Introduction
Autologous nerve grafting has long been considered
the gold standard for peripheral nerve defect repair (1). However, certain factors including donor
material limitations, functional limitations of the donor zone,
sensory axon dislocation growth, regeneration of axon dispersion,
and requirements for immunosuppression therapy severely restrict
its clinical application (2). Due to
these limitations, there is an urgent need to find an alternative
approach for repairing nerve defects and optimizing functional
recovery of the injured nerve. Several techniques and materials
have been tested, and one alternative was found using allografts
(3). Nerve conduits made from either
natural or synthetic materials are complex, having specific
demerits in their three-dimensional structure and biological
activity (4). These nerve conduits
have been reported to be the most promising method for bridging
injured peripheral nerves (5–9). For
example, Meek (10) used a
polyglycolic acid nerve conduit to treat 136 patients with nerve
damage. The patients expressed that the restoration process was
better than end-to-end nerve grafting; however, the repair was
limited to <3 cm in length. Suzuki et al (11) used a freeze-dried alginate conduit to
repair a 50-mm cat sciatic nerve defect. Postoperative histological
examination revealed newly generated nerve bundles, and the nerve
conduit was completely degraded.
Schwann and neural stem cells have an important role
in the repair and regeneration of peripheral nerve injury (12,13).
Neural stem cells are able to proliferate and differentiate into
neurons, astrocytes, and oligodendrocytes in in vitro and
in vivo transplantation conditions (14). Schwann cells secrete a variety of
nerve growth factors, neurotrophic factors, and neurite growth
factors, providing nutrition to the nerve and promoting axonal
regeneration, and so are widely used in experimental studies of
nerve repair (15,16). A pure neural stem cell culture in
vitro experiment found that although neural stem cells are able
to differentiate into neural cells, the majority differentiate into
oligodendrocytes and astrocytes, with few becoming neurons
(17). One study using rat neural
stem cells co-cultured with Schwann cells in vitro reported
that both symbiotic and Schwann cells promote neural stem cells to
differentiate into neuron-like cells (18). It has been speculated that this may
be due to the interaction of several neurotrophic factors that are
secreted by Schwann cells, including nerve growth factor,
brain-derived neurotrophic factor (BDNF), glial cell-derived
neurotrophic factor and basic fibroblast growth factor (19–21). Guo
et al (22) reported that
NT-3-modified Schwann cells co-transplanted with neural stem cells
were better able to promote neural survival and axonal regeneration
of spinal cord injuries compared with simple transplantation of
Schwann or neural stem cells alone. Clinically, the repair of
injured nerves requires neural stem cells to differentiate into
neurons more often than usual and also that well-differentiated
neurons survive and grow quickly prior to glial cells
proliferation, breaking through the injured area, and establishing
contact with the surrounding nerve cells (23). Schwann cells are able to secrete a
variety of neurotrophic factors that induce axons to build, extend,
and inhibit glial scar formation (24). Furthermore, Schwann cells promote
injured nerves to repair the structures and functions of tissues,
and so co-transplanting them together with neural stem cells may be
beneficial for repairing peripheral nerve injuries.
Some experiment results have demonstrated that the
transplantation of Schwann and neural stem cells has promising
effects for the treatment of central nervous system injuries
(25,26). Xia et al (25) cultured two types of cells into a
directional PLGA scaffold and transplanted it into a spinal cord
hemisection in a rat model. The results demonstrated that the
scaffolds provided a good environment for the regeneration of
neural stem cells and promoted the regeneration of axons, myelin
formation, and recovery of motor function. Chen et al
(26) reported that transplanted
neural stem cells were able to survive and migrate up to 24 weeks
following rat spinal cord injury, and were able to differentiate
into various neural cells. Co-transplantation of cells/PLGA
promotes the functional recovery of the injured spinal cord
(26). The effect of
co-transplanting neural stem cells and Schwann cells with PLGA is
better than transplanting neural stem cells combined PLGA alone
(26).
Based on these previous studies, it was presumed
that nerve conduits co-cultured with Schwann and neural stem cells
were able to promote the regeneration of recurrent laryngeal nerve
(RLN) injuries. To test the feasibility of this hypothesis, the
laminin-chitosan-PLGA nerve conduit was combined with Schwann and
neural stem cells to bridge injured laryngeal nerves in SD rats and
assess the regeneration of nerve structures and functions at
different time points.
Materials and methods
Experimental animals
A total of 132, 40-day-old female Sprague Dawley
(SD) rats (weighing ~150 g), provided by the Laboratory Animal
Center of the First People's Hospital of Shanghai Jiao Tong
University (Shanghai, China), were used to establish an animal
model of laryngeal nerve injury and were randomly divided into six
groups (n=22 in each): Co-culture of neural stem cells and Schwann
cells with a laminin-chitosan-PLGA nerve conduit (CO); Schwann
cells with a nerve conduit (SC); neural stem cells with a nerve
conduit (NSC); nerve conduit (NULL); autologous nerve grafts
(AUTOGRAFT); and sham operation (SHAM). Rats were maintained under
a 12-h light/dark cycle and were provided with standard mouse chow
and water ad libitum. The temperature was maintained at
18–23°C and humidity at 40–70%. All rat experiment protocols were
approved by the Ethics Committee of Shanghai General People's
Hospital, affiliated to the Shanghai Jiao Tong University School of
Medicine (Shanghai, China). All surgical procedures were performed
under aseptic conditions following general anesthesia
administration via an intraperitoneal injection of 10% choral
hydrate (400 mg/kg; Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China).
Cell culture
A total of 10 3-to 5-day-old Wistar rats (1:1 sex
ratio) were provided by the Laboratory Animal Center of the First
People's Hospital of Shanghai Jiao Tong University (~8 g) and
housed under a 12-h light/dark cycle with standard mouse chow and
water ad libitum. The temperature was maintained at 18–23°C
and humidity at 40–70%. Rats were sacrificed by decapitation and
the outer membrane of the sciatic nerve was gently removed under a
microscope (Leica Microsystems GmbH, Wetzlar, Germany;
magnification, ×10), and ophthalmic scissors were used to cut the
outer membrane into pieces. The enzyme digestion method was used
for the primary culture (27). The
culture was then purified to the second generation and identified
by S100 staining at room temperature for 2 h (Dako; Agilent
Technologies, Inc., Santa Clara, USA) as previously described
(27).
Caesarean sections were performed on 2 SD rats
(weight, ~220 g) provided by the First People's Hospital of
Shanghai Jiao Tong University Laboratory Animal Center on
gestational day 14. Rats were maintained under a 12-h light/dark
cycle and were provided with standard mouse chow and water ad
libitum. The temperature was maintained at 18–23°C and humidity
at 40–70%. The fetuses were obtained and decapitated, the
hemispheres were separated and the olfactory bulb was removed.
Diencephalon, cerebellum, and stripped vascular membrane were
observed under a inverted microscope (magnification, ×20). The left
cerebral cortex and the hippocampus of both sides were placed in a
15-ml centrifuge tube containing neural stem cells culture medium
[NSCM; DMEM/F12 (1:1; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA)], 2% B-27 Supplement Minus AO (Invitrogen; Thermo
Fisher Scientific, Inc.) 20 ng/ml epidermal growth factor, 20 ng/ml
basic fibroblast growth factor (both PeproTech, Inc., Rocky Hill,
NJ, USA), 50× L-glutamine (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and 1% penicillin-streptomycin] and the contents were
transferred to another 15-ml centrifuge tube and blown into
single-cell suspension. The contents were subsequently filtered by
a 40-µl mesh filter and seeded in the NSCM at a density of
1×105 cells/ml in a 25T bottle. NSCM was changed when
cells had been incubated at 37°C in an incubator containing 5%
CO2 for 2 days. The neural stem cells were identified
using nestin (BD Pharmingen; BD Biosciences, Franklin Lakes, NJ,
USA) (27).
Nerve conduit preparation
Chitosan-coated PLGA conduits were supplied by
Donghua University (Shanghai, China). In the present study, 10
nanofiber filaments were built in the nerve conduit of 0.6-mm inner
diameter, 0.2-mm tube wall thickness, and 2-cm length.
