Introduction
Mast cells are widely distributed in sites around
the body, including the skin, airways, gastrointestinal tract and
mucosa, and are able to quickly respond to internal and external
stimuli (1). Mast cells secrete a
number of bioactive mediators, including proteases, cytokines,
chemokines, β-hexosaminidase and histamine, which are associated
with the regulation of innate and acquired immune responses
(2). Mast cells serve a crucial role
in allergic disorders, including asthma, atopic dermatitis and
hypersensitivity (3), and are
associated with the allergy-induced inflammatory responses
(4). Mast cells present the
high-affinity immunoglobulin E (IgE) receptor (FcεRI) on their
surface, which is able to bind IgE and induce mast cell
degranulation following repeated-allergen stimulation (5). During the degranulation process, mast
cell-induced mediators are controlled by FcεRI-dependent signaling
pathways, including phosphoinositide3-kinase (PI3K) family members
(6). However, the underlying
mechanisms responsible for mast cell degranulation are complex and
remain to be fully elucidated.
Propofol is a widely used intravenous anesthetic
agent with rapid onset, short duration of action and rapid
elimination (7). A number of
pharmacological characteristics of propofol have been reported,
including antipruritic, anti-convulsant, anti-oxidant and
anti-inflammatory activities (8).
Furthermore, propofol has been demonstrated to inhibit adhesion,
proliferation, invasion and growth in various cancers, including
ovarian cancer, hepatocellular carcinoma, pancreatic cancer, glioma
and lung cancer (9–13). The anti-cancer effect of propofol is
typically associated with an upregulation or downregulation of
microRNA (miR or miRNA) (9,11,13). A
previous study indicated that propofol treatment attenuated
ischemia reperfusion injury in the small intestine via inhibiting
oxidative stress and mast cell degranulation (14), suggesting that propofol may function
by modulating mast cell activation. However, the underlying
molecular mechanisms remain unclear.
The aim of the present study was to investigate the
effect of propofol on IgE-activated mast cell degranulation and the
underlying mechanisms. The results indicate that propofol acts via
the miR-221/PI3K/protein kinase B (Akt)/Ca2+ pathway to
suppress mast cell activation, which suggests a possible target for
treating allergic diseases via inhibiting IgE-mediated mast cell
degranulation.
Materials and methods
Reagents
Propofol, LY294002, dimethyl sulfoxide (DMSO), MTT,
anti-2,4-dinitrophenyl (DNP) IgE and DNP-human serum albumin (HSA)
were purchased from Sigma Aldrich (Merck KGaA, Darmstadt, Germany.
Dulbecco's modified eagle medium (DMEM), TRIzol,
Lipofectamine® 2000 andfura-2/AM were obtained from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Fetal bovine serum (FBS), penicillin and streptomycin were obtained
from Gibco (Thermo Fisher Scientific, Inc.). The TaqMan MicroRNA
Reverse Transcription kit and TaqMan MicroRNA Assay kit were
acquired from Applied Biosystems (Thermo Fisher Scientific, Inc.).
ELISA kits (β-hexosaminidase cat. no. KT-17547; histamine cat. no.
KT-60094) were purchased from Kamiya Biomedical Co. (Tukwila, WA,
USA). Lysis buffer was obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA) and the BCA Protein Assay kit was acquired
from Pierce (Thermo Fisher Scientific, Inc.). Rabbit anti-rat
antibodies against Akt (cat. no. SC-8312), phosphorylated (p)-Akt
(s473) (cat. no. SC-33437), GAPDH (cat. no. SC-25778) and
horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary
antibodies (cat. no. SC-2030) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Unless otherwise stated,
other reagents were purchased from Sigma Aldrich (Merck KGaA).
RBL-2H3 cell culture and propofol
treatment
Rat basophilic leukemia RBL-2H3 cells were purchased
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). Cells were cultured in DMEM containing with 10%
heat-inactivated FBS, 100 IU/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified incubator containing 5%
CO2. Propofol was diluted with DMSO to obtain indicated
concentrations (5, 10, 15 or 20 µg/ml) and was added to the cells
for the indicated time (12, 24, 36 or 48 h).
The original RBL-2H3 cells were cultured in 12-well
plates at a density of 1×106 cells/well. After 24 h
following propofol treatment, 2×105 RBL-2H3 cells from
each treatment group were collected for further analysis. Cells
without propofol treatment or IgE stimulation were used as the
control (Con) group.
MTT assay
Cell viability was analyzed using an MTT assay.
RBL-2H3 cells were seeded in 96-well plates and treated with
propofol as described. A total of 5 mg/ml MTT was added to the
plates and incubated at 37°C for 4 h. DMSO was added to dissolve
the formazan crystals. Optical density (OD) was measured at 590 nm
using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The inhibitory rate of cell proliferation (%) was
measured using following formula: (OD of control group-OD of test
group) ×100/OD of control group (Table
I).
 | Table I.Inhibitory rate of propofol on the
proliferation of RBL-2H3 cells. |
Table I.
