Introduction
Primary hypertension is a common chronic
cardiovascular disease that causes cardiac and cerebrovascular
accidents (1,2). The incidence of primary hypertension is
increasing year by year, and the percentage of young patients
(<20 years of age) affected is significantly elevated (3). Early prevention of primary hypertension
and timely intervention of its development are of high significance
in controlling the incidence of primary hypertension and
alleviating the condition of affected patients. At present, the
molecular mechanisms of hypertension remain to be fully elucidated.
It has been reported that structural and functional abnormalities
of blood vessels are early events in the occurrence and development
of hypertension, including pathological processes, e.g. vascular
endothelial cell injury, proliferation and migration of vascular
smooth muscle cells, as well as vascular remodeling. Among these,
vascular endothelial cell injury has an important implication in
affecting vascular function in patients with hypertension (4). For instance, dysfunction of vascular
endothelial cells in patients with hypertension interrupts the
secretory homeostasis of nitric oxide and endothelin, induces
vasoconstriction and increases circulation resistance, finally
leading to the development of hypertension (5). Increased blood pressure promotes the
apoptosis of microvascular endothelial cells, decreases parallel
blood pathways and enhances peripheral circulation resistance,
finally leading to hypertension (6).
Although vascular endothelial cell injury induced by hypertension
has an important role in the occurrence and development of
hypertension, the molecular mechanisms remain to be fully
elucidated.
MicroRNAs (miRNAs or miRs) are a class of highly
conserved, small non-coding RNA molecules comprising 18-22
nucleotides (7). miRNAs regulate
gene expression at the post-transcriptional level. A miRNA binds
with the 3′-untranslated region (UTR) of its target mRNAs to form
silencing complexes and inhibit their translation (8). miRNAs regulate processes including cell
proliferation, differentiation, apoptosis, the cell cycle and aging
(9,10). It has been reported that the
expression of certain miRNAs is significantly changed in the
peripheral blood of patients with hypertension, and is closely
associated with myocardial hypertrophy, cerebral apoplexy and organ
injury induced by hypertension (11,12).
miR-199a is a newly discovered miRNA and its precursor gene is
located on chromosomes 1 and 19. After cleavage, it is converted
into its mature forms, miR-199a-5p and miR-199a-3p. To date,
miR-199a-5p has been more widely studied, revealing that it is
closely associated with the proliferation, invasion, migration and
autophagy of tumor cells (13). The
present study investigated the expression of miR-199a-5p in the
peripheral blood of patients with hypertension, and attempted to
elucidate its implication in vascular endothelial cell injury
induced by hypertension, as well as the underlying mechanism of
action.
Materials and methods
Patients
A total of 57 patients with primary hypertension,
who were treated at the Affiliated Hospital of Qingdao University
(Qingdao, China) between December 2014 and November 2015, were
included in the present study. Peripheral blood was collected from
all patients, as well as 31 age and gender-matched healthy subjects
(control group), who were recruited during physical examination at
the Affiliated Hospital of Qingdao University (Qingdao, China). The
57 patients included 34 males and 23 females, with an age range of
43–82 years, a mean age of 67.5 years and a median age of 64 years.
The 57 patients were divided into three groups (I, II and III)
according to the severity of hypertension determined according to
the hypertension staging standard (14), with patients in group III having the
most severe hypertension. The course of hypertension for each
patient was >5 years.
Cells
Human umbilical vein endothelial cells (HUVECs; Type
Culture Collection of the Chinese Academy of Sciences, Shanghai,
China) were cultured in Dulbecco's Modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Thermo Fisher Scientific, Inc.) at 37°C and
5% CO2. When reaching 70–90% confluency, the cells were
seeded into 24-well plates at 1×105 cells/well. The
HUVECs were divided into a negative control group (NC; transfected
with scrambled miRNA), miR-199a-5p mimics group (transfected with
miR-199a-5p mimics) and rescue group (co-transfected with
miR-199a-5p mimics and inhibitor; all obtained from HanBio
Biotechnology Co., Ltd., Shanghai, China).
Prior to transfection, HUVECs were seeded at a high
density and cultured in serum-free DMEM until reaching 70–90%
confluency. In the first vial, 2.5 µl miR-199a mimics (25 pmol/µl;
miR-199a mimics group), or a combination of 2.5 µl miR-199a mimics
(25 pmol/µl) and 2.5 µl miR-199a mimics inhibitor (25 pmol/µl;
rescue group) were mixed with 50 µl OptiMEM medium (Thermo Fisher
Scientific, Inc.). In the second vial, 1 µl
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.) was
mixed with 50 µl OptiMEM medium. After standing still for 5 min,
the two vials were combined, followed by incubation for 20 min at
room temperature. Subsequently, the mixtures were added to the
cells in the respective groups. Following 6 h of incubation, the
medium was replaced with DMEM containing 10% fetal bovine serum.
After cultivation for 48 h, the cells were collected for further
assays. To test how miR-199a-5p affects the adenosine
monophosphate-activated protein kinase (AMPK) signaling pathway,
HUVECs transfected with miR-199a mimics were incubated at 37°C with
the AMPK activator metformin (2 µM) for 4 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Peripheral blood (250 µl) was mixed with 0.75 ml
TRIzol (Thermo Fisher Scientific, Inc.) for lysis, while the cells
(1×105) were trypsinized and mixed with 0.5 ml TRIzol
for lysis. Total RNA was then extracted using the phenol chloroform
method. The purity of the RNA was determined by measuring the ratio
of the absorbance at 260 nm vs. that at 280 nm with an ultraviolet
spectrophotometer (Nanodrop ND1000; Thermo Scientific, Inc.).
Complementary (c)DNA was then obtained by RT of 0.5 µg RNA using
the Reverse Transcription System (Takara Bio, Inc., Dalian, China)
and stored at −20°C. The expression of miR-199a-5p was determined
with the SYBR PrimeScript miRNA RT-PCR Kit (Takara Bio, Inc.),
using U6 as an internal reference. The reaction system (20 µl)
contained 10 µl ‘qPCR-mix’, 0.5 µl upstream primer
(5′-CAGTGTCTTAGCTGGTTG-3′), 0.5 µl downstream universal primer, 2
µl cDNA and 7 µl double-distilled H2O. The reaction
protocol was as follows: Initial denaturation at 95°C for 10 min;
and 40 cycles of 95°C for 1 min and 60°C for 30 sec (iQ5; Bio-Rad
Laboratories, Hercules, CA, USA). The 2−ΔΔCq method
(15) was used to calculate the
relative expression of miR-199a-5p against U6. Each sample was
tested in triplicate.
Cell-counting kit 8 (CCK-8) assay
At 48 h after transfection, HUVECs were trypsinized
and seeded into 96-well plates at a density of
1×103/well. At 24, 48 and 72 h, the medium was
discarded, and the cells were washed with PBS twice, followed by
addition of DMEM and 10% CCK-8 reagent. After incubation at 37°C
for 1 h, the absorbance of each well was measured at 450 nm for
plotting cell proliferation curves.
Flow cytometry
At 48 h after transfection, HUVECs were trypsinized,
collected and washed with pre-cooled PBS twice. The apoptosis of
cells was examined by flow cytometry using BD Pharmingen FITC
Annexin V Apoptosis Detection kit I (cat. no. 556547; BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocol. Early apoptotic cells were identified by
positive staining with Annexin V only, necrotic cells were
indicated by positive staining with propidium iodide only, and late
apoptotic cells were identified by double-positive staining with
propidium iodide and Annexin V. In order to observe whether
autophagy regulates apoptosis in HUVECs, the cells were incubated
with autophagy inhibitor 3-methyladenine (3-MA; 50 nM) for 24 h
prior to flow cytometric analysis.
