Introduction
Stroke is a serious threat to human health around
the world and is characterized by high incidence, high morbidity
and high mortality (1,2). At present, the cure for ischemic stroke
is limited (3–5). For instance, recombinant tissue
plasminogen activator (rtPA), which is approved by the Food and
Drugs Administration (FDA), is effective only if its administration
is performed within 4.5 h after the stroke (6). Due to the narrow therapeutic window,
few patients benefit from it (6).
Hence, it is important to develop novel drugs for ischemic stroke
therapy.
As an important serine/threonine protein kinase,
AMP-activated protein kinase (AMPK) is responsible for the
peripheral energy balance (7,8). When
cellular energy supply is low, AMPK is activated, thereby enhancing
the energy production (9). In the
periphery, AMPK acutely modulates the homeostasis of cellular
metabolism by reducing energy storage and increasing energy
utilization. Furthermore, a high expression level of AMPK is
observed in neurons, and it is quickly activated in the brain upon
energy deprivation (10). In
addition, endothelial nitric oxide synthase (eNOS), phosphorylation
is enhanced by AMPK and NO production is hence increased, which
then leads to the regeneration of vessels (11–13).
Therefore, modulation of AMPK activity is an important intervention
method for the treatment of cerebral infarction.
Hydroxybutyrate (GHB), derived from γ-aminobutyric
acid, is a well-known neurotransmitter and neuromodulator
interacting with the GHB receptor and the γ-aminobutyric acid type
B receptor in the central nervous system (14). Generally, GHB is used by clinicians
to treat cataplexy, excessive daytime sleepiness and sleep
disturbance associated with narcolepsy (15,16).
Recent studies have shown that GHB is effective for protection
against ischemia/brain damage in rat models (17,18).
However, the specific underlying mechanism is poorly
understood.
In the current study, we mainly explored the GHB
effect on ischemic stroke in rats. Here, we demonstrated, for the
first time, that GHB enhanced the activation of AMPK and eNOS,
thereby improving recovery from cerebral infarction.
Materials and methods
Drug administration in middle cerebral
artery occlusion (MCAO) in rats
A total of 60 male Sprague Dawley (SD) rats weighing
200-240 g were used in this study. Rats were housed in the same
animal care facility during a 12-h light/dark cycle throughout the
duration of the protocol, with free access to food and water.
Briefly, the rats were anesthetized via 2% pentobarbital sodium (40
mg/kg) injected i.p. (19). The rats
were considered to be properly anesthetized once they were without
pain reflex. Arterial blood samples obtained via a femoral catheter
were collected to measure pO2, pCO2 and pH
with an AVL 998 Blood Gas Analyzer (Roche Diagnostics, Basel,
Switzerland). The rectal temperature was maintained at 37±0.5°C
during MCAO via a temperature-regulated heating lamp. A fiber-optic
probe was attached to the parietal bone overlaying the middle
cerebral artery territory 5 mm posterior and 5 mm lateral to the
bregma, and it was connected to a laser Doppler flowmeter (PeriFlux
System 5000; Perimed, Stockholm, Sweden) for continuous monitoring
of the cerebral blood flow (CBF). A 4-0 nylon monofilament suture
with a heat-blunted tip was introduced into the internal carotid
artery through the artery stump. It was gently advanced for a
distance of 18 mm from the common carotid artery bifurcation to
block the origin of the middle cerebral artery for 90 min and then
withdrawn to allow reperfusion. After the wound had been closed,
the animals were allowed to recover from anesthesia before they
were returned to their home cages. Sixty adult male SD rats were
randomly divided into a sham operation group, an
ischemia-reperfusion group (MCAO) and an MCAO+GHB treated group.
The rats were intraperitoneally treated with either 50 µl of saline
or 100 mg/kg GHB (Lipomed AG, Arlesheim, Switzerland) each day for
5 weeks starting from 24 h following the MCAO procedure.
All experimental protocols described in this study
were approved by the Ethics Review Committee for Animal
Experimentation of Zhongnan Hospital of Wuhan University Hospital
(no. XAERC-1002).
Assessment of neurological deficit
score and analysis of survival rates
For sham group (n=4 rats) and MCAO group (n=4 rats),
the neurological deficit score was evaluated after 24 h of
operation before the rats were sacrificed. For MCAO+GHB treated
group, the neurological deficit score was evaluated after the rats
received GHB therapy (n=4 rats) for 5 weeks before the rats were
sacrificed. Then, the neurological deficit score was evaluated. Two
examiners were blinded to the rat identities and the treatment
protocol. The following neurological deficit scoring (NDS) system
(20) was used: 0, no motor deficits
(normal); 1, forelimb weakness and torso turning to the ipsilateral
side when held by the tail (mild); 2, circling to the contralateral
side but normal posture at rest (moderate); 3, unable to bear
weight on the affected side at rest (severe); and 4, no spontaneous
locomotor activity or barrel rolling (critical). If no deficit was
observed 2 h post-recovery from anesthesia, the animal was removed
from further study.
Edema measurement
The ipsilateral and contralateral hemispheres were
dissected, and the wet weight of the tissue was determined. The
tissues were dried at 120°C for 24 h. The percent cerebral water
was determined as (wet weight-dry weight)/dry weight × 100.