A total of 3.5% shell syrup was used because of its
low viscosity and ability to penetrate into the yarn. The
composition of the chitosan syrup was as follows: 3.5% chitosan
(BBI Life Sciences, Shanghai, China), 4% acetic acid, and 92.5%
distilled water. Both ends of the 11-cm-long fabric conduit were
fixed by two metal clips, which were immersed in a 0.1% chitosan,
for 30 min at room temperature. The surface of the nerve conduit
was gently brushed with a fine brush. Excess shell syrup was
subsequently removed, and the nerve fabric conduit was dried at
room temperature. The fabric was shaped in an oven at 70°C for 15
min (Changzhou Textile Instrument Factory Co., Ltd., (Changzhou,
China). When the coating was dry and fixed, the core axis of the
conduit was gently drawn out, the conduit was cut into 7-mm
fragments, as per the requirements of the experiment, and
disinfected. The conduits were stored in a sealed pack, and
preserved at 0°C in a refrigerator.
Chitosan-PLGA tubes were soaked in the PEI solution
(1 mg/ml) for 20 min, and rinsed in running water twice.
Subsequently, the tubes were soaked in the laminin (LN) solution
(0.2 mg/ml) for another 20 min and rinsed in running water twice.
Thus, a double layer of polyethyleneimine/LN (PEI/LN) film was
formed. Repeating these steps allowed the formation of a multilayer
PEL/LN film on the surface of the PLGA tubes. To maintain the
activity of LN, the whole process was carried out in an ice bath
(0°C).
Surgical procedure
A bilateral incision was made in the right RLN of
all 132 SD rats that had previously been divided into six groups:
CO, SC, NSC, NULL, AUTOGRAFT and SHAM. All rats were subsequently
administered with an intraperitoneal injection of 10% chloral
hydrate (300 mg/kg) under sterile conditions. Each rat was
subsequently placed on the operating table and neck hair was
removed using a razor. The skin in the surgical area was
disinfected and, following the midline on the neck, a 2-cm-long
incision was made using a scalpel, and the skin and subcutaneous
tissue were exposed. Anterior muscles were dissected using a curved
hemostat to expose the larynx and tracheal rings. Subsequently, ~1
cm RLN, situated in the tracheoesophageal groove, was exposed and
disassociated. Microsurgical scissors were used to cut 5-mm-long
segments of the middle disassociated nerve.
In the CO group, the distal end of the nerve segment
was plugged into the laminin-chitosan-PLGA nerve conduit up to 1
mm. The nerve conduit and nerve segment were subsequently sutured
using 10-0 sutures. A mixture of 22.5 µl Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA), 3.75 µl Schwann cells
(~0.083×106 cells), and 3.75 µl neural stem cells
(~0.083×106 cells) was injected into the nerve conduit.
The proximal end of the nerve was treated in the same manner to
give a 5-mm distance between the two broken ends of the
conduit.
In the SC group, the same method was used as in the
CO group. The only change was that a mixture of 22.5 µl Matrigel
and 7.7 µl Schwann cells (~0.167×106 cells) was injected
into the nerve conduit. In the NSC group, the same method was used
as in the CO group. The only change was that a mixture of 22.5 µl
Matrigel and 7.7 µl neural stem cells (~0.167×106) was
injected into the conduit. In the NULL group, only 30 µl Matrigel
was injected into the conduit. In the AUTOGRAFT group, the proximal
and distal ends of the 5-mm nerve segment were swapped, and their
corresponding nerve adventitia sutured. In the SHAM group, the RLN
was located along the tracheoesophagea after dissection and no
further procedures were performed.
Electrophysiological examination
A total of 10 SD rats from each group were used for
this analysis at the 8th and 12th week post-surgery. Following the
induction of anesthesia (10% chloral hydrate), an anterior midline
incision was made and exposed, and the right RLN was freed. The
stimulation electrode (bipolar stimulation electrode) was hooked in
the proximal end of the regenerated nerve, and the recording
electrode was inserted into the middle of the thyroarytenoid muscle
via the cricothyroid membrane. Waveforms were recorded using a
Medtronic Keypoint electromyography machine (Medtronic,
Minneapolis, MN, USA), and the latency and amplitudes were
calculated and compared.
Electron microscopy examination
One SD rat was randomly selected from each group at
8 and 12 weeks post-surgery for toluidine blue staining and
transmission electron microscopy analysis. Following anesthesia
(10% chloral hydrate), the intermediate segment of the regenerated
nerve was cut and fixed in 2.5% glutaraldehyde at 4°C for 2 h.
Subsequently, the segment was dehydrated in graded ethanol and
embedded in epoxy resin. Later, the epoxy-embedded tissue was cut
into ultrathin sections (1 µm) using citrate staining (H7650;
Hitachi Ltd., Tokyo, Japan), and the thickness of the myelin sheath
and the diameter of the myelinated nerve fiber were measured using
an electron microscope. All images were processed using IPP
software (version 6.0; Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation and all statistical analyses were performed using
GraphPad Prism software (version 6.0; GraphPad Software, Inc., La
Jolla, CA, USA). Unpaired t-test with equal standard deviation was
used to test the differences between two samples in a group at
various time points. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell culture
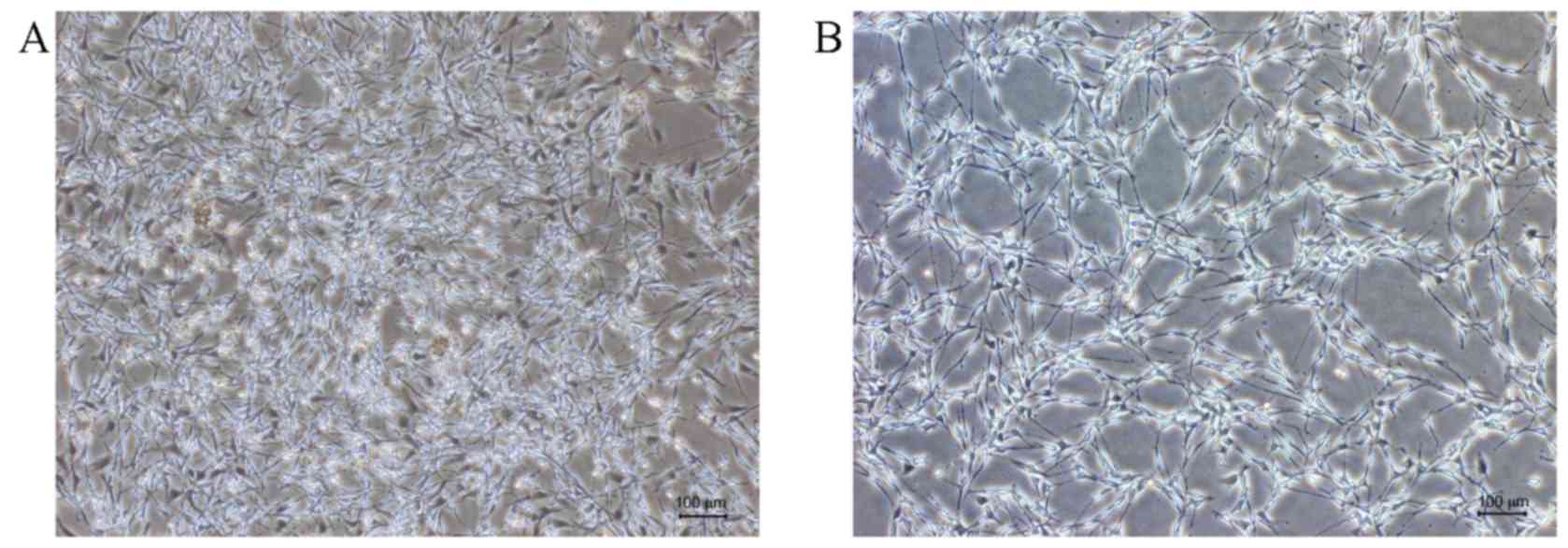
Previous in vitro results revealed that
Schwann cells were S100-positive (green), and fibroblasts revealed
only blue DAPI staining (14,28–30).
When passaged to P2 generation, only Schwann cells were visible
with few to no fibroblasts (Fig. 1).
