Introduction
Yes-associated protein 1 (YAP1) is a major
downstream effector of the Hippo pathway, which controls organ
size, normal tissue homeostasis and stem cell functions by
regulating cell proliferation, growth and apoptosis (1,2). YAP1 is
negatively regulated by Hippo and upon injury of the body; the
activation of YAP1 promotes the proliferation, differentiation and
regeneration of damaged tissues (3,4).
Overexpression of YAP1 has been found to exist in numerous
carcinoma types as an important oncoprotein (5–7); upon
its hyperactivation, it greatly promotes cell proliferation and
inhibits cell apoptosis. Studies have found that the functional
operation of YAP1 involves interactions with other factors. For
instance, YAP1 induced the expression of anti-apoptotic genes in
cancer cells through forming a complex with T-box 5 and β-catenin
(8). Furthermore, the synergy
between YAP1 and KRAS promoted cancer cell invasion and progression
(9) and upregulation of YAP1 with
abnormal Sonic hedgehog signaling existed in human medulloblastomas
(10).
Numerous studies have shown that aberrant Notch
signaling was closely associated with the genesis of various cancer
types, such as glioma, cervical cancer, melanoma and hepatocellular
carcinoma (HCC) (11–14). The complete Notch pathway includes
Notch receptors (Notch1, −2, −3 and −4), Notch ligands (Jagged1 and
−2 as well as Delta1, −3 and −4) and target genes (HES, HEY).
Tschaharganeh et al (15)
reported that overexpression of YAP1 led to upregulation of
Jagged-1 (JAG1), which resulted in activation of the Notch pathway
and promoted the proliferation of HCC cells in vitro and
in vivo; this YAP-dependent effect in HCC was consistent
with that in colorectal and pancreatic cancer.
Orr et al (16) demonstrated that YAP1 was highly
expressed in infiltrating astrocytomas and oligodendrogliomas and
promoted glioblastoma cell proliferation and metastasis. Notch1 and
JAG1 were also demonstrated to be overexpressed in glioma and to
have a role in maintaining tumor cell proliferation and survival
(17). Therefore, the present study
hypothesized that overexpression of YAP1 may promote glioma cell
metastasis via upregulation of JAG1 and activation of the Notch
pathway. The expression of YAP1, JAG1, NOTCH1 and HES1 in the U251
human glioma cell line and changes in the expression of these
proteins after transfection with small interfering (si)RNA
targeting YAP1 (siRNA-YAP1) and JAG1 or HES1 overexpression vector
were therefore assessed. In addition, the cellular migration
ability under these conditions was detected. The results indicated
that compared with that in normal astrocytes, YAP1 and proteins
associated with Notch signaling were upregulated in U251 cells,
which was downregulated by siRNA-YAP1 and compensated by
overexpression of JAG1 and HES1; these changes were found to be
associated with the migratory ability of U251 cells in
vitro.
Materials and methods
Cell lines
The U251 human glioma cell line was obtained from
the American Type Culture Collection (Manassas, VA, USA) and
cultured in Dulbecco's modified Eagle's medium (HyClone; GE
Healthcare, Little Chalfont, UK) supplemented with 10% fetal calf
serum (FCS; Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) in a humidified incubator at 37°C containing 5%
CO2. Normal astrocytes used in this study were human
astrocytes (Sciencell, Carlsbad, CA, USA), which were cultured in
complete Astrocyte Medium (Sciencell) in a humidified incubator at
37°C containing 5% CO2.
siRNA synthesis, overexpression vector
construction and cell transfection
Two siRNA sequences were designed to target YAP1
(siRNA1, 5′-CCACCAAGCUAGUAAAGAdTdT-3′; siRNA2,
5′-GGUCAGAGAUACUUCUUAAdTdT-3′) and a nonsense siRNA with a random
sequence (5′-UUCUCCGAACGUGUCACGUTT-3′) was used as a negative
control. These siRNAs were synthesized by GenePharma (Shanghai,
China). JAG1 and HES1 complementary (c)DNA were individually
sub-cloned into eukaryotic expression vector, as described
previously (18,19). U251 cells were transfected with siRNA
and recombinant plasmid using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's protocol. U251 cells were collected following 24 h
transfection and co-cultured with the siRNA and/or transfected with
recombinant plasmid for 48 h.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
In order to determine the relative expression level
of YAP1, JAG1, NOTCH1 and HES1 mRNA, total RNA was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) from
human astrocytes and U251 cells to perform RT-qPCR. cDNA synthesis
was performed using the PrimeScript™ RT kit (Takara
Biotechnology Co., Dalian, China) and qPCR was performed using
PrimeScript™ PCR Master Mix (Takara Biotechnology Co.)
in an ABI 7500 real-time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The PCR cycling conditions were as
follows: 40 cycles of 95°C for 30 sec and 60°C for 30 sec. The
primers used for PCR are listed in Table
I. The ΔΔCq method was used for the data analysis
(20).
 | Table I.Sequences of primers used for
polymerase chain reaction analysis. |
Table I.