Infarct analysis
At 72 h after stroke, the brain was removed and cut
into 5 2-mm slices and stained with 1.5% 2,3,5-triphenyltetrazolium
(TTC) for 30 min at 3°C. For sham group, MCAO group and MCAO+GHB
treated group (n=4 rats for each group), the rats were anesthetized
after the rats received saline or GHB therapy for 5 weeks via 2%
pentobarbital sodium (40 mg/kg) injected i.p. (19). The rats were considered to be
properly anesthetized once they were without pain reflex. Then,
they were perfused transcardially with cold PBS to assess
chronically post-stroke, followed by 4% paraformaldehyde; the brain
was post-fixed for 18 h and placed in cryoprotectant (30% sucrose).
The brain tissue was cut into 40-µm free-floating sections on a
freezing microtome and every eighth slice was stained by cresyl
violet stain for evaluation of ischemic cell damage. Infarct
volume, expressed as a percentage of whole-brain volume, was
measured by an image-processing and analysis system
(1.25×objective, Q570IW; Leica, Wetzlar, Germany) and calculated by
integration of the infarct area in each brain section along the
rostral-caudal axis.
Western blot analysis
Western blots were performed as described previously
(18). For sham group, MCAO group
and MCAO+GHB treated group (n=4 rats for each group), the rats were
sacrificed after the rats received saline or GHB therapy for 5
weeks. All rats in each group were sacrificed with an overdose of
10% chloral hydrate (400 mg/kg) and then were killed by cervical
dislocation (21). The mice were
considered to be dead once they were without breathing and
heartbeat. Then, brains were homogenized in the lysis buffer, and
proteins were separated in a 4-15% gradient SDS-polyacrylamide gel
and transferred to a polyvinylidene difluoride membrane. AMPK (cat.
no. 5831), p-AMPK (cat. no. 50081, Thr172), p-eNOS (cat. no. 9574,
Thr495) and eNOS (cat. no. 32027), β-actin (cat. no. 4970) proteins
(1:1,000 dilution; Cell Signaling Technology, Inc., Boston, MA,
USA) were used as an internal control controls. The blots were
incubated overnight with the primary antibodies at 4°C in TBS
containing 4% bovine serum albumin and 0.1% Tween-20. Secondary
antibodies (goat anti-rabbit IgG, 1:5,000 dilution; Zhongshan
Jinqiao Biotechnology Co., Ltd., Beijing) were diluted and
incubated with the blots, and an ECL (pico) detection kit (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used for signal
detection.
Immunostaining
Briefly, the brain sections were blocked with 10%
FBS for 1 h and then incubated with BrdU stain in 2 M HCl at 37°C
for 20 min and rinsed in 0.1 M borate buffer (pH 8.5) before
blocking. For CD31, lectin and nestin and von Willebrand factor
(1:50 dilution; Cell Signaling Technology, Inc.) staining, antigens
were retrieved with citrate buffer (10 mM, pH 6.5) for 20 min at
95°C before blocking at 4°C overnight. After washing, brain
sections were incubated with the appropriate secondary antibodies
for 1 h. Brain sections were examined using a confocal microscope
(Leica, Solms, Germany) and photographs were taken for further
analysis.
Cell culture
PC12 cells, a rat cell line derived from
phaeochromocytoma cells, were obtained from American Type Culture
Collection (Manassas, VA, USA). PC12 cells were cultured in DMEM
medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (HyClone; GE Healthcare
Life Sciences) at 37°C in a humidified atmosphere containing 5%
CO2.
Transient transfection
Cells were transfected with small interfering RNA
(siRNA) targeting AMPK (si-AMPK), or with negative control siRNA
(NC; 5′-ACUAGUCGAUCUAUGUGUGAUATT-3′) (Shanghai GenePharma Co.,
Ltd., Shanghai, China) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) at the indicated
concentrations, according to the manufacturer's protocol. In brief,
PC12 cells (1×106 cells/well) were seeded in a six well
plate with 2 ml RPMI-1640 medium. At the 60% confluence, the cells
were pretreated with 10 µg/ml GHB for 2 h. Subsequently, si-AMPK or
NC was mixed with Lipofectamine® 2000 at room
temperature for 20 min. Then, the mixture was added into each well
at a final concentration of 20 nM for 48 h. Then, the cells were
collected for further analysis.