Inhibitory rate of propofol on the
proliferation of RBL-2H3 cells.
| Propofol (µ
g/ml) | Optical density at
570 nm | Inhibitory rate
(%) |
|---|
| 0 | 0.943±0.006 | 0 |
| 1 | 0.898±0.012 |
4.416±0.363 |
| 5 | 0.713±0.014 | 23.038±0.798 |
| 10 | 0.585±0.018 | 36.846±1.093 |
| 15 | 0.463±0.015 | 49.675±1.261 |
| 20 | 0.212±0.011 | 76.035±0.782 |
Activation of mast cell
degranulation
RBL-2H3 cells were sensitized with 0.5 µg/ml
anti-DNP IgE overnight at 37°C to induce IgE-mediated allergic
responses. Cells were subsequently stimulated with 100 ng/ml
DNP-HSA at 37°C for 4 h to activate the degranulation of mast
cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol and RT was
performed with a TaqMan MicroRNA Reverse Transcription kit
according to the manufacturers' protocol (16°C for 30 min, 42°C for
30 min and 85°C for 5 min). To detect miR-221 expression, qPCR was
performed using the 7500 Fast Real-Time PCR System (Thermo Fisher
Scientific, Inc.) with TaqMan MicroRNA Assay kit. The thermocycling
conditions were as follows: 95°C for 2 min followed by 40 cycles of
95°C for 15 sec, 55°C for 30 sec, and 60°C for 1 min. The following
primers were purchased from Shanghai GenePharma Co., Ltd.,
(Shanghai, China): miR-221, forward 5′-CCCAGCATTTCTGACTGTTG-3′ and
reverse 5′-TGTGAGACCATTTGGGTGAA-3′; U6, forward
5′-CAAGGATGACACGCAAAT-3′ and reverse 5′-TGGTGTCGTGGAGTCG-3′. The
expression of U6 was used as endogenous control for miRNAs
analysis. Data were analyzed by the 2−ΔΔCq method
(15).
Transfection
RBL-2H3 cells were transfected with miR-221 mimic
(5′-AGCUACAUUGUCUGCUGGGUUUC-3′) and its negative control
(5′-UCACAACCUCCUAGAAAGAGUAGA-3′; both from Shanghai GenePharma Co.,
Ltd.) using Lipofectamine® 2000 reagent according to the
manufacturer's protocol. The final concentration of
oligonucleotides was 50 nM. Cells were subjected to RNA/protein
extraction or further experiments at 24 h following
transfection.
Detection of β-hexosaminidase
release
Following the activation of mast cell degranulation,
the cells were separated by centrifugation at 150 × g for 5 min at
4°C and then the supernatants were incubated in citrate buffer with
1 mM 4-nitrophenyl N-acetyl-β-D-glucosaminide at 37°C for 1 h. Cell
pellets were lysed with Tyrode's buffer containing 1% Triton X-100
and the reaction was stopped by adding 150 µl stop solution (0.1 M
Na2CO3-NaHCO3; pH=10). Absorbance
was measured at 405 nm using the microplate reader (ELX808; BioTek
Instruments, Inc., Winooski, VT, USA).
Analysis of histamine release
The levels of histamine in the supernatants of
RBL-2H3 cells were measured using the ELISA kit according to the
manufacturer's protocol.
Western blotting
RBL-2H3 cells were collected and lysed using lysis
buffer containing protease and phosphatase inhibitors on ice.
