Introduction
Ischemic cerebrovascular disease (ICVD) is a
disorder of blood supply to the brain based on the pathological
changes of the blood vessel wall. Cerebral ischemia in blood supply
area causes necrosis of the hypoxia, which in turn leads to local
or diffuse brain damage, resulting in a series of neurological
deficit syndromes (1,2). With the economic development,
improvement of people's living standard and acceleration of
population aging, incidence of ICVD in China is gradually
increasing. ICVD mainly includes cerebral infarction and transient
ischemic attack. Mortality and disability rate of ICVD are high,
seriously affecting human health and bringing financial burden to
patients' families and society (3).
The principle of ICVD treatment is to save lives, reduce brain
damage, promote the recovery of nerve function, and prevent
recurrence (4). Clinical treatment
of this disease is mainly based on the drug treatment in order to
achieve anticoagulant and antithrombotic effects, as well as
stabilize plaques, but the outcomes of drug treatment are usually
poor (5). Vascular interventional
therapy, which can directly remove occlusion of blood vessels to
promote blood supply return to normal, and induce vascular lumen
remodeling and restore blood supply to brain tissue, is novel
treatment of cerebrovascular disease (6). The application of endovascular stenting
has been widely studied. A controlled clinical trial has also
confirmed that this treatment method can effectively improve the
prognosis of ICVD, and reduce the incidence of cerebrovascular
events (7).
In this study, rat model of cerebral ischemia was
established, and rats were randomly divided into vascular
intervention group and aspirin combined defibrase drug treatment
group. The peak systolic velocity (Vs), end-diastolic velocity
(Vd), degree of neurological deficit and cerebrovascular disease
events of the two groups were compared before and after the
treatment to evaluate the therapeutic effect of vascular
intervention in ICVD, so as to provide the basis for clinical
treatment of ICVD.
Materials and methods
Experimental animals
A total of 90 healthy male SD rats aged 49–56 days
with body weight of 250–300 g were purchased from Animal Center of
Guangzhou University of Chinese Medicine (Guangzhou, China). Rats
were randomly divided into observation and control groups, 45 in
each group. After the establishment of cerebral ischemia model,
rats in observation group were treated with vascular intervention,
and rats in control group were treated with aspirin combined with
defibrase. Rats were housed in a temperature controlled room (21 ±
2°C) on a 12:12 h light/dark cycle (lights on at 06:00). All rats
had free access to water and food. The study was approved by the
Ethics Committee of The Third People's Hospital of Qingdao
(Qingdao, China).
Establishment of rat cerebral ischemia
model
After conventional anesthesia, rats were fixed and
an incision was made on the neck skin. Common carotid arteries were
separated. An incision was made on the middle region of posterior
face to expose bilateral first cervical transverse femoral holes.
Condensation of the vertebral artery was performed for 2–4 sec, and
then permanent bilateral occlusion of the vertebral artery was
done, and incisions were closed. After 24 h, carotid arteries on
both sides were closed with an artery clip for 20 min, followed by
reperfusion. Rats with subarachnoid hemorrhage, death or other
pathological changes were excluded from the study.
Method
Vascular intervention
Before surgery, rats in observation group were
subjected to gavage administration of aspirin enteric-coated
tablets (300 mg/day; state approval no. H41023324; Kaifeng Baiyun
Pharmaceutical Co., Ltd., Kaifeng, China) and clopidogrel bisulfate
tablets (75 mg/day; state approval no. H20000542; Shenzhen Xinlitai
Pharmaceutical Co., Ltd., Shenzhen, China) for 3 consecutive days.
Experimental animals were anesthetized; the right femoral artery
was punctured by Seldinger puncture; the 4F arterial sheath was
implanted; angiography was performed with a single bend
angiography, and arterial circulation was observed. The 4F
micro-guidewire tip was inserted into the carotid artery, and was
pushed to pass through the stenosis. Balloon dilatation at stenosis
was performed, and stent was placed in the stenosis. Angiography
was performed again to assess vascular circulation after stent
implantation. Postoperative treatment with aspirin (300 mg/day) and
clopidogrel sulfate tablets (75 mg/day) was performed for 6 months,
then changed to aspirin at a dose of 100 mg/day.
