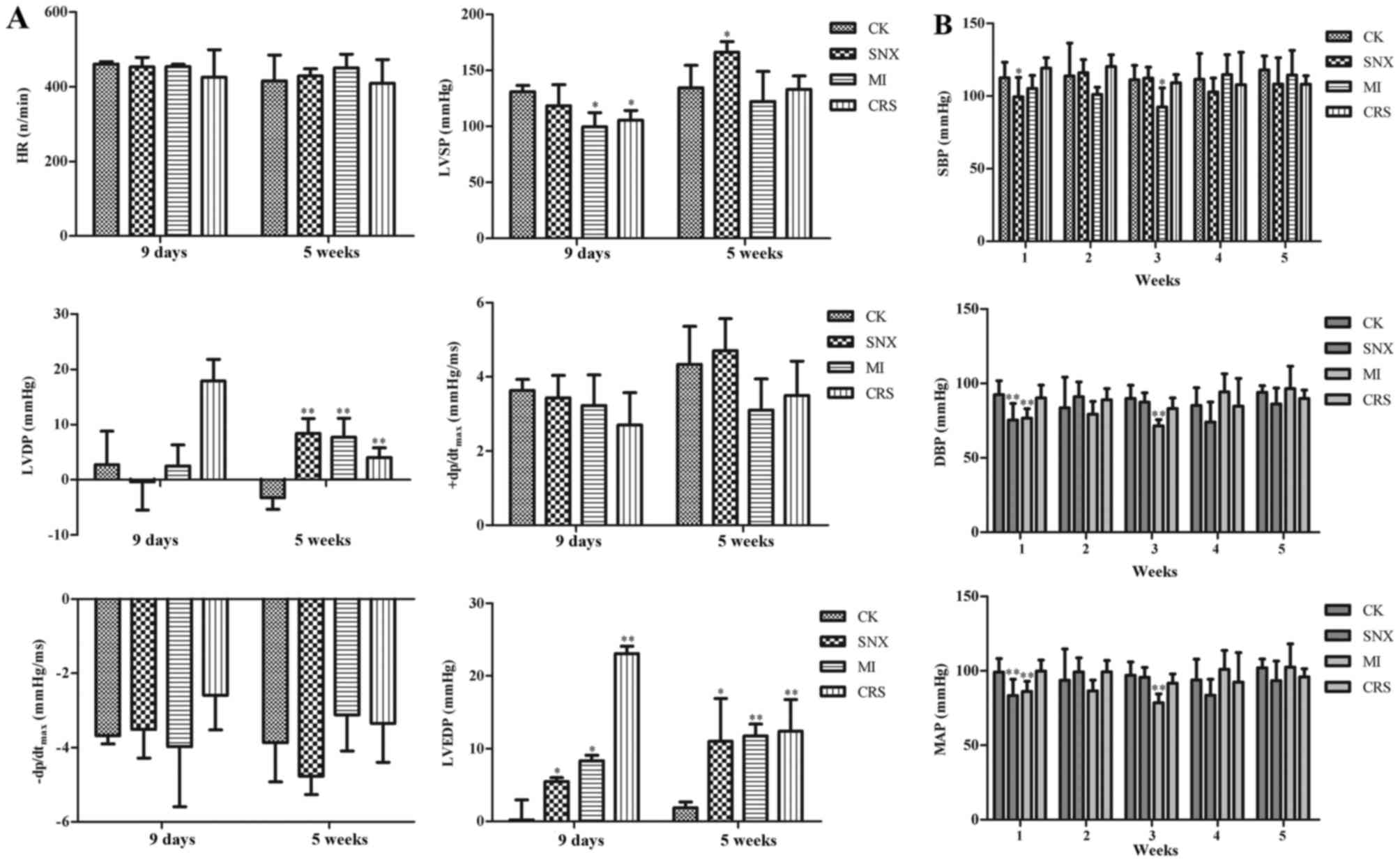
Cardiac function and BP changes in
each group
The results of hemodynamic test indicated that LVEDP
in the SNX group increased significantly in 9 days compared with CK
group (P<0.05). LVSP in the MI group decreased significantly in
9 days (P<0.05), and LVEDP increased significantly (P<0.05).