Following centrifugation at 400 × g for 5 min at 4°C, the
supernatants were collected. The protein concentrations in the
supernatants were measured using a BCA Protein Assay kit. Proteins
(20 µg) were separated by electrophoresis with 10% SDS-PAGE gel and
transferred onto polyvinylidene fluoride membranes. Membranes were
blocked with 5% skim milk at 37°C for 2 h and incubated with the
primary rabbit anti-rat antibodies against Akt, p-Akt (s473) and
GAPDH overnight at 4°C. Membranes were then incubated with
HRP-conjugated goat anti-rabbit secondary antibodies. Bands were
visualized using enhanced chemiluminescence detection kit (ECL; GE
Healthcare, Chicago, IL, USA) and scanned for analysis (Scanjet
7400C; Hewlett-Packard Co., Palo Alto, CA, USA).
Cytosolic calcium (Ca2+)
measurement
The supernatants of RBL-2H3 cells were collected and
incubated in Tyrode's buffer supplemented with fura-2/AM (2 µM
final concentration) at 37°C for 30 min. Absorbance was measured at
340 and 380 nm using a microplate fluorometer (Berthold
Technologies GmbH & Co., Bad Wildbad, Germany) and the
intracellular Ca2+ was shown as the 340/380 ratio.
Statistical analysis
Results are presented as the mean ± standard
deviation. All statistical analysis was performed using SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA). For multiple
comparisons of quantitative data, one-way analysis of variance was
used followed by Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Propofol suppresses the proliferation
of RBL-2H3 cells
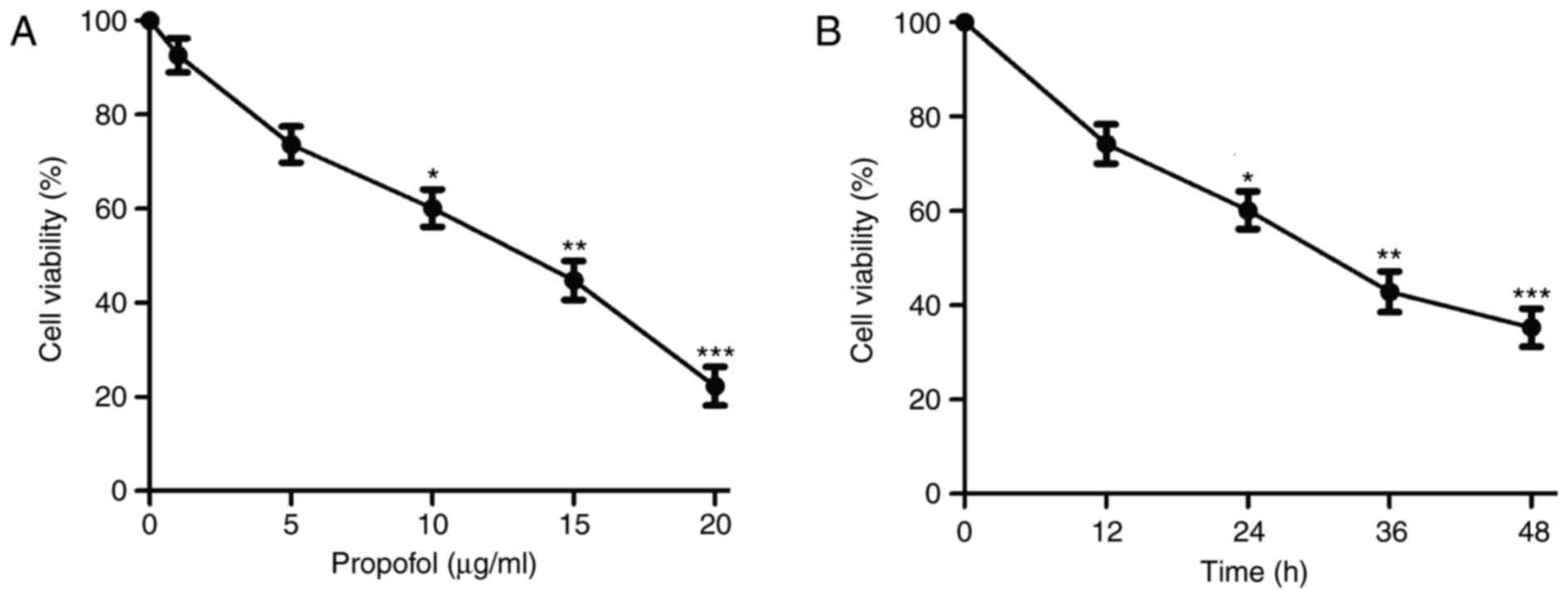
Following treatment with various concentrations of
propofol, the proliferation of RBL-2H3 cells was significantly
suppressed in a dose-dependent manner compared with the control
(Fig. 1A). As the IC50
was 13.380 µg/ml (Table I), 10 µg/ml
of propofol was selected as the highest concentration in further
experiments. Propofol treatment also resulted in a time-dependent
reduction in cell viability compared with the control (Fig. 1B). These results suggest that
propofol inhibits RBL-2H3 cell proliferation in a dose- and
time-dependent manner. Treatment duration of 24 h was selected for
subsequent experiments.
Propofol inhibits miR-221 expression
in RBL-2H3 cells
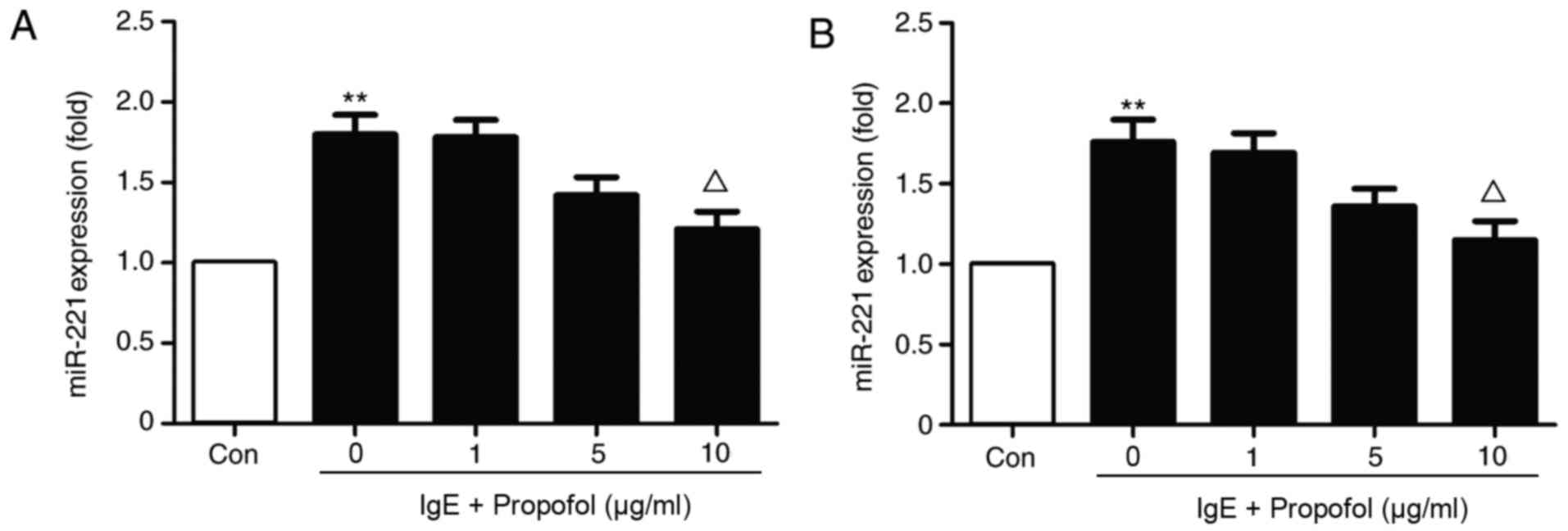
Treatment with 10 µg/ml propofol significantly
reduced miR-221 expression in IgE-activated RBL-2H3 cells (Fig. 2A). The results indicate that propofol
inhibits miR-221 expression in a dose-dependent manner. Because
propofol was demonstrated to inhibit the proliferation of RBL-2H3
cells, miR-221 expression was also detected in the same number of
RBL-2H3 cells obtained from a different group (Fig. 2B). The results suggest that miR-221
downregulation was independent of the decrease in RBL-2H3 cells
following propofol application.