Western blot analysis
HUVECs were trypsinized, collected and suspended
with 100 µl pre-cooled radioimmunoprecipitation assay lysis buffer
containing 1 mM phenylmethylsulfonyl fluoride for lysis at 4°C for
15 min. Subsequently, the cells were centrifuged at 12,000 × g for
5 min. The supernatant was used to determine protein concentration
(4 µg/µl), using a BCA protein concentration determination kit
(cat. no. RTP7102; Real-Times Biotechnology Co., Ltd., Beijing,
China). The samples were then mixed with 5× loading buffer. After
denaturation in a boiling water bath for 10 min, the samples (20
µg) were subjected to 10% SDS-PAGE at 100 V. The resolved proteins
were transferred to polyvinylidene difluoride membranes (Thermo
Fisher Scientific, Inc.) on ice (300 mA, 1 h) and blocked with 5%
skimmed milk in PBS containing Tween-20 at room temperature for 1
h. Subsequently, the membranes were incubated with rabbit
anti-human polyclonal primary antibodies to light chain (LC)3B
(1:1,000 dilution; cat. no. ab51520); p62 (1:1,000 diultion; cat.
no. ab91526) and GAPDH (1:5,000 dilution; cat. no. ab8245; Abcam,
Cambridge, UK) at 4°C overnight. After extensive washing with PBS
containing Tween-20 for 6 times (5 min each), the membranes were
incubated with horseradish peroxidase-conjugated goat anti-rabbit
polyclonal secondary antibody (1:8,000 dilution; cat. no. ab6721;
Abcam) for 1 h at room temperature prior to washing with PBS
containing Tween-20 for 6 times (5 min each). Subsequently, the
membranes were developed with an enhanced chemiluminescence
detection kit (Sigma-Aldrich; Merck KGaA) for imaging. Image lab
v3.0 software (Bio-Rad Laboratories) was used to acquire and
analyze imaging signals. Relative protein contents were quantified
against GAPDH.
Electron microscopy
At 48 h after transfection, HUVECs were trypsinized
and centrifuged, followed by re-suspension with PBS. After
discarding the supernatant, the cells were made into single
suspension and then fixed with 2.5% glutaraldehyde at 4°C
overnight. After washing with PBS once, the cells were fixed with
1% osmic acid for 1 h. After preparation of slides, autophagosomes
were observed under an electron microscope (JEM1230; JEOL, Tokyo,
Japan).
Laser scanning confocal
microscopy
HUVECs (1×105) were seeded onto
specialized cell culture dishes for laser scanning confocal
microscopy. At 24 h after transfection, the HUVECs were transfected
