Introduction
Intrauterine infection in pregnant women is a common
disease in gynecology and obstetrics and also an important factor
leading to neonatal premature birth, resulting in ~40% of premature
infants (1), and it causes
hypoplasia of neonatal organ function, or even death (2). Ways leading to intrauterine infection
are mainly vaginal and cervical elevation, placental infection,
retrograde infection in abdominal cavity and invasive operation.
The most severe stage of intrauterine infection elevation is fetal
infection, causing neonatal sepsis or even death (3).
Preterm birth has serious adverse reactions on the
integrity of neonatal brain structure and function (4). A study showed that (5), gray matter and white matter volumes in
brains of premature infants are less than those in brains of
normally born infants, which may cause cognitive and neural defects
in pediatric patients when the pathology is severe. Increasing
number of people think that inflammation is a key factor for normal
development and damage outcome of immature brains. Perinatal
neuroinflammation can increase risks of nerve system diseases and
neuropsychiatric disorders in childhood and adult stage (6).
Micro-ribonucleic acid-182 (miR-182) is a highly
conserved polycistronic miR cluster located within the 5-kb region
of human chromosome 7q32.2. Studies have shown that (7,8) miR-182
is aberrantly expressed in multiple tumors, and is directly
involved in the occurrence and development of human cancers, but
the mechanism of action of miR-182 in tumors remains unclear. It
can act as an oncogene or tumor suppressor gene, depending on the
type, location and stage of the cancer.
The present study detected the expression level of
miR-182 in amniotic fluid of the pregnant women to explore its
associations with intrauterine infection and brain injury in
premature infants.
Subjects and methods
Subjects
A total of 257 premature infants delivered in
obstetrics department of Jinan Maternity and Child Care Hospital
from February 2015 to February 2017 were selected, including 140
male infants and 117 female infants, with an average time of
pregnancy termination of 34.1±2.5 weeks and a mean weight of
premature infants of 2.4±0.9 kg. Premature infants who had a time
of pregnancy termination of shorter than 35 weeks and whose mother
did not have preeclampsia or diabetes mellitus were included in
this study. This study was approved by medical Ethics Committee of
Jinan Maternity and Child Care Hospital (Shandong, China). Parents
of the patients signed the informed consent.
Methods
Main instruments and reagents used are listed in
Table I.
 | Table I.Main instruments and reagents. |
Table I.
Main instruments and reagents.
| Instrument and
reagent | Manufacturer |
|---|
| H&E kit | Beyotime, Shanghai,
China |
| SonoSite portable
color ultrasound | SonoSite Inc.,
Bothell, WA, USA |
| GE prospeed CT | Beijing Deanren
Technology Co., Ltd. (Beijing, China) |
| PCR
instrumentation | Bio-Rad Laboratories,
Inc., Hercules, CA, USA |
| TRIzol kit | Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA |
| TaqMan®
miR reverse transcription kit | Thermo Fisher
Scientific Inc., Beijing, China |
| Agarose | Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany |
| U6 internal reference
primer | GenScript, Jiangsu,
China |
H&E staining: Puerperal placental tissues were
collected from pregnant women and subjected to H&E staining.
After staining, extent of inflammatory response in samples was
observed under an optical microscope and expressed as leukocyte
infiltration degree.
Diagnostic basis of intrauterine infection (9): leukocyte infiltration 2+ or above;
heart rate of the pregnant woman >100 beats/min or fetal heart
rate >160 beats/min, body temperature of the pregnant woman
>37.5°C; leukocyte count >15×109/l; peculiar smell
was found in amniotic fluid; and there was uterine body tenderness
(positive).
Diagnostic basis of brain injury (10): imageological examination was used as
the diagnostic basis, and fetuses received craniocerebral magnetic
resonance imaging, computed tomography (CT), ultrasound diagnosis
of brain injury within one week after birth. Brain injury could be
considered if one of the above was met.