miR-221 has no significant effect on
RBL-2H3cell proliferation
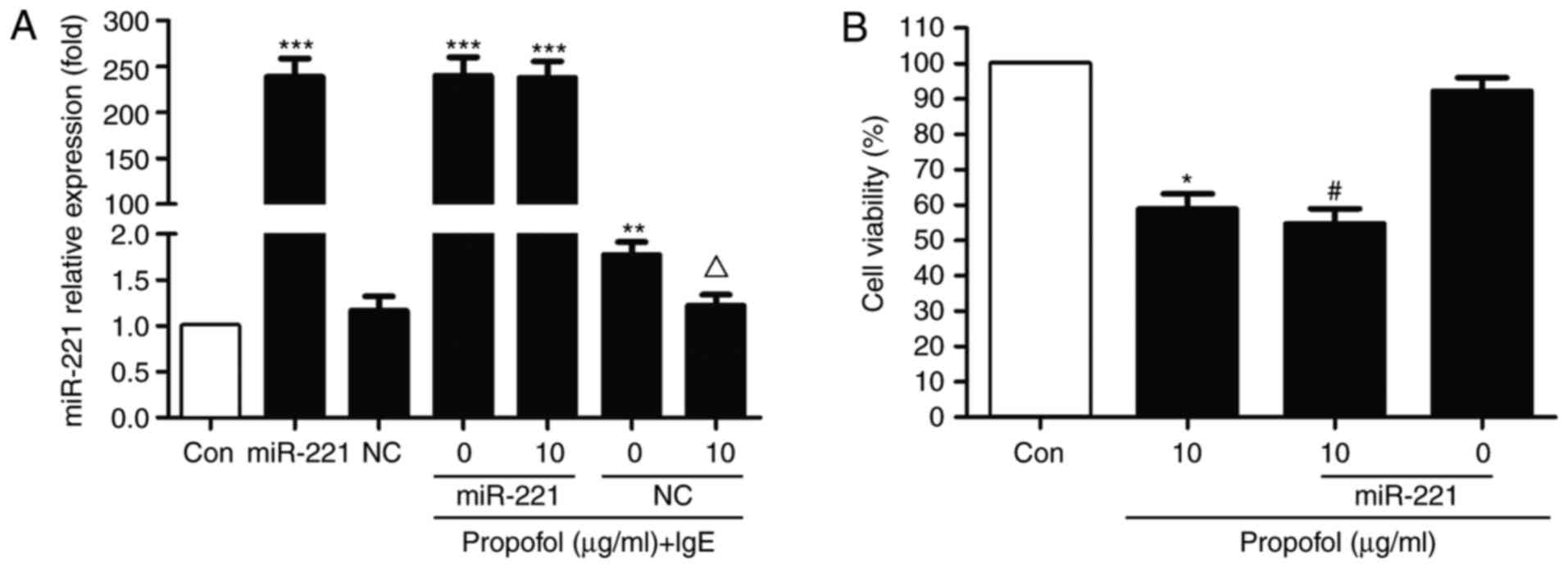
To investigate whether miR-221 is associated with
the action of propofol in RBL-2H3 cells, an miR-221 overexpression
model was constructed via transfection with miR-221 mimics. miR-221
notably abrogated the inhibitive effect of propofol in
IgE-activated RBL-2H3 cells (Fig.
3A). However, no significant difference was observed compared
with the NC group. Furthermore, following transfection with
miR-221, cell viability was markedly reduced compared with the
control. No statistical differences were observed in the presence
of propofol, suggesting that miR-221 has no effect on RBL-2H3 cell
proliferation (Fig. 3B).
miR-221 and the effect of propofol on
mast cell degranulation
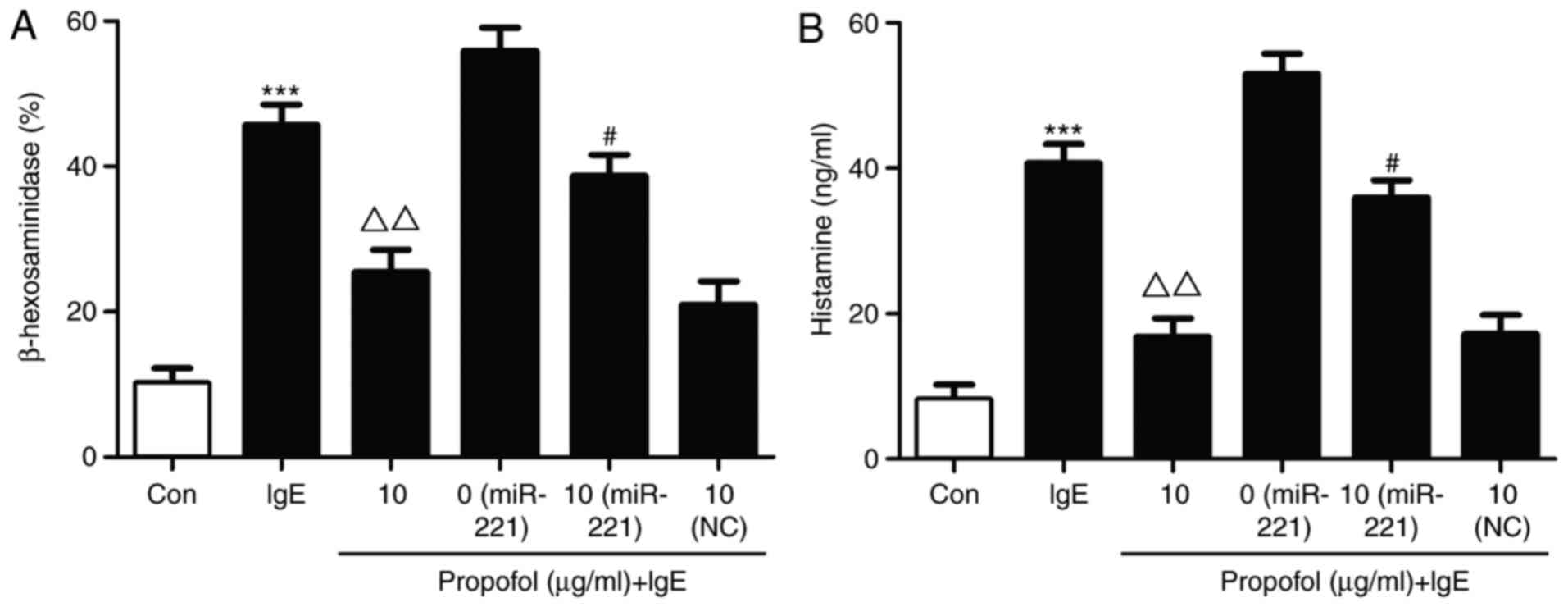
β-hexosaminidase and histamine release are typically
used as indicators of mast cell granulation (16). In the present study, β-hexosaminidase
and histamine release were significantly increased in the miR-221
overexpression group compared with the IgE stimulated group
(Fig. 4). Following propofol
treatment, β-hexosaminidase release was obviously decreased.