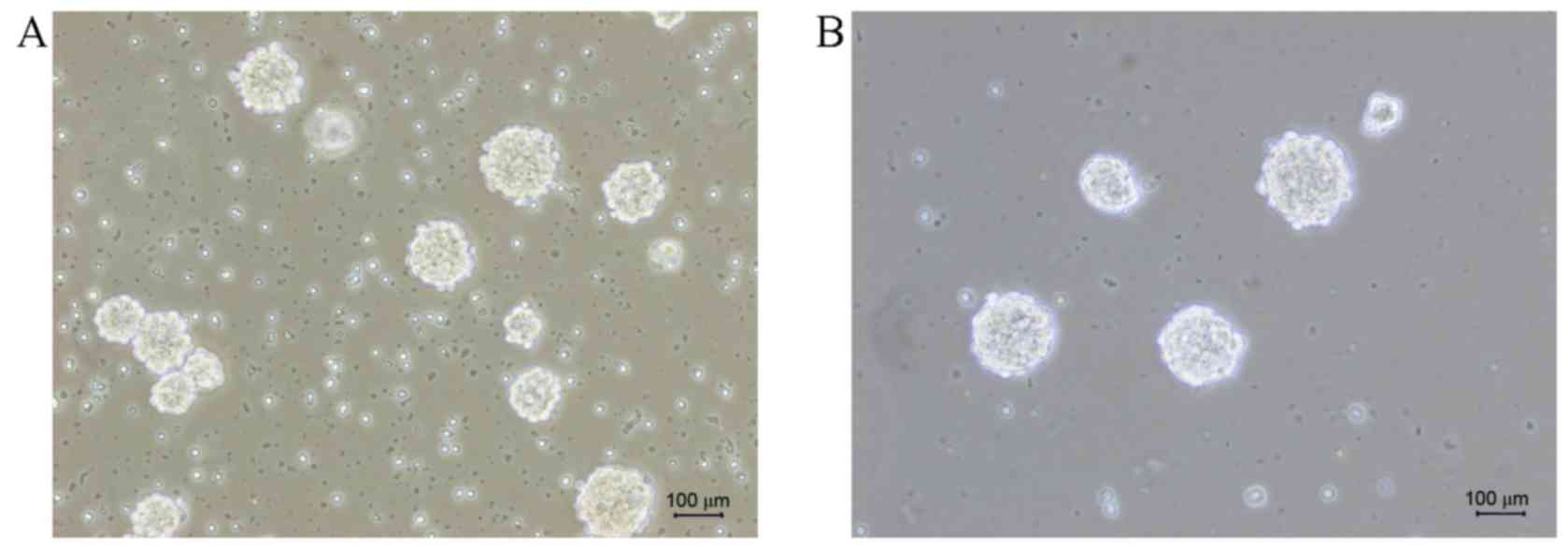
The P2 generation of neural stem cells was nestin-positive (red)
under the microscope, and it can be observed that neural stem cells
formed suspended spheres with nonadherent growth (Fig. 2). These indicated that the
cultivation of Schwann and neural stem cells were successful.
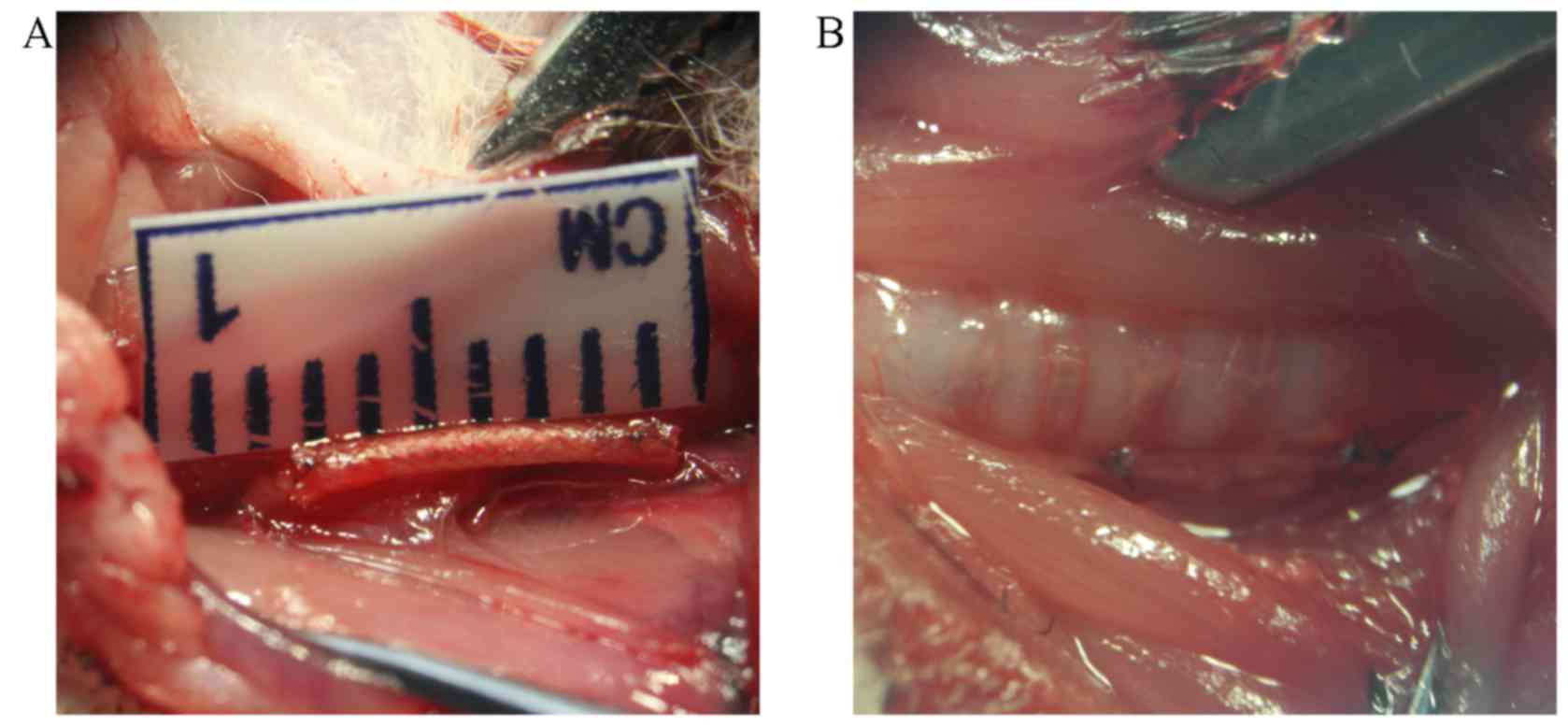
Animal model
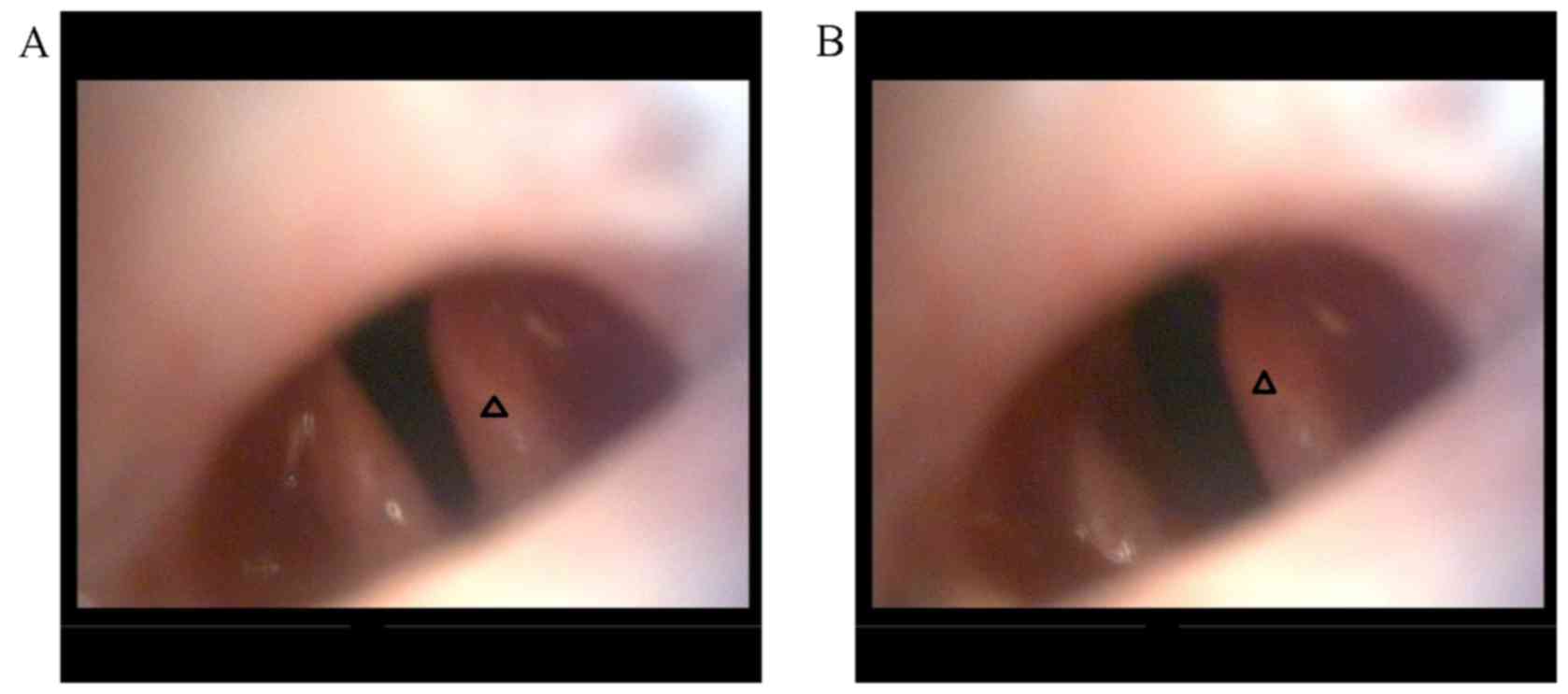
Surgery was successful in all groups (Fig. 3). The immediate post-surgical
laryngoscopy revealed that the vocal cord on the operative side was
immovable, whereas the other side had good movement (Fig. 4), indicating successful establishment
of animal models. All experimental animals survived without any
complications, including infections. Surgical wounds healed
well.