with Ad-green fluorescent protein-red fluorescent rotein-LC3
adenovirus (multiplicity of infection, 20; cat. no. HB-AP210 0001;
HanBio Biotechnology Co., Ltd.). At 12 h after transfection, the
medium was replenished. At 72 h, the cells in each group were fixed
with 4% polyoxymethylene and washed with PBS. The cells were
observed under an SP8 laser scanning confocal microscope (Leica
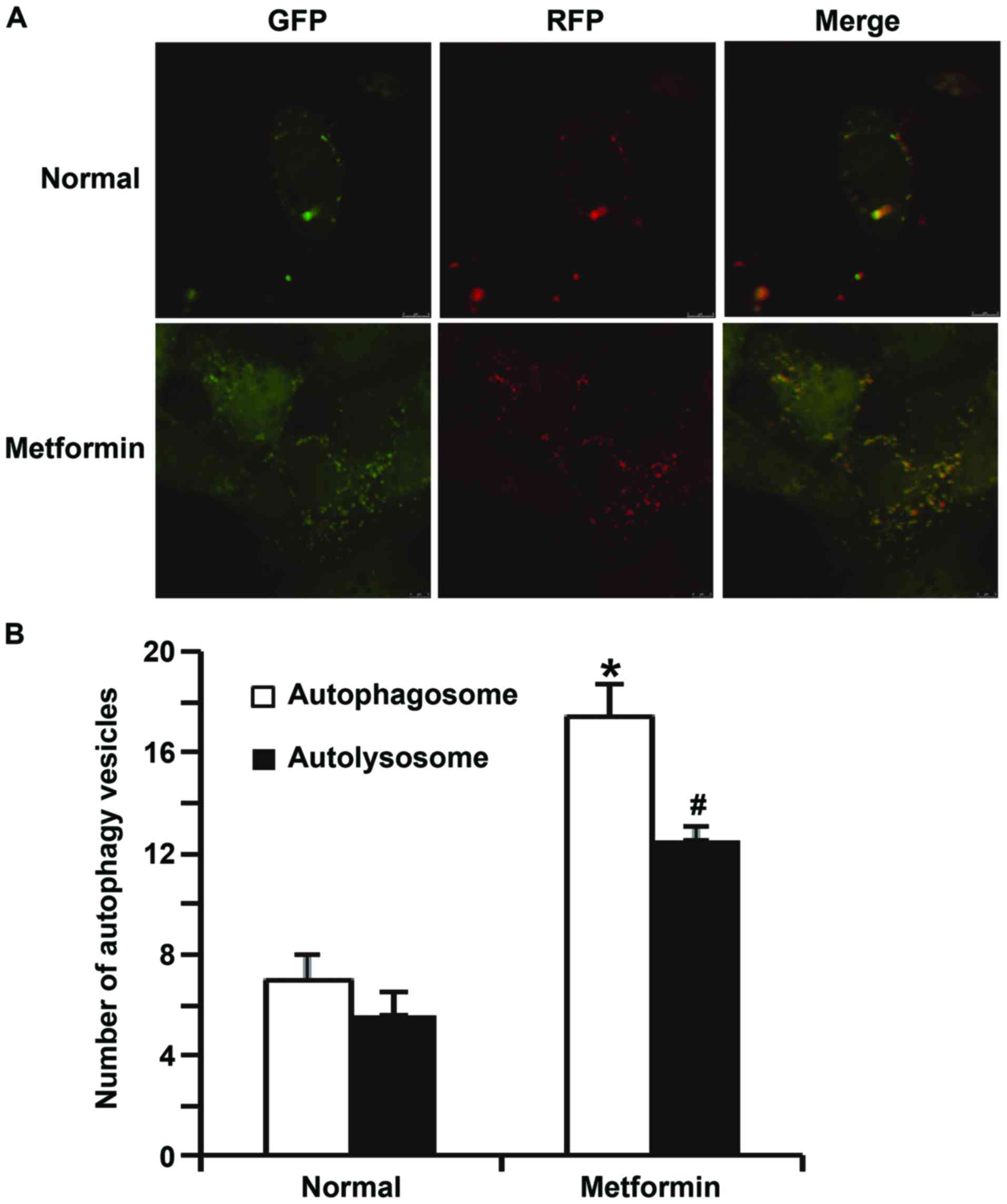
Microsystems, Wetzlar, Germany). Green vesicles represented
autophagosomes and red vesicles represented autolysosomes. Cells in
5 fields were individually counted to obtain a mean value.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). Measurement data were expressed as
the mean ± standard deviation. Data were compared between two
groups by using a Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR-199a-5p in
peripheral blood is positively associated with the progression of
hypertension
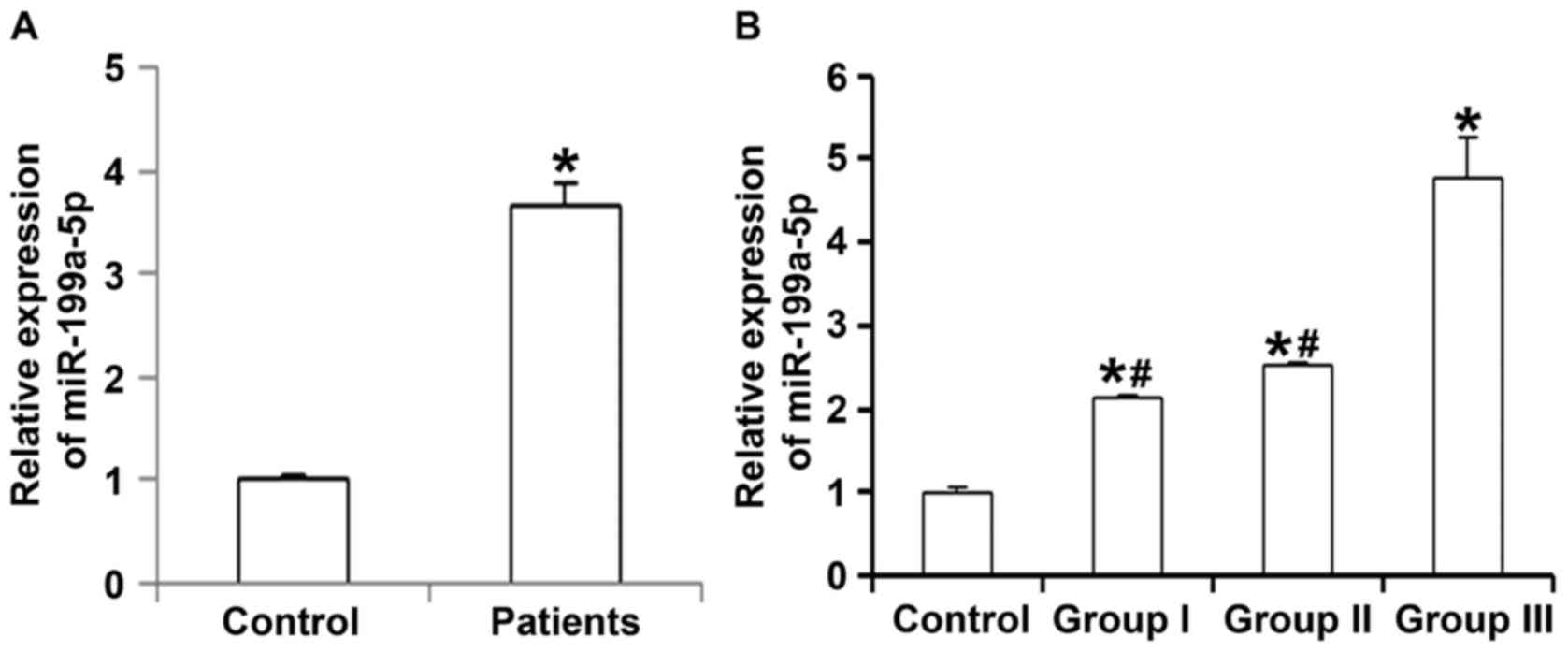
To measure the expression of miR-199a-5p in
peripheral blood of patients with hypertension, RT-qPCR was
employed. The results indicated that the level of miR-199a-5p in
peripheral blood from patients with primary hypertension were
significantly higher than that in healthy subjects (P<0.05;
Fig. 1A). In addition, the serum
levels of miR-199a-5p in patients with grade I or II hypertension
were significantly lower than those from patients with grade III
hypertension (P<0.05), while they were not significantly
different between patients with grade I and those with grade II
hypertension (P>0.05; Fig. 1B).
These results suggest that the expression of miR-199a-5p in
peripheral blood is associated with the progression of
hypertension.
Overexpression of miR-199a-5p inhibits
the proliferation of HUVECs
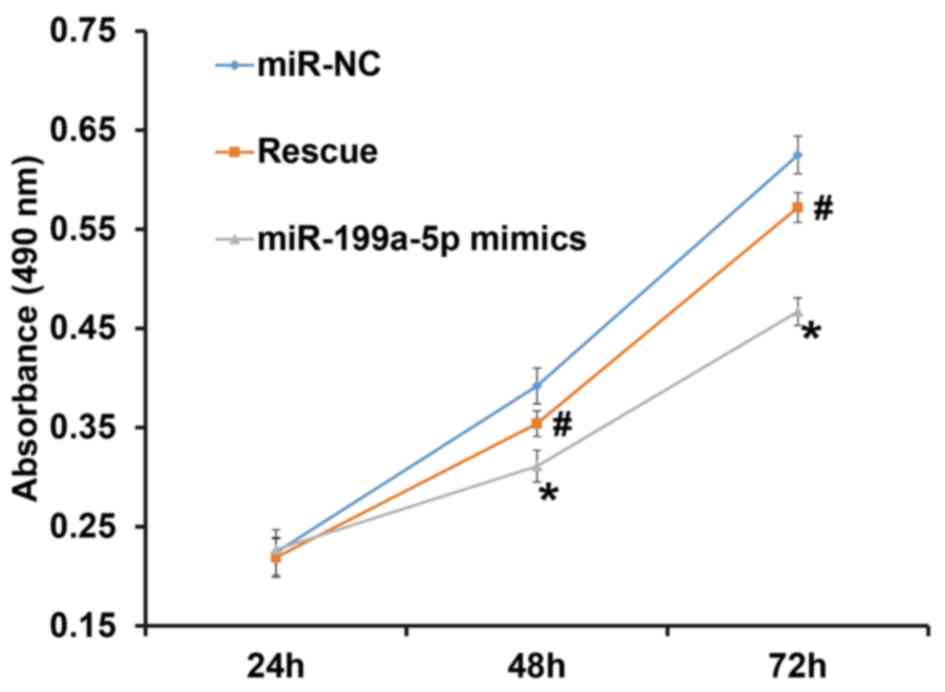
To assess the proliferation of HUVECs, a CCK-8 assay
was performed. The results indicated that the number of HUVECs in
the group transfected with miR-199a-5p mimics was significantly
lower than that in the NC group at 48 and 72 h (P<0.05). In
addition, the number of HUVECs in the rescue group was
significantly higher than that in the miR-199a-5p mimics group at
48 and 72 h (P<0.05), but was not different from that in the NC
group (P>0.05; Fig. 2). The
result indicates that overexpression of miR-199a-5p inhibits the
proliferation of HUVECs.
