Introduction
Obesity is a worldwide epidemic and the fifth
leading cause of mortality (1).
Obesity is also a well-documented risk factor for non-alcoholic
fatty liver disease (NAFLD), which occurs in approximately one
third of the population in the developed countries (2). Liver steatosis, which is common in
NAFLD patients, is a chronic liver disease characterized by a
spectrum of hepatic pathologies that can lead to cirrhosis
(3).
Pregnant women may experience preterm delivery, and
~7% of them are treated with synthetic glucocorticoids to improve
neonatal outcomes. However, prenatal exposure to glucocorticoids
has been reported to be associated with negative health
consequences for the offspring that persist into adulthood
(4,5). It has become increasingly clear that
early environmental influences also have a long-lasting impact on
the development and physiology of the fetus. For instance, prenatal
glucocorticoid overexposure may result in liver steatosis (6). Previous studies have observed that
prenatal exposure to dexamethasone increased lipid accumulation in
the liver in rats on a high-fat diet, while the deleterious effects
of the high-fat diet in the perinatal and post-weaning periods
persisted into adulthood (7,8). The present study aimed to expand upon
the results of a previous study by our group, which also
investigated the effect of melatonin (9). In addition, our previous study
identified that postnatal high-fat diet increased liver steatosis
and apoptosis exacerbated by prenatal dexamethasone exposure via an
oxidative effect (6). High-fat diet
is an environmental risk factor for disease progression in patients
with NAFLD subsequent to prenatal stress. Thus, it is important to
identify a strategy to prevent liver steatosis in the perinatal
period.
Melatonin, an indoleamine secreted from the pineal
gland, has a wide range of physiological and pharmacological
functions, as well as beneficial effects in metabolic diseases
(10–12). The administration of melatonin
reduced the metabolic pathologies associated with the intake of
high-fat diet, suggesting its protective role (13). A further animal study demonstrated
the therapeutic amelioration caused by melatonin, which improved
the metabolic syndrome induced by high fructose intake in rats
(14). In addition, melatonin
maintains the biological membrane fluidity, and functions as an
antioxidant and reactive oxygen species scavenger at the
mitochondrial level (15). Melatonin
also counteracts adipogenesis by stimulating thermogenesis, insulin
sensitivity and glucose uptake, as well as by improving the liver
function in different metabolic and physiological conditions
(16). Furthermore, the
hepatoprotective effect of melatonin in suppressing steatosis and
oxidative stress has been observed in human patients with NAFLD
(17) and in animal models of
obesity (18). Certain investigators
observed that the use of pentoxifylline and melatonin in
combination with pioglitazone ameliorated experimental NAFLD, thus
indicating that pentoxifylline and melatonin can be used as
adjuvant therapies in the clinical management of NAFLD (19). Finally, it was previously observed
that melatonin alleviated liver steatosis and oxidative injury
(8). However, its roles and
mechanisms of action are not fully understood in the context of
environmental exposure in the perinatal period.
The aim of the present study was to determine
whether melatonin protects the liver from NAFDL induced by prenatal
dexamethasone exposure and postnatal high-fat diet. The study
focused on the investigation of the liver morphology, redox state,
apoptosis and metabolic markers.
Materials and methods
Animals
The present study followed the Guide for the Care
and Use of Laboratory Animals of the National Institutes of Health
(version 8) (20). The protocol was
approved by the Institutional Animal Care and Use Committee of the
Kaohsiung Chang Gung Memorial Hospital (Kaohsiung, Taiwan). A total
of 15 Sprague-Dawley rats (12–16 weeks old; 200–250 mg;
male:female, 1:2; BioLasco Taiwan Co., Ltd., Taipei, Taiwan) were
housed in the animal care facility of the Kaohsiung Chang Gung
Memorial Hospital at 22°C and a 12 h light/dark cycle (light from 7
a.m.). Pregnant rats were checked for litters daily at 10 a.m.
Sprague-Dawley female rats were allowed to mate with male rats for
24 h. After 1 day, female rats were separated from the male rats
and housed individually in a standard plastic home cage. Following
confirmation of pregnancy on day 14 after mating, pregnant females
were randomly subjected to prenatal steroid exposure or left
undisturbed until delivery. The day of birth was designated as
postnatal day 0 (PND 0). Rat pups were weaned at PND 21 and had
free access to standard chow and water.
Grouping
The offspring rats were divided into five groups
(n=6/group), as follows: Vehicle group, vehicle + postnatal
high-fat diet group (VHF), prenatal dexamethasone exposure group
(DEX), prenatal dexamethasone exposure + postnatal high-fat diet
group (DHF), and prenatal dexamethasone exposure + postnatal
high-fat diet + melatonin group (DHFM).
Postnatal high fat diet
Offspring rats in the VHF, DHF and DHFM groups
received a 58% high-fat diet (D12331; Research Diet, Inc., New
Brunswick, NJ, USA) from weaning to 6 months of age. The vehicle
and DEX groups received control diet, consisting of 23.5% protein,
4.5% fat, 5.0% crude fiber, 7.0% crude ash and 13% water (Taiwan
Fwusow Industry Co., Ltd., Taichung, Taiwan).
Prenatal dexamethasone or vehicle
exposure
Pregnant Sprague-Dawley rats at gestational days
14–21 were administered dexamethasone (0.1 mg/kg/day; D4902;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or normal saline via
intraperitoneal injection in the DEX and vehicle groups,
respectively, as reported in a previous study (21).
Melatonin administration
Melatonin was prepared three times a week by
dissolving 16 mg dry melatonin in 100% ethanol (1 ml; M5250;
Sigma-Aldrich; Merck KGaA), followed by dilution with distilled
water to a final concentration of 40 mg/l (0.01%). The bottles were
covered with aluminum foil to protect them from light. Rats were
estimated to consume ~25 ml of water per day; thus the mean daily
intake of melatonin to the rat mothers in the DHFM group was
estimated to be 1 mg/kg/day from gestational days 14–21 until
weaning ended and to the offspring rats in the DHFM group was
estimated to be 1 mg/kg/day until they were sacrificed, as
previously reported (6). This
melatonin regimen has previously been used in our laboratory, and
rats have demonstrated good compliance with beneficial results
(6,22,23).