Statistical analysis
Data were expressed as the means ± SD. Statistics
were performed with one-way analysis of variance with Tukey's
post-hoc test for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
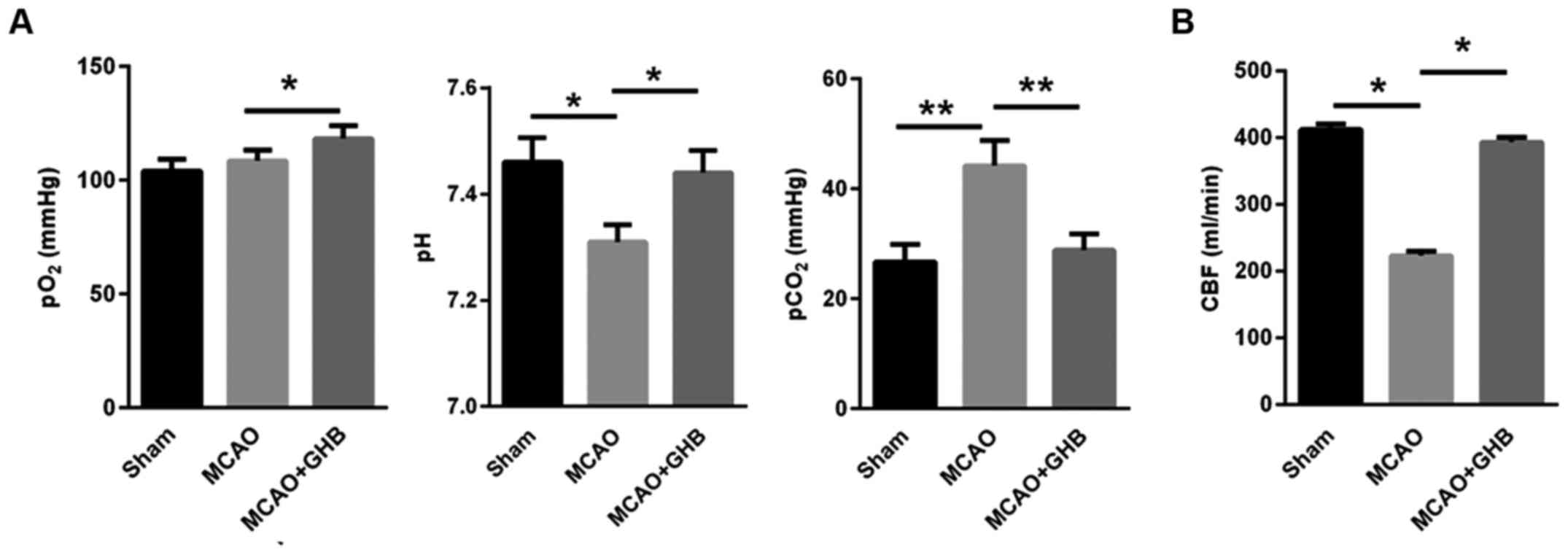
The improvement of blood parameters
and CBF by GHB in MCAO rats
The blood parameters, including pO2, pH
and pCO2, were measured in rats. Our data showed that
the pO2 values were stable after MCAO, but the pH values
decreased after MCAO, and the pCO2 was enhanced 24 h
post-MCAO, indicating that MCAO resulted in the respiratory
depression (Fig. 1A). However, the
5-week-long GHB therapy enhanced pO2, reduced
pCO2 and increased pH to the levels observed in the sham
group (Fig. 1A). In addition, CBF in
rats of the MCAO group was significantly decreased. However, after
GHB treatment for 5 weeks, CBF was significantly increased,
indicating that the MCAO-induced shortage of brain blood supply
could be partially restored to the normal level by GHB treatment
(Fig. 1B).
Cerebral infarction was relieved in
rats after the GHB treatment
Next, TTC and H&E staining were carried out to
evaluate the effects of GHB on cerebral infarction. Not
surprisingly, cerebral infarction was obvious in the MCAO group,
but the infarction volume was decreased after GHB treatment
(Fig. 2A). In addition, decreased
neurological deficits, cerebral infarct volume and brain edema rate
were found in the MCAO group, but GHB treatment improved these
parameters (Fig. 2B-D). Notably,
reduced neuron counts were identified in the MCAO group, but GHB
treatment significantly restored the number of neurons (Fig. 2E), suggesting the protective role of
GHB in cerebral infarction.
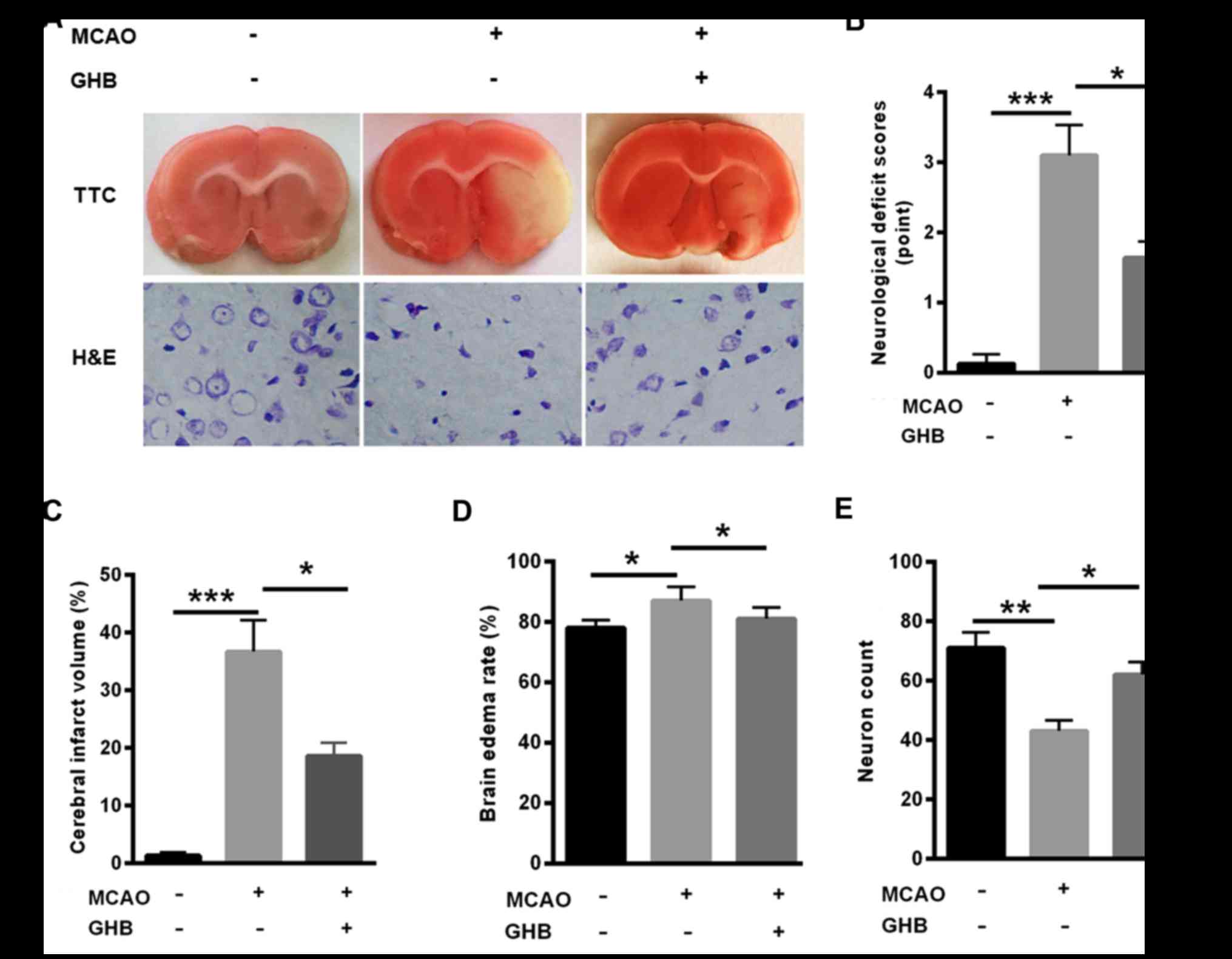 | Figure 2.Cerebral infarction was relieved in
rats after GHB treatment. (A) TTC staining TTC staining and H&E
staining (magnification, ×100) indicated that cerebral infarction
was obvious in the MCAO group, but the infarction volume was
decreased after GHB treatment. (B) Decreased neurological deficits,
(C) cerebral infarct volume and (D) brain edema rate were
determined in all groups; GHB treatment improved these parameters.