LVSP in the CRS group decreased significantly in 9 days
(P<0.05), and LVEDP increased significantly (P<0.01). The
results of BP estimation demonstrated that systolic BP (SBP),
diastolic BP (DBP) and MAP (mean pressure) in the SNX group
decreased significantly in 1 week compared with the CK group
(P<0.05). SBP, DBP, and MAP in the MI group decreased
significantly in 3 weeks (P<0.05, P<0.01, and P<0.01,
respectively), whereas no statistically significant difference in
SBP, DBP, and MAP was observed in the CRS group at each time point
(P>0.05; Fig. 1).
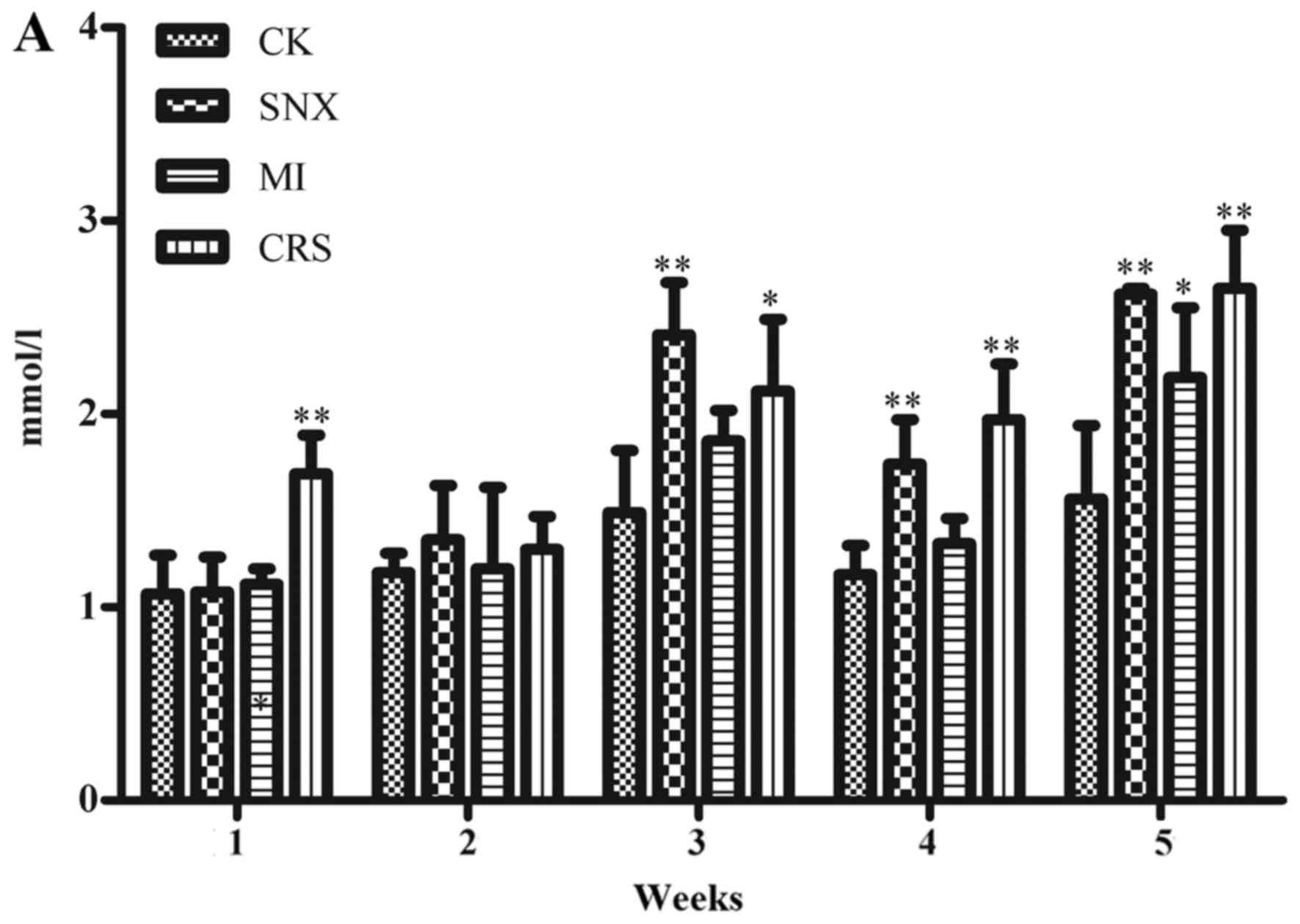
Renal function changes in each
group
The results showed that compared with the CK group,
serum BUN in the MI group elevated significantly in 5 weeks after
modeling (P<0.05), serum BUN in the SNX group elevated
significantly in 3–5 weeks after modeling (P<0.01), serum BUN in
the CRS group elevated significantly in 1 week and 3–5 weeks after
modeling (P<0.01, P<0.05). SCr in the SNX group increased
significantly in 3 weeks and 5 weeks (P<0.05), and UCr increased
significantly in 1–4 weeks (P<0.01, P<0.05). UCr in the MI
group increased significantly in 1–2 weeks and 5 weeks (P<0.01,
P<0.05). UCr in the CRS group increased significantly in 1–5
weeks (P<0.01; Fig. 2).