However, miR-221 overexpression markedly restored the suppressive
effect of propofol on β-hexosaminidase in IgE-stimulated mast cell
degranulation (Fig. 4A). Similar
results were observed for histamine (Fig. 4B).
miR-221-induced PI3K/Akt signaling is
associated with the suppressive effect of propofol
Compared with the control group, Akt phosphorylation
was significantly increased in IgE-activated RBL-2H3 cells.
However, treatment with propofol significantly reduced Akt
phosphorylation compared with cells treated with IgE alone
(Fig. 5). miR-221 overexpression
significantly reversed the suppressive effect of propofol on
PI3K/Akt signaling, suggesting that miR-221 inhibits propofol via
targeting its mechanistic pathway.
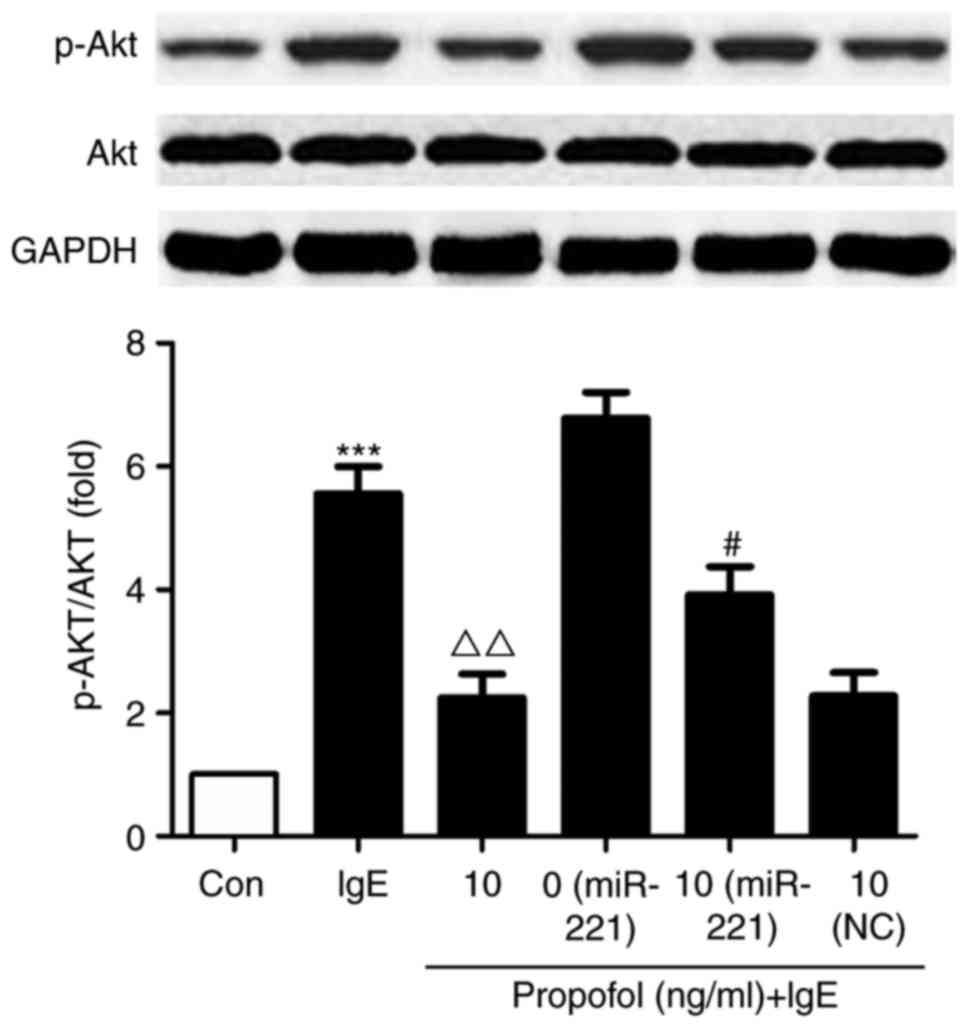 | Figure 5.Effect of propofol on Akt
phosphorylation. RBL-2H3 cells were transfected with miR-221 mimic
or NC miRNA and treated with 10 µg/ml propofol. Cells were
activated with anti-DNP IgE and DNP-HSA, following which the
expression of Akt and p-Akt was assessed using western blotting.