Overexpression of miR-199a-5p promotes
the apoptosis of HUVECs
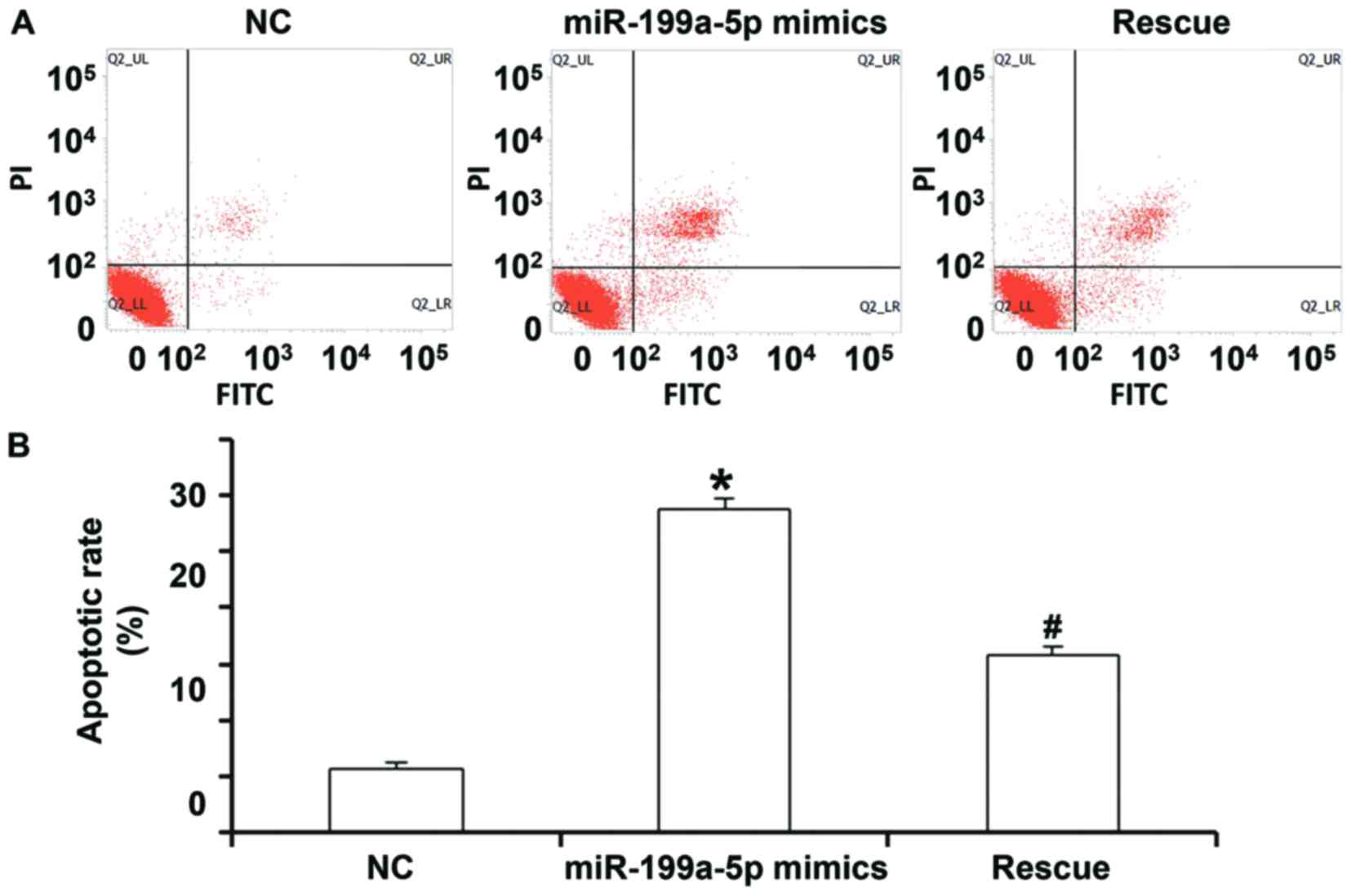
To detect apoptosis of HUVECs, a flow cytometric
assay was performed. In the NC group, the percentages of necrotic
cells, as well as cells in the early and late stage of apoptosis
were 1.86, 1.47 and 4.27%, respectively, while they were 2.10, 8.80
and 18.50%, respectively, in the miR-199a-5p group. In the rescue
group, the percentages were 1.30, 6.50 and 10.80%, respectively
(Fig. 3A). Quantification of the
percentage of total apoptotic cells (early stage from lower right
quadrant and late stage from upper right quadrant) indicated that
the apoptotic rate of HUVECs transfected with miR-199a-5p mimics
was significantly higher than that in the NC group (P<0.05).
Furthermore, the apoptotic rate of HUVECs in the rescue group was
significantly lower than that in the miR-199a-5p mimics group
(P<0.05), but significantly higher than that in the NC group
(P>0.05; Fig. 3A and B). These
results suggest that overexpression of miR-199a-5p promotes the
apoptosis of HUVECs.
miR-199a-5p aggravates vascular
endothelial injury by inhibiting autophagy and promoting
apoptosis
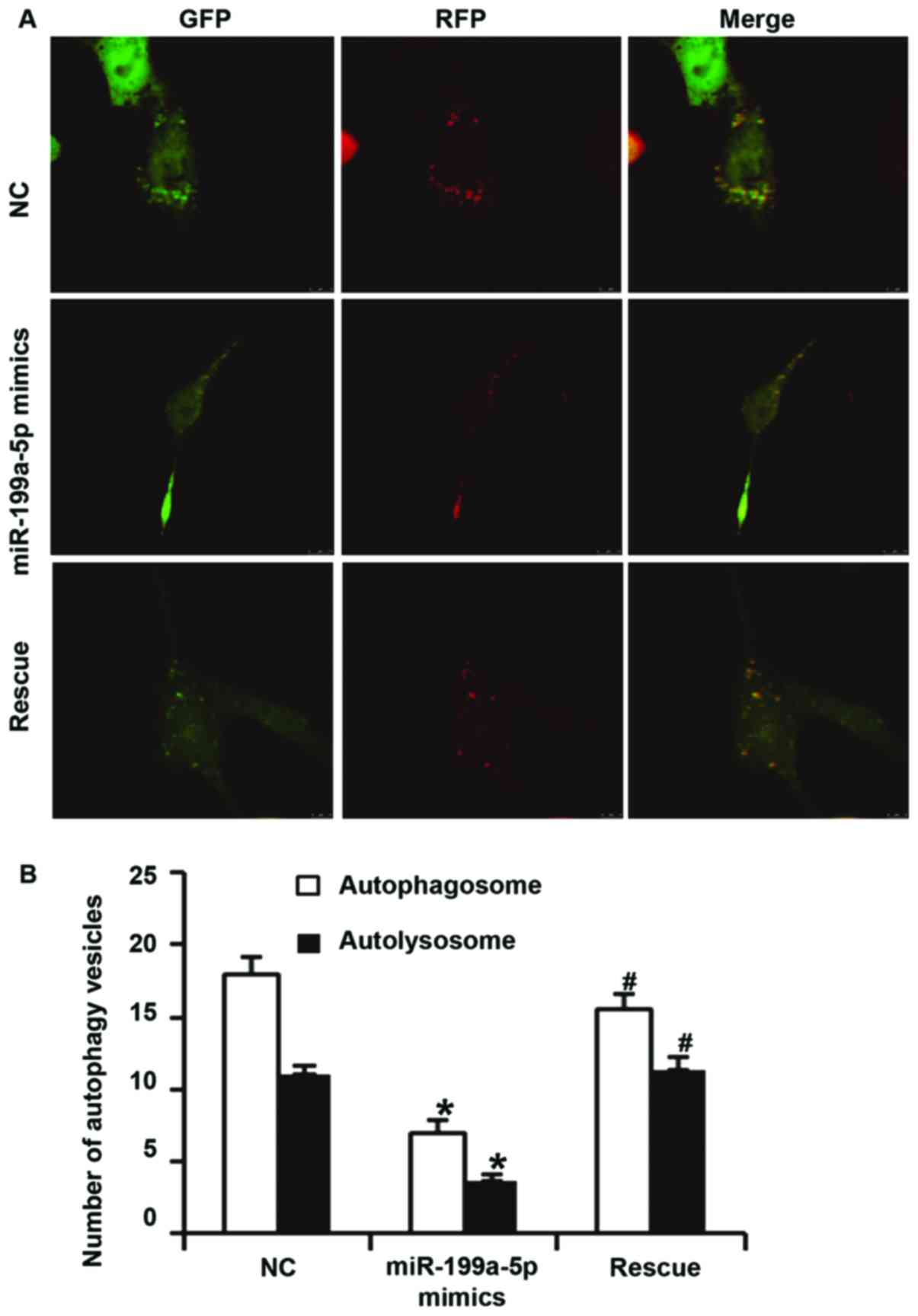
To examine the effect of miR-199a-5p overexpression
on autophagy of HUVECs, laser scanning confocal microscopy, western
blot analysis, electron microscopy and flow cytometry were
utilized. Confocal microscopy indicated that the numbers of