(E) Reduced neuron counts were identified in the MCAO group but GHB
treatment significantly increased the number of neurons. n=4
biological replicates. Analysis of variance was performed.
*P<0.05, **P<0.01, ***P<0.001 as indicated. TTC,
2,3,5-triphenyltetrazolium; H&E, haematoxylin and eosin; GHB,
hydroxybutyrate; MCAO, middle cerebral artery occlusion. |
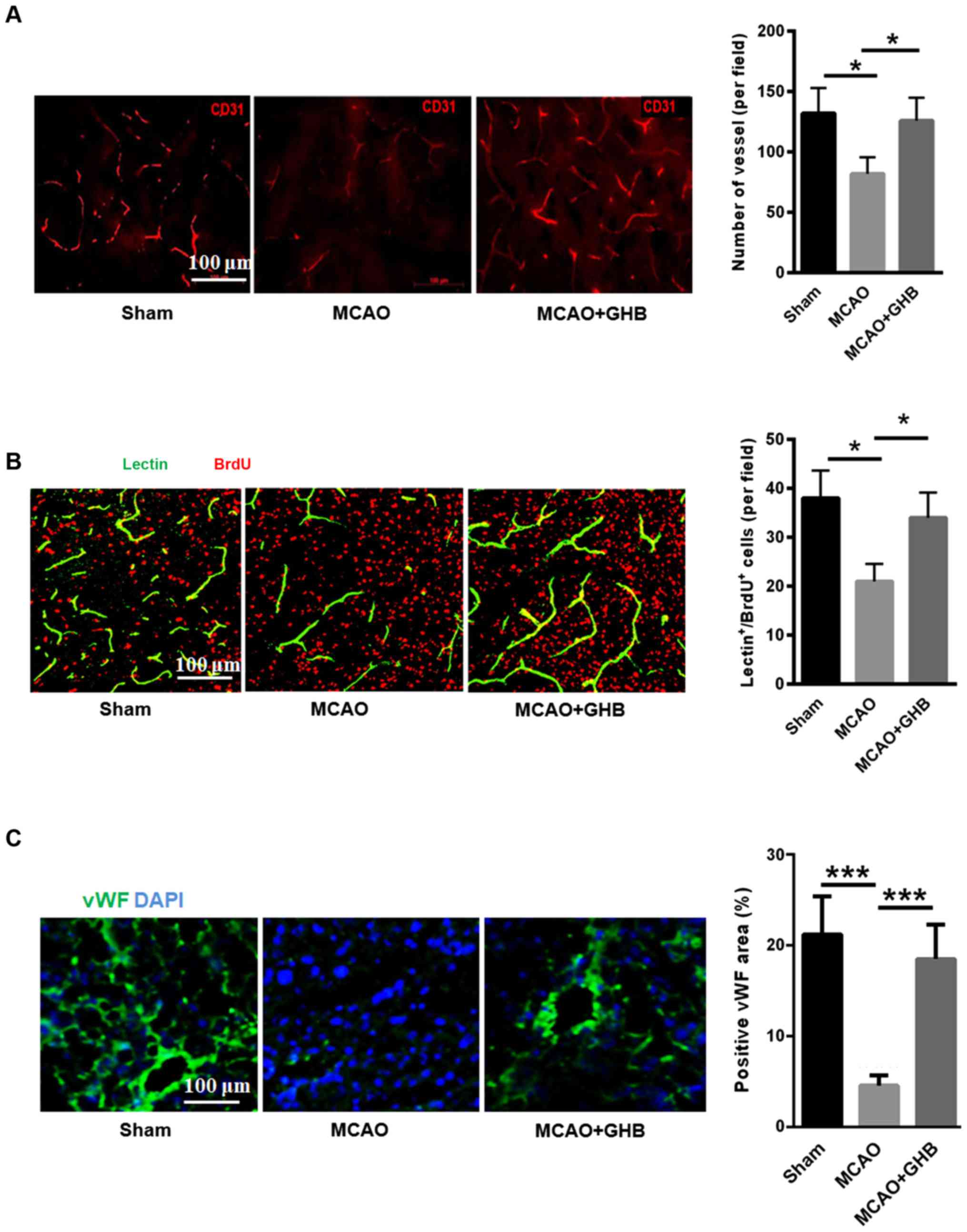
Angiogenesis was enhanced after GHB
treatment
Vascular regeneration after ischemia can increase
the perfusion of brain tissue and improve the repair of ischemic
tissue. Here, we evaluated the density of blood vessels in the
ischemic area by CD31-immunofluorescence staining. The decreased
blood vessel density was found in the MCAO group, but GHB treatment
enhanced the cerebral vascular density in the ischemic area
(Fig. 3A). The BrdU/lectin staining
was also carried out to evaluate the GHB effect on angiogenesis. As
shown in Fig. 3B, MCAO surgery
reduced the number of functional vessels, but GHB treatment
enhanced the number of BrdU/lectin double-positive cells,
suggesting the promotion of angiogenesis by GHB. Additionally, we
applied endothel-specific marker (von willebrand factor, vWF) to
analyze angiogenesis. As shown in Fig.
