Introduction
Osteoarthritis (OA) is one of the most prevalent
chronic arthritic diseases and typically occurs in middle aged and
elderly patients (1). A recent study
revealed that OA is a leading cause of disability, with 10% of men
and 13% of women over 60 years of age suffering from symptomatic OA
of the knee (2). The incidence of OA
is higher in women compared with men, and aging, obesity, genetics
and biomechanical predisposing factors are risk factors for the
initiation and progression of OA (3,4).
At present, the conservative therapies available for
OA include oral analgesics, non-steroidal anti-inflammatory drugs
(NSAIDs) and viscosupplementation, which provide short-term
treatment effects (5). For patients
with cardiovascular or gastrointestinal comorbidities, a number of
systemic drugs, including analgesics and NSAIDs are not recommended
due to their cardiovascular side effects (6). As such, injection of therapeutic agents
directly into the OA joint may be more advantageous for reducing
systemic complications.
A number of clinicians have confirmed that
hyaluronic acid (HA) and corticosteroid (CS) supplementation is an
effective means of controlling the symptoms of OA in the knee
(7–9). However, the recent OA treatment
guidelines from the American Academy of Orthopedic Surgeons
strongly discourage the use of HA, while there is little conclusive
evidence to support the use of CS injections (10). More clarity on the intra-articular
supplementation of HA and CS should be determined by future studies
regarding the discrepancies.
Recently, a number of studies have demonstrated that
HA and CS injections are safe and effective treatments that can
reduce pain and improve joint functionality in patients with OA of
the knee (11–13). CS has been reported to be more
effective in reducing acute pain compared with HA, due to its
anti-inflammatory effect. However, the duration of pain relief is
shorter in CS compared with HA (14). As such, the combined use of HA and CS
may be more effective and have longer-lasting analgesic effects
than either agent used alone. However, long-term use and a high
frequency of injections of either agent may cause unnecessary
injury and even infection in the arthritic joint. Furthermore, it
has been reported that CS increases the apoptotic progression of
cartilage cells when injected into the OA joint (15). Clinicians must therefore delicately
balance the amount of CS used to avoid doing more damage than
good.
The aim of the present study was to determine
whether a single-shot co-injection of HA and CS resulted in a
longer duration of pain relief and better functional improvement
compared with the use of HA alone.
Materials and methods
Study design
The present study was designed as a single-center,
prospective, randomized, double blind trial with parallel groups.
The Ethics Committee of The Affiliated Zhongda Hospital of
Southeast University (Nanjing, China) approved the present study
prior to patient enrollment (approval no. 2017zdSYLL012-Y01). The
study protocol was registered at ClinicalTrials.gov (registration no. NCT03047096). All
enrolled subjects provided informed written consent prior to
inclusion in the study. The investigation was performed at The
Affiliated Zhongda Hospital of Southeast University (Nanjing,
China) between February and November 2017.
Samples
Patients that presented themselves to the Department
of Orthopaedics at The Affiliated Zhongda Hospital of Southeast
University with knee pain were diagnosed radiographically and those
who were suffering from knee OA with a duration of >3 months
were graded as stage II–IV by a senior radiologist based on the
Kellgren-Lawrence (KL) grade (16).
Symptomatic knee OA was diagnosed based on the American Rheumatism
Association classification criteria for knee OA (17). Patients were excluded if they had a
diagnosis of rheumatoid arthritis or other inflammatory OA, trauma
or pain-causing diseases, had received treatment with oral
medications within 3 days, or had received physiotherapy or
intra-articular injections of HA or CS within 6 months.
Participants with severe diabetes were excluded due to the
increased risk of developing serious side effects. Participants who
were allergic to any of the medications used in the present study
or were diagnosed with current systemic infection were also
excluded.
Interventions
A total of 120 patients met the inclusion criteria
and were randomized into two groups (n=60/group) via the following
method. The age range of the enrolled patients was 45–80 years
(mean, 63.05±6.40 years) and 75% of the patients were female
(90/120). A computer-generated list of random numbers was used.