Treatment using aspirin combined with
defibrase
Control rats were subjected to a simple drug
treatment program including clopidogrel sulfate (75 mg/day) and
aspirin (300 mg/day) for 6 months, then changed to aspirin (100
mg/day). The drug used in control and observation group was
produced from the same factory.
Observation index
The observation time of both groups was 12 months.
Observation indexes included Vs and Vd before and after 12 months
of treatment, incidence of cerebrovascular disease events (cerebral
infarction, transient ischemic attack) and mortality at 6 and 12
months after treatment. Zea longa 5-point method was used to assess
the neurological deficit score (NDS) before and after 6 and 12
months of treatment in each group (8). Zero points: No symptoms of neurological
deficit; 1 point: Unable to fully extend the right forelimb; 2
points: Horner sign appears, turning right when crawling; 3 points:
The body tilts to the right when crawling; 4 points: Unable to
crawl. Higher NDS score indicated more severe neurological
dysfunction in rats.
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Normal distribution quantitative
data were compared by using t-test and ANOVA with SNK-Q test as a
post hoc test. Intragroup comparisons were performed by using
paired t-test and repeated measures ANOVA with Fisher's exact test
as a post hoc test. Qualitative data were treated with
χ2 test. Data with theoretical frequency <5 were
subjected to Fisher's exact probability method. All P-values
represented bilateral probability, and test level α was 0.05
Results
Comparison of general information
between two groups
Two rats in observation group were excluded due to
the failure in model construction, and 43 rats (male, n=21; female,
n=22) with an average bodyweight of 274.83±3.24g were finally
included in subsequent analysis. A total of 45 rats (male, n=23;
female, n=22) with an average bodyweight of 276.59±3.71g were
included in control group. There were 21 males (48.84%) in
observation group and body weight was 274.83±3.24 g. There were 23
males (51.11%) in control group and body weight was 276.59±3.71 g.
There was no significant difference in male ratio and body weight
between two groups (p>0.05) (Table
I).
 | Table I.Comparison of general information
between two groups. |
Table I.
Comparison of general information
between two groups.
| Groups | Male (%) | Weight (g) | Age (days) |
|---|
| Observation
(n=43) | 21 (48.84) | 274.83±3.24 | 54.00±0.28 |
| Control (n=45) | 23 (51.11) | 276.59±3.71 | 55.00±0.31 |
| t/χ2 | 0.045 | 1.151 | 0.892 |
| P-value | 0.831 | 0.653 | 0.112 |
Comparison of Vs between two groups
before and after treatment
There was no significant difference in Vs between
observation group and control group before treatment (p=0.281).
After treatment, Vd value of observation group was lower than that
of control group (p=0.001). After treatment, Vs value of
observation group was significantly reduced (p<0.05), while no
significant changes of Vs value in control group were observed
(p>0.05) (Table II).
 | Table II.Comparison of Vs between two groups
(mean ± standard deviation, cm/sec). |
Table II.
Comparison of Vs between two groups
(mean ± standard deviation, cm/sec).
| Groups | Before treatment | After treatment | t-test | P-value |
|---|
| Observation
(n=43) | 151.23±21.37 |
112.57±23.41a | 10.877 | <0.001 |
| Control (n=45) | 150.43±20.17 | 148.32±24.92 | 2.686 | 0.560 |
| t-test | 0.878 | 8.495 |
|
|
| P-value | 0.281 | 0.001 |
|
|
Comparison of Vd between two groups
before and after treatment
There was no significant difference in Vd between
observation group and control group before treatment (p=0.827).
After treatment, Vd in observation group was significantly lower
than that of control group (p<0.001). After treatment, Vd of
observation group was significantly reduced (p<0.05). After
treatment, there were no significant changes in Vd in the control
group (p>0.05) (Table III).
 | Table III.Comparison of Vd between two groups
(mean ± standard deviation, cm/sec). |
Table III.