RNA extraction
TRIzol reagents were used for the extraction of
total RNA in amniotic fluid, and steps were carried out according
to instructions provided by Invitrogen (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). An ultraviolet spectrophotometer was
utilized to analyze the concentration and purity of the extracted
RNA, and 3% agarose gel electrophoresis was applied to analyze the
integrity of RNA. Total RNA was taken to synthesize complementary
deoxyribonucleic acid (cDNA) using the RT Revert Aid First Strand
cDNA Synthesis kit and Moloney Murine Leukemia Virus (M-MLV)
Reverse Transcriptase (both from Thermo Fisher Scientific,
Inc.)
cDNA synthesis
cDNA was synthesized with reverse transcriptase
based on relevant instructions. Reaction: 37°C for 45 min and 95°C
for 5 min. The product was stored at −20°C.
Quantitative PCR (qPCR)
The reaction system volume was in total 25 µl,
pre-denaturation at 95°C for 5 min, denaturation at 95°C for 30
sec, annealing at 60°C for 45 sec, extension at 72°C for 3 min,
with 35 cycles, and then extension at 72°C for 5 min. PCR products
were stored at 4°C. Upstream primer of miR-182 was
5′-TGCGGTTTGGCAATGGTAGAAC-3′, and its downstream primer was
5′-CCAGTGCAGGGTCCGAGGT-3′; U6 was used as the internal inference of
reaction. Quantitative analysis was carried out using the ABI 7500
fluorescence PCR amplification instrument (Applied Biosystems;
Thermo Fisher Scientific, Inc.). All samples were repeated on 3
wells and the results were analyzed using 2−ΔΔCq method
(11).
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 software package (IBM Corp., Armonk, NY, USA) was used to
analyze data obtained from this study. Chi-square test was used for
enumeration data. Measurement data were expressed as mean ±
standard deviation. Independent Student's t-test was employed for
data comparison between two groups. COX regression analysis was
utilized to analyze associations of miR-182 expression level with
intrauterine infection and brain injury in premature infants.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General information
A total of 257 premature infants born in Jinan
Maternity hospital from February 2015 to February 2017 were
selected. They were divided into infected group (n=113), with a
gestational age of 33.7±2.4 weeks and a body weight of 2.2±0.8 kg,
and uninfected group (n=144), with a gestational age of 34.5±3.3
weeks and a body weight of 2.8±0.7 kg based on pathological
diagnoses. There was no difference in sex between the groups
(P>0.05). There was no difference in sex, gestational age and
body weight between the two groups (P>0.05), and the IL-6 level,
heart rate and white blood cell count in the infected group were
significantly higher than those in the non-infected group
(P<0.05) (Table II).
 | Table II.Comparison of clinical data of
premature infants in infected group and uninfected group. |
Table II.
Comparison of clinical data of
premature infants in infected group and uninfected group.
| Clinical data | Infected group
(n=113) | Uninfected group
(n=144) | P-value |
|---|
| Sex
(male/female) | 62/51 | 65/79 | 0.568 |
| Mother's body
temperature (°C) | 38.1±0.2 | 37.2±0.3 | 0.824 |
| Gestational age
(weeks) | 33.7±2.4 | 34.5±3.3 | 0.775 |
| Body weight (kg) | 2.2±0.8 | 2.8±0.7 | 0.698 |
| Method of
delivery |
|
| 0.856 |
|
Spontaneous labor | 68 (60.18) | 88 (61.11) |
|
| Cesarean
section | 45 (39.82) | 56 (38.89) |
|
| Premature infant with
brain injury | 61 (54.0%) | 28 (19.4%) | 0.023 |
| IL-6 (µg/l) | 6.82±3.59 | 2.61±1.22 | 0.024 |
| Heart rate of
premature infants (beats/min) | 181.2±10.3 | 144.5±11.6 | 0.031 |
| Leukocyte count
(×109/) in cord blood | 18.2±1.3 | 9.5±1.9 | 0.027 |
Diagnostic results of infection in
pregnant women
A total of 61 premature infants with brain injury
were found in infected group, and the incidence rate was 54.0%;
while in uninfected group, there were 28 premature infants with
brain injury, with an incidence rate of 19.4%; 3 had placental
infection caused by intrauterine infection in pregnant women, and
all premature infants had brain injury (Table III).
 | Table III.Diagnostic results of infections in
pregnant women. |
Table III.