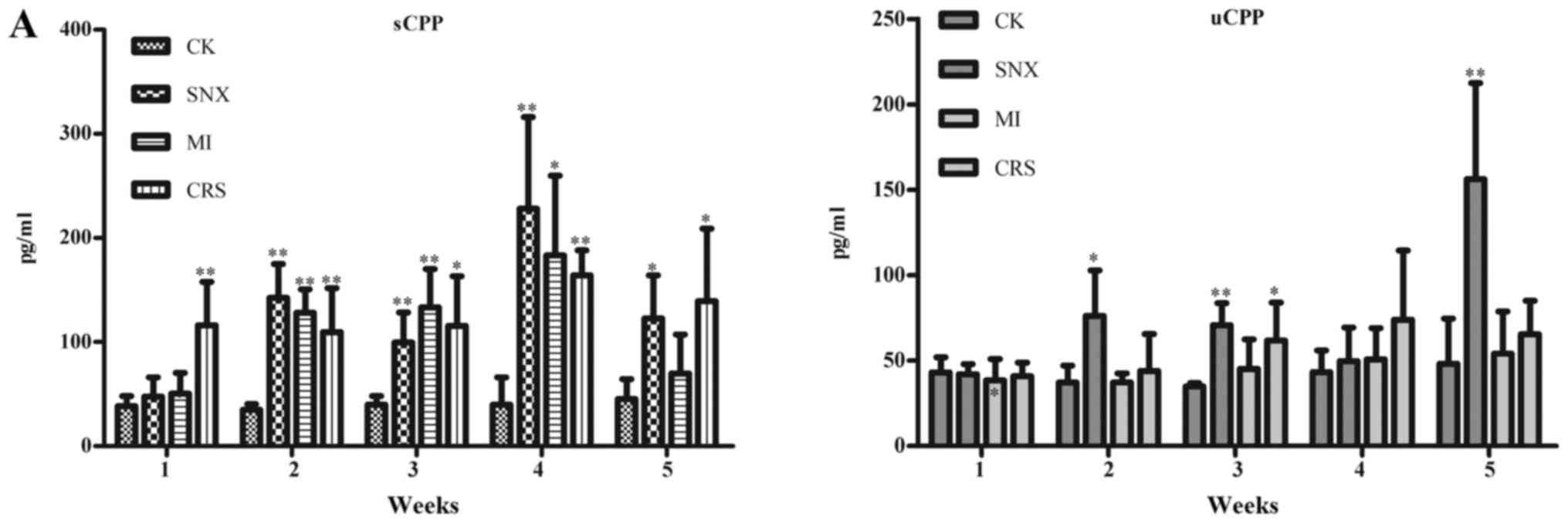
Changes in CPP and BNP levels as well
as their relationship with other indicators
Serum CPP in the SNX group increased significantly
in 2–5 weeks after modeling compared with the CK group (P<0.05,
P<0.01), and urine CPP increased significantly in 2–3 weeks and
5 weeks after modeling (P<0.05, P<0.01). Serum CPP in the MI
group increased significantly in 2–4 weeks after modeling
(P<0.05, P<0.01). Serum CPP in the CRS group increased
significantly in 1–5 weeks after modeling (P<0.05, P<0.01),
and urine CPP increased significantly in 3 weeks after modeling
(P<0.05). Meanwhile, compared with the CK group, serum BNP in
the SNX group increased significantly in 1–3 weeks after modeling
(P<0.05, P<0.01), while urine BNP increased significantly in
4–5 weeks after modeling (P<0.01). Urine BNP in the MI group
increased significantly in 1 week, 3–5 weeks after modeling
(P<0.05, P<0.01). Serum BNP in the CRS group increased
significantly in 1 week and 3 weeks after modeling (P<0.01),
while urine BNP increased significantly in 4–5 weeks after modeling
(P<0.05; Fig. 3).