Sacrifice and sample collection
Animals in all five groups were sacrificed at PND
180 by intramuscular injection of ketamine (10 mg/kg; Pfizer,
Hsinchu, Taiwan), with efforts made to minimize suffering.
Immediately after sacrificing, the liver was resected, weighed,
embedded in paraffin and stored at −80°C. A blood sample was also
collected by cardiocentesis and placed into an EDTA-containing
vial. The levels of cholesterol, aspartate aminotransferase (AST)
and alanine aminotransferase (ALT) levels in the blood were
determined using a standard autoanalyzer (Hitachi 7450; Hitachi,
Ltd., Tokyo, Japan).
Immunohistochemical localization of
Oil Red O
Frozen liver samples obtained from 6 rats in each
group were cut into 2–3-µm-thick sections, which were mounted on
coating slides. Tissue sections were then incubated with 3%
hydrogen peroxide for 10 min to block any endogenous peroxidase
activity. Next, the sections were boiled in citrate buffer in the
microwave for 12 min for antigen retrieval, stained with Oil Red O
(cat. no. 1.02419; EMD Millipore, Billerica, MA, USA), dissolved in
60% isopropanol for 15 min at room temperature, and rinsed in 60%
isopropanol and washing in dH2O. The tissue was then
counterstained by hematoxylin, washed thoroughly in dH2O
and transferred to Aqua-Mount mounting medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The labeling index of
hepatocytes was defined as the mean ± standard error of number of
positively stained nuclei among 500 hepatocytes in the liver
section. Sections containing at least three portal tracts were used
for the labeling index in at least three non-overlapping fields
under a light microscope (magnification, −100).
Western blot analysis
Liver tissues from the five rat groups were
dissected and frozen immediately in liquid N2. The
tissue was homogenized in lysis buffer (cat. no. 17081; iNtRON
Biotechnology, Seongnam, Korea) and then centrifuged at 14,000 × g
at 4°C for 5 min. Total protein concentration was detected by
Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Protein (40 µg) from the
supernatant of each sample was separated by 10–15% SDS-PAGE and
transferred to polyvinylidene difluoride membranes by
electrophoresis. Next, the membranes were blocked in Tris-buffered
saline/Tween-20 buffer containing 5% low-fat milk powder for 1 h at
room temperature. Immunoblotting was then performed by incubation
at 4°C for overnight with specific primary antibodies. The
monoclonal primary antibodies included cleaved caspase-3 (cat. no.
9661; Cell Signaling Technology, Inc., Danvers, MA, USA), tumor
necrosis factor-α (TNF-α; cat. no. 3707; Cell Signaling Technology,
Inc.), suppressor of cytokine signaling 3 (SOCS3; cat. no. ab16030;
Abcam, Cambridge, MA, USA), phosphoinositide 3-kinase (PI3K; cat.
no. ab40755; Abcam), antioxidant manganese superoxide dismutase
(MnSOD; cat. no. sc133134; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), malondialdehyde (MDA; ab27642; Abcam), and the internal
control, GAPDH (cat. no. ab9485; Abcam). The membranes were then
incubated for 1 h at room temperature with an alkaline phosphatase
(AP) conjugated anti-rabbit (1:5,000; cat. no. S3731; Promega
Corp., Madison, WI, USA) or mouse (1:10,000; cat. no. 715–055-150;
Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA)
secondary antibody (6,24,25).
Subsequently, the western blots were visualized using the ProtoBlot
II AP System (Promega Corp.), The western blotting results were
quantified by the colorimetric method using Quantity One software
(version 4.5.2; Bio-Rad Laboratories, Inc.).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
In order to investigate cellular apoptosis in the
liver tissue, TUNEL staining was performed as previously reported
(26). ApopTag Plus Peroxidase in
situ Apoptosis Detection kit (Chemicon; EMD Millipore) was used
for TUNEL assay. Briefly, deparaffinized sections obtained from 6
rats in each group were washed with distilled water and treated
with a protease for 15 min at 37°C. Sections containing at least
three portal tracts were used for counting the labeling index in at
least three non-overlapping fields under a microscope, and the mean
± standard error of these counts was considered as a labeling
index. A total of 500 hepatocytes in each rat were used to count
positively stained cells.
Statistical analysis
SPSS software (version 15.0; SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. Statistical analysis
was conducted using analysis of variance with a Bonferroni post hoc
test. The data are presented as the mean ± standard error of the
mean. Statistically significant differences were indicated by
values of P<0.05.
Results
AST and ALT levels, and the body
weight of the rats are increased in the DHF group and decreased in
the DHFM group
As demonstrated in Table
I, the body weight was significantly higher in the DHF group as
compared with that in the DHFM and DEX groups. There was no
significant difference in the liver weight among the DHF, vehicle
and DHFM groups. By contrast, rats in the DHF group exhibited a
higher body weight and a lower liver/body ratio compared with the
DEX group. In addition, the DHF group had a significantly lower
liver/body ratio comparing with the DEX group, but no significant
difference when compared with the Vehicle, VHF and DHFM groups.
 | Table I.Weight and biochemical parameters of
animals in the experimental groups. |
Table I.
Weight and biochemical parameters of
animals in the experimental groups.
| Parameter | Vehicle | VHF | DEX | DHF | DHFM |
|---|
| Weight (mg) |
623.0±17.1a |
759.0±17.9b |
658.2±20.0a |
804.4±35.1b,c |
699.2±13.9a |
| Liver weight
(mg) | 17.5±0.5 | 18.9±0.4 |
20.7±0.8b | 20.3±0.9 | 18.7±0.2 |
| Liver/body (%) |
2.63±0.1c |
2.48±0.1c |
3.09±0.1a,b |
2.52±0.1c |
2.68±0.1c |
| AST (U/l) |
95.0±9.0a |
148.3±7.1a |
128.9±19.5a | 413.1±
64.2b,c |
163.8±17.7a |
| ALT (U/l) |
35.8±1.8a |
76.0±8.3a |
57.2±10.5a |
336.1±67.2b,c |
90.2±10.2a |
| Cholesterol
(mg/dl) |
74.3±2.6c | 79.3±4.0 |
102.9±8.6b | 88.2±7.1 |
59.8±6.8a,c |
Animals in the DHF group presented the highest AST
and ALT levels (Table I).