Sequences of primers used for
polymerase chain reaction analysis.
| Gene | Direction | Sequences of primers
(5′-3′) |
|---|
| YAP1 | F |
TAGCCCTGCGTAGCCAGTTA |
|
| R |
GGTTCGAGGGACACTGTAGC |
| JAG1 | F | AAGTGCACCCGCGACG |
|
| R |
ATTACTGGAATCCCACGCCTC |
| NOTCH1 | F |
TGAATGGCGGGAAGTGTGAA |
|
| R |
ATAGTCTGCCACGCCTCTG |
| HES1 | F |
GATAGCTCGCGGCATTCCAA |
|
| R |
TCGGTATTAACGCCCTCGC |
| β-actin | F |
ACCTTCTACAATGAGCTGCG |
|
| R |
CCTGGATAGCAACGTACATGG |
Western blot analysis
The protein levels of YAP1, JAG1, NOTCH1,
intracellular domain of Notch protein (NICD) and HES1 in each
sample were detected using western blot analysis. Total proteins
were extracted with radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Inc., Shanghai, China) and
the protein concentration was determined using a BCA Protein Assay
kit (Beyotime Institute of Biotechnology, Inc.). The same quantity
of total protein (20 µg) was separated using SDS-PAGE with 7.5%
polyacrylamide prior to transfer onto a nitrocellulose membrane
(Biodee Biotechnology, Beijing, China). The membrane was then
blocked in Tris-buffered saline containing 0.1% Tween-20 (TBST) and
5% nonfat dry milk followed by incubation with primary antibodies
overnight at 4°C. Primary antibodies were anti-YAP1 (cat. no.
ab56701), anti-JAG1 (cat. no. ab109536), anti-NOTCH1 (cat. no.
ab52627), anti-NICD (cat. no. ab8925), anti-HES1 (cat. no.
ab108937) and anti-β-actin (cat. no. ab1801) (all from Abcam,
Cambridge, MA, USA) and were used at the dilutions recommended by
the supplier. Subsequent to washing for three times with TBST,
membranes were incubated with the secondary antibodies: Horseradish
peroxidase-conjugated goat anti-mouse immunoglobulin (Ig)G
(dilution, 1:2,000; ab6789) and goat anti-rabbit IgG (dilution,
1:2,000; ab6721) (both from Abcam). Finally, the membranes were
washed three times and the protein bands were visualized using an
EasyBlot ECL kit (Sangon Biotech Co., Ltd., Shanghai, China)
according to the manufacturers protocol and images of the blots
were captured using X-ray film.
Cell migration assay
U251 cells with YAP1 knockdown and overexpression of
JAG1 or HES1 were used for cell migration assays using the
Transwell method. Cells were suspended in serum-free medium (100
µl; 5×105/ml) and inoculated into the upper chamber with
8-µm pore membranes (Corning, NY, USA). Medium containing 10% FCS
was added to the lower chamber, followed by conventional culture
for 6 h. The cells were fixed with 4% formaldehyde and stained with
crystal violet after washing with PBS. The medium and cells in the
upper chamber were discarded for observing and counting cells on
the lower surface of the chamber with a microscope (magnification,
×400).
Statistical analysis
Data were analyzed using GraphPad Prism 5.0 software
(GraphPad Inc., La Jolla, CA, USA). Values are expressed as the
mean ± standard deviation from at least three separate experiments.
The significance of differences between two groups was detected
using Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of YAP1, JAG1, NOTCH1 and
HES1 in U251 cell line
RT-qPCR and western blot analyses were used to
detect the mRNA and protein levels of YAP1, JAG1, NOTCH1 and HES1
in normal astrocytes and U251 glioma cells. As presented in
Fig. 1, not only the mRNA levels of
YAP1 in U251 cells were obviously higher than those in human
astrocytes (P<0.05), but the mRNA levels of JAG1, NOTCH1 and
HES1 in U251 cells were also significantly higher than those in
human astrocytes (P<0.01). Similarly, the corresponding protein
levels were higher in U251 cells, particularly those of YAP1, JAG1
and HES1 (P<0.01).