This method created 120 study cards, which were titled as either
HA&CS or HA. Each card was sealed in an opaque envelope. An
assistant nurse opened each envelope and assigned patients to the
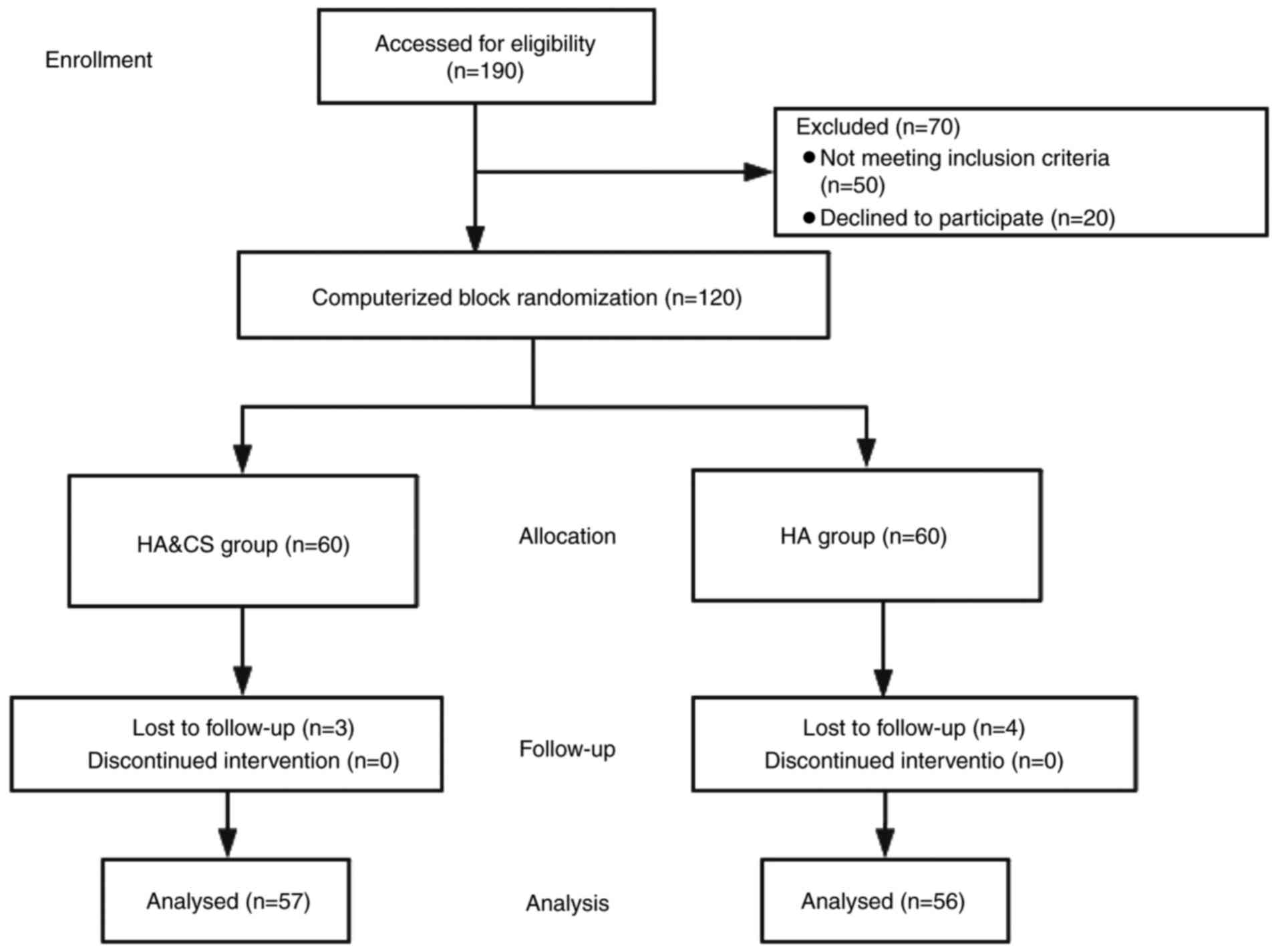
corresponding group. The disposition of the patients in the present
study was processed according to a Consolidated Standards of
Reporting Trials flow diagram (Fig.