***P<0.001 vs. Con. #P<0.05 vs. miR-221 + 0 µg/ml
propofol. ΔΔP<0.01 vs. IgE + 0 µg/ml propofol. Akt,
protein kinase B; miR or miRNA, microRNA; NC, negative control;
DNP, 2,4-dinitrophenyl; Ig, immunoglobulin; HSA, human serum
albumin; p, phosphorylated; Con, control. |
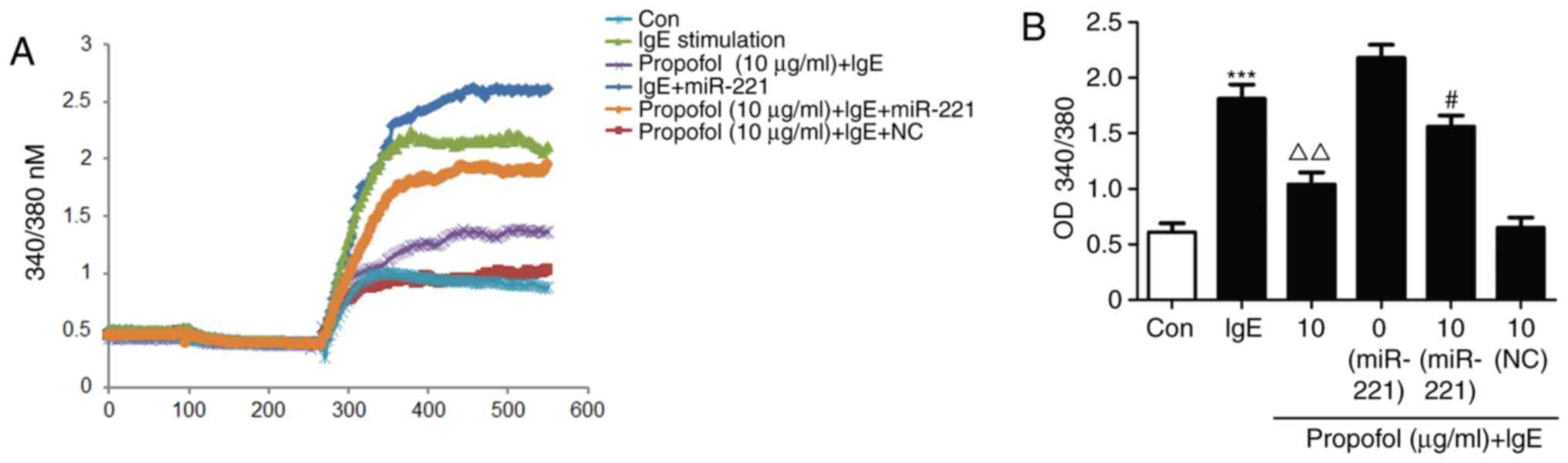
Decreased Ca2+ influx
regulates the effects of propofol in mast cell degranulation
As Ca2+ is associated with mast cell
degranulation, fura-2 induced changes in Ca2+ were
measured. Ca2+ influx was significantly upregulated in
IgE-activated RBL-2H3 cells. However, propofol treatment
significantly inhibited Ca2+ influx (Fig. 6A). Furthermore, miR-221 treatment was
able to reverse the suppressive effect of propofol (Fig. 6B), suggesting that Ca2+
participates in the regulation of propofol in mast cell
degranulation.
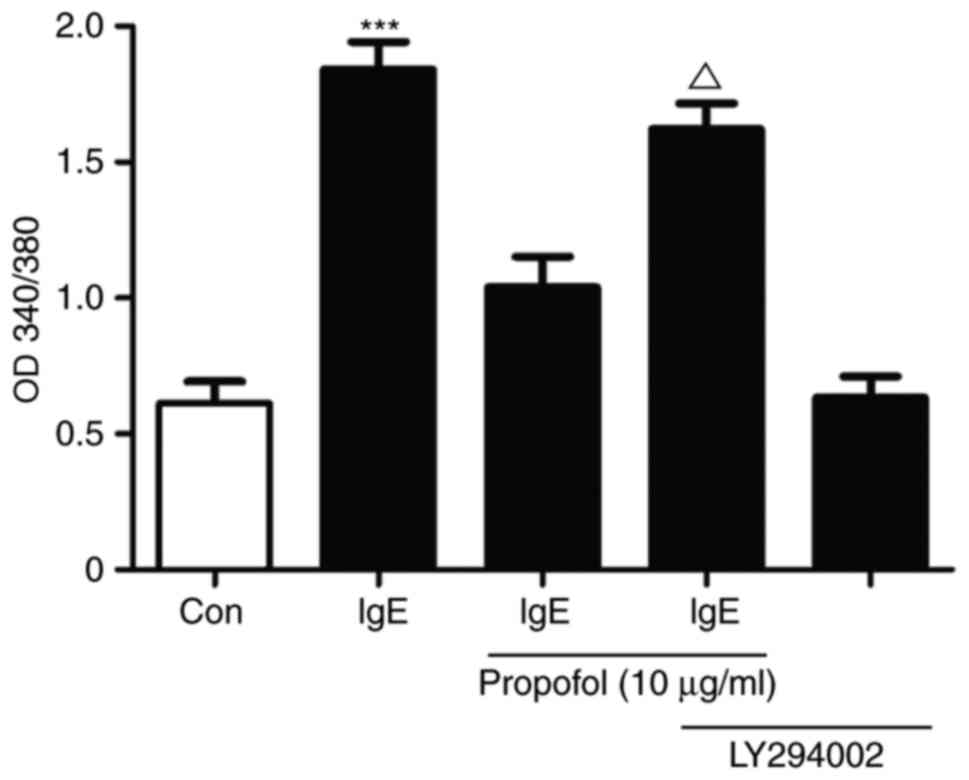
Propofol attenuates mast cell
degranulation via inhibiting the miR-221/PI3K/Akt/Ca2+
pathway
The association between PI3K/Akt signaling and
Ca2+ influxwas investigated in propofol-treated
IgE-mediated mast cell activation (Fig.
7). Treatment with propofol reduced Ca2+ influx in
activated RBL-2H3 cells compared with the control cells. LY294002,
a specific PI3K inhibitor, reversed the propofol-induced reduction
in Ca2+ influx, suggesting that propofol attenuates mast
cell degranulation via inhibiting the
miR-221/PI3K/Akt/Ca2+ pathway (Fig. 7).