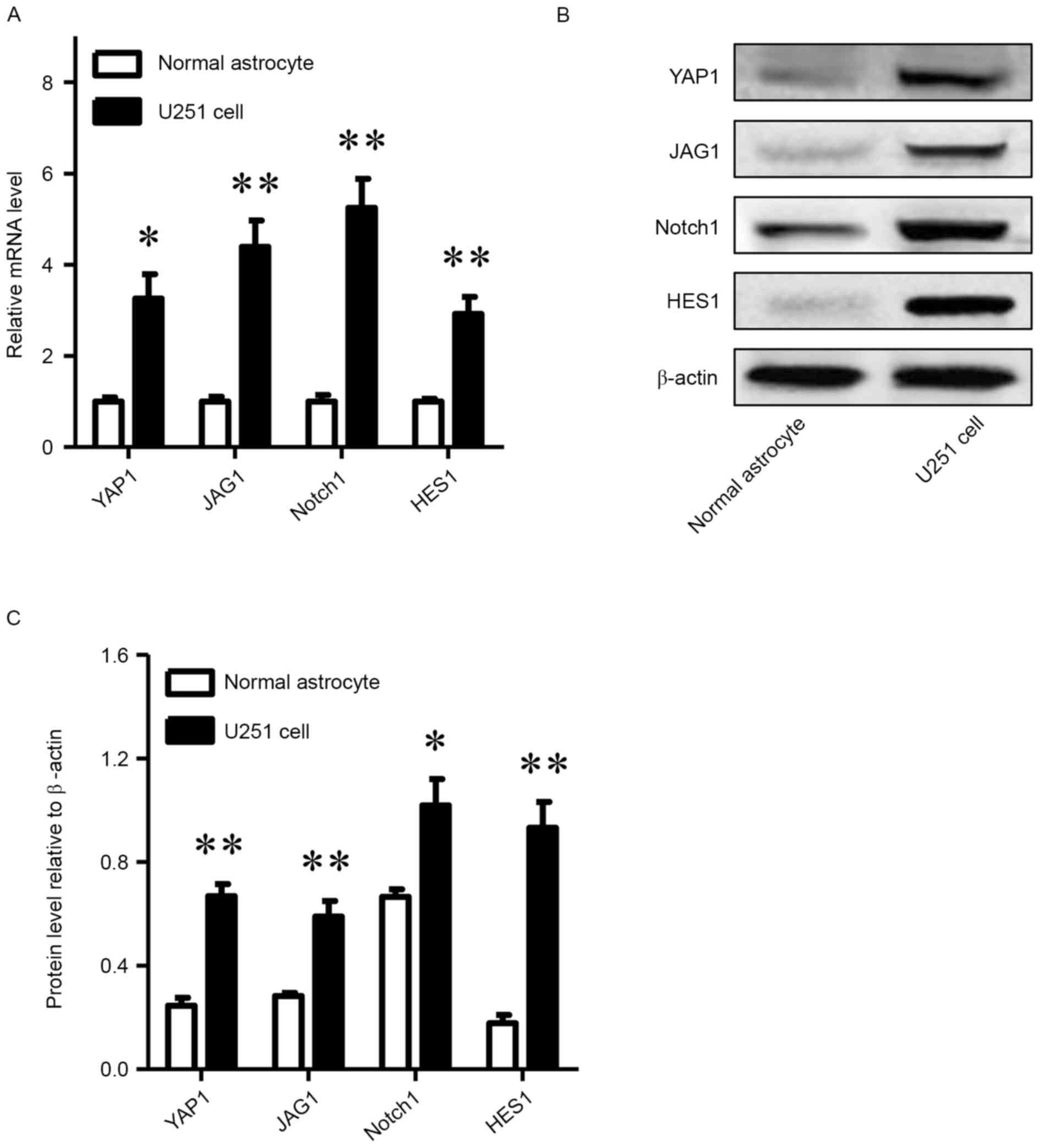 | Figure 1.YAP1, JAG1, NOTCH1 and HES1 are
upregulated in glioma cells compared with human astrocytes. (A)
Relative mRNA levels of YAP1, JAG1, NOTCH1 and HES1 in U251 glioma
cells compared with human astrocytes. (B) Western blot analysis of
YAP1, JAG1, NOTCH1 and (C) protein levels relative to β-actin HES1
in U251 glioma cells and human astrocytes. *P<0.05, **P<0.01,
compared with normal astrocytes. YAP-1, Yes-associated protein 1;
JAG1, Jagged 1. |
Effects of YAP1 knockdown on protein
levels in U251 cell line
After 24 h of transfection with YAP1 siRNA, U251
cells were collected to detect the expression levels of associated
proteins using western blot analysis. As presented in Fig. 2A and B, YAP1 siRNA-1 and YAP1 siRNA-2
interfered the YAP1 protein expression, which was obviously lower
than that in the blank group and scramble siRNA group (P<0.01).