1). In the HA group, 4 ml high molecular weight
(600,000–1,500,000 g/mol) HA (Shandong FREDA Pharmaceutical
Industry Group Co., Ltd., Jinan, China) was administered to
patients. In the HA&CS group, 4 ml HA was administered,
followed by 3 ml compound betamethasone solution (Schering-Plough
Labo NV, Heist-op-den-Berg, Belgium), which comprised 1 ml compound
betamethasone (2 mg betamethasone sodium phosphate and 5 mg
betamethasone dipropionate) and 2 ml 0.9% normal saline.
All procedures were performed in the injection room
of the Orthopedic Department at The Affiliated Zhongda Hospital of
Southeast University. An experienced orthopedic surgeon
administered injections. Patients were placed in sitting position
with the eyes covered. Knees were flexed to ~90 degrees and
sterilized prior to injection. A 22-gauge needle (external
diameter, 0.71 mm) was used to puncture the lateral soft spot above
the joint line into the joint capsule. A small amount of air was
injected to ensure that the needle was accurately positioned in the
joint capsule. The 22-gauge needle was left in the injection spot
for further synovial fluid aspiration and drug delivery. The
effusion, if present, was removed using the inserted 22-gauge
needle prior to administration.
In the present study, both the evaluators and
patients were blinded. Evaluators were not informed of the patient
allocations to reduce bias in the assessment. Patients were blinded
using an eye mask during the injection process and were unaware
which group they were assigned to. The use of any additional
medications associated with OA, including NSAIDs and analgesics,
was prohibited following treatment.
Measures
The primary outcomes of treatment were evaluated at
a 6-month follow-up. Pain in the knee with OA was measured using a
100-mm visual analog scale (VAS), whereas knee function was
measured using 3 dimensions of the Western Ontario and McMaster
Universities Osteoarthritis Index (WOMAC) (18). The WOMAC score was validated in
Chinese language. VAS and WOMAC questionnaires were completed at
the baseline (prior to treatment), and at week 1 and months 1, 3
and 6 following treatment. An independent, blinded therapist
assessed the active flexion motion of the treated knees using a
goniometer at the baseline, week 1 and months 1, 3 and 6.
Statistical analysis
A calculation was performed as previously described
to determine the sample size needed to provide 80% power to
demonstrate a difference of >1.2 points in the VAS score at the
5% significance level in a 2-sided hypothesis test (19). A total of 50 patients were required
from each group to ensure adequate power to detect a similar
between-group difference. As such, the study was designed to enroll
120 participants at the baseline (n=60/group), anticipating that
20% may drop out. The analysis was performed on the
intention-to-treat populations. Data are presented as mean ±
standard deviation in tables and as mean ± standard error of the
mean in figures. Demographic variables were tested using a
χ2 test. VAS score, WOMAC score and the range of motion
observed at each time point was evaluated using independent
Student's t-tests. Comparisons in each group prior to the injection
and 6 months following the injection were performed using paired
Student's t-tests. Data were analyzed using SPSS 19 (IBM Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics and
follow-up
A total of 120 patients were enrolled in the present
study and allocated to the HA or HA&CS treatment groups
(n=60/group). No significant differences were observed in basic
characteristics, including sex distribution, mean age, mean VAS
score, mean body mass index, mean WOMAC score and mean knee range
of motion, between the groups (Table
I). At the end of the trial, the majority of enrolled patients
underwent clinical assessments (57 patients in the HA&CS group
and 56 patients in the HA group). In the HA&CS group, 3
patients were lost to follow-up at month 6. In the HA group, 2
patients were lost to follow-up at month 3 and 2 patients were lost
to follow-up at month 6.
 | Table I.Baseline demographic data and clinical
parameters of patients. |
Table I.