Comparison of Vd between two groups
(mean ± standard deviation, cm/sec).
| Groups | Before treatment | After treatment | t-test | P-value |
|---|
| Observation
(n=43) | 41.72±9.66 |
20.17±8.01a | 14.217 | <0.001 |
| Control (n=45) | 41.92±9.42 | 38.28±10.48 | 2.198 | 0.490 |
| t-test | 0.098 | 3.688 |
|
|
| P-value | 0.827 | <0.001 |
|
|
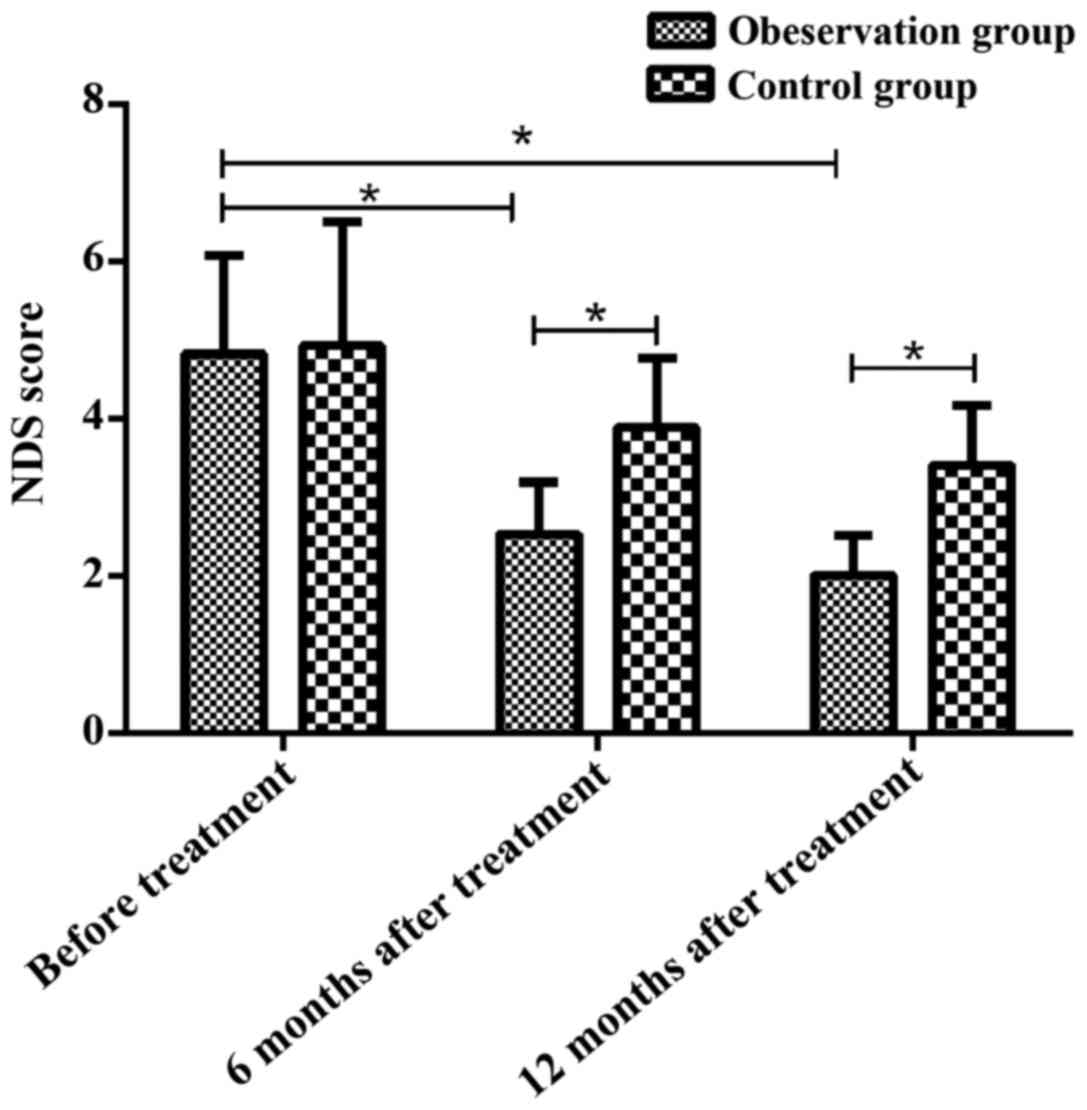
Comparison of neurological deficits
between two groups before and after treatment
There was no significant difference in NDS score
between two groups before treatment (4.82±1.25 vs. 4.92±1.58;
p=0.756). NDS score in observation group was significantly lower
than that in control group at 6 months (2.53±0.66 vs. 3.88±0.89;
p<0.05) and 12 months (2.01±0.51 vs. 3.41±0.76; p<0.05) after
treatment. There were no significant changes in NDS score at 6
months after treatment compared with that at 12 months after
treatment in control group (p>0.05). NDS score in the
observation group was significantly reduced at 6 and 12 months
after treatment compared with pretreatment values (p<0.05)
(Fig. 1).