Furthermore, the NICD and HES1 protein levels in the U251 cells
transfected with YAP1 siRNA showed a noticeable decline
(P<0.01); however, the difference in NOTCH1 protein levels
between YAP1 siRNA group, blank group and scramble siRNA group was
not significant.
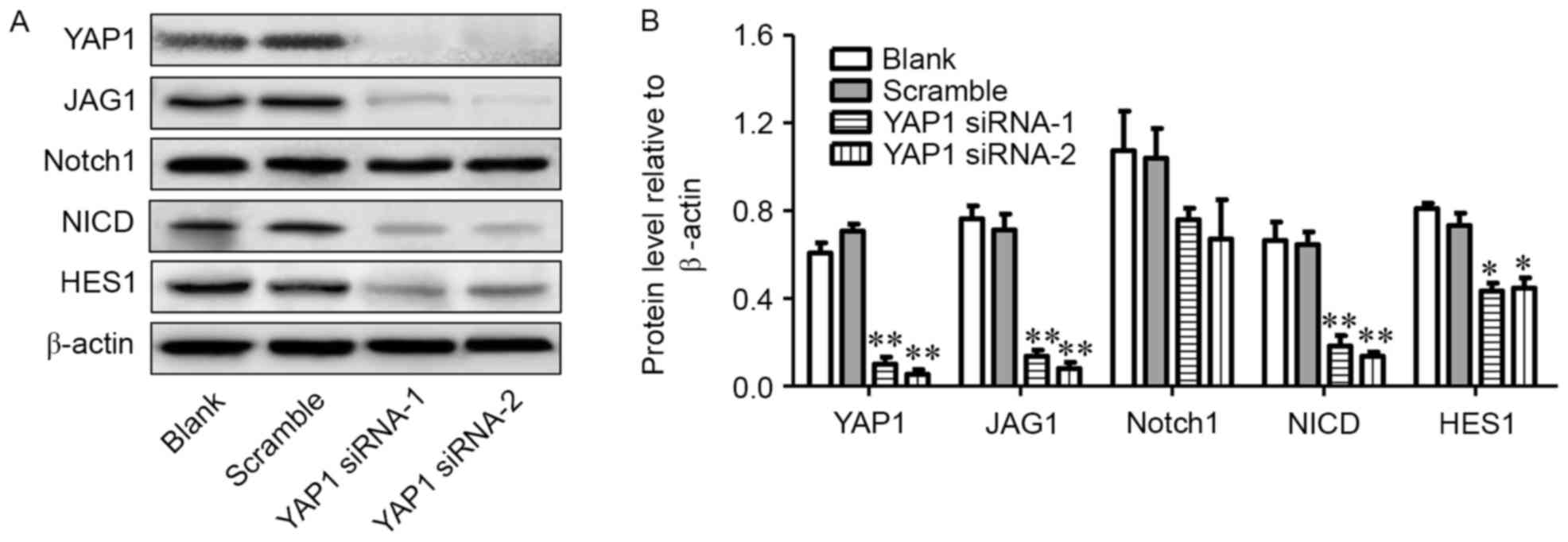 | Figure 2.Knockdown of YAP1 leads to
downregulation of JAG1, NOTCH1, NICD and HES1 proteins. (A and B)
Protein levels of YAP1, JAG1, NOTCH1, NICD and HES1 in blank,
scramble siRNA, YAP1 siRNA1 and YAP1 siRNA2 groups. *P<0.05,
**P<0.01, compared with scramble group. YAP-1, Yes-associated
protein 1; JAG1, Jagged 1; siRNA, small interfering RNA; NICD,
intracellular domain of NOTCH protein. |
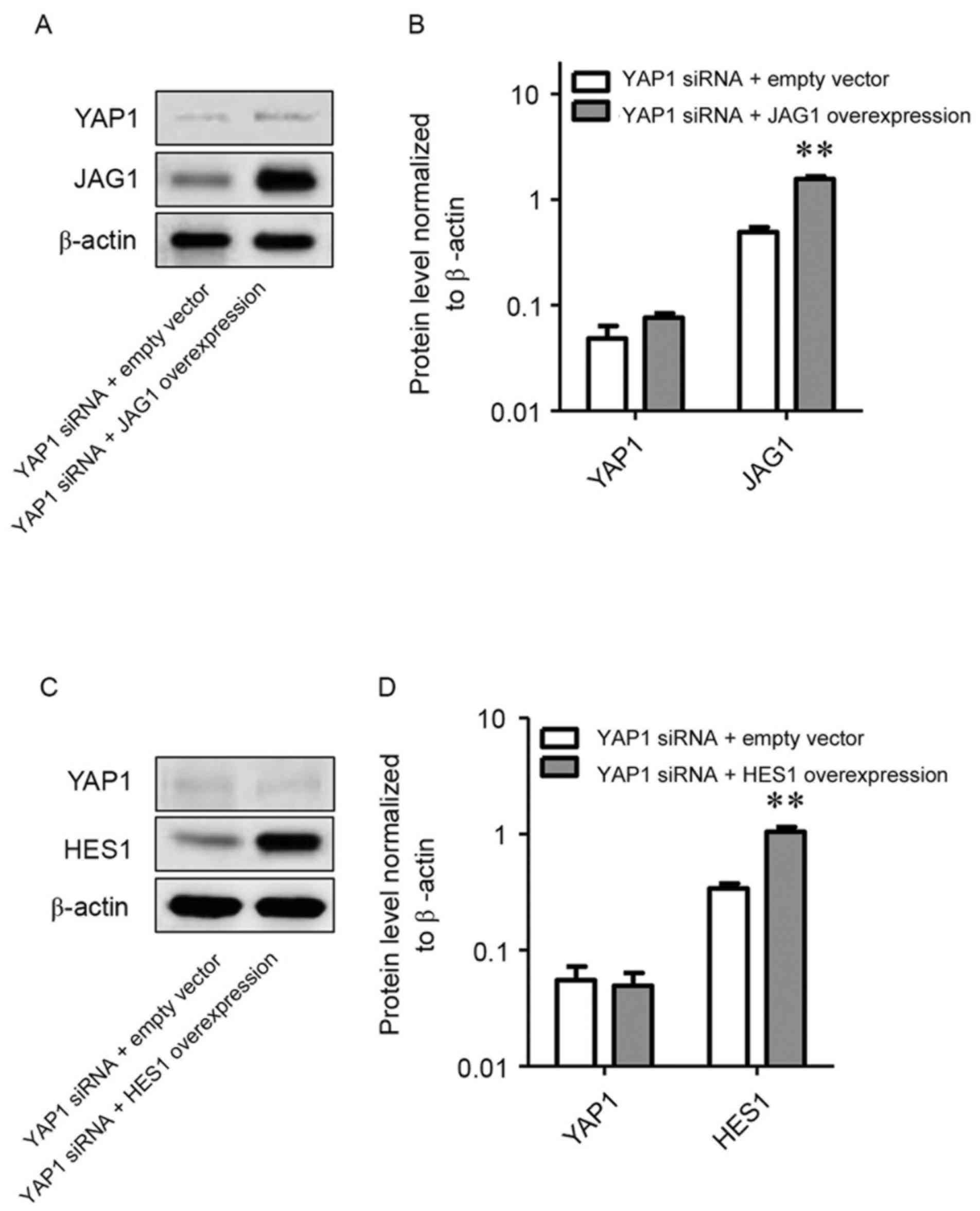
Effects of overexpression of JAG1 and
HES1 on U251 cells following YAP1 gene knockdown
Recombinant plasmids for overexpressing JAG1 or HES1
were respectively transfected into U251 cells following YAP1 gene
knockdown and the expression of YAP1, JAG1 and HES1 protein were
investigated. When U251 cells were co-transfected with recombinant
plasmids and siYAP1, overexpression of JAG1 or HES1 replenished the
decrease of JAG1 and HES1 in U251 cells following YAP1 gene
knockdown (P<0.01). However, neither overexpression of JAG1 or
HES1 reversed the downregulation of YAP1 (Fig. 3A-D).
Migration of the U251 cell line
following YAP1 gene knockdown and overexpression of JAG1 and
HES1
The migration ability of U251 cells was assessed via
the Transwell method. Following knockdown of YAP1, the number of
migrated cells per field of view was significantly reduced compared
with that in the blank group and scrambled siRNA group (12 or 15
vs. 36 and 38, respectively; P<0.01; Fig. 4A and B). This reduction caused by
knockdown of YAP1 was compensated by overexpression of JAG1 or HES1
when cells were co-transfected with siYAP1 and recombinant plasmids
and there were significant increases between empty vector and JAG1
overexpression (18 vs. 64; P<0.01) or empty vector and HES1
overexpression groups (20 vs. 46; P<0.05; Fig. 4C and D).