Discussion
Although propofol has been demonstrated to have
anti-oxidant, anti-inflammatory and anti-tumor properties, few
studies have investigated the effect of propofol on mast cell
degranulation (8). The results of
the present study demonstrate that propofol is able to affect
RBL-2H3 cell proliferation, reduce miR-221 expression, suppress the
release of β-hexosaminidase and histamine, limit activation of the
PI3K/Akt signaling pathway and decrease Ca2+ influx in
mast cells. These findings suggest that propofol may have potential
as an anesthetic for surgical procedures associated with allergic
responses.
MicroRNAs (miRs) are a group of small,
single-stranded non-coding RNAs that regulate gene expression at
the post-transcriptional level (17). Through binding to the 3′-untranslated
region of target mRNAs, miRs are able to induce mRNA degradation or
translational inhibition (18).
miR-221 has been reported to be associated with the modulation of
mast cell degranulation (19). A
previous study demonstrated that miR-221 influenced the cell cycle,
the extent of degranulation, cytokine production and the actin
cytoskeleton in activated bone marrow-derived mast cells (20). It has been indicated that miR-221,
which was overexpressed in a murine asthma model, is able to
stimulate interleukin-4 secretion in mast cells through a pathway
involving phosphatase and tensin homolog, p38 and nuclear factor
(NF)-Κb (21). In line with these
results, propofol treatment inhibited miR-221 expression in
IgE-stimulated RBL-2H3 cells, suggesting that miR-221 may serve a
role in the suppressive effect of propofol. These results were
further confirmed by cell transfection with the miR-221 mimic.
The effect of PI3K/Akt signaling in mast cells has
previously been investigated (22,23), as
has the association between miR expression and PI3K/Akt signaling
in mast cell degranulation (24,25).
Although miR-223 expression was upregulated in IgE-mediated mast
cells, miR-223 downregulation was demonstrated to promote mast cell
degranulation and apoptosis via the PI3K/Akt pathway by targeting
insulin-like growth factor 1 receptor in mast cells (25,26).
Helicobacter pylori neutrophil-activating protein induced
the release of histamine and interleukin-6 in human mast cell
line-1 via the G protein-mediated mitogen-activated protein kinase
(MAPK) and PI3K/Akt pathways (27).
These studies indicated that PI3K/Akt signaling is associated with
the regulation of mast cell activation, which may contribute to the
inhibitive biological properties of propofol. The results of the
present study also confirmed that propofol treatment restricted
mast cell degranulation, as evidenced by the downregulation of
β-hexosaminidase and histamine. Propofol treatment results in
decrease Akt phosphorylation, suggesting that the PI3K/Akt
signaling pathway serves a role in the suppressive effect of
propofol on mast cell degranulation.
Generally, mast cell activation results in the
degranulation of preformed mediators, including histamine, and the
secretion of newly synthesized mediators, including leukotrienes
and inflammatory cytokines (28). An
influx of extracellular Ca2+ is essential for mast cell
mediator release (29). It has been
reported that Ca2+ mobilization is associated with the
regulation of mast cell function (29). A previous study demonstrated that
Ca2+ influx served a key role in modulating the
spontaneous motility and directional migration of mast cells
towards stimulating antigens (30).
Furthermore, it was reported that miR-221 promoted the IgE-mediated
activation of mast cell degranulation via the
PI3K/Akt/PLCγ/Ca2+ signaling pathway in a non-NF-κB
dependent manner (31). Consistent
with the above findings, propofol treatment resulted in reduced
Ca2+ influx, miR-221 and Akt phosphorylation, which were
abrogated by the specific PI3K-inhibitor LY294002. This suggests
that the miR-221/PI3K/Akt/Ca2+ pathway is responsible
for the suppressive effect of propofol.
In conclusion, the results of the present study
demonstrate that propofol attenuates the IgE-mediated activation of
mast cell degranulation via inhibiting the
miR-221/PI3K/Akt/Ca2+ pathway. Although the present
study provides a novel insight into the biological effect of
propofol and suggests a potential molecular target for the
treatment of mast cell-associated allergic diseases. However, there
were various limitations to the present study. Firstly,
miR-221−/− derived from animal or bone marrow mast cells
were not utilized. Use of these cells in future studies may provide
results to support the conclusion of the present study. In
addition, interactions with different signaling pathways including
MAPK and NF-κB, or its involvement with miR-221-associated mast
cell degranulation should be elucidated for clarification in future
studies.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZhiyongY, WL, and GL conceived the experimental
design; ZhiyongY, ZhipanY, KH, YC and CX performed the experiments;
YL and QL performed Ca2+ measurement and analysis;
ZhipanY and SZ aided in data analysis; WL and GL reviewed and
approved the final draft of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Humbles AA, Lu B, Friend DS, Okinaga S,
Lora J, Al-Garawi A, Martin TR, Gerard NP and Gerard C: The murine
CCR3 receptor regulates both the role of eosinophils and mast cells
in allergen-induced airway inflammation and hyperresponsiveness.