Furthermore, the levels of AST, ALT and cholesterol were
significantly reduced in the DHFM group compared with the DHF
group. The cholesterol level in the DHF group was not statistically
significant compared with the Vehicle, VHF, DEX and DHFM groups
(Table I).
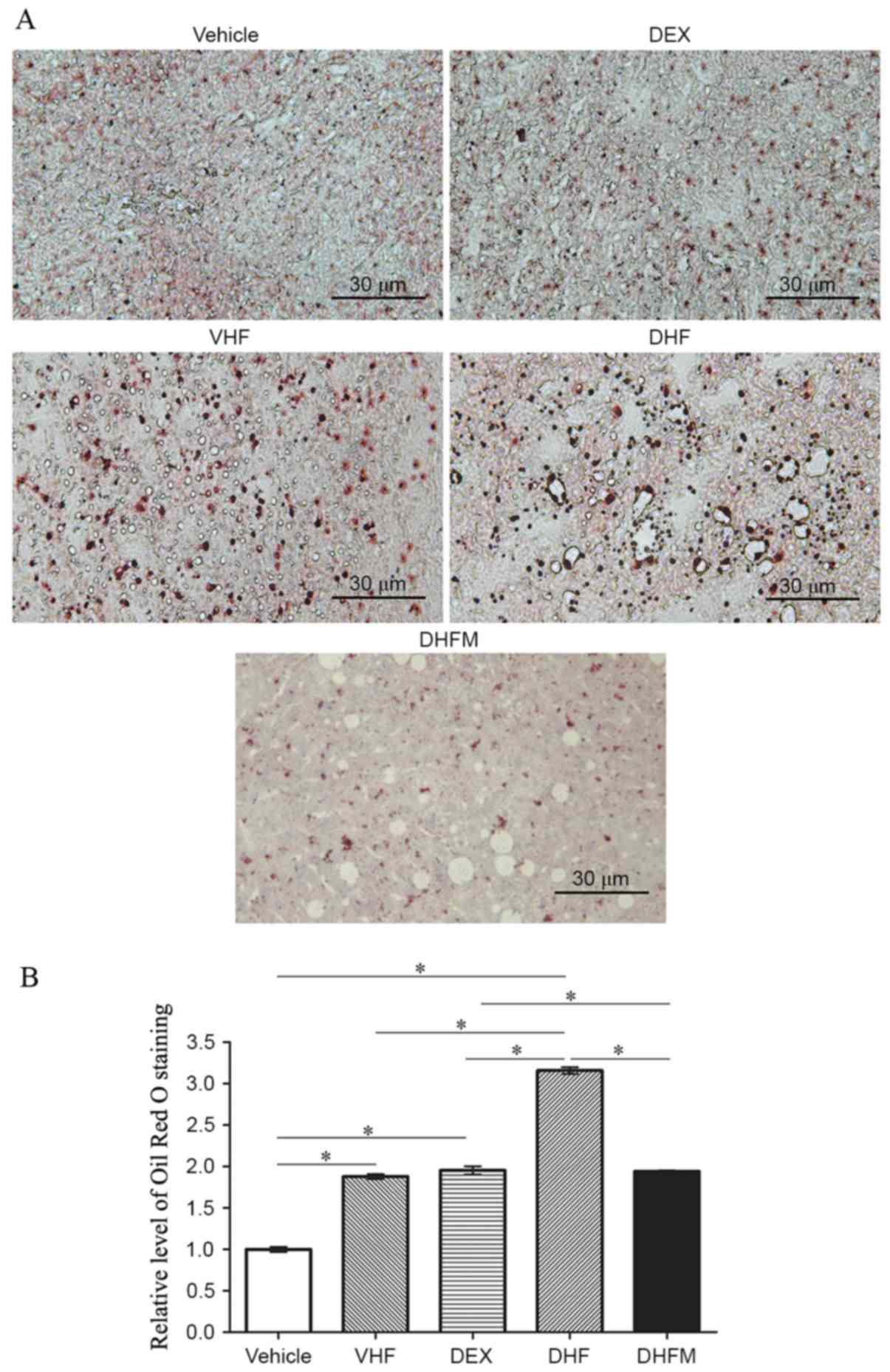
Liver steatosis in the DHFM group
Oil Red O staining of the liver tissues (Fig. 1A) exhibited a stronger intensity in
the DHF group compared with that in the other four groups,
indicating a synergistic effect between prenatal dexamethasone
exposure and postnatal high-fat diet. In addition, the results
demonstrated that melatonin administration reduced the Oil Red O
staining level in the DHFM group as compared with the DHF group
(P<0.05; Fig. 1B). This suggested
that melatonin was efficient in reducing the liver lipid storage by
attenuating liver steatosis in rats with postnatal high-fat diets
and exposed to prenatal dexamethasone.