Diagnostic results of infections in
pregnant women.
| Infections | Infected group
(n=113) | Uninfected group
(n=144) |
| Leukocyte
infiltration degree | 2+/3+ | −/+ |
| Heart rate of the
pregnant woman | 112.3±12.5 | 90.6±10.1 |
| Fetal heart rate | 181.2±10.3 | 144.5±11.6 |
| Body temperature of
the pregnant woman | 38.1±0.2 | 37.2±0.3 |
| Leukocyte count | 18.2±1.3 | 9.5±1.9 |
| Peculiar smell in
amniotic fluid | Positive | Negative |
| Uterine body
tenderness | Positive | Negative |
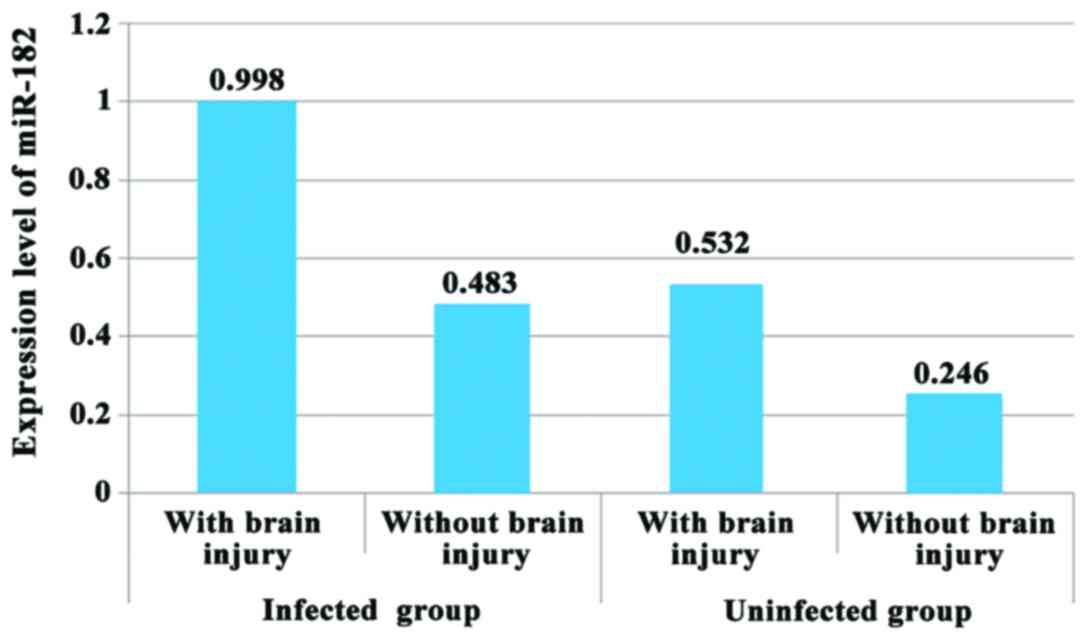
Expression level of miR-182 in
infected and uninfected groups
The difference in miR-182 level between infected
group and uninfected group was statistically significant, and
infected group had a higher miR-182 level in comparison with
uninfected group (P<0.05). In both infected group and uninfected
group, expression level of miR-182 in premature infants with brain
injury was higher than that in premature infants without brain
injury (P<0.05) (Fig. 1).
Multivariate COX regression analysis
of brain injury in premature infants
The median of miR-182 expression level in 89
premature infants with brain injury was 0.723, these infants were
divided into high expression group with miR-182 ≥0.723 and low
expression group with miR-182 <0.723; expression level of
miR-182 and placental infection were independent risk factors of
brain injury in premature infants; intrauterine infection was
closely related to brain injury in premature infants; and risk
value of brain injury in premature infants caused by intrauterine
infection was hazard ratio (HR) = 2.226, P=0.003 (Tables IV and V).
 | Table IV.Univariate COX regression analysis of
brain injury in premature infants. |
Table IV.