Proc Natl Acad Sci USA. 99:1479–1484. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cardamone C, Parente R, Feo GD and
Triggiani M: Mast cells as effector cells of innate immunity and
regulators of adaptive immunity. Immunol Lett. 178:10–14. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galli SJ and Tsai M: IgE and mast cells in
allergic disease. Nat Med. 18:693–704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amin K: The role of mast cells in allergic
inflammation. Respir Med. 106:9–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gelfand EW, Joetham A, Wang M, Takeda K
and Schedel M: Spectrum of T-lymphocyte activities regulating
allergic lung inflammation. Immunol Rev. 278:63–86. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu-Kuo JM, Fruman DA, Joyal DM, Cantley LC
and Katz HR: Impaired kit-but not FcepsilonRI-initiated mast cell
activation in the absence of phosphoinositide 3-kinase p85alpha
gene products. J Biol Chem. 275:6022–6029. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hari Keerthy P, Balakrishna R, Srungeri
KM, Singhvi N, John J and Islam M: Comparitive evaluation of
propofol and midazolam as conscious sedatives in minor oral
surgery. J Maxillofac Oral Surg. 14:773–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vasileiou I, Xanthos T, Koudouna E, Perrea
D, Klonaris C, Katsargyris A and Papadimitriou L: Propofol: A
review of its non-anaesthetic effects. Eur J Pharmacol. 605:1–8.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang X, Teng Y, Yang H and Ma J: Propofol
inhibits invasion and growth of ovarian cancer cells via regulating
miR-9/NF-κB signal. Braz J Med Biol Res. 49:e57172016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ou W, Lv J, Zou X, Yao Y, Wu J, Yang J,
Wang Z and Ma Y: Propofol inhibits hepatocellular carcinoma growth
and invasion through the HMGA2-mediated Wnt/β-catenin pathway. Exp
Ther Med. 13:2501–2506. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Z, Zhang J, Hong G, Quan J, Zhang L
and Yu M: Propofol inhibits growth and invasion of pancreatic
cancer cells through regulation of the miR-21/Slug signaling
pathway. Am J Transl Res. 8:4120–4133. 2016.PubMed/NCBI
|
|
12
|
Wang XY, Li YL, Wang HY, Zhu M, Guo D,
Wang GL, Gao YT, Yang Z, Li T, Yang CY and Chen YM: Propofol
inhibits invasion and proliferation of C6 glioma cells by
regulating the Ca2+ permeable AMPA receptor-system
xc− pathway. Toxicol In Vitro. 44:57–65.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu WZ and Liu N: Propofol inhibits lung
cancer A549 cells growth and epithelial-mesenchymal transition
process by up-regulation of microRNA-1284. Oncol Res. Feb
5–2018.(Epub ahead of print).
|
|
14
|
Zhao W, Zhou S, Yao W, Gan X, Su G, Yuan D
and Hei Z: Propofol prevents lung injury after intestinal
ischemia-reperfusion by inhibiting the interaction between mast
cell activation and oxidative stress. Life Sci. 108:80–87. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moon TC, Befus AD and Kulka M: Mast cell
mediators: Their differential release and the secretory pathways
involved. Front Immunol. 5:5692014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hombach S and Kretz M: Non-coding RNAs:
Classification, biology and functioning. Adv Exp Med Biol.
937:3–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chua JH, Armugam A and Jeyaseelan K:
MicroRNAs: biogenesis, function and applications. Curr Opin Mol
Ther. 11:189–199. 2009.PubMed/NCBI
|
|
19
|
Montagner S, Orlandi EM, Merante S and
Monticelli S: The role of miRNAs in mast cells and other innate
immune cells. Immunol Rev. 253:12–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayoral RJ, Deho L, Rusca N, Bartonicek N,
Saini HK, Enright AJ and Monticelli S: miR-221 influences effector
functions and actin cytoskeleton in mast cells. PLoS One.
6:e261332011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Y, Yang Q, Xu H, Zhang J, Deng H, Gao
H, Yang J, Zhao D and Liu F: miRNA-221-3p enhances the secretion of
Interleukin-4 in mast cells through the phosphatase and tensin
homolog/p38/Nuclear Factor-kappaB Pathway. PLoS One.
11:e01488212016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weichhart T and Säemann MD: The
PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic
applications. Ann Rheum Dis. 67 Suppl 3:iii70–iii74. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin H, Zheng C, Li J, Yang C and Hu L:
Lentiviral shRNA against KCa3.1 inhibits allergic response in
allergic rhinitis and suppresses mast cell activity via PI3K/AKT
signaling pathway. Sci Rep. 5:131272015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Biethahn K, Orinska Z, Vigorito E,
Goyeneche-Patino DA, Mirghomizadeh F, Föger N and Bulfone-Paus S:
miRNA-155 controls mast cell activation by regulating the PI3Kγ
pathway and anaphylaxis in a mouse model. Allergy. 69:752–762.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Q, Zhao DY, Xu H, Zhou H, Yang QY,
Liu F and Zhou GP: Down-regulation of microRNA-223 promotes
degranulation via the PI3K/Akt pathway by targeting IGF-1R in mast
cells. PLoS One. 10:e01235752015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao H, Deng H, Xu H, Yang Q, Zhou Y, Zhang
J, Zhao D and Liu F: MicroRNA-223 promotes mast cell apoptosis by
targeting the insulin-like growth factor 1 receptor. Exp Ther Med.
11:2171–2176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsai CC, Kuo TY, Hong ZW, Yeh YC, Shih KS,
Du SY and Fu HW: Helicobacter pylori neutrophil-activating protein
induces release of histamine and interleukin-6 through G
protein-mediated MAPKs and PI3K/Akt pathways in HMC-1 cells.
Virulence. 6:755–765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bulfone-Paus S, Nilsson G, Draber P, Blank
U and Levi-Schaffer F: Positive and negative signals in mast cell
activation. Trends Immunol. 38:657–667. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holowka D, Wilkes M, Stefan C and Baird B:
Roles for Ca2+ mobilization and its regulation in mast cell
functions: Recent progress. Biochem Soc Trans. 44:505–509. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee J, Veatch SL, Baird B and Holowka D:
Molecular mechanisms of spontaneous and directed mast cell
motility. J Leukoc Biol. 92:1029–1041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu H, Gu LN, Yang QY, Zhao DY and Liu F:
miR-221 promotes IgE-mediated activation of mast cells
degranulation by PI3K/Akt/PLCγ/Ca(2+) pathway. J Bioenerg Biomembr.
48:293–299. 2016. View Article : Google Scholar : PubMed/NCBI
|