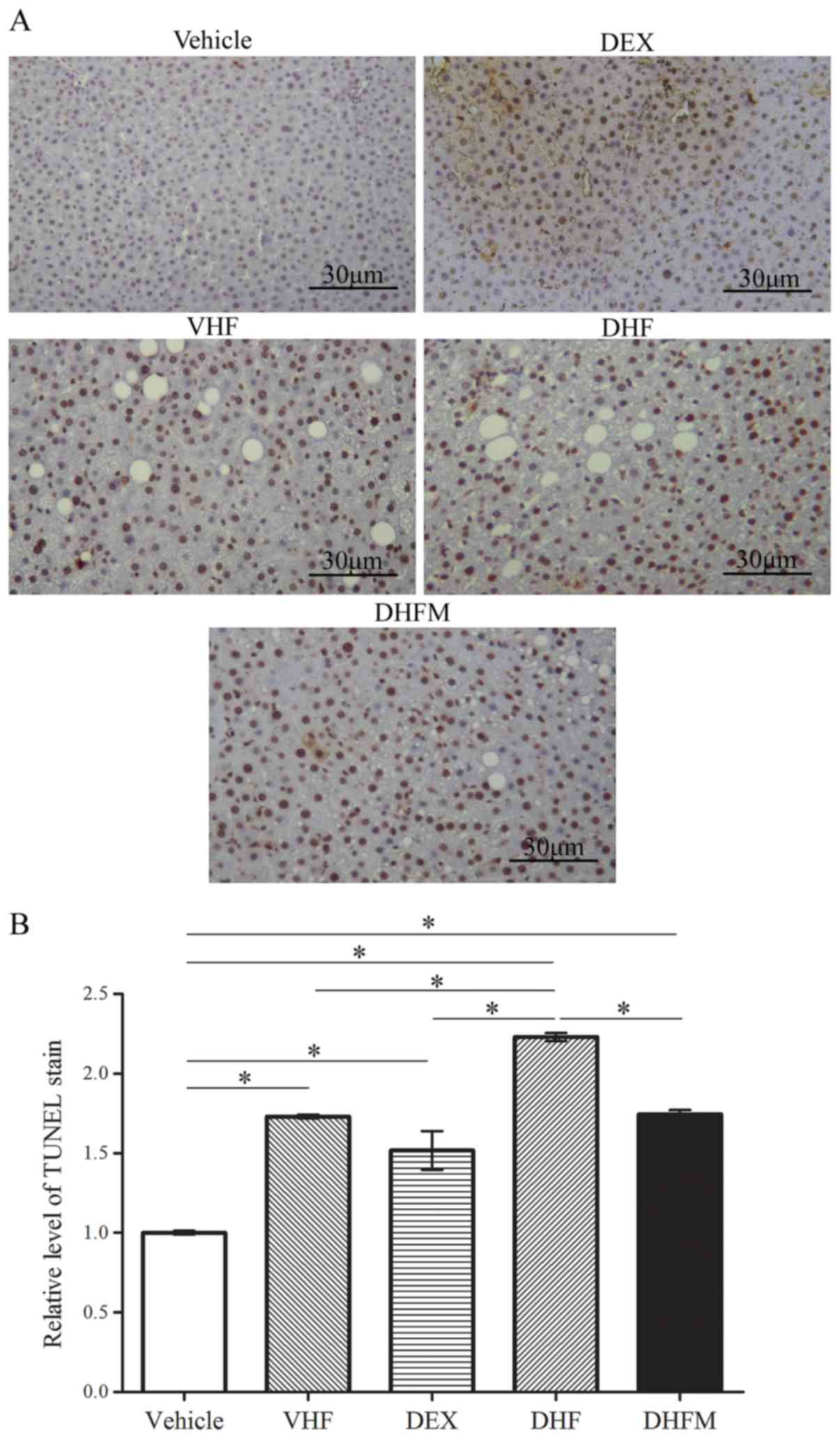
Apoptosis in the DHFM group
Activation of apoptotic pathways was detected based
on the extent of TUNEL staining (Fig.
2), as well as the level of cleaved caspase-3 (Fig. 3A and B). TUNEL staining revealed a
significantly greater proportion of apoptotic cells in the DHF
group compared with that in the other four groups, indicating a
synergistic effect between prenatal dexamethasone exposure and
postnatal high-fat diet (Fig. 2).
Following melatonin administration to the offspring rats in the
DHFM group, the degree of TUNEL staining was decreased in
comparison with that in the DHF group (P<0.05; Fig. 2B). These findings suggested that
melatonin was efficient in reducing liver cell apoptosis in rats
with prenatal dexamethasone exposure and postnatal high-fat
diet.
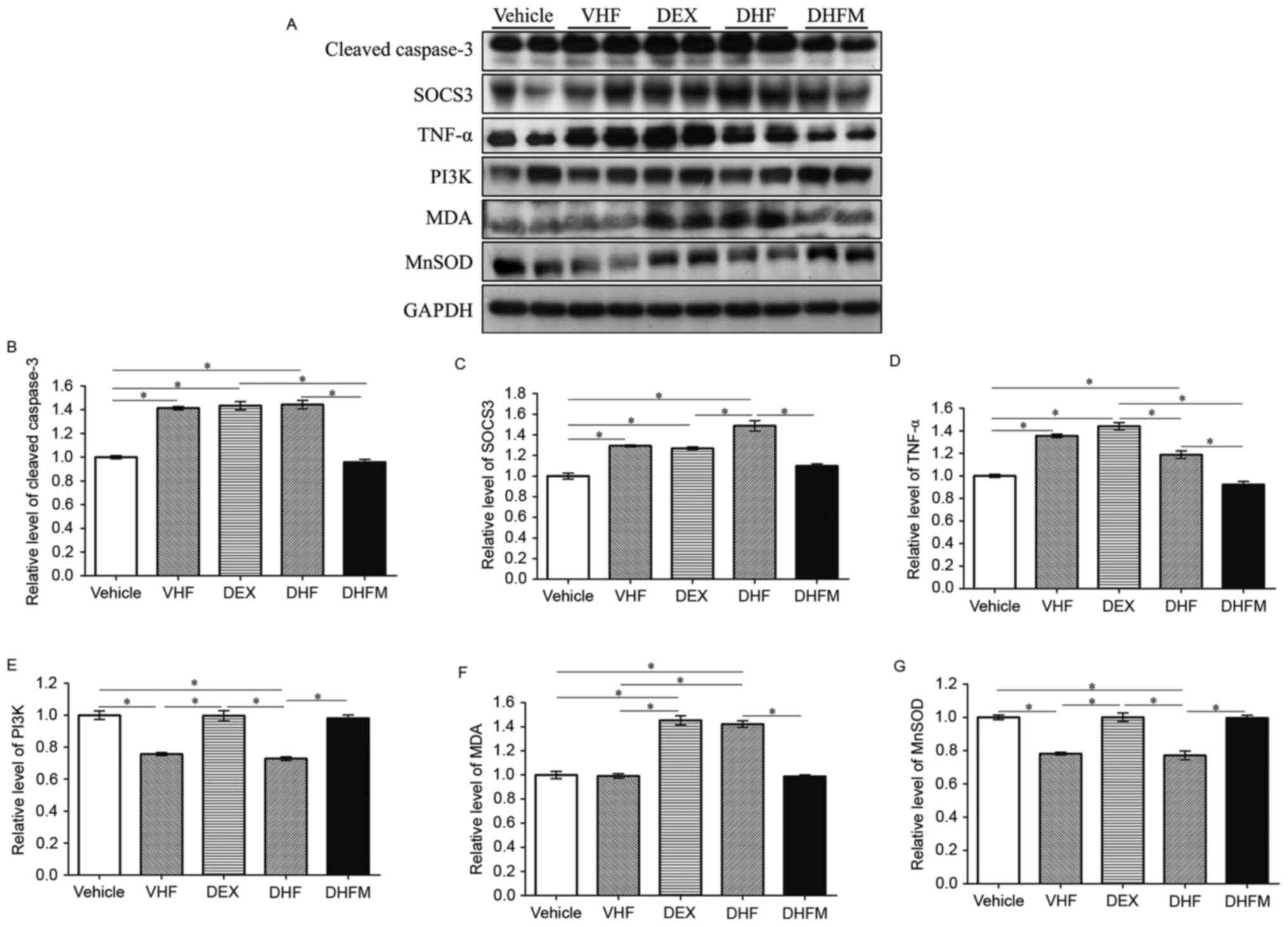 | Figure 3.Western blotting and analyses of (A)
western blotting results revealed the changes in protein expression
in the five groups. Protein expression levels of (B) cleaved
caspase-3, (C) SOCS3, (D) TNF-α, (E) PI3K, (F) MDA and (G) MnSOD,
examined by western blot analysis. Higher expression levels of
cleaved caspase-3, TNF-α, SOCS3 and MDA were observed in the DHF
group and lower levels in the DHFM group. By contrast, the
expression of PI3K and MnSOD was decreased in the DHF group
compared with that of the DHFM group. All values are expressed as
the mean ± standard error (n=6). *P<0.05. VHF, postnatal
high-fat diet; DEX, prenatal dexamethasone; DHF, prenatal
dexamethasone + postnatal high-fat diet; DHFM, prenatal
dexamethasone + postnatal high-fat diet + melatonin; SOCS3,
suppressor of cytokine signaling 3; MDA, malondialdehyde; TNF-α,
tumor necrosis factor α; MnSOD, manganese superoxide dismutase;
PI3K, phosphoinositide-3-kinase. |
The level of cleaved caspase-3 was significantly
higher in the DHF group as compared with Vehicle and DHFM groups
(Fig. 3B). Following melatonin
administration, the level in the DHFM group was significantly
decreased compared with the DHF group (P<0.05), suggesting that
melatonin was efficient in reducing the level of cleaved caspase-3
and thus decreasing apoptosis via insulin resistance and oxidative
stress in rats subjected to prenatal dexamethasone exposure and
postnatal high-fat diet.
Insulin resistance and inflammation in
the DHFM group
Western blot analysis (Fig. 3A) also revealed significantly higher
SOCS3 levels in the DHF group in comparison with that in the DHFM,
DEX and Vehicle groups (Fig. 3C).
Following melatonin administration, SOCS3 expression was decreased