The correlation analysis demonstrated that serum
CPP, BNP, and BUN in the CRS group in 1 week after modeling were
not correlated (r=0.109 and 0.683, respectively; P>0.05).
Moreover, urine CPP, BNP, and BUN were also not correlated
(r=−0.342 and 0.293, respectively; P>0.05).
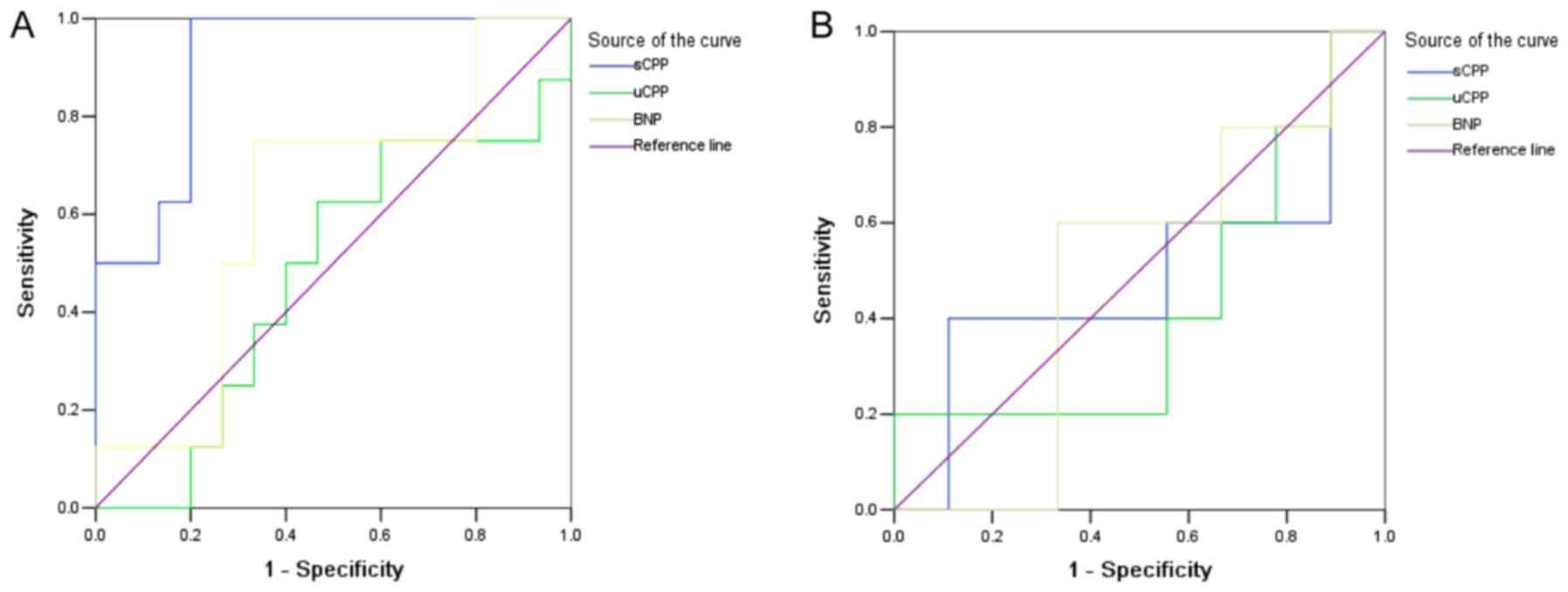
CPP and BNP levels in the early
diagnosis of CRS
Test results of serum CPP (sCPP), urine CPP (uCPP),
and BNP in 1 week and 3 weeks in each model group were selected as
the test variables to diagnose CRS, and the groups were used as the
state variables. Moreover, SNX and MI groups were defined as 0; CRS
group was defined as 1; and ROC curve analysis was performed. The
results revealed that the area under the curve (AUC) of sCPP in 1
week was 0.908 (95% CI: 0.789–1.028). With 56.59 pg/ml as the
cutoff point, the diagnostic sensitivity was 87.5% and the
specificity was 80.0%. However, the test results of sCPP in 3 weeks
and uCPP and BNP in 1 week and 3 weeks were not statistically
significant (P>0.05; (Fig. 4 and
Table I).