Nerve conduit
Surgical areas were re-exposed at 8 and 12 weeks
post-surgery to observe the RLN conduit bridging defects. At 8
weeks, the catheter appeared thinner in all the experimental groups
with some meager vascular membranes observed on the surface of the
nerve conduit. No adhesion was observed between the conduit and the
surrounding muscles.
At week 12, the nerve conduit in the CO group was
wrapped with fibrous connective tissues, with no adhesion to
surrounding tissue. The conduit became thinner, with newly formed
blood capillaries on its surface. The connection of the nerve
tissues was intact. Incising the conduit longitudinally revealed
newly generated RLN connecting the proximal nerve to the distal
nerve ends. The diameter of the middle part of the regenerated
conduit was observed to be slightly smaller than the normal nerve.
No obvious scars or swelling were observed on the nerve connection,
and nerve conduit body neoplasia was not observed.
No significant atrophy was found in the muscle and
muscle thyroarytenoid after freeing the throat body. Local swelling
was observed in the neural stem cell group, which was surrounded by
fibrous connective tissue. Mild adhesion was observed in the
autograft group and nerve anastomosis was smooth without any
swelling. The transplanted nerve was intact and had a soft
texture.
Electrophysiological evaluation
Thyroarytenoid muscle electromyography (EMG) test
results at 8 and 12 weeks post-surgery are presented in Figs. 5–8.
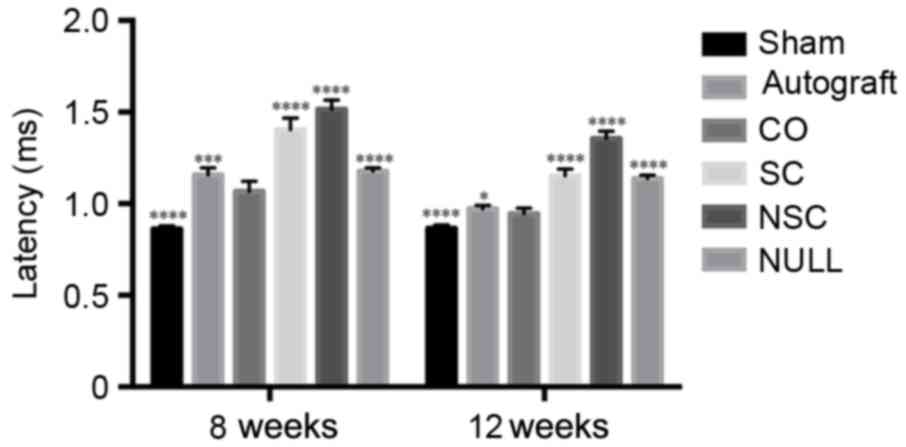
The amplitude of the CO group was lower compared with the SHAM
group (P<0.0001; Fig. 7);
however, it was significantly higher than all other groups
(P<0.01; Fig. 7). The amplitude
of CO group at 12th week recovered to 70% of that of the SHAM
group. At weeks 8 and 12, the CO group had a longer latency period
(P<0.0001; Fig. 8) compared with
the SHAM group and a shorter period compared with all other groups
(P<0.05; Fig. 8).
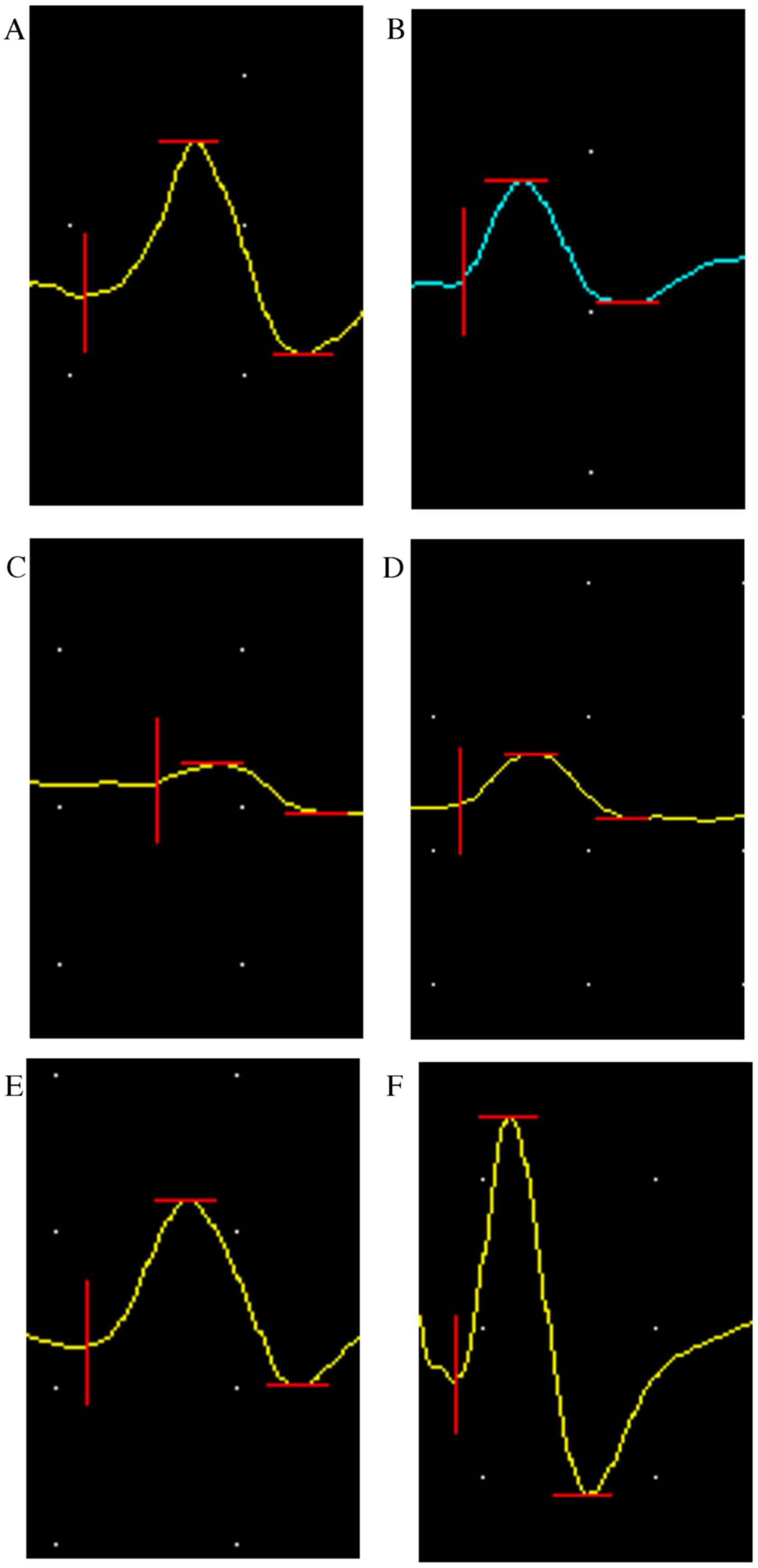
 | Figure 8.Latency of SHAM, AUTOGRAFT, CO, SC,
NSC, and NULL groups was calculated and statistically analyzed 8
and 12 weeks post-surgery, respectively. *P<0.05, ***P<0.001
and ****P<0.0001 vs. CO group. CO, co-culture of neural stem
cells and Schwann cells with a
laminin-chitosan-poly(lactic-co-glycolic acid) nerve conduit; SC,
Schwann cells with a nerve conduit; NSC, neural stem cells with a
nerve conduit; NULL, nerve conduit; AUTOGRAFT, autologous nerve
grafts; SHAM, sham operation. |
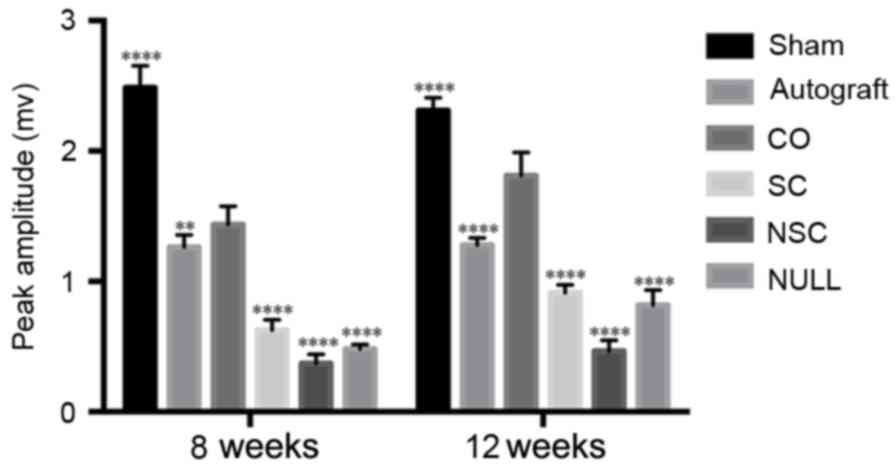 | Figure 7.Peak amplitudes of SHAM, AUTOGRAFT,
CO, SC, NSC, and NULL groups were calculated and statistically
analyzed at 8 and 12 weeks post-surgery. **P<0.01 and