(DHF vs. DHFM groups; P<0.05), suggesting that melatonin
effectively reduced the SOCS3 protein expression in rats with
prenatal dexamethasone exposure and postnatal high-fat diet.
TNF-α is a cell signaling protein involved in
systemic inflammation that has been proposed to be associated with
insulin resistance (27) and to
cause insulin resistance (28). Rats
in the DHF group exhibited significantly higher levels of TNF-α
compared with animals in the DHFM and Vehicle groups (Fig. 3D). These results suggest that
melatonin was efficient in reducing the TNF-α level and this
insulin resistance in rats with prenatal dexamethasone exposure and
postnatal high-fat diet.
PI3 kinases are key components of the insulin
signaling pathway (29,30). PI3 kinases are also associated with
oxidative stress (31). Rats in the
DHF group demonstrated reduced PI3K protein expression compared
with animals in the Vehicle, DEX and DHFM groups (Fig. 3E), indicating a synergistic effect
between prenatal dexamethasone exposure and postnatal high-fat
diet. Furthermore, melatonin administration increased the PI3K
expression (DHF vs. DHFM groups; P<0.05), suggesting that
melatonin was efficient in restoring the PI3K protein expression in
rats with prenatal dexamethasone exposure and postnatal high-fat
diet.
Anti-oxidative stress in the DHFM
group
MDA results from lipid peroxidation of
polyunsaturated fatty acids and is used as a biomarker to measure
the level of oxidative stress (32,33).
Rats in the DHF group presented higher MDA levels in comparison
with animals in the Vehicle, VHF and DHFM groups (Fig. 3F). Subsequent to melatonin
administration in DHFM rats, the MDA level was decreased compared
with the DHF group (P<0.05), suggesting that melatonin was
efficient in reducing oxidative stress in rats with prenatal
dexamethasone exposure and postnatal high-fat diet.
The crucial role of MnSOD in protecting cells
against oxidative stress is well known (34). In the present study, rats in the DHF
group exhibited a decreased MnSOD level in comparison with that in
animals of the other four groups, indicating a synergistic effect
between prenatal dexamethasone exposure and postnatal high-fat diet
(Fig. 3G). However, melatonin
administration increased the MnSOD level (DHF vs. DHFM groups;
P<0.05), suggesting that melatonin was efficient in restoring
the MnSOD protein expression, which exhibits the anti-oxidative and
protective effects, in rats with prenatal dexamethasone exposure
and postnatal high-fat diet. Therefore inflammation was
reduced.