 | Table I.ROC curve analysis of sCPP, uCPP and
BNP in the diagnosis of cardiorenal syndrome. |
Table I.
ROC curve analysis of sCPP, uCPP and
BNP in the diagnosis of cardiorenal syndrome.
| Indicators | AUC | SE | P-value | 95% CI | Cutoff value | Sensitivity | Specificity |
|---|
| sCPP |
|
|
|
|
|
|
|
| 1
week | 0.908 | 0.061 | 0.002 | 0.789–1.028 | 56.590 | 0.875 | 0.800 |
| 3
weeks | 0.489 | 0.181 |
0.947 | 0.134–0.844 | 84.455 | 0.600 | 0.444 |
| uCPP |
|
|
|
|
|
|
|
| 1
week | 0.475 | 0.130 | 0.846 | 0.221–0.729 | 45.815 | 0.625 | 0.533 |
| 3
weeks | 0.422 | 0.173 |
0.641 | 0.083–0.761 | 60.665 | 0.400 | 0.444 |
| BNP |
|
|
|
|
|
|
|
| 1
week | 0.617 | 0.126 | 0.366 | 0.370–0.863 | 181.250 | 0.625 | 0.667 |
| 3
weeks | 0.489 | 0.164 | 0.947 | 0.168–0.810 | 167.480 | 0.600 | 0.556 |
Pathological changes in cardiac and
renal tissues in each group
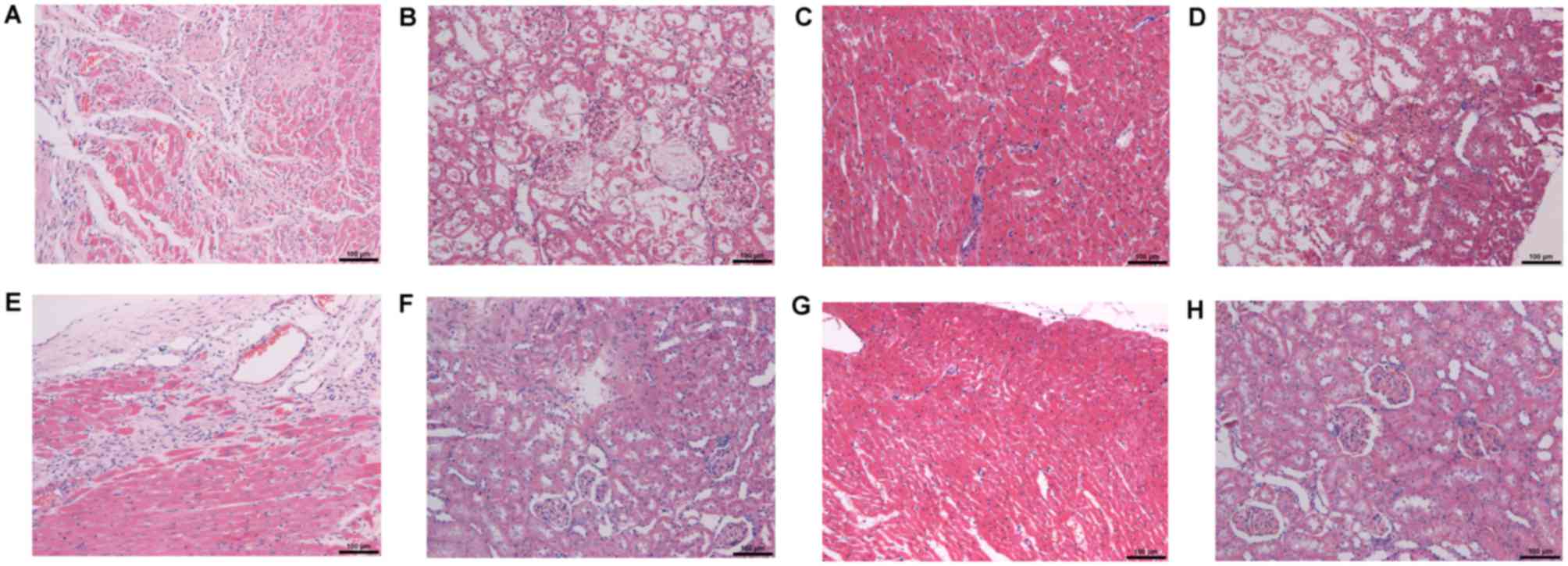
H&E staining of cardiac tissue showed no
significant pathological changes in the CK group, while SNX, MI,
and CRS groups showed myocardial tissue disorder, some myocardial
degeneration, and dissolution and rupture together with congestion
and expansion, which were significant in the CRS group (Fig. 5A, C, E and G). H&E staining of
renal tissue demonstrated that the CK group had no significant
pathological change; however, the SNX and MI groups showed mild
injury, and the CRS group was the most serious (Fig. 5B, D, F and H).