****P<0.0001 vs. CO group. CO, co-culture of neural stem cells
and Schwann cells with a laminin-chitosan-poly(lactic-co-glycolic
acid) nerve conduit; SC, Schwann cells with a nerve conduit; NSC,
neural stem cells with a nerve conduit; NULL, nerve conduit;
AUTOGRAFT, autologous nerve grafts; SHAM, sham operation. |
Electron microscopy
The electron microscopy examination results at 8 and
12 weeks post-surgery are displayed in Figs. 9 and 10. At 8 weeks post-surgery, a large number
of regenerated nerve fibers were observed in the CO group. They
were thick with a thick myelin sheath and had less connective
tissue between the beams. The regenerated myelin sheath matured
well with consistent thickness. Regenerated axons also developed
well and were arranged in an orderly manner. Regenerated nerve
fibers in the SC and NULL groups were smaller; they were scattered,
twisted, and irregular, and the myelin sheath was thinner. A large
amount of connective tissue, inflammatory cells, fragmentation of
nuclei, and unabsorbed Matrigel were observed in the conduit of the
NSC group. However, no significant newly generated myelin was
observed. The regenerated myelin sheath was markedly thicker in the
CO group than in other groups; however, the sheath was thinner in
the CO group compared with the AUTOGRAFT and SHAM groups.
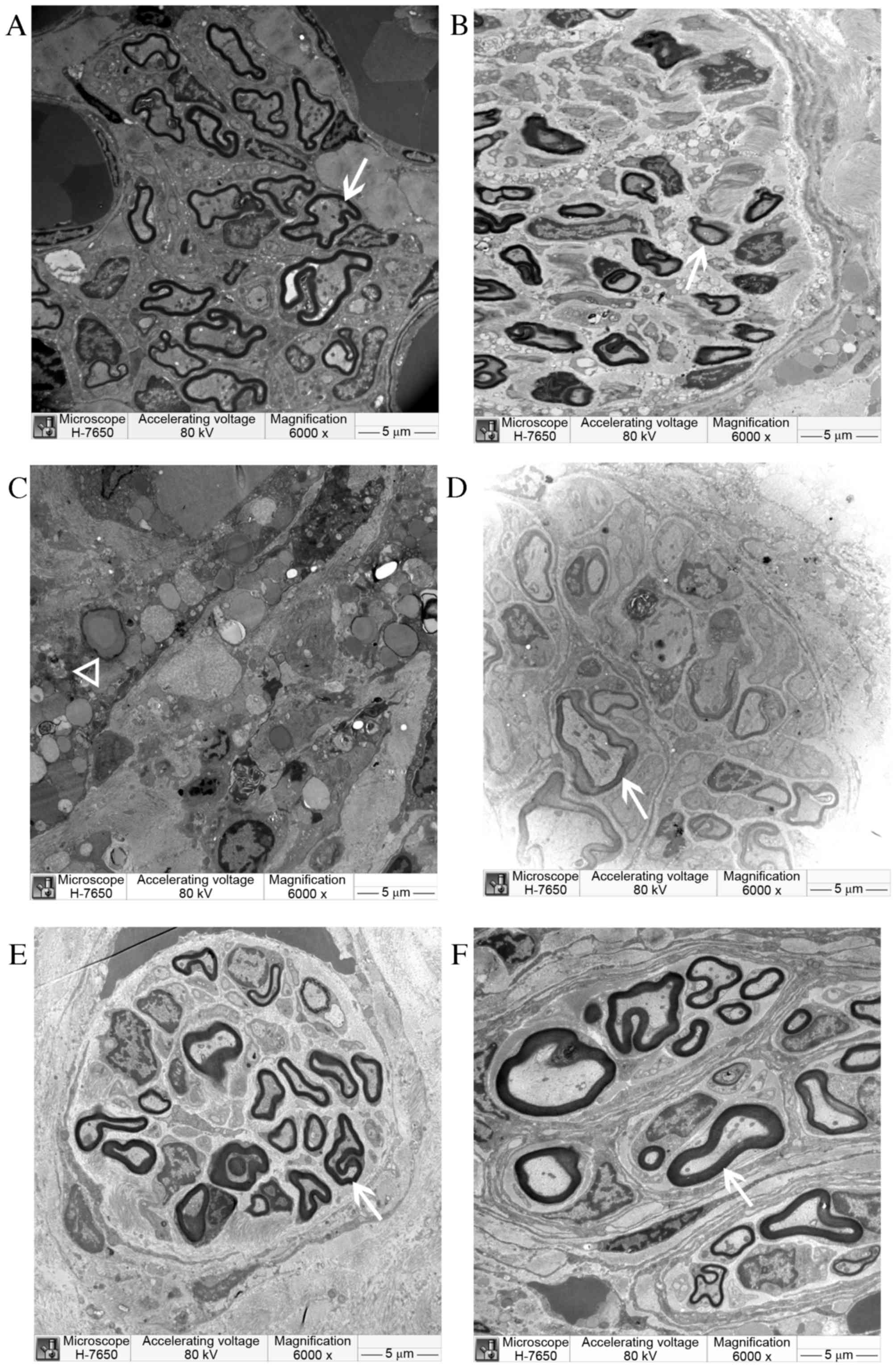 | Figure 9.Semi-thin cross-sections of
regenerating laryngeal nerves of (A) CO, (B) SC, (C) NSC (D) NULL,
(E) AUTOGRAFT and (F) sham operation groups under electron
microscopy at 8 weeks post-surgery. New myelin sheath (arrow) was
observed under an electron microscope in CO, SC, NULL and AUTOGRAFT
groups. In the NSC group, unabsorbed Matrigel (triangle) was
observed, but no myelin sheath. Magnification, ×6,000. CO,
co-culture of neural stem cells and Schwann cells with a
laminin-chitosan-poly (lactic-co-glycolic acid) nerve conduit; SC,
Schwann cells with a nerve conduit; NSC, neural stem cells with a
nerve conduit; NULL, nerve conduit; AUTOGRAFT, autologous nerve
grafts. |
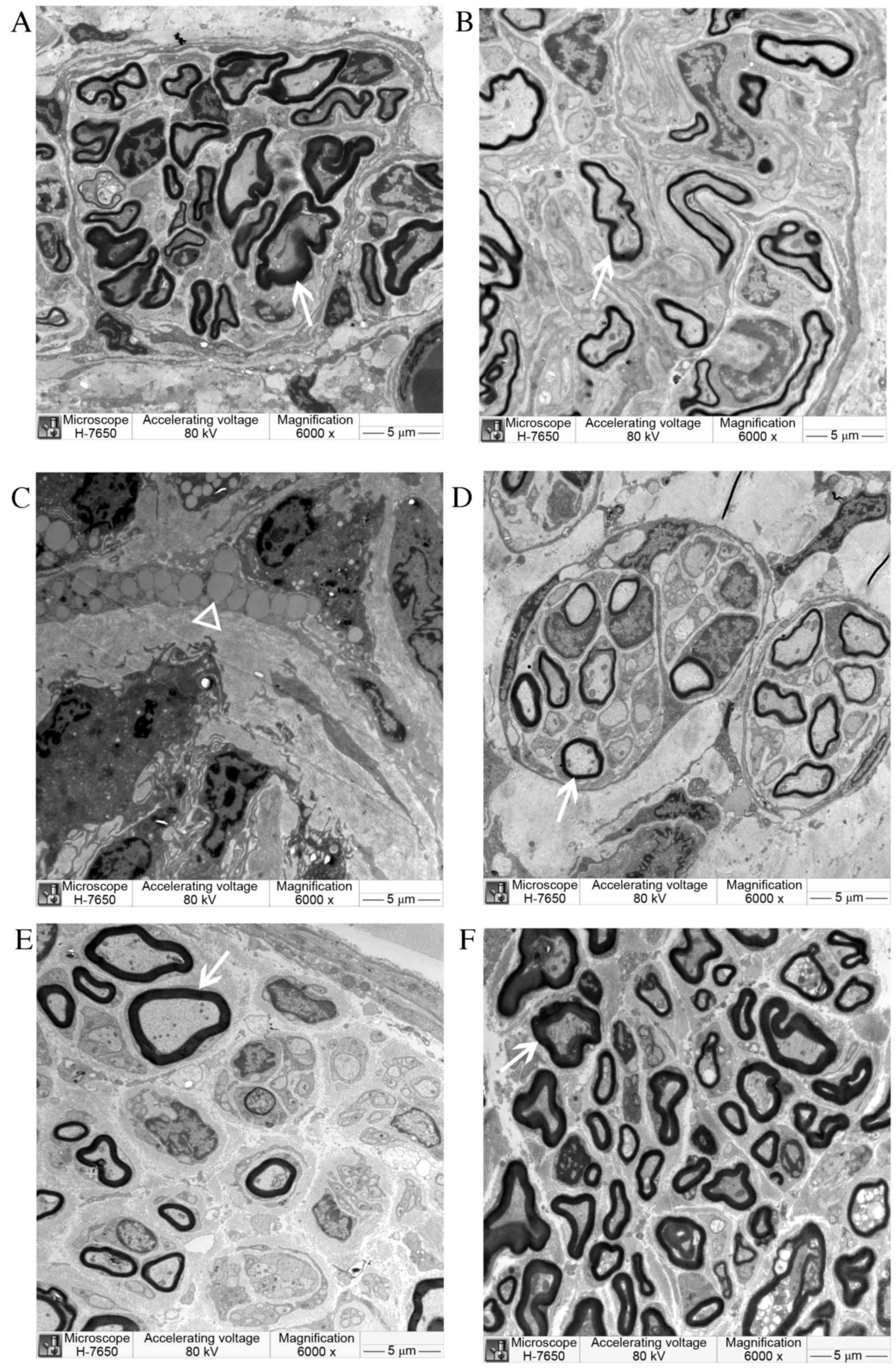 | Figure 10.Semi-thin cross-sections of
regenerating laryngeal nerves of (A) CO, (B) SC, (C) NSC (D) NULL,
(E) AUTOGRAFT and (F) sham operation groups under electron
microscopy at 12 weeks post-surgery. The newly regenerated myelin
sheath (arrow) was observed in the CO, SC, NULL and AUTOGRAFT
groups. In the NSC group, only unabsorbed Matrigel (triangle) was
observed. Magnification, ×6,000. CO, co-culture of neural stem
cells and Schwann cells with a
laminin-chitosan-poly(lactic-co-glycolic acid) nerve conduit; SC,
Schwann cells with a nerve conduit; NSC, neural stem cells with a