autophagosomes and autolysosomes in HUVECs transfected with
miR-199a-5p mimics were significantly lower than those in the NC
group (P<0.05). In addition, the numbers of autophagosomes and
autolysosomes in HUVECs of the rescue group were significantly
higher than those in the miR-199a-5p mimics group (P<0.05). In
addition, the number of autophagosomes in HUVECs of the rescue
group was significantly lower than that in NC group (P<0.05),
but the number of autolysosomes in HUVECs of the rescue group were
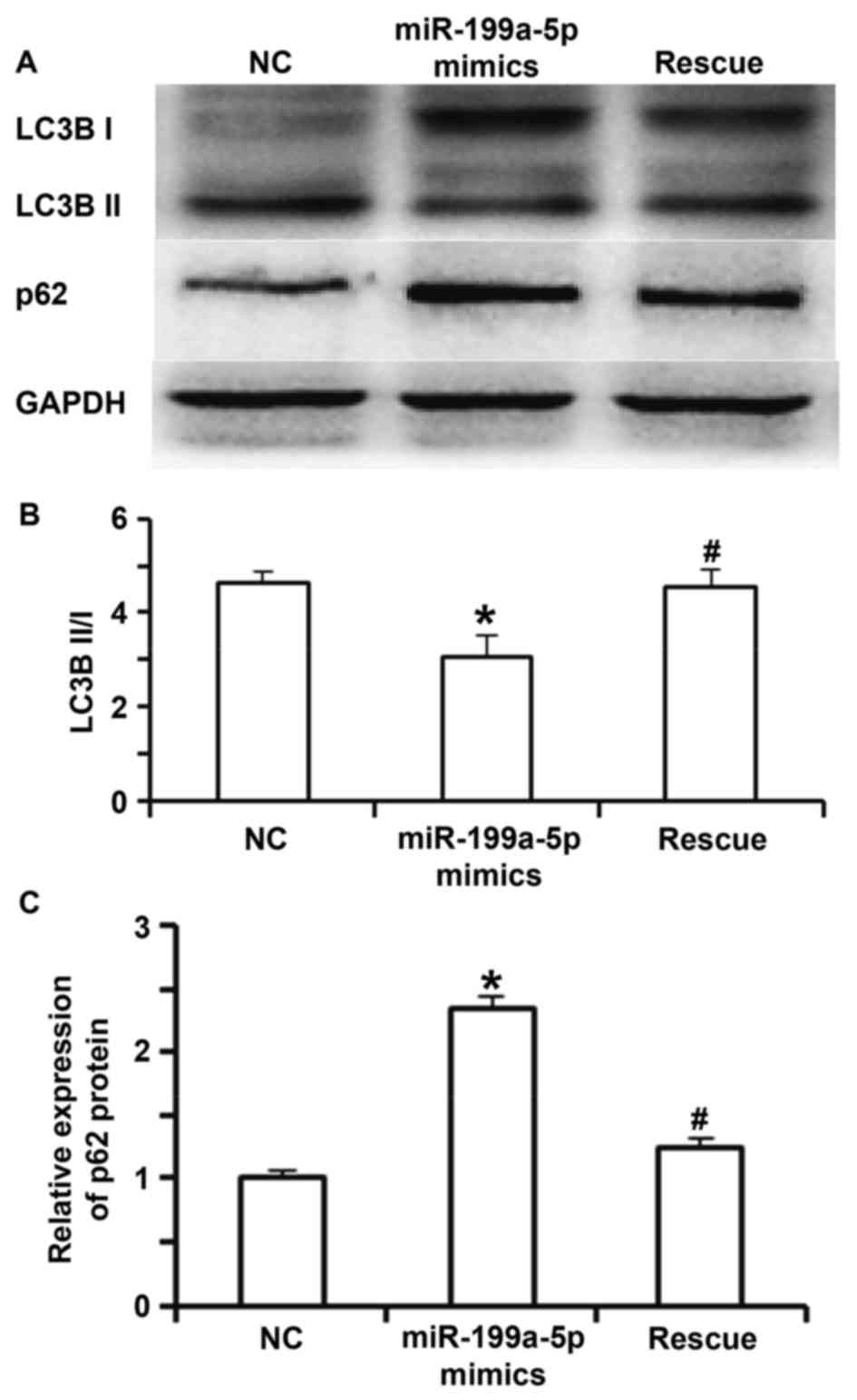
not different from those in the NC group (P>0.05; Fig. 4A and B). Western blot analysis
indicated that the ratio of LC3BII to LC3BI in the miR-199a-5p
mimics group was significantly lower than that in the NC group
(P<0.05). In the rescue group, the ratio of LC3BII to LC3BI was
significantly increased compared with that in the miR-199a-5p
mimics group (P<0.05), but was not different from that in the NC
group (P>0.05; Fig. 5A and B). In
addition, the expression of p62 protein in the miR-199a-5p mimics
group was significantly higher than that in the NC group and the
rescue group (P<0.05; Fig. 5A and
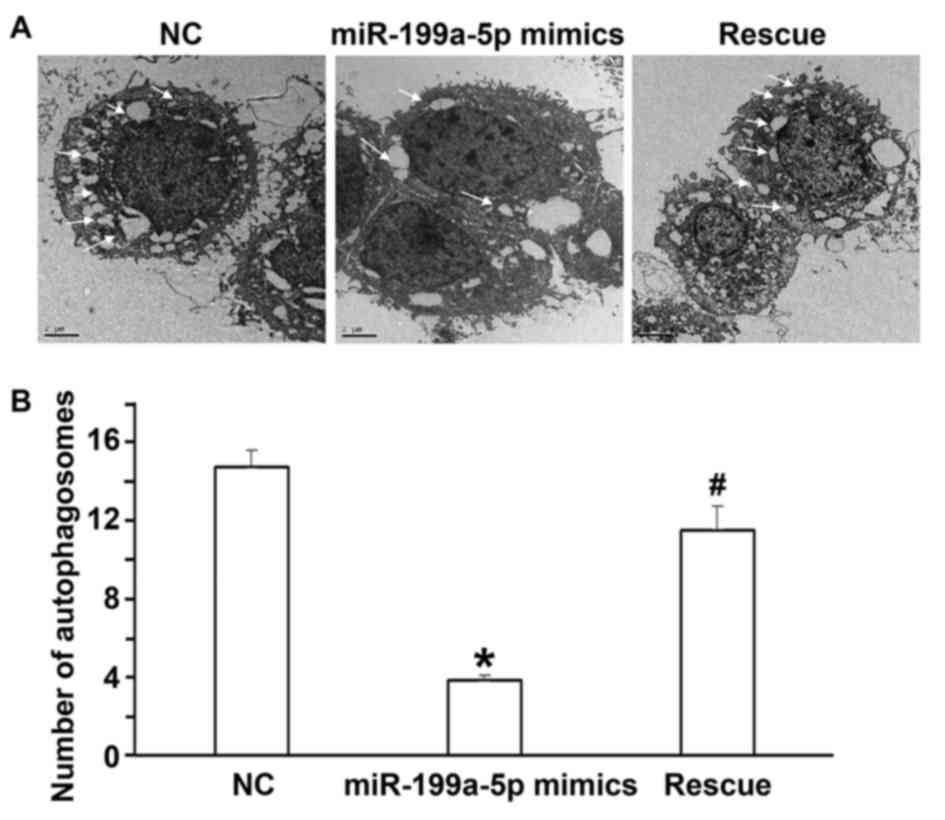
C). Electron microscopy indicated that the number of
autophagosomes in the miR-199a-5p mimics group was lower than that
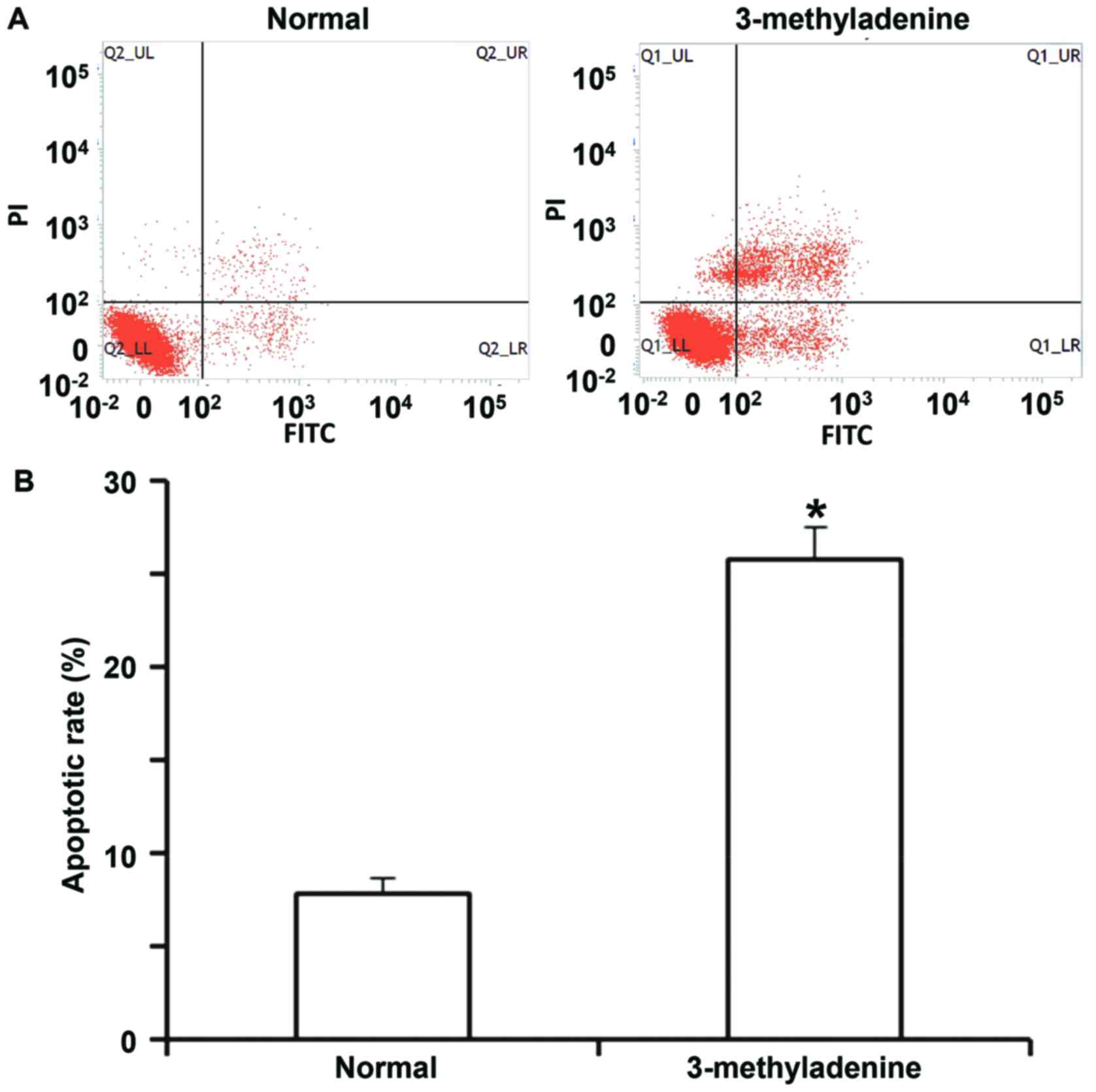
in the NC group and the rescue group (P<0.05; Fig. 6A and B). The flow cytometry assay