3C, positive vWF area was significantly decreased in the MCAO
group than that of sham group, but GHB treatment induced higher
positive vWF area in the brains of MCAO rats.
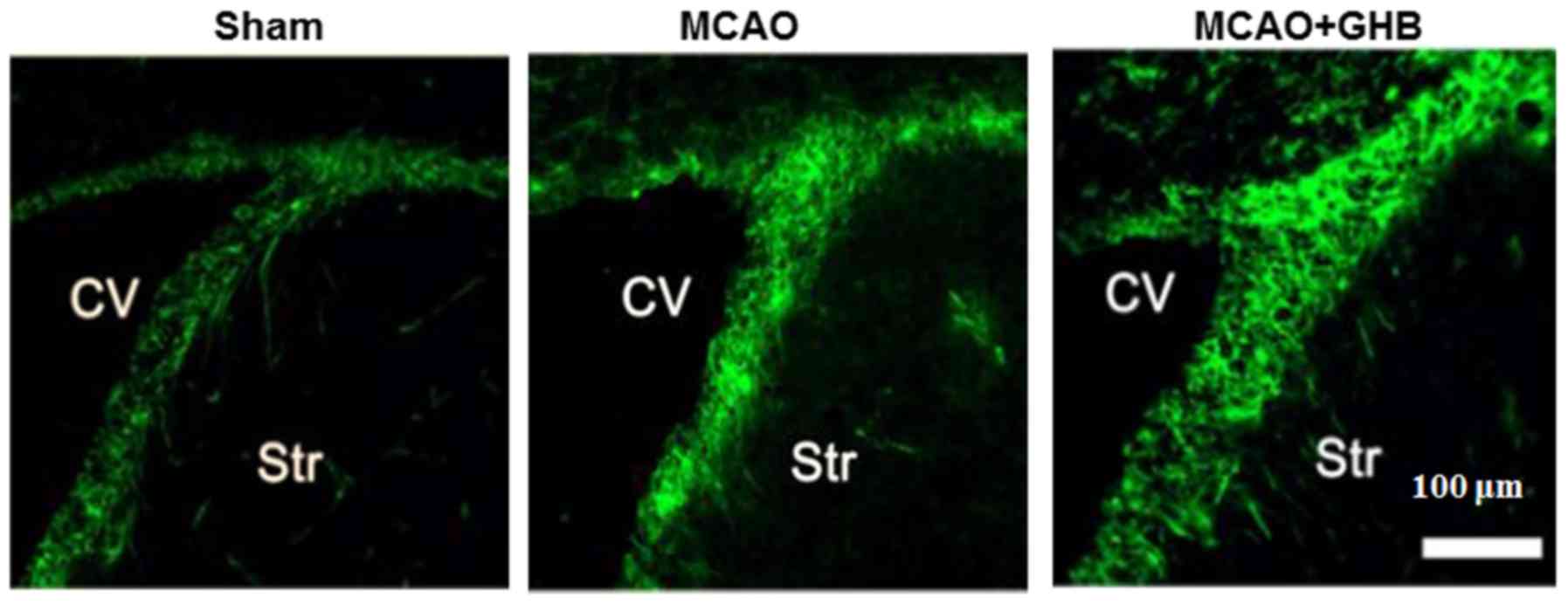
Enhanced nestin-positive cells in the
subventricular zone (SVZ) after GHB therapy
The presence of nestin, the marker of neural stem
cells, was examined in the SVZ after ischemia. In the sham group,
nestin-positive cells were sparse in the SVZ region. However, they
were more obvious seven days after MCAO in the ischemic hemisphere
(Fig. 4), indicating a spontaneous
recovery. Interestingly, more numerous nestin-positive cells were
identified in the SVZ region of the MCAO+GHB group (Fig. 4), suggesting the proliferation of
neural stem cells after GHB treatment.