Univariate COX regression analysis of
brain injury in premature infants.
|
| Single factor |
|---|
| Indicator | HR | 95% CI | P-value |
|---|
| miR-182 (high vs.
low) | 1.674 | 1.134–2.869 | 0.01 |
| Gestational age
(weeks) | 1.051 | 0.989–1.038 | 0.061 |
| Sex (male vs.
female) | 0.893 | 0.274–2.452 | 0.779 |
| Body weight | 0.832 | 0.376–1.864 | 0.672 |
| Intrauterine
infection | 2.226 | 0.937–147.46 | 0.003 |
| Placental
infection | 3.053 | 1.233–7.345 | 0.026 |
 | Table V.Multivariate COX regression analysis
of brain injury in premature infants. |
Table V.
Multivariate COX regression analysis
of brain injury in premature infants.
|
| Multi-factor |
|---|
|
|
|
|---|
| Indicator | HR | 95% CI | P-value |
|---|
| miR-182 (high vs.
low) | 1.969 | 0.951–4.077 | 0.012 |
| Intrauterine
infection | 1.639 | 0.832–3.695 | 0.002 |
| Placental
infection | 1.268 | 0.918–2.471 | 0.001 |
Discussion
Despite the average neonatal mortality rate has
declined year by year since 1990, there are still ~3,000,000
neonates who die every year (12).
Intrauterine infection is one of the important risk factors for
neonatal sepsis and is a common cause of neonatal infant mortality
and morbidity, especially for premature infants (13). Intrauterine infection is a leading
cause of premature birth and brain injury, bacterial invasions in
chorion and amnion or placenta can lead to fetal inflammatory
response, which has significant adverse effects on growth of fetal
brains (14).
This study explored relationships of miR-182
expression level with intrauterine infection and brain injury in
premature infants via examinations of miR-182 expression level in
amniotic fluid. In this study, premature infants who had a time of
pregnancy termination of <35 weeks and whose mother did not have
preeclampsia and diabetes mellitus were included. This study was
approved by the medical Ethics Committee of the hospital. Patients
or their families signed the informed consent.
In this study, the analysis of expression level of
miR-182 via qPCR showed that pregnant women with intrauterine
infection had a clearly higher miR-182 expression level in amniotic
fluid in comparison with those without intrauterine infection
(P<0.05), suggesting that miR-182 expression level is associated
with intrauterine infection. At present, no studies on association
of miR-182 expression level with intrauterine infection and brain
injury in premature infants are found. This study is for reference
only. The study of Hanke et al (15) showed that the level of miR-182
expression in bladder epithelial carcinoma complicated with urinary
tract infection was also increased. Therefore, miR-182 is closely
related to infection. Kelada et al (16) found that in schistosome and
leishmania-related inflammatory stages, IL-4 can upregulate miR-182
expression level via the transcription factor macrophage activating
factor (cMaf), phosphorylation status of cMaf and other pathways
including IL-2 and other transcriptional regulators may also have
impact on miR-182. Stittrich et al (17) indicated that miR-182 is closely
related to the activity of helper T lymphocytes. IL-2 induces the
increase of miR-182 level and promotes the expansion of helper T
lymphocytes. Later, further studies are needed to confirm whether
intrauterine infection in pregnant women can also regulate miR-182
expression level through this mechanism. Present studies have
confirmed (18–22) that intrauterine infection can cause
brain damage in premature infants. Therefore, expression level of
miR-182 is closely related to brain injury in premature infants.
The results of this study also revealed that miR-182 expression
level in infants with brain injury was higher than that in infants
without brain injury (P<0.05). In this study, differences in
miR-182 expression levels in preterm infants and extremely preterm
infants as well as various degrees of brain damage were not
investigated, which are worth exploring in the future.
The results of this study also indicated that among
infants delivered by pregnant women without intrauterine infection,
infants with brain injury had a higher miR-182 expression level in
comparison with those without brain injury (P<0.05), suggesting
that miR-182 may have an independent impact on brain injury in
premature infants; COX regression analysis also revealed that
miR-182 expression level is an independent risk factor for brain
injury in premature infants. miR-182 is a member of miR-183 cluster
(miR-183, miR-182 and miR-96). Yi et al (23) found that miR-182 aggravates cerebral
ischemic injury by targeting an inhibitor of apoptosis-stimulating
protein of p53 (iASPP) the ASPP family. Ding et al (24) established models of rats with brain
injury and also found abnormalities in miR-182 expression. These
have further confirmed that miR-182 is involved in the occurrence
of brain injury.