Comparison of incidence rates of
cerebrovascular events between two groups
The incidence of cerebral infarction, transient
ischemic attack and mortality in the observation group were
significantly lower than those in control group at 12 months after
treatment (p<0.05) (Table
IV).
 | Table IV.Comparison of incidence rates of
cerebrovascular events between two groups (n, %). |
Table IV.
Comparison of incidence rates of
cerebrovascular events between two groups (n, %).
|
| Cerebral
infarction | Transient ischemic
attack | Mortality |
|---|
|
|
|
|
|
|---|
| Groups | 6 months | 12 months | 6 months | 12 months | 6 months | 12 months |
|---|
| Observation group
(n=43) | 2 (4.65) | 3 (6.98) | 0 (0.00) | 2 (4.65) | 3 (6.98) | 6 (13.33) |
| Control group
(n=45) | 4 (8.89) | 10 (22.22) | 1 (2.78) | 11 (23.91) | 6 (13.33) | 17 (36.96) |
| χ2 | – | 4.059 | – | 6.842 | 0.968 | 6.136 |
| P-value | 0.677a | 0.044 | 0.456a | 0.009 | 0.328 | 0.013 |
Discussion
ICVD is a class of diseases characterized by local
neurological dysfunction as a result of cerebral blood flow
disorders caused by cerebral arterial thrombosis. Ischemic brain
tissue can be irreversibly damaged in a short time and cause
systemic reactions (9–12). The key point of clinically treating
ICVD is to open the occluded vessels as soon as possible, so as to
restore blood flow to the brain to improve the patient's prognosis
(13,14). Main treatment of ICVD, including
thrombolysis, fibrinolysis, anticoagulant, anti-platelet
aggregation and other drug treatment, usually provide poor
treatment outcomes (15). Vascular
interventional therapy, including stenosis angioplasty, acute
arterial occlusive thrombolysis and recanalization of chronic
occlusion arteries, is achieved by unblocking the obstructed blood
vessel by placing a stent in the obstructed blood vessel to restore
blood flow (16). Interventional
therapy, as a new treatment, has the advantages of simple
operation, high tolerance and few complications, which can reduce
the morbidity and mortality of the disease (17,18).
In this study, rat models of cerebral ischemia were
established. Rats were randomly divided into vascular intervention
group and aspirin combined with defibrase drug treatment group. The
Vs, Vd and neurological deficit of the vascular lesion and the
severity of cerebrovascular disease events were compared to explore
the therapeutic values of vascular intervention in the treatment of
ICVD. All experiments were performed by using scientific and
standardized methods with strict internal quality control, so the
results had high accuracy and reliability.
Vascular intervention and aspirin combined defibrase
were used in the treatment of cerebral ischemia and therapeutic
effects were compared. Before treatment, no significant differences
in Vs and Vd at vascular lesions were found between the groups.
After treatment, Vs and Vd were lower in observation group than
those in control group. After treatment, values of Vs and Vd in
observation group were significantly reduced, while no significant
changes of Vs and Vd were found in control group. The data suggest
that vascular intervention can reduce blood flow resistance to
improve ICVD. Wang et al (19) and Singh et al (20) showed that interventional therapy can
increase patients' survival and improve their quality of life.
Neurological deficits of rats in observation group
and control group were scored before and after 6 and 12 months of
treatment. Results showed that NDS score of observation group was
lower than that of control group at 6 and 12 months after
treatment. NDS score in control group was not significantly changed
at 6 and 12 months after treatment compared with pretreatment
values, while NDS score in observation group was significantly
reduced at 6 and 12 months after treatment compared with
pretreatment values. The data show that interventional treatment of
blood vessels can improve neurological function in patients, which
is consistent with the findings reported by Ha et al
(21). Vascular intervention can
expand stenosed blood vessels, thereby restoring brain tissue blood
supply, promoting the repair of damaged brain regeneration and
improving patient's neurological function. Rankine-Mullings et
al (22) showed that the
cerebral blood flow was improved, and the incidence of neuronal
damage in brain tissue decreased after interventional
treatment.