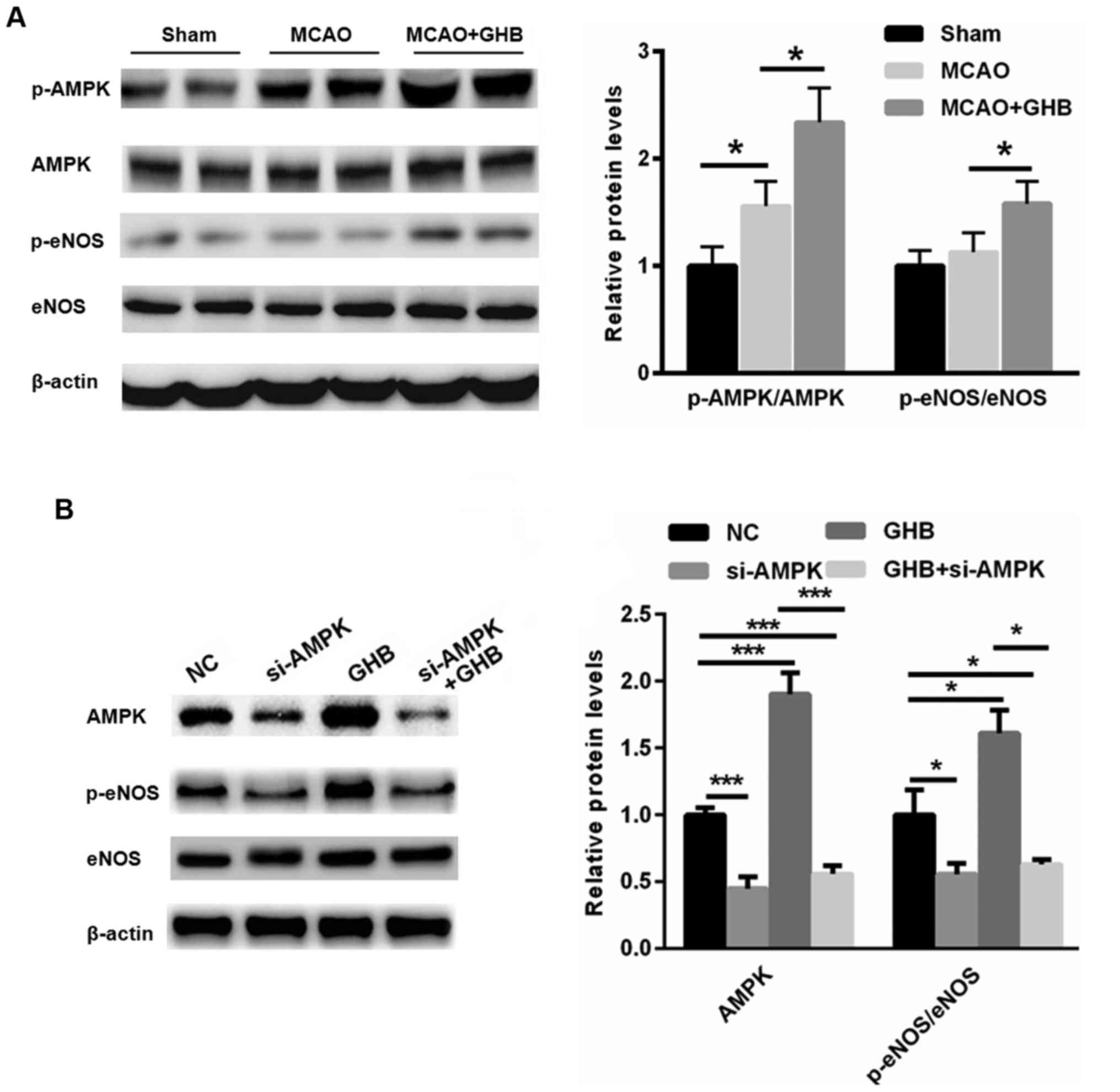
GHB may improve the recovery of nerve
function by activating AMPK/eNOS signaling
The general involvement of the AMPK signaling
pathway in ischemic brain injury is well accepted (22). Compared with the sham group, AMPK
activation was observed in the MCAO rats, suggesting that
stress-mediated AMPK activation occurs after ischemia (Fig. 5A). Furthermore, the Western blot
assay showed that GHB treatment further resulted in the activation
of AMPK and eNOS, suggesting an enhanced energy supply. To explore
the causal link between AMPK/eNOS phosphorylation and the
beneficial effect of GHB, a specific siRNA targeting AMPK was
selected. As shown in Fig. 5B,
knockdown of AMPK reduced the phosphorylation of eNOS even in the
presence of GHB. These data suggested that GHB may improve the
recovery of nerve function by activating AMPK/eNOS signaling.
Discussion
GHB is an important component of Japanese green tea
(Camellia sinensis), which is shown to reduce the size of cerebral
infarcts following MCAO for 4 h in rats (1). In line with the previous studies, our
data showed that GHB improves CBF and physiological variables,
including pH, pCO2 and pO2. Moreover, TTC and
H&E staining indicated that cerebral infarction and neuronal
death were decreased in the SVZ region after GHB treatment. These
findings suggest that GHB directly protects rats from cerebral
ischemia.
The SVZ is an important region for nerve
regeneration in the adult mammalian brain (23,24).
Under normal conditions, the neurons migrate to the olfactory bulb
and hippocampus from SVZ. Previous research has shown that
increased nerve regeneration is identified in the circumstances of
cerebral ischemia and hemorrhage (24). In the recovery process of stroke,
post-ischemic neurogenesis plays an important role (25). Hence, the proliferation, migration,
and differentiation of neural stem cells are the key therapeutic
targets for the functional improvement after stroke (26). For the first time, we showed that GHB
enhanced nestin-positive cells in the SVZ area, indicating enhanced
neuron repair after ischemia. In line with the nerve regeneration
and neuronal plasticity, there is a complex vascular remodeling
during the recovery phase of ischemia. Angiogenesis is found in
brain tissue after ischemia in both humans and rats (27). CD31-staining showed that GHB
treatment increased the density of blood vessels in the ischemic
area. Hence, GHB improves nerve regeneration and angiogenesis in
the ischemic rat brains.