nerve conduit; NULL, nerve conduit; AUTOGRAFT, autologous nerve
grafts. |
Discussion
To resolve peripheral nerve defects, various
materials including collagen, silk, cellulose, veins, muscles, and
other manufacturing nerve conduits have been used to bridge nerve
damage; however, no effective substitute for nerve graft has been
identified that can be widely used in clinics (31). The laminin-chitosan-PLGA nerve
conduits used in the present study were made by the Donghua
University. The specific thermal setting process made the structure
more stable, the built-in nanofiber filaments supported the
conduit, and the conduits had a certain compressive strength and
elasticity. Preliminary results demonstrated that
laminin-chitosan-PLGA exhibited good adhesion with Schwann and
neural stem cells. LN protein is one of the main components of the
extracellular matrix (32).
Recently, a number of studies in the field of organ development
have reported that LN protein is able to induce differentiation of
embryonic stem cells and neural stem cells (33). This suggests that
laminin-chitosan-PLGA conduit is a good choice for repairing
peripheral nerve damage.
Previous experimental results have demonstrated that
EMG is useful for evaluating the degree of nerve regeneration
(34), as the amplitude correlates
with the number of muscle fibers. If a nerve is injured, some nerve
fibers will be unable to transmit the nerve impulse, and the
amplitude and latency will be affected (35–38). The
waveform amplitude was proportional to the degree of damage
following injury to the RLN; that is, the more serious the nerve
injury, the smaller the amplitude, to the point where no waveform
is observed. However, the latency is inversely proportional to the
degree of nerve damage. The present experimental results
demonstrated that the amplitude was significantly higher in the CO
group compared with other groups, with the exception of the SHAM
group. Furthermore, latency was significantly shorter in the CO
group compared with all other groups, except for the SHAM group.
These results suggest that nerve recovery was superior in the CO
group when compared with the SC, NSC, NULL, and AUTOGRAFT
groups.
In a separate neural stem cell + conduit group, a
large number of fibroblasts, unabsorbed Matrigel, inflammatory
cells, and fragmentation of cell nucleus caused by the death of
neural stem cells were observed. It has been reported that neural
stem cells have a low survival rate after transplantation in
vivo; An et al (39)
reported that Schwann cell secretions significantly support the
growth of human neural stem cells; however, if they lose the
support of Schwann cell secretions, nerve stem cells gradually die.