indicated that the apoptotic rate of HUVECs incubated with
3-methyladenine was significantly higher than that of normal HUVECs
(P<0.05; Fig. 7A and B). These
results suggest that miR-199a-5p aggravates vascular endothelial
injury by inhibiting autophagy and inducing apoptosis, and that the
downregulation of autophagy itself promotes HUVEC apoptosis.
miR-199a-5p inhibits autophagy and
promotes apoptosis of HUVECs via the adenosine monophosphate kinase
(AMPK)/unc-51 like autophagy activating kinase 1 (ULK1) 1 signaling
pathway
To determine the effect of miR-199a-5p expression on
the AMPK/ULK1 signaling pathway, western blot analysis, flow
cytometry and laser scanning confocal microscopy were performed.
Western blot analysis indicated that the phosphorylation levels of
AMPK-α, acetyl-CoA carboxylase (ACC) and ULK1 in the miR-199a-5p
mimics group were reduced compared with those in the NC or rescue
group (data not shown). In addition, treatment with metformin
enhanced the phosphorylation levels of AMPK-α, ACC and ULK1
compared with those in the miR-199a-5p mimics group (data not
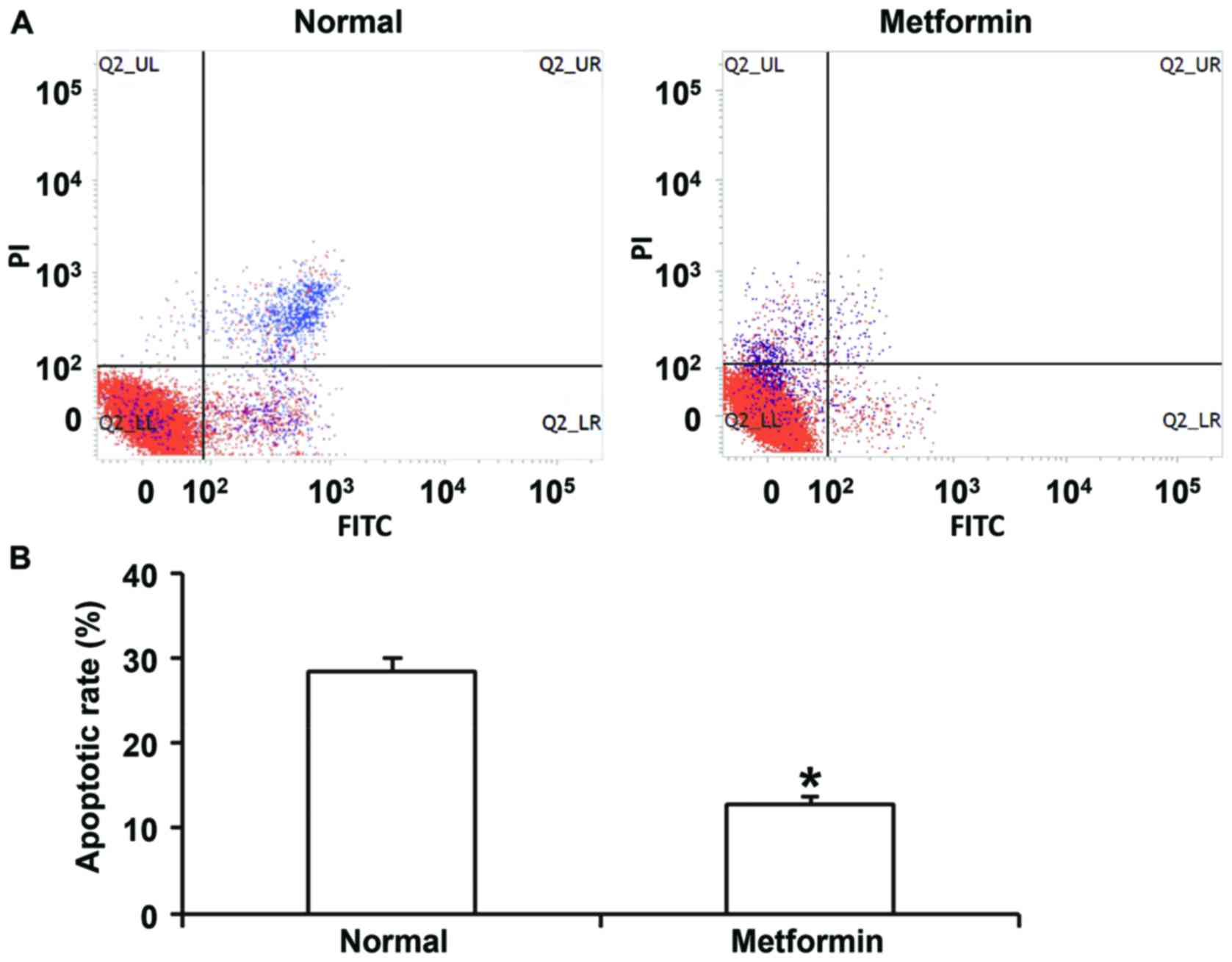
shown). Flow cytometry indicated that metformin treatment inhibited
apoptosis of HUVECs transfected with miR-199a mimics (Fig. 8). In addition, confocal microscopy
demonstrated that metformin treatment activated autophagy of HUVECs
transfected with miR-199a mimics (Fig.