The role of AMPK in stroke is controversial
(22). For instance, several studies
have shown that the acute AMPK activation is detrimental in the
progression of cerebral infarction, while some other studies
indicated that it is protective (28–30). For
instance, statins enhance angiogenesis partially by activating AMPK
and enhancing the regeneration of vessels after cerebral ischemia
(31). Moreover, AMPK could also
activate eNOS in the peripheral vascular tissue (32,33). In
line with the previous studies, our data showed that AMPK was
activated in the brains of MCAO rats. Our data indicated that the
activation of AMPK and eNOS could be further enhanced by GHB, thus
resulting in improved nerve vascular regeneration. Hence, we
propose that activation of AMPK by GHB improves nerve regeneration,
angiogenesis and brain function in the chronic recovery phase of
cerebral infarction.
In conclusion, for the first time, our data showed
that GHB may improve the recovery of nerve function and
angiogenesis by activating AMPK/eNOS signaling during the recovery
period of ischemia. In the current study, it is not sufficient to
demonstrate whether this mechanism underlies the recovery of nerve
function following ischemia. In order to demonstrate this,
functional assays, such as cell growth/viability in response to
si-AMPK and/or GHB treatment, would need to be performed in an
in vitro model of ischemic stroke.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from Zhongnan
Hospital of Wuhan University Hospital (grant no. 2312087).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW performed the experiments and analyzed the data.
PG, XL, ZJ and XY performed the animal experiments. YW designed the
experiments, analyzed the data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All experimental protocols described in this study
were approved by the Ethics Review Committee for Animal
Experimentation of Zhongnan Hospital of Wuhan University Hospital
(no. XAERC-1002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Basu P, Jenkins H, Tsang K and Vakharia
VN: National survey of neurosurgeons and stroke physicians on
decompressive hemicraniectomy for malignant middle cerebral artery
infarction. World Neurosurg. 102:320–328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedrich B, Lobsien D, Maegerlein C,
Wunderlich S, Zimmer C, Kaesmacher J and Kleine J: Distance to
Thrombus in acute middle cerebral artery stroke predicts basal
ganglia infarction after mechanical thrombectomy. Oncotarget.
7:85813–85818. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Tang G, Li Y, Wang Y, Chen X, Gu X,
Zhang Z, Wang Y1 and Yang GY: Metformin attenuates blood-brain
barrier disruption in mice following middle cerebral artery
occlusion. J Neuroinflammation. 11:1772014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bujak R, Błażejewski J, Biedermann A,
Sinkiewicz W, Karasek D, Banach J and Dobosiewicz M: Severe,
thromboembolic pulmonary hypertension with recurrent pulmonary
embolism and right heart thrombi in a patient with past myocardial
infarction, cerebral ischaemic stroke and small intestine necrosis.
Kardiol Pol. 69:61–66. 2011.(In Polish). PubMed/NCBI
|
|
5
|
De Schryver EL and Halkes PH: No role for
oral anticoagulants (target INR: 2.0-3.0) after transient ischaemic
attack or cerebral infarction of arterial origin; the
‘European/Australasian stroke prevention in reversible ischaemia
trial’ (ESPRIT). Ned Tijdschr Geneeskd. 152:445–453. 2008.(In
Dutch). PubMed/NCBI
|
|
6
|
Kleindorfer D, Lindsell CJ, Brass L,
Koroshetz W and Broderick JP: National US estimates of recombinant
tissue plasminogen activator use: ICD-9 codes substantially
underestimate. Stroke. 39:924–928. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Huang Y, Xu Y, Ruan W, Wang H,
Zhang Y, Saavedra JM, Zhang L, Huang Z and Pang T: A Dual AMPK/Nrf2
activator reduces brain inflammation after stroke by enhancing
microglia M2 polarization. Antioxid Redox Signal. 28:141–163. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiu J, Wang M, Zhang J, Cai Q, Lu D, Li Y,
Dong Y, Zhao T and Chen H: The neuroprotection of Sinomenine
against ischemic stroke in mice by suppressing NLRP3 inflammasome
via AMPK signaling. Int Immunopharmacol. 40:492–500. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen H, Liu H, Zou H, Chen R, Dou Y, Sheng
S, Dai S, Ai J, Melson J, Kittles RA, et al: Evaluation of plasma
miR-21 and miR-152 as diagnostic biomarkers for common types of
human cancers. J Cancer. 7:490–499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang LM, Wang YJ, Cui M, Luo WJ, Wang XJ,
Barber PA and Chen ZY: A dietary polyphenol resveratrol acts to
provide neuroprotection in recurrent stroke models by regulating
AMPK and SIRT1 signaling, thereby reducing energy requirements
during ischemia. Eur J Neurosci. 37:1669–1681. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garcia-Prieto CF, Hernández-Nuño F, Rio
DD, Ruiz-Hurtado G, Aránguez I, Ruiz-Gayo M, Somoza B and
Fernández-Alfonso MS: High-fat diet induces endothelial dysfunction
through a down-regulation of the endothelial AMPK-PI3K-Akt-eNOS
pathway. Mol Nutr Food Res. 59:520–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han F, Guo Y, Xu L, Hou N, Han F and Sun
X: Induction of haemeoxygenase-1 directly improves endothelial
function in isolated aortas from obese rats through the
Ampk-Pi3k/Akt-Enos pathway. Cell Physiol Biochem. 36:1480–1490.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han L, Yu Y, Sun X and Wang B: Exendin-4
directly improves endothelial dysfunction in isolated aortas from
obese rats through the cAMP or AMPK-eNOS pathways. Diabetes Res
Clin Pract. 97:453–460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao B, Kilic E, Baumann CR, Hermann DM and
Bassetti CL: Gamma-hydroxybutyrate accelerates functional recovery
after focal cerebral ischemia. Cerebrovasc Dis. 26:413–419. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamal RM, van Noorden MS, Franzek E,
Dijkstra BA, Loonen AJ and De Jong CA: The neurobiological
mechanisms of gamma-hydroxybutyrate dependence and withdrawal and
their clinical relevance: A review. Neuropsychobiology. 73:65–80.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liechti ME, Quednow BB, Liakoni E,
Dornbierer D, von Rotz R, Gachet MS, Gertsch J, Seifritz E and
Bosch OG: Pharmacokinetics and pharmacodynamics of
γ-hydroxybutyrate in healthy subjects. Br J Clin Pharmacol.