This study also found that incidence of cerebral
infarction, transient ischemic incidence and mortality in
observation group were significantly lower than those of control
group at 12 months after treatment, indicating that vascular
intervention can reduce the incidence of cerebrovascular disease
events. Interventional therapy can restore normal blood flow to
ischemic brain tissue, promote recovery of neurological function,
and reduce the incidence of cerebrovascular events by implanting
scaffolds at stenotic sites of diseased vessels and dilating
stenosed vessels (23). Endovascular
stents also promote the repair of damaged intima and prevent the
shedding of arterial plaque and thrombus formation (24,25).
In conclusion, intracranial vascular interventional
therapy can achieve satisfactory outcomes in the treatment of
cerebral ischemia, and can effectively improve the patient's
neurological function, reduce the incidence of cerebrovascular
disease, extend the patient's survival time and improve the quality
of life. Therefore, this technique should be popularized.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL conceived the study and wrote the paper. SG and
YD interpreted the results. YD revised and finalized the paper. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Third People's Hospital of Qingdao (Qingdao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang YS, Koo M, Chen JC and Hwang JH: The
association between tinnitus and the risk of ischemic
cerebrovascular disease in young and middle-aged patients: A
secondary case-control analysis of a nationwide, population-based
health claims database. PLoS One. 12:e01874742017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bondonno CP, Blekkenhorst LC, Prince RL,
Ivey KL, Lewis JR, Devine A, Woodman RJ, Lundberg JO, Croft KD,
Thompson PL, et al: Association of vegetable nitrate intake with
carotid atherosclerosis and ischemic cerebrovascular disease in
older women. Stroke. 48:1724–1729. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lai SW, Lin HF, Lin CL and Liao KF:
Long-term effects of pioglitazone on first attack of ischemic
cerebrovascular disease in older people with type 2 diabetes: A
case-control study in Taiwan. Medicine (Baltimore). 95:e44552016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang GQ, Xie YM, Liu H, Zhang Y, Jia PP
and Zhuang Y: Drug combination characteristics of Shenxiong glucose
injection in treating ischemic cerebrovascular disease in real
world. Zhongguo Zhong Yao Za Zhi. 42:2808–2813. 2017.(In Chinese).
PubMed/NCBI
|
|
5
|
Ikeda-Sakai Y, Sasaki M and Nakase T:
Effects with and without clopidogrel loading treatment for acute
ischemic cerebrovascular disease patients: A retrospective cohort
study. J Stroke Cerebrovasc Dis. 26:2901–2908. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zuo FT, Liu H, Wu HJ, Su N, Liu JQ and
Dong AQ: The effectiveness and safety of dual antiplatelet therapy
in ischemic cerebrovascular disease with intracranial and
extracranial arteriostenosis in Chinese patients: A randomized and
controlled trail. Medicine (Baltimore). 96:e54972017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kurz T and Thiele H: Interventional
therapy for aortic valve stenosis. MMW Fortschr Med. 158:49–52.
2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qian LH, Li NG, Tang YP, Zhang L, Tang H,
Wang ZJ, Liu L, Song SL, Guo JM and Ding AW: Synthesis and
bio-activity evaluation of scutellarein as a potent agent for the
therapy of ischemic cerebrovascular disease. Int J Mol Sci.
12:8208–8216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharma VK, Tsivgoulis G, Lao AY and
Alexandrov AV: Role of transcranial Doppler ultrasonography in
evaluation of patients with cerebrovascular disease. Curr Neurol
Neurosci Rep. 7:8–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hira RS, Cowart JB, Akeroyd JM, Ramsey DJ,
Pokharel Y, Nambi V, Jneid H, Deswal A, Denktas A, Taylor A, et al:
Risk factor optimization and guideline-directed medical therapy in
US veterans with peripheral arterial and ischemic cerebrovascular
disease compared to veterans with coronary heart disease. Am J
Cardiol. 118:1144–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Franzone A, Piccolo R, Gargiulo G, Ariotti
S, Marino M, Santucci A, Baldo A, Magnani G, Moschovitis A,
Windecker S, et al: Prolonged vs short duration of dual
antiplatelet therapy after percutaneous coronary intervention in
patients with or without peripheral arterial disease: A subgroup
analysis of the PRODIGY randomized clinical trial. JAMA Cardiol.