Oil Red O staining and western blot analyses of
cleaved caspase-3, TNF-α, SOCS3, MDA, MnSOD and PI3K demonstrated
that their expression levels were similar in the Vehicle and DHFM
groups following melatonin administration. In the VHF group,
cleaved caspase-3, TNF-α, SOCS3 expression levels and TUNEL
staining were increased, and PI3K and MnSOD expression levels were
decreased compared with the Vehicle group as inflammation and the
level of apoptosis were found to be higher in the postnatal high
fat diet.
Discussion
The present study demonstrated that prenatal
melatonin administration in rats exposed to dexamethasone
prenatally and receiving a high-fat diet postnatally was efficient
in: i) Reducing the liver lipid storage; ii) decreasing the
expression levels of cleaved caspase-3, TNF-α, SOCS3 and MDA in the
liver; and iii) restoring the liver PI3K and MnSOD protein
expression levels in the liver.
Animals in all five groups were sacrificed at PND
180, and body weight measurement indicated a higher weight in the
DHF group as compared with that in the DHFM and DEX groups
(Table I). However, there was no
significant difference in the liver weight among the DHF, vehicle
and DHFM groups. In addition, higher body weight and lower
liver/body ratio were detected in the DHF group as compared with
the DEX group. In the present study, it was also observed that the
cholesterol in the DEX group was higher than that in the DHF group,
although these groups presented a higher cholesterol level when
compared with the vehicle group. These results are similar to the
observations of our previous study (9). Additionally, the DHFM group presented
the lowest cholesterol level, it was significantly less compared
with the DEX and DHF group. Furthermore, animals in the DHF group
exhibited the highest AST and ALT levels. The results demonstrate
that melatonin treatment led to reduced the levels of AST, ALT and
cholesterol.
In our previous study (9), it was observed that rats in the DHF
group had stronger liver lipid accumulation as compared with rats
in the VHF and DEX groups. Therefore, a high-fat diet in
combination with prenatal dexamethasone exposure may lead to more
severe lipid accumulation and liver injury. In the present study,
melatonin administration reduced the lipid storage in the DHF
group. To the best of our knowledge, this is the first study to
demonstrate that melatonin administration is able to prevent liver
steatosis in adult rats induced by a combination of prenatal
dexamethasone exposure and postnatal high-fat diet.
Kupffer cells are major producers of cytokines,
modulating the levels of TNF-α, and a higher TNF-α expression is
correlated with Kupffer cell dysfunction or activation (35,36).
Thus, higher TNF-α expression during prenatal dexamethasone
administration may indicate inflammation. Decreased TNF-α
expression in the DHFM group compared with the DHF group indicates
that prenatal melatonin treatment reduced inflammation.
Apoptosis is the main process contributing to
disease progression in NAFLD (37).
Our previous study revealed increased liver apoptosis in rats with
prenatal dexamethasone exposure that were receiving a high-fat diet
postnatally (8). In addition, there
is growing evidence that melatonin may directly affect the pathways
associated with apoptosis (38–40). In
the present study, it was demonstrated that melatonin reduced the
apoptosis by decreasing the level of cleaved caspase-3 in rats with
prenatal dexamethasone exposure and postnatal high-fat diet.
Oxidative stress is another major contributor to
disease progression in NAFLD (27),
and previous studies indicated that the hepatic MDA level was
increased in high-fat diet-induced NAFLD (41,42).
Furthermore, MDA levels were increased in rats with NAFLD, and the
PI3K level was decreased during oxidative stress in rats on a
high-fat diet (31,43). The results of a previous study by our
group are consistent with the observations of the present study
(9), it revealed that a higher level
of oxidative stress in the DHF group was accompanied by increased
MDA and decreased MnSOD levels, which was not observed in the DEX
group. In addition, the level of PI3K in the DHF group was
decreased, suggesting that it participates in the pathogenesis of
oxidative stress. In the present study, it was demonstrated that
melatonin administration reduced the oxidative stress by lowering
the MDA level, as well as increasing the MnSOD and PI3K levels, in
rats with prenatal dexamethasone exposure receiving a high-fat diet
postnatally.
SOCS3 contributes to leptin and insulin resistance,
and certain studies have demonstrated that removal of the SOCS gene
prevents insulin resistance in obesity (44,45). A
previous study also observed that overexpression of SOCS3 in
adipocytes led to a reduction in insulin signaling activation, and
diminished the glucose uptake and lipogenesis in mice that were
resistant to the development of diet-induced obesity and associated
insulin resistance (46). SOCS3 is
known to serve two functions in insulin resistance in the liver.
Hepatic SOCS3 expression is able to mediate insulin resistance in
the liver, whereas the lack of SOCS3 in the liver may stimulate
nuclear factor-κB-dependent chronic inflammation, which may also
result in systemic insulin resistance (47,48). In
the present study, SOCS3 was overexpressed in the liver of animals
in the DHF group, therefore insulin resistance was increased, and
its level was significantly decreased following melatonin
administration. Melatonin may decrease insulin resistance by
decreasing SOCS3.
In conclusion, the present study demonstrated that a
high-fat postnatal diet exacerbated the effect of prenatal
dexamethasone exposure and led to enhanced liver steatosis in adult
offspring rats, which was reversed by prenatal melatonin
administration.
Acknowledgements
The present study was supported by grants from the
Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan (nos.
CMRPG8C0841, CMRPG8F0131 and CMRPG8E0641).
Glossary
Abbreviations
Abbreviations:
|
NAFLD
|
non-alcoholic fatty liver disease
|
|
TUNEL
|
TdT-mediated dUTP-biotin nick
end-labeling
|
|
SOCS3
|
suppressor of cytokine signaling 3
|
|
MDA
|
malondialdehyde
|
|
MnSOD
|
manganese superoxide dismutase
|
|
PI3K
|
phosphoinositide 3-kinase
|
References
|
1
|
Malik VS, Willett WC and Hu FB: Global
obesity: Trends, risk factors and policy implications. Nat Rev
Endocrinol. 9:13–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen JC, Horton JD and Hobbs HH: Human
fatty liver disease: Old questions and new insights. Science.