Baseline demographic data and clinical
parameters of patients.
| Parameters | HA&CS group
(n=60) | HA group (n=60) | P-value |
|---|
| Sexa |
|
| 0.833 |
|
Female | 46 (76.67) | 44 (73.33) |
|
| Male | 14 (23.33) | 16 (26.67) |
|
| Ageb | 63.60±6.24 | 62.50±6.55 | 0.348 |
| Body mass index
(kg/m2)b | 25.30±3.22 | 26.00±4.15 | 0.304 |
|
Smokinga |
|
| 1.000 |
|
Yes | 5 (8.33) | 6 (10.00) |
|
| No | 55 (91.67) | 54 (90.00) |
|
| Living
situationa |
|
| 1.000 |
| Live
with partner/spouse | 54 (90.00) | 55 (8.33) |
|
| Live
alone | 6 (10.00) | 5 (91.67) |
|
| Kellgren-Lawrence
gradea |
|
| 0.872 |
| II | 12 (20.00) | 10 (16.67) |
|
|
III | 37 (61.67) | 39 (65.00) |
|
| IV | 11 (18.33) | 11 (18.33) |
|
| VAS pain (mean
points)b | 7.13±1.00 | 7.15±0.99 | 0.927 |
| WOMAC score (mean
points)b | 40.95±8.016 | 41.08±6.828 | 0.922 |
| Active knee range
of motion (mean flexion°)b | 126.40±6.35 | 125.93±6.31 | 0.455 |
Pain relief and VAS score
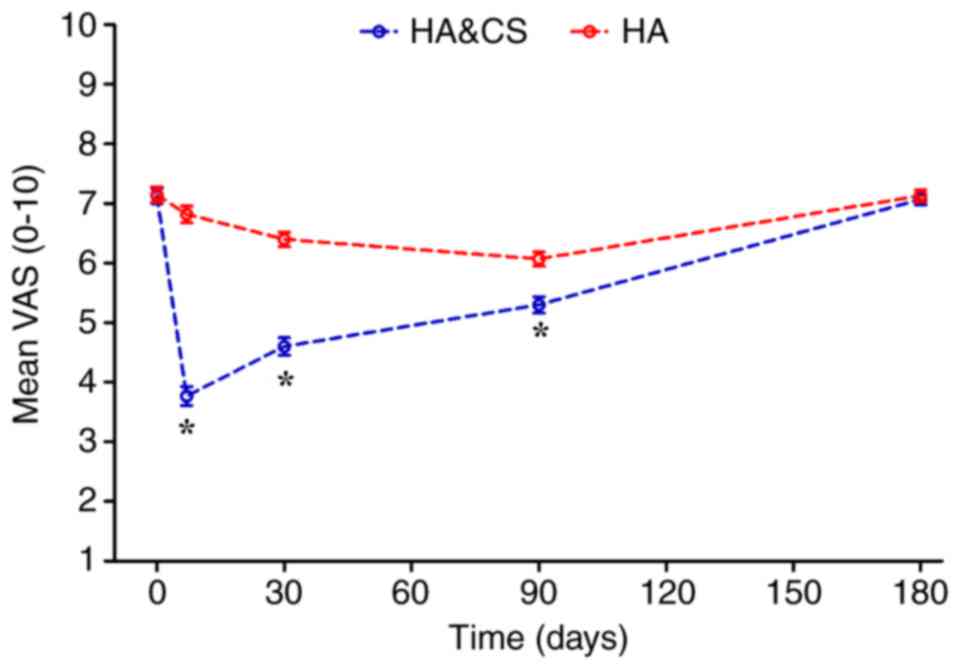
Prior to treatment, no significant difference in VAS
score was observed between the HA&CS and HA groups (7.45±2.05
vs. 7.30±1.96, respectively; Table
II). Following treatment, the VAS score in the HA&CS group
decreased significantly compared with the HA group (Fig. 2). At month 6, the mean VAS score in
both groups increased and were not significantly different.
Patients in both groups exhibited decreased VAS scores following
injection; however, there was no significant difference in the VAS
score at month 6 compared with the baseline in either group
(Table III).
 | Table II.Differences in mean outcome scores
between groups. |
Table II.