This suggests that the demand for neural stem cells on local
micro-environmental requirements is higher, and cultured alone they
may easily die. Schwann cells in the CO group are able to secrete
neurotrophic factors that prevent neural stem cell death and induce
differentiation into neurons.
The results of the present study suggest that
Schwann and neural stem cells co-cultured and transplanted with a
nerve conduit are effective at repairing RLN injuries in rats. The
conduit provides a good microenvironment for planted nerve cells
and promotes axonal regeneration and myelination.
Before these findings can be applied clinically,
there are some limitations to be addressed. Firstly, the approach
of establishing animal models and assessing the regenerated nerve
varies and lacks uniform standards. This makes it difficult to
directly compare the results of different studies. Furthermore,
obtaining enough Schwann cells of high purity, with high biological
activity, no immune rejection, and limited proliferation is
difficult. The in vitro culture, amplification, and
purification of Schwann cells is complicated, and they have been
reported to rapidly lose their phenotypic characteristics (40–49). As
the passage number increases, the form and function of Schwann
cells may change significantly. The results of the present study
demonstrated that various types of stem cells have the potential
ability to differentiate into Schwann-like cells and may assist in
peripheral nerve regeneration. For example, adipose tissue-derived
stem cells, skin mesenchymal precursors, human umbilical
cord-derived mesenchymal stem cells, embryonic stem cell-derived
neural crest cells, human embryonic stem cell-derived neurospheres,
and amniotic mesenchymal stem cells and mesenchymal stem cells have
all been reported to have this ability (40–49). A
study by Dezawa (50) revealed that
bone marrow stromal cells can be induced and differentiated into
Schwann cells, promoting regeneration of the peripheral nervous
system in a rat model. Based on these reports, stem cells are
expected to become an important source of Schwann cells.
At present, many studies are in the experimental
stage using animal models, and there is a big difference between
in vitro and in vivo experiments. Zhang et al
(51) observed that when Schwann and
neural stem cells differentiated into nerve cells, morphological
and functional detection demonstrated good results; however, in
in vivo experiments they found that the in vitro
pre-induction only slightly promoted the differentiation of neural
stem cells. Therefore, to apply the results obtained from animal or
in vitro experiments in a clinical setting, further research
is required.
Although autologous nerve grafting is still an
option, the use of a nerve conduit with co-cultured neural stem
cells and Schwann cells was found to be superior in terms of the
electrophysiological recovery and myelin sheath thickness of the
regenerated nerve. If other neurotrophic factors were added to
future experiments and the suturing techniques were improved, nerve
repair may continue to advance. Further testing should also be
utilized, such as using immunofluorescence, using reverse
transcription polymerase chain reaction to detect BDNF RNA
expression of the regenerated nerve and muscle, and observing vocal
movement using a laryngoscope. To the best of our knowledge,
cellular and molecular therapies directed at peripheral nerve
repair have not yet advanced beyond the laboratory stage, and their
translation to a clinical setting has been beset with challenges,
such as the type and quantity of cells or factors and their
delivery, cell viability or factor activity, cell phenotypic
stability, timing of treatment, regulatory issues, and high costs
(52). Although current clinical
tissue engineering technology has not fully replaced autologous
nerve graft and nerve stump anastomosis, with further research,
tissue engineering in the field of neural defects may offer wider
clinical applications.
In conclusion, the laminin-chitosan-PLGA nerve
conduit combined with co-transplantation of Schwann and neural stem
cells was found to effectively promote rat RLN regeneration in the
present study, both by guiding the regenerated axons and
contributing cells to the reconstruction.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81170925). The authors would
like to thank Donghua University, College of Textiles for their
technical support.
References
|
1
|
Zhu L, Liu T, Cai J, Ma J and Chen AM:
Repair and regeneration of lumbosacral nerve defects in rats with
chitosan conduits containing bone marrow mesenchymal stem cells.
Injury. 46:2156–2163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sinis N, Haerle M, Becker ST,
Schulte-Eversum C, Vonthein R, Rösner H and Schaller HE: Neuroma
formation in a rat median nerve model: Influence of distal stump
and muscular coating. Plast Reconstr Surg. 119:960–966. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gu X, Ding F, Yang Y and Liu J:
Construction of tissue engineered nerve grafts and their
application in peripheral nerve regeneration. Prog Neurobiol.
93:204–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arslantunali D, Dursun T, Yucel D, Hasirci
N and Hasirci V: Peripheral nerve conduits: Technology update. Med
Devices (Auckl). 7:405–424. 2014.PubMed/NCBI
|
|
5
|
Siemionow M and Sonmez E: Nerve allograft
transplantation: A review. J Reconstr Microsurg. 23:511–520. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brooks DN, Weber RV, Chao JD, Rinker BD,
Zoldos J, Robichaux MR, Ruggeri SB, Anderson KA, Bonatz EE,
Wisotsky SM, et al: Processed nerve allografts for peripheral nerve
reconstruction: A multicenter study of utilization and outcomes in
sensory, mixed, and motor nerve reconstructions. Microsurgery.
32:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang P, Kilic A, Konopka G, Regalbuto R,
Akelina Y and Gardner T: Histologic and functional outcomes of
nerve defects treated with acellular allograft versus cabled
autograft in a rat model. Microsurgery. 33:460–467. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brya DJ, Holway H, Wang KK, Silva AE,
Trantolo DJ, Wise D and Summerhayes IC: Influence of glia growh
factor and Schwann cells in a bioresorbable guidance channel on
periphera nerve regeneration. Tissue Eng. 6:129–138. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Evans GR, Brandt K, Nidebichler AD,
Chauvin P, Herrman S, Bogle M, Otta L, Wang B and Patrick CW Jr:
Clinical long-term in vivo evaluation of poly(l-lactic aid) porous
conduits for peripheral nerve regeneration. J Bioater Sci Polym Ed.
11:869–878. 2000. View Article : Google Scholar
|
|
10
|
Meek MF: A randomized prospective study of
polyglycolic acid conduits for digital nerve reconstruction in
humans. Plast Reconstr Surg. 108:1087–1088. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki Y, Tanihara M, Ohnishi K, Suzuki K,
Endo K and Nishimura Y: Cat peripheral nerve regeneration across 50
mm gap repaired with a novel nerve guide composed of freeze-dried
alginate gel. Neurosci Lett. 259:75–78. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong MM and Yi TH: Stem cell and
peripheral nerve injury and repair. Facial Plast Surg. 26:421–427.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma MS, Boddeke E and Copray S: Pluripotent
stem cells for Schwann cell engineering. Stem Cell Rev. 11:205–218.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sher F, Rössler R, Brouwer N,
Balasubramaniyan V, Boddeke E and Copray S: Differentiation of
neural stem cells into oligodendrocytes: Involvement of the
polycomb group protein Ezh2. Stem Cells. 26:2875–2883. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ansselin AD, Fink T and Davey DF:
Peripheral nerve regeneration through nerve guides seeded with
adult Schwann cells. Neuropathol Appl Neurobiol. 23:387–398. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mimura T, Dezawa M, Kanno H, Sawada H and
Yamamoto I: Peripheral nerve regeneration by transplantation of
bone marrow stromal cell-derived Schwann cells in adult rats. J
Neurosurg. 101:806–812. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carpenter MK, Cui X, Hu ZY, Jackson J,
Sherman S, Seiger A and Wahlberg LU: In vitro expansion of a
multipotent population of human neural progenitor cells. Exp
Neurol. 158:265–278. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan H, An Y, Zhang Z, Zhang Y and Wang Z:
Differentiation of rat embryonic neural stem cells promoted by
co-cultured Schwann cells. Chin Med J (Engl). 116:428–431.