At present, studies on miR-182 have also found that
it affects the occurrence and development of tumors. Spitschak
et al (25) found that
miR-182 can promote cancer invasion by rearranged during
transfection (RET) oncogene-activated nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB) and HES1/Notch1
regulatory pathway. Xu et al (26) also found that miR-182, the target
gene of the long non-coding RNA death associated protein kinase1
(DAPK1), participates in the invasion and metastasis of pancreatic
cancer by modulating Ras homolog gene family-associated coiled-coil
containing protein kinase 1 (ROCK-1)/ Rho, member A (RhoA)
signaling pathway. Yu et al (27) found that miR-182 promotes the
development of breast cancer via targeted regulation of forkhead
box protein F2 (FOXF2). These are part of the latest studies on
associations of miR-182 with the occurrence and development of
tumors so far, suggesting that miR-182 is a multifunctional
molecule. Whether it also plays roles in other diseases is worth
exploring further.
In conclusion, intrauterine infection can cause an
increase in miR-182 level; growth in miR-182 level and brain injury
in premature infants are closely related.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FG, XJ and KF conceived and designed the study. FG
and QL were responsible for the collection and analysis of the
patient data. FG and XJ interpreted the data and drafted the
manuscript. KF revised the manuscript critically for important
intellectual content. All authors read and approved the final
study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Jinan Maternity and Child Care Hospital (Shandong, China). Signed
informed consents were obtained from the parents of the
infants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kemp MW: Preterm birth, intrauterine
infection, and fetal inflammation. Front Immunol. 5:5742014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sarno M, Sacramento GA, Khouri R, do
Rosário MS, Costa F, Archanjo G, Santos LA, Nery N Jr, Vasilakis N,
Ko AI, et al: Zika virus infection and stillbirths: A case of
hydrops fetalis, hydranencephaly and fetal demise. PLoS Negl Trop
Dis. 10:e00045172016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Higgins RD, Saade G, Polin RA, Grobman WA,
Buhimschi IA, Watterberg K, Silver RM and Raju TN: Chorioamnionitis
Workshop Participants: Evaluation and management of women and
newborns with a maternal diagnosis of chorioamnionitis: Summary of
a workshop. Obstet Gynecol. 127:426–436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salmaso N, Jablonska B, Scafidi J,
Vaccarino FM and Gallo V: Neurobiology of premature brain injury.
Nat Neurosci. 17:341–346. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ball G, Boardman JP, Rueckert D, Aljabar
P, Arichi T, Merchant N, Gousias IS, Edwards AD and Counsell SJ:
The effect of preterm birth on thalamic and cortical development.
Cereb Cortex. 22:1016–1024. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hagberg H, Mallard C, Ferriero DM,
Vannucci SJ, Levison SW, Vexler ZS and Gressens P: The role of
inflammation in perinatal brain injury. Nat Rev Neurol. 11:192–208.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weeraratne SD, Amani V, Teider N,
Pierre-Francois J, Winter D, Kye MJ, Sengupta S, Archer T, Remke M,
Bai AH, et al: Pleiotropic effects of miR-183~96~182 converge to
regulate cell survival, proliferation and migration in
medulloblastoma. Acta Neuropathol. 123:539–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song L, Liu L, Wu Z, Li Y, Ying Z, Lin C,
Wu J, Hu B, Cheng SY, Li M, et al: TGF-β induces miR-182 to sustain
NF-κB activation in glioma subsets. J Clin Invest. 122:3563–3578.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim CJ, Romero R, Chaemsaithong P,
Chaiyasit N, Yoon BH and Kim YM: Acute chorioamnionitis and
funisitis: Definition, pathologic features, and clinical
significance. Am J Obstet Gynecol. 213 Suppl:S29–S52. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roth TL, Nayak D, Atanasijevic T, Koretsky
AP, Latour LL and McGavern DB: Transcranial amelioration of
inflammation and cell death after brain injury. Nature.