Discussion
This study confirmed that the combination of SNX +
MI aggravated organ damage to establish a CRS rat model, which
could reflect the two-way interaction of heart and kidney and could
be used to evaluate the diagnostic effect of CRS. It had been
confirmed by dynamically observing changes in serum and urine CPP
concentrations 1–5 weeks after modeling that the detection of the
serum CPP level of CRS rats was conducive to early detection of
cardiac and renal damage, which displayed a warning effect.
Moreover, CPP could be used as a potential biomarker for the early
prediction of CRS with high sensitivity and specificity.
The relationship between cardiac diseases and renal
diseases has been widely explored with the proposal of CRS concept
in recent years. A stable and reliable animal model is an urgent
need for further clinical studies. The CRS rat model was first
introduced in 2004, and myocardial infarction combined with
unilateral nephrectomy (UNX + MI) was used, but this model did not
find any progressive impairment of cardiac function combined with
renal function degeneration (12).
However, the SNX + MI method used in this study was designed by
Entin-Meer et al (13) in the
study of CRS model, which demonstrated its advantages by partial
nephrectomy combined with MI, indicating the two-way interaction of
heart and kidney. This study found, through the dynamical
observation of hemodynamics, BP, and renal function 1–5 weeks after
modeling, that the degeneration of cardiac and renal function
occurred 1 week after modeling, suggesting that rats developed
cardiac and renal damage in a short time. Thus, this model could
highly stimulate the angiographic features of clinical CRS,
providing experimental methods for exploring the pathophysiological
mechanism and for drug research of CRS. The myocardium of some rats
with renal injury due to nephrectomy was more prone to ischemic
injury, and the MI area of uremic rats increased significantly.
These findings were consistent with the finding that the mortality
rate of patients with renal diseases combined with MI was higher.
Due to partial nephrectomy, erythropoietin secreted by kidney
decreased, blood oxygen capacity decreased, cardiac ejection
aggravated, blood return of heart and various organs increased,
vessels contracted, cardiac hypertrophy aggravated, and BP
increased. Moreover, endothelial damage led to a decrease in
nutritional function of the coronary artery, aggravating myocardial
ischemia. Meanwhile, cardiac dysfunction could, in turn, accelerate
the inherent damage of kidney (14).
The occurrence of CRS is accompanied by the
corresponding changes in different biomarkers, which plays an
important role in the early diagnosis, risk assessment, and
prognosis. Due to capacity load and sodium retention as well as the
inappropriate activation of atrial natriuretic peptide, AVP, and
renin-angiotensin system, water metabolism disorder caused by CRS
is more prominent. Among them, the AVP system may be another
neuroendocrine system closely related to the poor prognosis of
acute myocardial infarction (AMI) or cardiac failure (15). The CPP test is easy and convenient,
and its results are stable, which can be carried out at all levels
of hospitals. Moreover, the CPP test can replace the direct
measurement of AVP. This study dynamically detected changes in
serum and urine CPP concentrations 1–5 weeks after modeling and
found that serum and urine CPP in 1 week and 3 weeks after modeling
increased significantly in the CRS group, and only serum CPP
increased in 5 weeks after modeling. The ROC curve analysis showed
that serum CPP in 1 week after modeling had higher specificity
(80%) and sensitivity (87%) in predicting CRS, suggesting that CPP
changed in the early stage of cardiac and renal damage, but it
tended to be stable in the late stage, which was expected to
provide a new and good biomarker for the early diagnosis of CRS.