2003.PubMed/NCBI
|
|
19
|
Koh HS, Yong T, Chan CK and Ramakrishna S:
Enhancement of neurite outgrowth using nano-structured scaffolds
coupled with laminin. Biomaterials. 29:3574–3582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang YC, Huang CC, Huang YY and Chen KS:
Surface modification and characterization of chitosan or PLGA
membrane with laminin by chemical and oxygen plasma treatment for
neural regeneration. J Biomed Mater Res A. 82:842–851. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsumoto K, Ohnishi K, Kiyotani T, Sekine
T, Ueda H, Nakamura T, Endo K and Shimizu Y: Peripheral nerve
regeneration across an 80-mm gap bridged by a polyglycolic acid
(PGA)-collagen tube filled with laminin-coated collagen fibers: A
histological and electrophysiological evaluation of regenerated
nerves. Brain Res. 868:315–328. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo JS, Zeng YS, Li HB, Huang WL, Liu RY,
Li XB, Ding Y, Wu LZ and Cai DZ: Cotransplant of neural stem cells
and NT-3 gene modified Schwann cells promote the recovery of
transected spinal cord injury. Spinal Cord. 45:15–24. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan Hua: In vitro co culture and growth
characteristics of neural stem cells and Schwann cells in
combination with spinal cord injury. Med Univ Tianjin. 2003.
|
|
24
|
Madduri S and Gander B: Schwann cell
delivery of neurotrophic factors for peripheral nerve regeneration.
J Peripher Nerv Syst. 15:93–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia L, Wan H, Hao SY, Li DZ, Chen G, Gao
CC, Li JH, Yang F, Wang SG and Liu S: Co-transplantation of neural
stem cells and Schwann cells within poly (L-lactic-co-glycolic
acid) scaffolds facilitates axonal regeneration in hemisected rat
spinal cord. Chin Med J (Engl). 126:909–917. 2013.PubMed/NCBI
|
|
26
|
Chen G, Hu YR, Wan H, Xia L, Li JH, Yang
F, Qu X, Wang SG and Wang ZC: Functional recovery following
traumatic spinal cord injury mediated by a unique polymer scaffold
seeded with neural stem cells and Schwann cells. Chin Med J (Engl).
123:2424–2431. 2010.PubMed/NCBI
|
|
27
|
Yu Z, Men Y and Dong P: Schwann cells
promote the capability of neural stem cells to differentiate into
neurons and secret neurotrophic factors. Exp Ther Med.
13:2029–2035. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Filip S, Mokrý J, Karbanová J, Vávrová J,
Vokurková J, Bláha M and English D: The transplantation of neural
stem cells and predictive factors in hematopoietic recovery in
irradiated mice. Transfus Apher Sci. 32:157–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blakemore WF: The case for a central
nervous system (CNS) origin for the Schwann cells that remyelinate
CNS axons following concurrent loss of oligodendrocytes and
astrocytes. Neuropathol Appl Neurobiol. 31:1–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heath CA: Cells for tissue engineering.
Trends Biotechnol. 18:17–19. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kolar MK and Kingham PJ: Regenerative
effects of adipose-tissue-derived stem cells for treatment of
peripheral nerve injuries. Biochem Soc Trans. 42:697–701. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zilic L, Wilshaw SP and Haycock JW:
Decellularisation and histological characterisation of porcine
peripheral nerves. Biotechnol Bioeng. 113:2041–2053. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahmad I and Akhtar MS: Use of vein conduit
and isolated nerve graft in peripheral nerve repair: A comparative
study. Plast Surg Int. 2014:5879682014.PubMed/NCBI
|
|
34
|
Zheng H, Zhou S, Chen S, Li Z and Cuan Y:
An experimental comparison of different kinds of laryngeal muscle
reinnervation. Otolaryngol Head Neck Surg. 119:540–547. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Randolph GW and Dralle H: International
Intraoperative Monitoring Study Group, Abdullah H, Barczynski M,
Bellantone R, Brauckhoff M, Carnaille B, Cherenko S, Chiang FY,
et al: Electrophysiologic recurrent laryngeal nerve
monitoring during thyroid and parathyroid surgery: International
standards guideline statement. Laryngoscope. 121 Suppl 1:S1–S16.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chiang FY, Lee KW, Chen HC, Chen HY, Lu
IC, Kuo WR, Hsieh MC and Wu CW: Standardization of intraoperative
neuromonitoring of recurrent laryngeal nerve in thyroid operation.
World J Surg. 34:223–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu CW, Lu IC, Randolph GW, Kuo WR, Lee KW,
Chen CL and Chiang FY: Investigation of optimal intensity and
safety of electrical nerve stimulation during intraoperative
neuromonitoring of the recurrent laryngeal nerve: A prospective
porcine model. Head Neck. 32:1295–1301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chiang FY, Lu IC, Kuo WR, Lee KW, Chang NC
and Wu CW: The mechanism of recurrent laryngeal nerve injury during
thyroid surgery-the application of intraoperative neuromonitoring.
Surgery. 143:743–749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
An YH, Wan H, Zhang ZS, Wang HY, Gao ZX,
Sun MZ and Wang ZC: Effect of rat Schwann cell secretion on
proliferation and differentiation of human neural stem cells.
Biomed Environ Sci. 16:90–94. 2003.PubMed/NCBI
|
|
40
|
Banerjee A, Nürnberger S, Hennerbichler S,
Riedl S, Schuh CM, Hacobian A, Teuschl A, Eibl J, Redl H and
Wolbank S: In toto differentiation of human amniotic membrane
towards the Schwann cell lineage. Cell Tissue Bank. 15:227–239.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang TM, Yang ZJ, Kong CZ and Zhang HT:
Schwann-like cells can be induction from human nestin-positive
amniotic fluid mesenchymal stem cells. In Vitro Cell Dev Biol Anim.
46:793–800. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kingham PJ, Kalbermatten DF, Mahay D,
Armstrong SJ, Wiberg M and Terenghi G: Adipose-derived stem cells
differentiate into a Schwann cell phenotype and promote neurite
outgrowth in vitro. Exp Neurol. 207:267–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Krause MP, Dworski S, Feinberg K, Jones K,
Johnston AP, Paul S, Paris M, Peles E, Bagli D, Forrest CR, et al:
Direct genesis of functional rodent and human schwann cells from
skin mesenchymal precursors. Stem Cell Reports. 3:85–100. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee JH, Chung WH, Kang EH, Chung DJ, Choi
CB, Chang HS, Lee JH, Hwang SH, Han H, Choe BY and Kim HY: Schwann
cell-like remyelination following transplantation of human
umbilical cord blood (hUCB)-derived mesenchymal stem cells in dogs
with acute spinal cord injury. J Neurol Sci. 300:86–96. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pan Y and Cai S: Current state of the
development of mesenchymal stem cells into clinically applicable
Schwann cell transplants. Mol Cell Biochem. 368:127–135. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Razavi S, Mardani M, Kazemi M, Esfandiari
E, Narimani M, Esmaeili A and Ahmadi N: Effect of leukemia
inhibitory factor on the myelinogenic ability of Schwann-like cells
induced from human adipose-derived stem cells. Cell Mol Neurobiol.
33:283–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Reid AJ, Sun M, Wiberg M, Downes S,
Terenghi G and Kingham PJ: Nerve repair with adipose-derived stem
cells protects dorsal root ganglia neurons from apoptosis.
Neuroscience. 199:515–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ren YJ, Zhang S, Mi R, Liu Q, Zeng X, Rao
M, Hoke A and Mao HQ: Enhanced differentiation of human neural
crest stem cells towards the Schwann cell lineage by aligned
electrospun fiber matrix. Acta Biomater. 9:7727–7736. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ziegler L, Grigoryan S, Yang IH, Thakor NV
and Goldstein RS: Efficient generation of schwann cells from human
embryonic stem cell-derived neurospheres. Stem Cell Rev. 7:394–403.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dezawa M: Central and peripheral nerve
regeneration by transplantation of Schwann cells and
transdifferentiated bone marrow stromal cells. Anat Sci Int.
77:12–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang X, Zeng Y, Zhang W, Wang J, Wu J and
Li J: Co-transplantation of neural stem cells and
NT-3-overexpressing Schwann cells in transected spinal cord. J
Neurotrauma. 24:1863–1877. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gu Y, Zhu J, Xue C, Li Z, Ding F, Yang Y
and Gu X: Chitosan/silk fibroin-based, Schwann cell-derived
extracellular matrix-modified scaffolds for bridging rat sciatic
nerve gaps. Biomaterials. 35:2253–2263. 2014. View Article : Google Scholar : PubMed/NCBI
|