9). These results suggest that miR-199a-5p inhibits autophagy
and promotes apoptosis of HUVECs via the AMPK/ULK1 signaling
pathway.
Discussion
Primary hypertension is a common chronic
cardiovascular disease that causes vascular endothelial cell injury
and inflammation in patients, leading to various complications
(16). Early diagnosis and timely
intervention of vascular endothelial injury have great significance
in the prevention and delay of damage to tissues and organs. In the
development of hypertension and its complications, the expression
of multiple miRNA molecules is changed, suggesting their clinical
and diagnostic value for injuries of the heart, brain and blood
vessels (17). In the present study,
the expression of miR-199a-5p in the peripheral blood of patients
with primary hypertension was indicated to be elevated, and
miR-199a-5p was demonstrated to inhibit autophagy of HUVECs by
downregulating the activity of the AMPK/ULK1 signaling pathway,
finally leading to damage of vascular endothelial cells.
Vascular endothelial cells are important barriers in
the vascular lumen. They not only maintain the stability of
hemodynamics and material exchange, but also secrete inflammatory
cytokines, as well as vascular relaxation and contraction factors,
which regulate blood pressure. Abnormal structure and function of
vascular endothelial cells are important factors that promote the
development of hypertension (18).
Persistent hypertension may cause injury of vascular endothelial
cells, further influencing the relaxation and contraction of blood
vessels, and facilitating the development of hypertension. It has
been reported that various miRNAs have important roles in the
injury of vascular endothelial cells in normal or tumor tissues.
For instance, miR-129-1 and miR-133 have been demonstrated to
promote the proliferation of vascular endothelial cells (19). In addition, miR-506 inhibits tumor
angiogenesis in hepatocellular carcinoma (20). miR-141 reduces the expression of
intercellular adhesion molecule-1 in endothelial cells, and
alleviates myocardial ischemia/reperfusion injury (21). The newly discovered miR-199a-5p has
been reported to inhibit the proliferation, invasion and migration
of various types of tumor cells, and its expression was
downregulated in certain tumor types (13). Reduced expression of miR-199a-5p is
associated with methylation of its promoter (22). In cardiovascular diseases,
downregulation of miR-199a expression is closely associated with
the hypertrophy of cardiac muscle cells (23). The results of the present study
indicate that miR-199a-5p expression is upregulated in patients
with hypertension, and that miR-199a-5p mimics inhibit the
proliferation and promote the apoptosis of HUVECs. Furthermore,
miR-199a-5p expression is also increased in smooth muscle cells and
promotes hypertension. For instance, Hashemi Gheinani et al
(24), reported that miR-199a-5p
regulates smooth muscle cell proliferation and morphology by
targeting the WNT2 signaling pathway. Liu et al (25) confirmed that miR-199a-5p was
increased in human pulmonary artery smooth muscle cells, and that
it was associated with pulmonary artery hypertension. The results
of the present study reveal that miR-199a-5p expression in HUVECs
is decreased compared with that in human vascular smooth muscle
cells.
Autophagy is an important biological process that is
evolutionarily conserved in eukaryotic cells and maintains
homeostasis of circulating intracellular substances. It has a
variety of roles in inflammation, immunity and tumors. For
instance, the nucleotide-binding oligomerization domain-containing
protein 2 gene controls bacterial infection of intestinal
epithelial cells by regulating autophagy (26), and nuclear factor (erythroid-derived
2)-like 2 participates in acute lung injury by regulating
autophagic activity (27). Under
stress conditions, autophagy is significantly increased and this
process is dependent on the regulation of a series of
autophagy-associated genes. It has been reported that miR-183,
miR-376b and miR-106a participate in the regulation of autophagy.
In addition, Li et al (28)
reported that miR-199a-5p inhibits autophagy via the mammalian
target of rapamycin signaling pathway, finally leading to
cardiomyocyte hypertrophy. Xu et al (29) discovered that downregulation of
miR-199a-5p expression in hepatocellular carcinoma activates
autophagy of tumor cells, and enhances resistance to chemotherapy.
The present study reported that miR-199a-5p inhibits AMPK signaling
in vascular endothelial cells. It is known that activation of the
AMPK signaling pathway leads to the phosphorylation of ULK1 and the
activation of autophagy (30). In
addition, treatment with metformin, an AMPK activator, inhibited
apoptosis and enhanced autophagy of HUVECs induced by miR-199a-5p.
These results suggest that miR-199a-5p aggravates vascular
endothelial injury by inhibiting autophagy and promoting apoptosis
via the suppression of the AMPK signaling pathway and inhibition of
ULK1 phosphorylation.
In conclusion, the present study demonstrates that
miR-199a-5p expression is enhanced in peripheral blood of patients
with hypertension. miR-199a-5p regulates the AMPK signaling
pathway, inhibits autophagy and promotes vascular endothelial
injury induced by hypertension, and may be a suitable marker for
the clinical diagnosis as well as a target for the treatment of
hypertension.
Acknowledgements
The authors would like to thank the Affiliated
Hospital of Qingdao University (Shandong, China) for their
support.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XT, CY and YA designed the study; XT, CY, LS, DL,
XC, DX, JZ, WX, CM and LG performed experiments; XT, CY, DL and YA
analyzed the data. The final version of the manuscript has been
read and approved by all authors, and each author believes that the
manuscript represents honest work.