1:795–803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Della-Morte D, Pacifici F and Rundek T:
Genetic susceptibility to cerebrovascular disease. Curr Opin
Lipidol. 27:187–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Pan Y, Wu Y, Zhao X, Li H, Wang D,
Johnston SC, Liu L, Wang C, Meng X, et al: CHANCE Investigators:
Effect of estimated glomerular filtration rate decline on the
efficacy and safety of clopidogrel with aspirin in minor stroke or
transient ischemic attack: CHANCE substudy (clopidogrel in
high-risk patients with acute nondisabling cerebrovascular events).
Stroke. 47:2791–2796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Senoo K, Lau YC, Dzeshka M, Lane D,
Okumura K and Lip GY: Efficacy and safety of non-vitamin K
antagonist oral anticoagulants vs. warfarin in Japanese patients
with atrial fibrillation - meta-analysis. Circ J. 79:339–345. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen CH, Lin CL and Kao CH: Subtotal
gastrectomy with billroth II anastomosis is associated with a low
risk of ischemic stroke in peptic ulcer disease patients: A
nationwide population-based study. Medicine (Baltimore).
95:e34812016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Menon BK, Sajobi TT, Zhang Y, Rempel JL,
Shuaib A, Thornton J, Williams D, Roy D, Poppe AY, Jovin TG, et al:
Analysis of workflow and time to treatment on thrombectomy outcome
in the ESCAPE randomized controlled trial. Circulation.
133:2279–2286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ouyang F, Chen Y, Zhao Y, Dang G, Liang J
and Zeng J: Selection of patients and anesthetic types for
endovascular treatment in acute ischemic stroke: A meta-analysis of
randomized controlled trials. PLoS One. 11:e01512102016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang S, Jiang J, Qu C, Wang C and Sun Z:
Predictive value of serum pregnancy-associated plasma protein A for
patients with ischemic cerebrovascular disease. J Clin Lab Anal.
31:22091–22092. 2017. View Article : Google Scholar
|
|
20
|
Singh AG, Crowson CS, Singh S, Denis M,
Davis P, Maradit-Kremers H, Matteson EL and Chowdhary VR: Risk of
cerebrovascular accidents and ischemic heart disease in cutaneous
lupus erythematosus: A population-based cohort study. Arthritis
Care Res (Hoboken). 68:1664–1670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ha M, Choi CH, Lee JI, Cha SH, Lee SW and
Ko JK: The efficacy of single barrel superficial temporal
artery-middle cerebral artery bypass in treatment of adult patients
with ischemic-type moyamoya disease. J Cerebrovasc Endovasc
Neurosurg. 18:239–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rankine-Mullings AE, Little CR, Reid ME,
Soares DP, Taylor-Bryan C, Knight-Madden JM, Stuber SE, Badaloo AV,
Aldred K, Wisdom-Phipps ME, et al: EXpanding treatment for existing
neurological disease (EXTEND): An open-label phase II clinical
trial of hydroxyurea treatment in sickle cell anemia. JMIR Res
Protoc. 5:e1852016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hira RS, Cowart JB, Akeroyd JM, Ramsey DJ,
Pokharel Y, Nambi V, Jneid H, Deswal A, Denktas A, Taylor A, et al:
Risk factor optimization and guideline-directed medical therapy in
US veterans with peripheral arterial and ischemic cerebrovascular
disease compared to veterans with coronary heart disease. Am J
Cardiol. 118:1144–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nussbaum ES and Erickson DL:
Extracranial-intracranial bypass for ischemic cerebrovascular
disease refractory to maximal medical therapy. Neurosurgery.
46:37–42. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamazaki M, Ohnishi T, Hosokawa K,
Yamaguchi K, Yoneyama T, Kawashima A, Okada Y, Kitagawa K and
Uchiyama S: Measurement of residual platelet thrombogenicity under
arterial shear conditions in cerebrovascular disease patients
receiving antiplatelet therapy. J Thromb Haemost. 14:1788–1797.
2016. View Article : Google Scholar : PubMed/NCBI
|