Therefore, CPP had a broad application prospect. In fact, CPP has
recently been proved to have a predictive value in multiple
clinical diseases, including CV diseases, such as heart failure,
myocardial infarction, and resuscitation (6,16,17).
However, few reports are available on kidney-related diseases. Only
Kim et al (6) confirmed that
CPP could be a useful indicator for diagnosing left ventricular
dysfunction in patients receiving hemodialysis. The ROC analysis
showed that AUC was 0.737, the cutoff point was 125.48 pg/ml, and
the sensitivity and specificity were 0.7 and 0.8, respectively.
However, it was believed that the potential to predict the risk of
CV events in perioperative CV surgery for patients with chronic
kidney disease was insufficient (18), or in neonatal and perinatal asphyxia
animal models, CPP was not a specific marker for diagnosing acute
renal injury (19). Further
correlation analysis of this study showed that serum or urine CPP,
BNP, and BUN were not correlated, which might be related to the
complex pathophysiology of CRS. BNP was publicly recognized as a
clinical diagnostic marker of heart failure. Although BNP was
reported to have a certain application value in the early diagnosis
of CRS (2,20,21),
whether it would accumulate in the body of patients with CRS due to
heart failure or the decrease in glomerular filtration rate, as
well as its diagnostic value for renal insufficiency, still needed
to be further studied (22).
CRS is a complex clinical syndrome with various
difficulties in its treatment due to the uncertainty of its
definition and pathophysiological mechanism. Early diagnosis is one
of the most effective ways to improve its efficacy, and the
development of new biomarkers is the key to early diagnosis. In
conclution, present study confirmed that the combination of SNX +
MI could be used to establish a CRS rat model with cardiac and
renal injury. Moreover, CPP could be used as a potentially
sensitive and specific biomarker for the early diagnosis of
CRS.
Acknowledgements
Not applicable.
Funding
This study was supported by Experimental Animals
Science and Technology Project of Zhejiang Province in China (grant
no. 2015C37088).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XS, LL and MD conceived and coordinated the study,
designed, performed and analyzed the experiments, and wrote the
paper. FG and SL carried out the data collection and data analysis.
HC and YF performed and analyzed the experiments. All authors
reviewed the results and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of the JinHua Center of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bongartz LG, Braam B, Gaillard CA, Cramer
MJ, Goldschmeding R, Verhaar MC, Doevendans PA and Joles JA: Target
organ cross talk in cardiorenal syndrome: Animal models. Am J
Physiol Renal Physiol. 303:F1253–F1263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hou FF and Yang X: Advances in the
management of acute cardiorenal syndrome in China: Biomarkers for
predicting development and outcomes. Kidney Dis (Basel). 2:145–150.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niizuma S, Iwanaga Y, Yahata T and
Miyazaki S: Renocardiovascular biomarkers: From the perspective of
managing chronic kidney disease and cardiovascular disease. Front
Cardiovasc Med. 4:102017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morgenthaler NG, Struck J, Jochberger S
and Dünser MW: Copeptin: Clinical use of a new biomarker. Trends
Endocrinol Metab. 19:43–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morawiec B and Kawecki D: Copeptin: A new
marker in cardiology. J Cardiovasc Med (Hagerstown). 14:19–25.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JS, Yang JW, Chai MH, Lee JY, Park H,
Kim Y, Choi SO and Han BG: Copeptin in hemodialysis patients with
left ventricular dysfunction. Yonsei Med J. 56:976–980. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Velho G, Bouby N, Hadjadj S, Matallah N,
Mohammedi K, Fumeron F, Potier L, Bellili-Munoz N, Taveau C,
Alhenc-Gelas F, et al: Plasma copeptin and renal outcomes in
patients with type 2 diabetes and albuminuria. Diabetes Care.