332:1519–1523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stacchiotti A, Favero G, Lavazza A, Golic
I, Aleksic M, Korac A, Rodella LF and Rezzani R: Hepatic
macrosteatosis is partially converted to microsteatosis by
melatonin supplementation in ob/ob mice non-alcoholic fatty liver
disease. PLoS One. 11:e01481152016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kapoor A, Petropoulos S and Matthews SG:
Fetal programming of hypothalamic-pituitary-adrenal (HPA) axis
function and behavior by synthetic glucocorticoids. Brain Res Rev.
57:586–595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Varcoe TJ, Boden MJ, Voultsios A, Salkeld
MD, Rattanatray L and Kennaway DJ: Characterisation of the maternal
response to chronic phase shifts during gestation in the rat:
Implications for fetal metabolic programming. PLoS One.
8:e538002013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tiao MM, Huang LT, Chen CJ, Sheen JM, Tain
YL, Chen CC, Kuo HC, Huang YH, Tang KS, Chu EW and Yu HR: Melatonin
in the regulation of liver steatosis following prenatal
glucocorticoid exposure. Biomed Res Int. 2014:9421722014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drake AJ, Raubenheimer PJ, Kerrigan D,
McInnes KJ, Seckl JR and Walker BR: Prenatal dexamethasone programs
expression of genes in liver and adipose tissue and increased
hepatic lipid accumulation but not obesity on a high-fat diet.
Endocrinology. 151:1581–1587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tamashiro KL, Terrillion CE, Hyun J,
Koenig JI and Moran TH: Prenatal stress or high-fat diet increases
susceptibility to diet-induced obesity in rat offspring. Diabetes.
58:1116–1125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang YH, Chen CJ, Tang KS, Sheen JM, Tiao
MM, Tain YL, Chen CC, Chu EW, Li SW, Yu HR and Huang LT: Postnatal
high-fat diet increases liver steatosis and apoptosis threatened by
prenatal dexamethasone through the oxidative effect. Int J Mol Sci.
17:3692016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Favero G, Lonati C, Giugno L, Castrezzati
S, Rodella LF and Rezzani R: Obesity-related dysfunction of the
aorta and prevention by melatonin treatment in ob/ob mice. Acta
Histochem. 115:783–788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Govender J, Loos B, Marais E and
Engelbrecht AM: Mitochondrial catastrophe during
doxorubicin-induced cardiotoxicity: A review of the protective role
of melatonin. J Pineal Res. 57:367–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan DX, Manchester LC, Fuentes-Broto L,
Paredes SD and Reiter RJ: Significance and application of melatonin
in the regulation of brown adipose tissue metabolism: Relation to
human obesity. Obes Rev. 12:167–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hussein MR, Ahmed OG, Hassan AF and Ahmed
MA: Intake of melatonin is associated with amelioration of
physiological changes, both metabolic and morphological pathologies
associated with obesity: An animal model. Int J Exp Pathol.
88:19–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitagawa A, Ohta Y and Ohashi K: Melatonin
improves metabolic syndrome induced by high fructose intake in
rats. J Pineal Res. 52:403–413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
García JJ, López-Pingarrón L,
Almeida-Souza P, Tres A, Escudero P, García-Gil FA, Tan DX, Reiter
RJ, Ramírez JM and Bernal-Pérez M: Protective effects of melatonin
in reducing oxidative stress and in preserving the fluidity of
biological membranes: A review. J Pineal Res. 56:225–237. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Luxán-Delgado B, Caballero B, Potes Y,
Rubio-González A, Rodríguez I, Gutiérrez-Rodríguez J, Solano JJ and
Coto-Montes A: Melatonin administration decreases adipogenesis in
the liver of ob/ob mice through autophagy modulation. J Pineal Res.
56:126–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Celinski K, Konturek PC, Slomka M,
Cichoz-Lach H, Brzozowski T, Konturek SJ and Korolczuk A: Effects
of treatment with melatonin and tryptophan on liver enzymes,
parameters of fat metabolism and plasma levels of cytokines in
patients with non-alcoholic fatty liver disease-14 months follow
up. J Physiol Pharmacol. 65:75–82. 2014.PubMed/NCBI
|
|
18
|
Hatzis G, Ziakas P, Kavantzas N,
Triantafyllou A, Sigalas P, Andreadou I, Ioannidis K, Chatzis S,
Filis K, Papalampros A and Sigala F: Melatonin attenuates high fat
diet-induced fatty liver disease in rats. World J Hepatol.
5:160–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaitone S, Hassan N, El-Orabi N and
El-Awady el-S: Pentoxifylline and melatonin in combination with
pioglitazone ameliorate experimental non-alcoholic fatty liver
disease. Eur J Pharmacol. 662:70–77. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 8th edition. National Acadamies
Press; Washington, DC; 2011, PubMed/NCBI
|
|
21
|
Hauser J, Feldon J and Pryce CR: Direct
and dam-mediated effects of prenatal dexamethasone on emotionality,
cognition and HPA axis in adult Wistar rats. Horm Behav.
56:364–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lui CC, Hsu MH, Kuo HC, Chen CC, Sheen JM,
Yu HR, Tiao MM, Tain YL, Chang KA and Huang LT: Effects of
melatonin on prenatal dexamethasone-induced epigenetic alterations
in hippocampal morphology and reelin and glutamic acid
decarboxylase 67 levels. Dev Neurosci. 37:105–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tain YL, Huang LT, Lin IC, Lau YT and Lin
CY: Melatonin prevents hypertension and increased asymmetric
dimethylarginine in young spontaneous hypertensive rats. J Pineal
Res. 49:390–398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin TK, Huang LT, Huang YH, Tiao MM, Tang
KS and Liou CW: The effect of the red wine polyphenol resveratrol
on a rat model of biliary obstructed cholestasis: Involvement of
anti-apoptotic signalling, mitochondrial biogenesis and the
induction of autophagy. Apoptosis. 17:871–879. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tiao MM, Wang FS, Huang LT, Chuang JH, Kuo
HC, Yang YL and Huang YH: MicroRNA-29a protects against acute liver
injury in a mouse model of obstructive jaundice via inhibition of
the extrinsic apoptosis pathway. Apoptosis. 19:30–41. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tiao MM, Lin TK, Kuo FY, Huang CC, Du YY,
Chen CL and Chuang JH: Early stage of biliary atresia is associated
with significant changes in 8-hydroxydeoxyguanosine and
mitochondrial copy number. J Pediatr Gastroenterol Nutr.