81:980–988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lavyne MH, Hariri RJ, Tankosic T and
Babiak T: Effect of low dose gamma-butyrolactone therapy on
forebrain neuronal ischemia in the unrestrained, awake rat.
Neurosurgery. 12:430–434. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vergoni AV, Ottani A, Botticelli AR, Zaffe
D, Guano L, Loche A, Genedani S, Gessa GL and Bertolini A:
Neuroprotective effect of gamma-hydroxybutyrate in transient global
cerebral ischemia in the rat. Eur J Pharmacol. 397:75–84. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang C, Wang H, Zhang S, Cheng Y and Sun
J: Neuroprotective effects of NKN on focal cerebral ischemia in
rats. Turk Neurosurg. 22:1–6. 2012.PubMed/NCBI
|
|
20
|
Zhang X, Chen L, Dang X, Liu J, Ito Y and
Sun W: Neuroprotective effects of total steroid saponins on
cerebral ischemia injuries in an animal model of focal
ischemia/reperfusion. Planta Med. 80:637–644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang HT, Zhang P, Gao Y, Li CL, Wang HJ,
Chen LC, Feng Y, Li RY, Li YL and Jiang CL: Early VEGF inhibition
attenuates blood-brain barrier disruption in ischemic rat brains by
regulating the expression of MMPs. Mol Med Rep. 15:57–64. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ran QQ, Chen HL, Liu YL, Yu HX, Shi F and
Wang MS: Electroacupuncture preconditioning attenuates ischemic
brain injury by activation of the adenosine monophosphate-activated
protein kinase signaling pathway. Neural Regen Res. 10:1069–1075.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Wang X, Zhang J, Dang C, Liu G,
Liang Z, Huang G, Zhao W and Zeng J: Tongxinluo enhances
neurogenesis and angiogenesis in peri-infarct area and
subventricular zone and promotes functional recovery after focal
cerebral ischemic infarction in hypertensive rats. Evid Based
Complement Alternat Med. 2016:85495902016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Q, Sanai N, Jin WN, La Cava A, Van
Kaer L and Shi FD: Neural stem cells sustain natural killer cells
that dictate recovery from brain inflammation. Nat Neurosci.
19:243–252. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bellenchi GC, Volpicelli F, Piscopo V,
Perrone-Capano C and di Porzio U: Adult neural stem cells: An
endogenous tool to repair brain injury? J Neurochem. 124:159–167.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Christie KJ and Turnley AM: Regulation of
endogenous neural stem/progenitor cells for neural repair-factors
that promote neurogenesis and gliogenesis in the normal and damaged
brain. Front Cell Neurosci. 6:702013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao Y, Zheng XR, Zhang SS, Wang X, Yu XH,
Tan JL and Yang YJ: Transplantation of vascular endothelial growth
factor-modified neural stem/progenitor cells promotes the recovery
of neurological function following hypoxic-ischemic brain damage.
Neural Regen Res. 11:1456–1463. 2016.PubMed/NCBI
|
|
28
|
Ma Y, Bu J, Dang H, Sha J, Jing Y,
Shan-jiang AI, Li H and Zhu Y: Inhibition of adenosine
monophosphate-activated protein kinase reduces glial cell-mediated
inflammation and induces the expression of Cx43 in astroglias after
cerebral ischemia. Brain Res. 1605:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nam HG, Kim W, Yoo DY, Choi JH, Won MH,
Hwang IK, Jeong JH, Hwang HS and Moon SM: Chronological changes and
effects of AMP-activated kinase in the hippocampal CA1 region after
transient forebrain ischemia in gerbils. Neurol Res. 35:395–405.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gasbarrino K, Zheng H, Hafiane A, Veinot
JP, Lai C and Daskalopoulou SS: Decreased adiponectin-mediated
signaling through the AdipoR2 pathway is associated with carotid
plaque instability. Stroke. 48:915–924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rockberg J, Jørgensen L, Taylor B, Sobocki
P and Johansson G: Risk of mortality and recurrent cardiovascular
events in patients with acute coronary syndromes on high intensity
statin treatment. Prev Med Rep. 6:203–209. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu JW, Deng YP, Han X, Ren GF, Cai J and
Jiang GJ: Metformin improves the angiogenic functions of
endothelial progenitor cells via activating AMPK/eNOS pathway in
diabetic mice. Cardiovasc Diabetol. 15:882016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang C, Liao Y, Li Q, Chen M, Zhao Q,
Deng R, Wu C, Yang A, Guo Z, Wang D and He X: Recombinant
adiponectin ameliorates liver ischemia reperfusion injury via
activating the AMPK/eNOS pathway. PLoS One. 8:e663822013.
View Article : Google Scholar : PubMed/NCBI
|