36:3639–3645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Windt WA, Henning RH, Kluppel AC, Xu Y, de
Zeeuw D and van Dokkum RP: Myocardial infarction does not further
impair renal damage in 5/6 nephrectomized rats. Nephrol Dial
Transplant. 23:3103–3110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng DF, Tang SY, Hu YJ, Chen J and Yang
L: Pathophysiological model of chronic heart failure complicated
with renal failure caused by three-quarter nephrectomy and
subcutaneous injection of isoprenaline. Exp Ther Med. 5:835–839.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pang L, Lian X, Li Y, Nan L and Ma H:
Efficacy and safety of parecoxib sodium after renal
transplantation. African J Pharm Pharmacol. 5:2467–2473. 2011.
|
|
11
|
Aydin S, Eren MN, Kuloglu T, Aydin S,
Yilmaz M, Gul E, Kalayci M, Yel Y, Cakmak T and Bico S: Alteration
of serum and cardiac tissue adropin, copeptin, irisin and TRPM2
expressions in DOX treated male rats. Biotech Histochem.
90:197–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Dokkum RP, Eijkelkamp WB, Kluppel AC,
Henning RH, van Goor H, Citgez M, Windt WA, van Veldhuisen DJ, de
Graeff PA and de Zeeuw D: Myocardial infarction enhances
progressive renal damage in an experimental model for cardio-renal
interaction. J Am Soc Nephrol. 15:3103–3110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Entin-Meer M, Ben-Shoshan J,
Maysel-Auslender S, Levy R, Goryainov P, Schwartz I, Barshack I,
Avivi C, Sharir R and Keren G: Accelerated renal fibrosis in
cardiorenal syndrome is associated with long-term increase in urine
neutrophil gelatinase-associated lipocalin levels. Am J Nephrol.
36:190–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu S, Kompa AR, Kumfu S, Nishijima F,
Kelly DJ, Krum H and Wang BH: Subtotal nephrectomy accelerates
pathological cardiac remodeling post-myocardial infarction:
implications for cardiorenal syndrome. Int J Cardiol.
168:1866–1880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lipinski MJ, Escárcega RO, D'Ascenzo F,
Magalhães MA, Baker NC, Torguson R, Chen F, Epstein SE, Miró O,
Llorens P, et al: A systematic review and collaborative
meta-analysis to determine the incremental value of copeptin for
rapid rule-out of acute myocardial infarction. Am J Cardiol.
113:1581–1591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boeckel JN, Oppermann J, Anadol R,
Fichtlscherer S, Zeiher AM and Keller T: Analyzing the release of
copeptin from the heart in acute myocardial infarction using a
transcoronary gradient model. Sci Rep. 6:208122016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Broessner G, Hasslacher J, Beer R, Lackner
P, Lehner GF, Harler U, Schiefecker A, Helbok R, Pfausler B,
Hammerer-Lercher A and Joannidis M: Outcome prediction and
temperature dependency of MR-proANP and copeptin in comatose
resuscitated patients. Resuscitation. 89:75–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schrimpf C, Gillmann HJ, Sahlmann B,
Meinders A, Larmann J, Wilhelmi M, Aper T, Rustum S, Lichtinghagen
R, Theilmeier G and Teebken OE: Renal function interferes with
copeptin in prediction of major adverse cardiac events in patients
undergoing vascular surgery. PLoS One. 10:e01230932015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baumert M, Surmiak P, Więcek A and
Walencka Z: Serum NGAL and copeptin levels as predictors of acute
kidney injury in asphyxiated neonates. Clin Exp Nephrol.
21:658–664. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Palazzuoli A, Ruocco G, Pellegrini M,
Martini S, Del Castillo G, Beltrami M, Franci B, Lucani B and Nuti
R: Patients with cardiorenal syndrome revealed increased
neurohormonal activity, tubular and myocardial damage compared to
heart failure patients with preserved renal function. Cardiorenal
Med. 4:257–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beltrami M, Ruocco G, Ibrahim A, Lucani B,
Franci B, Nuti R and Palazzuoli A: Different trajectories and
significance of B-type natriuretic peptide, congestion and acute
kidney injury in patients with heart failure. Intern Emerg Med.
12:593–603. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Santos-Araújo C, Leite-Moreira A and
Pestana M: Clinical value of natriuretic peptides in chronic kidney
disease. Nefrologia. 35:227–233. 2015. View Article : Google Scholar : PubMed/NCBI
|