45:329–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nieto-Vazquez I, Fernández-Veledo S,
Krämer DK, Vila-Bedmar R, Garcia-Guerra L and Lorenzo M: Insulin
resistance associated to obesity: The link TNF-alpha. Arch Physiol
Biochem. 114:183–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kwon H and Pessin JE: Adipokines mediate
inflammation and insulin resistance. Front Endocrinol (Lausanne).
4:712013.PubMed/NCBI
|
|
29
|
D'Souza K, Kane DA, Touaibia M, Kershaw
EE, Pulinilkunnil T and Kienesberger PC: Autotaxin is regulated by
glucose and insulin in adipocytes. Endocrinology. 158:791–803.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rashid K, Das J and Sil PC: Taurine
ameliorate alloxan induced oxidative stress and intrinsic apoptotic
pathway in the hepatic tissue of diabetic rats. Food Chem Toxicol.
51:317–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y and Yang JH: Activation of the
PI3K/Akt pathway by oxidative stress mediates high glucose-induced
increase of adipogenic differentiation in primary rat osteoblasts.
J Cell Biochem. 114:2595–2602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Davey MW, Stals E, Panis B, Keulemans J
and Swennen RL: High-throughput determination of malondialdehyde in
plant tissues. Anal Biochem. 347:201–207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Del Rio D, Stewart AJ and Pellegrini N: A
review of recent studies on malondialdehyde as toxic molecule and
biological marker of oxidative stress. Nutr Metab Cardiovasc Dis.
15:316–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Candas D and Li JJ: MnSOD in oxidative
stress response-potential regulation via mitochondrial protein
influx. Antioxid Redox Signal. 20:1599–1617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Diehl AM: Nonalcoholic steatosis and
steatohepatitis IV. Nonalcoholic fatty liver disease abnormalities
in macrophage function and cytokines. Am J Physiol Gastrointest
Liver Physiol. 282:G1–G5. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tacke F, Luedde T and Trautwein C:
Inflammatory pathways in liver homeostasis and liver injury. Clin
Rev Allergy Immunol. 36:4–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Canbakan B, Senturk H, Canbakan M, Toptas
T, Tabak O, Balci H, Olgac V and Ozbay G: Is alanine
aminotransferase level a surrogate biomarker of hepatic apoptosis
in nonalcoholic fatty liver disease? Biomark Med. 4:205–214. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guha M, Maity P, Choubey V, Mitra K,
Reiter RJ and Bandyopadhyay U: Melatonin inhibits free
radical-mediated mitochondrial-dependent hepatocyte apoptosis and
liver damage induced during malarial infection. J Pineal Res.
43:372–381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cruz A, Padillo FJ, Torres E, Navarrete
CM, Muñoz-Castañeda JR, Caballero FJ, Briceño J, Marchal T, Túnez
I, Montilla P, et al: Melatonin prevents experimental liver
cirrhosis induced by thioacetamide in rats. J Pineal Res.
39:143–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Padillo FJ, Cruz A, Navarrete C, Bujalance
I, Briceño J, Gallardo JI, Marchal T, Caballero R, Túnez I, Muntané
J, et al: Melatonin prevents oxidative stress and hepatocyte cell
death induced by experimental cholestasis. Free Radic Res.
38:697–704. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Heeba GH and Morsy MA: Fucoidan
ameliorates steatohepatitis and insulin resistance by suppressing
oxidative stress and inflammatory cytokines in experimental
non-alcoholic fatty liver disease. Environ Toxicol Pharmacol.
40:907–914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Y, Song A, Zang S, Wang C, Song G, Li
X, Zhu Y, Yu X, Li L, Wang Y and Duan L: Jinlida reduces insulin
resistance and ameliorates liver oxidative stress in high-fat fed
rats. J Ethnopharmacol. 162:244–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang Y, Chen L, Wang H, Narisi B and Chen
B: Li-Gan-Shi-Liu-Ba-Wei-San improves non-alcoholic fatty liver
disease through enhancing lipid oxidation and alleviating oxidation
stress. J Ethnopharmacol. 176:499–507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jorgensen SB, O'Neill HM, Sylow L,
Honeyman J, Hewitt KA, Palanivel R, Fullerton MD, Öberg L,
Balendran A, Galic S, et al: Deletion of skeletal muscle SOCS3
prevents insulin resistance in obesity. Diabetes. 62:56–64. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang G, Badeanlou L, Bielawski J, Roberts
AJ, Hannun YA and Samad F: Central role of ceramide biosynthesis in
body weight regulation, energy metabolism, and the metabolic
syndrome. Am J Physiol Endocrinol Metab. 297:E211–E224. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shi H, Cave B, Inouye K, Bjørbaek C and
Flier JS: Overexpression of suppressor of cytokine signaling 3 in
adipose tissue causes local but not systemic insulin resistance.
Diabetes. 55:699–707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Buzzelli MD, Navaratnarajah M, Ahmed T,
Nagarajan M, Shumate ML, Lang CH and Cooney RN: Nuclear factor
kappaB mediates the inhibitory effects of interleukin-1 on growth
hormone-inducible gene expression. J Trauma. 64:1427–1436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Torisu T, Sato N, Yoshiga D, Kobayashi T,
Yoshioka T, Mori H, Iida M and Yoshimura A: The dual function of
hepatic SOCS3 in insulin resistance in vivo. Genes Cells.
12:143–154. 2007. View Article : Google Scholar : PubMed/NCBI
|