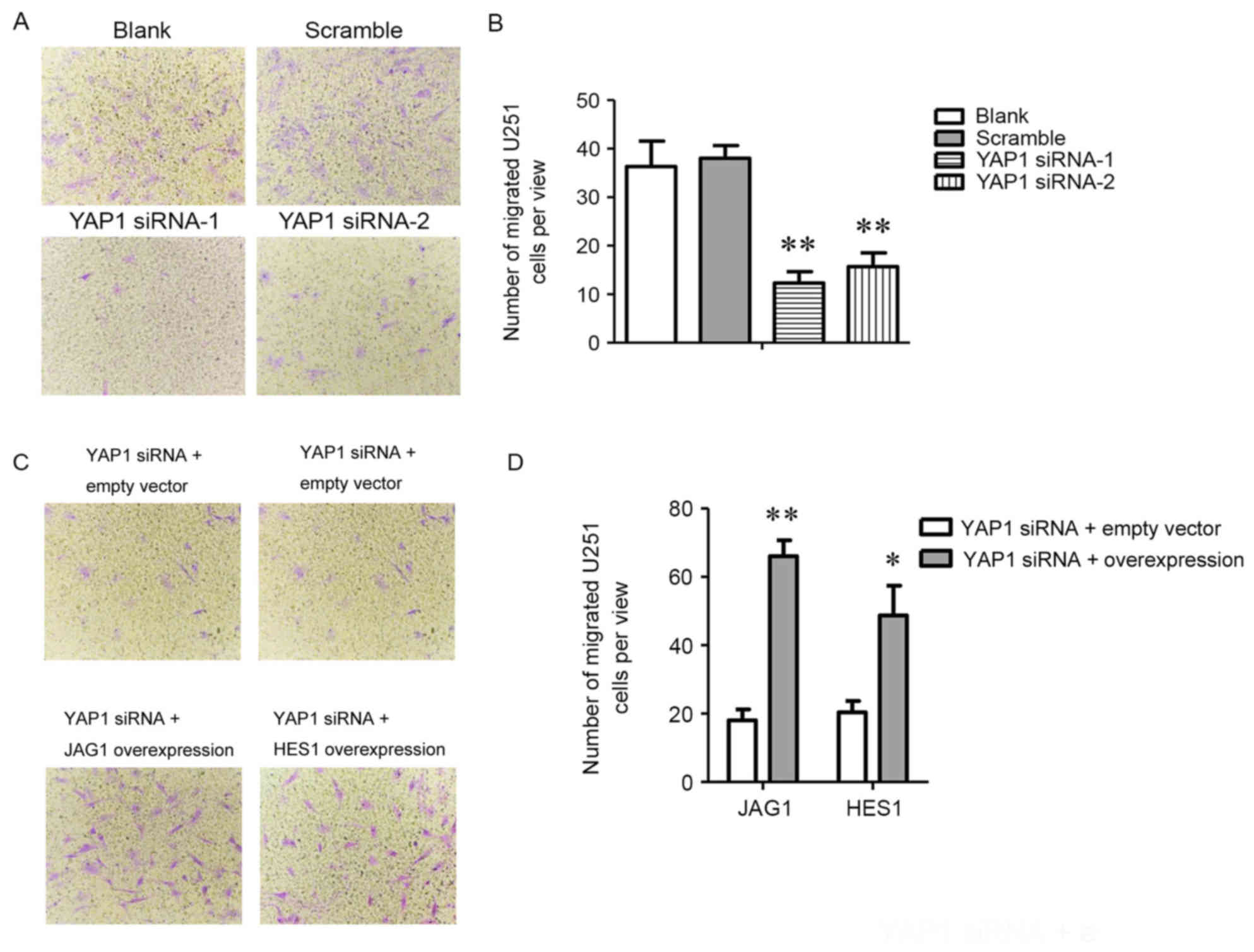 | Figure 4.Overexpression of JAG1 or HES1
promotes migration of U251 cells transfected with YAP1 siRNA. (A)
U251 cell migration in blank, scramble siRNA, YAP1 siRNA1 and YAP1
siRNA2 groups (magnification, 200×); (B) Number of migrated U251
cells per field of view in blank, scramble siRNA, YAP1 siRNA1 and
YAP1 siRNA2 groups. (C) U251 cell migration in empty vector group,
JAG1 overexpression group and HES1 overexpression group with
knockdown of YAP1 in U251 cells (magnification, ×200); (D) number
of migrated U251 cells per field of view in empty vector group,
JAG1 overexpression group and HES1 overexpression group with
knockdown of YAP1 in U251 cells. *P<0.05, **P<0.01, compared
with the group transfected with empty vector or blank group. YAP-1,
Yes-associated protein 1; JAG1, Jagged 1. |
Discussion
YAP1 exerts its growth stimulatory effects via
forming a complex with Tafazzin, which promotes proliferation and
inhibits apoptosis. Phosphorylation of YAP1 suppresses
proliferation and accelerates apoptosis. Overexpression of YAP1
accounts for the overproliferation in numerous solid tumor types
and has an important role in tumorigenesis and tumor progression
(21,22). The present study demonstrated that
YAP1 is highly expressed in U251 glioma cells compared with that in
normal astrocyte cells, which conformed to the overexpression of
YAP1 in infiltrating astrocytomas and oligodendrogliomas (16). Furthermore, compared with that in
normal astrocytes, the U251 cell line was found to exhibit high
expression of JAG1, NOTCH1 and HES1 mRNA and protein, which are
ligand, receptor and downstream response gene of Notch signaling,
respectively (23,24). The Notch signaling pathway is
deregulated in numerous types of solid tumor and it directly or
indirectly influences cancer. NOTCH1 has been previously reported
to be expressed at a high level in human glioma and to rise with
the pathological grade (25).