505:223–228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lawn JE, Blencowe H, Oza S, You D, Lee AC,
Waiswa P, Lalli M, Bhutta Z, Barros AJ, Christian P, et al: Lancet
Every Newborn Study Group: Every Newborn: Progress, priorities, and
potential beyond survival. Lancet. 384:189–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greksova K, Parrak V, Chovancova D, Stencl
P, Oravec J, Marsik L, Sysak R, Fuchs D, Peskova Z and Borovsky M:
Procalcitonin, neopterin and C-reactive protein in diagnostics of
intrauterine infection and preterm delivery. Bratisl Lek Listy.
110:623–626. 2009.PubMed/NCBI
|
|
14
|
Paton MCB, McDonald CA, Allison BJ, Fahey
MC, Jenkin G and Miller SL: Perinatal brain injury as a consequence
of preterm birth and intrauterine inflammation: Designing targeted
stem cell therapies. Front Neurosci. 11:2002017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanke M, Hoefig K, Merz H, Feller AC,
Kausch I, Jocham D, Warnecke JM and Sczakiel G: A robust
methodology to study urine microRNA as tumor marker: microRNA-126
and microRNA-182 are related to urinary bladder cancer. Urol Oncol.
28:655–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kelada S, Sethupathy P, Okoye IS, Kistasis
E, Czieso S, White SD, Chou D, Martens C, Ricklefs SM, Virtaneva K,
et al: miR-182 and miR-10a are key regulators of Treg
specialisation and stability during Schistosome and
Leishmania-associated inflammation. PLoS Pathog. 9:e10034512013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stittrich AB, Haftmann C, Sgouroudis E,
Kühl AA, Hegazy AN, Panse I, Riedel R, Flossdorf M, Dong J,
Fuhrmann F, et al: The microRNA miR-182 is induced by IL-2 and
promotes clonal expansion of activated helper T lymphocytes. Nat
Immunol. 11:1057–1062. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Masaoka N, Nakajima Y, Morooka M, Tashiro
H, Wada M, Maruta K, Iwane E and Yamashiro M: The impact of
intrauterine infection on fetal brain damage assessed by S100B
protein concentrations in umbilical cord arteries. J Matern Fetal
Neonatal Med. 29:2464–2469. 2016.PubMed/NCBI
|
|
19
|
Sameshima H and Ikenoue T: Developmental
effects on neonatal mortality and subsequent cerebral palsy in
infants exposed to intrauterine infection. Early Hum Dev.
83:517–519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao J, Chen Y, Xu Y and Pi G: Effect of
intrauterine infection on brain development and injury. Int J Dev
Neurosci. 31:543–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burd I, Balakrishnan B and Kannan S:
Models of fetal brain injury, intrauterine inflammation, and
preterm birth. Am J Reprod Immunol. 67:287–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elovitz MA, Brown AG, Breen K, Anton L,
Maubert M and Burd I: Intrauterine inflammation, insufficient to
induce parturition, still evokes fetal and neonatal brain injury.
Int J Dev Neurosci. 29:663–671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yi H, Huang Y, Yang F, Liu W, He S and Hu
X: MicroRNA-182 aggravates cerebral ischemia injury by targeting
inhibitory member of the ASPP family (iASPP). Arch Biochem Biophys.
620:52–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding X, Sun B, Huang J, Xu L, Pan J, Fang
C, Tao Y, Hu S, Li R, Han X, et al: The role of miR-182 in
regulating pineal CLOCK expression after hypoxia-ischemia brain
injury in neonatal rats. Neurosci Lett. 591:75–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spitschak A, Meier C, Kowtharapu B,
Engelmann D and Pützer BM: miR-182 promotes cancer invasion by
linking RET oncogene activated NF-κB to loss of the HES1/Notch1
regulatory circuit. Mol Cancer. 16:242017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu X, Wang X and Geng C: Long-chain
non-coding RNA DAPK1 targeting miR-182 regulates pancreatic cancer
invasion and metastasis through ROCK-1/rhoa signaling pathway. Int
J Clin Exp Pathol. 10:9273–9283. 2017.
|
|
27
|
Yu J, Shen W, Gao B, Zhao H, Xu J and Gong
B: MicroRNA-182 targets FOXF2 to promote the development of
triple-negative breast cancer. Neoplasma. 64:209–215. 2017.
View Article : Google Scholar : PubMed/NCBI
|