Ethical approval and consent to
participate
All procedures were approved by the Ethics Committee
of Qingdao University (Qingdao, China). Written informed consent
was obtained from all patients or their families.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kjeldsen SE, Os I and Redon J: Treatment
of hypertension and the price to pay; adverse events and
discontinuation from randomized treatment in clinical trials. J
Hypertens. 34:1489–1491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhuo Y, Zeng Q, Zhang P, Li G, Xie Q and
Cheng Y: Functional polymorphism of lncRNA MALAT1 contributes to
pulmonary arterial hypertension susceptibility in Chinese people.
Clin Chem Lab Med. 55:38–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rudemiller NP and Crowley SD: Interactions
between the immune and the renin-angiotensin systems in
hypertension. Hypertension. 68:289–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taddei S and Bruno RM: Endothelial
dysfunction in hypertension: Achievements and open questions. J
Hypertens. 34:1492–1493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Yang Y, Yang D, Tong G, Lv S, Lin
X, Chen C and Dong W: Tetrandrine prevents monocrotaline-induced
pulmonary arterial hypertension in rats through regulation of the
protein expression of inducible nitric oxide synthase and cyclic
guanosine monophosphate-dependent protein kinase type 1. J Vasc
Surg. 64:1468–1477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao Y, Jiang Z, Zeng Z, Liu Y, Gu Y, Ji Y,
Zhao Y and Li Y: Bcl-2 silencing attenuates hypoxia-induced
apoptosis resistance in pulmonary microvascular endothelial cells.
Apoptosis. 21:69–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Santovito D and Weber C: Zooming in on
microRNAs for refining cardiovascular risk prediction in secondary
prevention. Eur Heart J. 38:524–528. 2017.PubMed/NCBI
|
|
8
|
Shyu YC, Lee TL, Lu MJ, Chen JR, Chien RN,
Chen HY, Lin JF, Tsou AP, Chen YH, Hsieh CW and Huang TS:
miR-122-mediated translational repression of PEG10 and its
suppression in human hepatocellular carcinoma. J Transl Med.
14:2002016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang C, Chen X, Wang X, Ji A, Jiang L,
Sang F and Li F: miR-135a acts as a tumor suppressor in gastric
cancer in part by targeting KIFC1. Onco Targets Ther. 9:3555–3563.
2016.PubMed/NCBI
|
|
10
|
Zhang H, Cao H, Xu D and Zhu K:
MicroRNA-92a promotes metastasis of nasopharyngeal carcinoma by
targeting the PTEN/AKT pathway. Onco Targets Ther. 9:3579–3588.
2016.PubMed/NCBI
|
|
11
|
Wang P, Xu J, Hou Z, Wang F, Song Y, Wang
J, Zhu H and Jin H: miRNA-34a promotes proliferation of human
pulmonary artery smooth muscle cells by targeting PDGFRA. Cell
Prolif. 49:484–493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elia L and Condorelli G: MicroRNAs and
pulmonary hypertension: A tight link. Cardiovasc Res. 111:163–164.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo Z, Feng C, Hu P, Chen Y, He XF, Li Y
and Zhao J: Serum microRNA-199a/b-3p as a predictive biomarker for
treatment response in patients with hepatocellular carcinoma
undergoing transarterial chemoembolization. Onco Targets Ther.
9:2667–2674. 2016.PubMed/NCBI
|
|
14
|
Wang J, Sun W, Wells GA, Li Z, Li T, Wu J,
Zhang Y, Liu Y, Li L, Yu Y, et al: Differences in prevalence of
hypertension and associated risk factors in urban and rural
residents of the northeastern region of the people's republic of
china: A cross-sectional study. PLoS One. 13:e01953402018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vashukova ES, Glotov AS, Fedotov PV,
Efimova OA, Pakin VS, Mozgovaya EV, Pendina AA, Tikhonov AV,
Koltsova AS and Baranov VS: Placental microRNA expression in
pregnancies complicated by superimposed preeclampsia on chronic
hypertension. Mol Med Rep. 14:22–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Samanta S, Balasubramanian S, Rajasingh S,
Patel U, Dhanasekaran A, Dawn B and Rajasingh J: MicroRNA: A new
therapeutic strategy for cardiovascular diseases. Trends Cardiovasc
Med. 26:407–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pang Y and Thomas P: Additive effects of
low concentrations of estradiol-17β and progesterone on nitric
oxide production by human vascular endothelial cells through shared
signaling pathways. J Steroid Biochem Mol Biol. 165:258–267. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soufi-Zomorrod M, Hajifathali A, Kouhkan
F, Mehdizadeh M, Rad SM and Soleimani M: MicroRNAs modulating
angiogenesis: miR-129-1 and miR-133 act as angio-miR in HUVECs.
Tumour Biol. 37:9527–9534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu Z, Zhang W, Gao S, Jiang Q, Xiao Z, Ye
L and Zhang X: MiR-506 suppresses liver cancer angiogenesis through
targeting sphingosine kinase 1 (SPHK1) mRNA. Biochem Biophys Res
Commun. 468:8–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu RR, Li J, Gong JY, Kuang F, Liu JY,
Zhang YS, Ma QL, Song CJ, Truax AD, Gao F, et al: MicroRNA-141
regulates the expression level of ICAM-1 on endothelium to decrease
myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ
Physiol. 309:H1303–H1313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang X, Lei S, Long J, Liu X and Wu Q:
MicroRNA-199a-5p inhibits tumor proliferation in melanoma by
mediating HIF-1α. Mol Med Rep. 13:5241–5247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Li S, Zhou Q, Sun Q, Shen S, Zhou
Y, Bei Y and Li X: Qiliqiangxin attenuates phenylephrine-induced
cardiac hypertrophy through downregulation of mir-199a-5p. Cell
Physiol Biochem. 38:1743–1751. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hashemi Gheinani A, Burkhard FC, Rehrauer
H, Aquino Fournier C and Monastyrskaya K: MicroRNA MiR-199a-5p
regulates smooth muscle cell proliferation and morphology by
targeting WNT2 signaling pathway. J Biol Chem. 290:7067–7086. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Liu G, Zhang H and Wang J:
MiRNA-199a-5p influences pulmonary artery hypertension via
downregulating Smad3. Biochem Biophys Res Commun. 473:859–866.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Negroni A, Colantoni E, Vitali R, Palone
F, Pierdomenico M, Costanzo M, Cesi V, Cucchiara S and Stronati L:
NOD2 induces autophagy to control AIEC bacteria infectiveness in
intestinal epithelial cells. Inflamm Res. 65:803–813. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rojo de la Vega M, Dodson M, Gross C,
Mansour HM, Lantz RC, Chapman E, Wang T, Black SM, Garcia JG and
Zhang DD: Role of Nrf2 and autophagy in acute lung injury. Curr
Pharmacol Rep. 2:91–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Z, Song Y, Liu L, Hou N, An X, Zhan D,
Li Y, Zhou L, Li P, Yu L, et al: miR-199a impairs autophagy and
induces cardiac hypertrophy through mTOR activation. Cell Death
Differ. 24:1205–1213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu N, Zhang J, Shen C, Luo Y, Xia L, Xue F
and Xia Q: Cisplatin-induced downregulation of miR-199a-5p
increases drug resistance by activating autophagy in HCC cell.
Biochem Biophys Res Commun. 423:826–831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan XY, Tian C, Wang H, Xu Y, Ren K, Zhang
BY, Gao C, Shi Q, Meng G, Zhang LB, et al: Activation of the
AMPK-ULK1 pathway plays an important role in autophagy during prion
infection. Sci Rep. 5:147282015. View Article : Google Scholar : PubMed/NCBI
|