A previous study showed that YAP1 upregulated JAG1
and activated the Notch signaling in HCC cells (15). In order to elucidate the association
between YAP1, JAG1 and Notch signaling in U251 cells, the present
study used a knockdown approach with siRNA to directly target YAP1
mRNA in the U251 cell line, which led to a decrease of YAP1, JAG1,
NICD and HES1. NICD is the activated form of NOTCH1 after binding
to its ligand and has a role in the transmission of signals via
entry into the nucleus (26). This
indicated that YAP1 positively regulated JAG1 and Notch signaling
in U251 cells in vitro.
The Notch pathway has been proved to take part in
the invasion and metastasis of tumors; for instance, high
expression of JAG1 and NOTCH1 promoted the invasion and metastasis
of breast cancer cells (27) and
overexpression of NOTCH1 and HES1 was also responsible for the
invasion and metastasis of HCC cells (28). The present study demonstrated that
knockdown of YAP1 decreased U251 cell migration and that
overexpression of JAG1 or HES1 enhanced cell migration of U251
cells transfected with YAP1 siRNA, which indicated that Notch
signaling is associated with U251 cell migration in vitro.
At the same time, YAP1 knockdown led to downregulation of JAG1 and
inactivated Notch signaling, while overexpression of JAG1 or HES1
did not influence the expression of YAP1.
In conclusion, the present study demonstrated that
YAP1, JAG1, NOTCH1 and HES1 were highly expressed in U251 cells
compared to normal astrocytes and that the expression of JAG1, NICD
and HES1 was significantly reduced in U251 cells with knockdown of
YAP1, which also reduced the cell migratory capacity in
vitro. Overexpression of JAG1 or HES1 enhanced cell migration
in vitro, but did not impact the expression of YAP1. Taken
together, these findings indicated that upregulation of JAG1
activated Notch signaling and promoted U251 cell migration in
vitro. Furthermore, YAP1 was overexpressed in U251 cells and
associated with high JAG1 expression to take part in the activation
of Notch signaling and cell migration in vitro. These
findings contributed to the understanding of the mechanisms of
glioma cell metastasis and may provide approaches for the treatment
of gliomas.
Acknowledgements
This study was financially supported by the Shanghai
Natural Science Foudation (grant no. 16ZR1406300) and the Natural
Science Foundation of China (grant no. 81572921).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Camargo FD, Gokhale S, Johnnidis JB, Fu D,
Bell GW, Jaenisch R and Brummelkamp TR: YAP1 increases organ size
and expands undifferentiated progenitor cells. Curr Biol.
17:2054–2060. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao X, Pfaff SL and Gage FH: YAP regulates
neural progenitor cell number via the TEA domain transcription
factor. Genes Dev. 22:3320–3334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson R and Halder G: The two faces of
Hippo: Targeting the Hippo pathway for regenerative medicine and
cancer treatment. Nat Rev Drug Discov. 13:63–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai J, Zhang N, Zheng Y, de Wilde RF,
Maitra A and Pan D: The Hippo signaling pathway restricts the
oncogenic potential of an intestinal regeneration program. Genes
Dev. 24:2383–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu H, Jiang D, Chi F and Zhao B: The
Hippo pathway regulates stem cell proliferation, self-renewal, and
differentiation. Protein Cell. 3:291–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cander. 13:246–257. 2013.
View Article : Google Scholar
|
|
7
|
Zhao B, Tumaneng K and Guan KL: The Hippo
pathway in organ size control, tissue regeneration and stem cell
self-renewal. Nat Cell Biol. 13:877–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenbluh J, Nijhawan D, Cox AG, Li X,
Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, et
al: β-catenin-driven cancers require a YAP1 transcriptional complex
for survival and tumorigenesis. Cell. 151:1457–1473. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shao DD, Xue W, Krall EB, Bhutkar A,
Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, et
al: KRAS and YAP1 converge to regulate EMT and tumor survival.
Cell. 158:171–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fernandez LA, Northcott PA, Dalton J,
Fraga C, Ellison D, Angers S, Taylor MD and Kenney AM: YAP1 is
amplified and up-regulated in hedgehog-associated medulloblastomas
and mediates Sonic hedgehog-driven neural precursor proliferation.
Genes Dev. 23:2729–2741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katoh M: Networking of WNT, FGF, Notch,
BMP, and Hedgehog signaling pathways during carcinogenesis. Stem
Cell Rev. 3:30–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao J, Duan L, Fan M, Yuan J and Wu X:
Notch1 induces cell cycle arrest and apoptosis in human cervical
cancer cells: Involvement of nuclear factor kappa B inhibition. Int
J Gynecol Cancer. 17:502–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu ZJ, Xiao M, Balint K, Smalley KS,
Brafford P, Qiu R, Pinnix CC, Li X and Herlyn M: Notch1 signaling
promotes primary melanoma progression by activating
mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt
pathways and up-regulating N-cadherin expression. Cancer Res.
66:4182–4190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan RH, Li J, Wu N and Chen PS: Late SV40
factor: A key mediator of Notch signaling in human
hepatocarcinogenesis. World J Gastroenterol. 17:3420–3430. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tschaharganeh DF, Chen X, Latzko P, Malz
M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, et al:
Yes-associated protein up-regulates Jagged-1 and activates the
Notch pathway in human hepatocellular carcinoma. Gastroenterology.
144:1530–1542e12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Orr BA, Bai H, Odia Y, Jain D, Anders RA
and Eberhart CG: Yes-associated protein 1 is widely expressed in
human brain tumors and promotes glioblastoma growth. J Neuropathol
Exp Neurol. 70:568–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Purow BW, Haque RM, Noel MW, Su Q, Burdick
MJ, Lee J, Sundaresan T, Pastorino S, Park JK, Mikolaenko I, et al:
Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1,
is critical for glioma cell survival and proliferation. Cancer Res.
65:2353–2363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li BY, Ma CG, Zhao HY, Zhao CL, Xu QY and
Gao FL: Construction of eukaryotic expression vector containing rat
Hes1 gene and expression in neural precursor cells for the study of
its differentiation functions. Acta Anatomica Sinica. 40:187–192.
2009.
|
|
19
|
Qian DH, Wu XJ, Jiang H, Kuang CY, Wang K,
Song MB and Huang L: Construction of eukaryotic vector expressing
Jagged1 gene and its effect on proliferation and apoptosis of
vascular smooth muscle cells. J Third Mil Med Univ. 33:1095–1098.
2011.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steinhardt AA, Gayyed MF, Klein AP, Dong
J, Maitra A, Pan D, Montgomery EA and Anders RA: Expression of
Yes-associated protein in common solid tumors. Hum Pathol.
39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT,
Zender L, Lowe SW, Poon RT and Luk JM: Yes-associated protein is an
independent prognostic marker in hepatocellular carcinoma. Cancer.
115:4576–4585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dohda T, Maljukova A, Liu L, Heyman M,
Grandér D, Brodin D, Sangfelt O and Lendahl U: Notch signaling
induces SKP2 expression and promotes reduction of p27Kip1 in T-cell
acute lymphoblastic leukemia cell lines. Exp Cell Res.
313:3141–3152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bridges E, Oon CE and Harris A: Notch
regulation of tumor angiogenesis. Future Oncol. 7:569–588. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yi HB, Shi SS, Yang WZ and Chen CM: The
expression and significance of notch-1 gene in human gliomas.
Cancer Res Prev Treat. 33:701–703. 2006.(In Chinese).
|
|
26
|
Wang LX and Hua ZC: Research progress of
the Notch signaling pathway. Chin Med Biotechnol. 4:224–226.
2009.(In Chinese).
|
|
27
|
Reedijk M, Odorcic S, Chang L, Zhang H,
Miller N, McCready DR, Lockwood G and Egan SE: High-level
coexpression of JAG1 and NOTCH1 is observed in human breast cancer
and is associated with poor overall survival. Cancer Res.
65:8530–8537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cantarini MC, de la Monte SM, Pang M, Tong
M, D'Errico A, Trevisani F and Wands JR: Aspartyl-asparagyl beta
hydroxylase over-expression in human hepatoma is linked to
activation of insulin-like growth factor and notch signaling
mechanisms. Hepatology. 44:446–457. 2006. View Article : Google Scholar : PubMed/NCBI
|