Differences in mean outcome scores
between groups.
| Treatment | HA&CS group
(n=60) | HA group
(n=60) | P-value |
|---|
| VAS pain
(points) |
|
|
|
|
Baseline | 7.13±1.00 | 7.15±0.99 | 0.927 |
| 1
week | 3.77±1.23 | 6.82±1.10 | <0.001 |
| 1
month | 4.60±1.20 | 6.40±0.96 | <0.001 |
| 3
months | 5.30±1.11 | 6.07±1.06 | <0.001 |
| 6
months | 7.07±0.73 | 7.13±0.81 | 0.638 |
| WOMAC score
(points) |
|
|
|
|
Baseline | 40.95±8.02 | 41.08±6.83 | 0.922 |
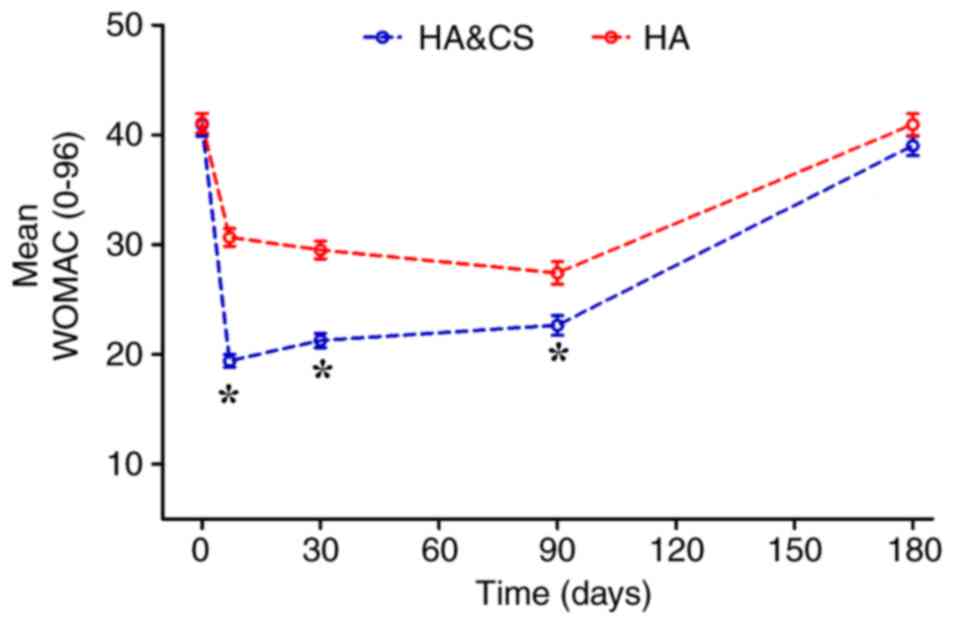
| 1
week | 19.42±4.49 | 30.67±6.37 | <0.001 |
| 1
month | 21.27±5.00 | 29.52±6.31 | <0.001 |
| 3
months | 22.65±7.01 | 27.43±8.12 | 0.008 |
| 6
months | 39.02±6.88 | 40.95±7.70 | 0.150 |
| Flexion motion of
knee (°) |
|
|
|
|
Baseline | 126.37±6.35 | 125.50±6.31 | 0.455 |
| 1
week | 129.83±4.73 | 129.16±4.53 | 0.432 |
| 1
month | 130.17±4.78 | 129.63±4.40 | 0.526 |
| 3
months | 132.20±4.71 | 131.93±4.58 | 0.754 |
| 6
months | 126.90±4.52 | 126.73±4.29 | 0.559 |
 | Table III.Differences in mean outcome scores
over time. |
Table III.
Differences in mean outcome scores
over time.
| Treatment | HA&CS group
(n=60) | HA group
(n=60) |
|---|
| VAS pain
(points) |
|
|
|
Baseline | 7.13±1.00 | 7.15±0.99 |
| 6
months | 7.07±0.73 | 7.13±0.81 |
|
P-value | 0.677 | 0.927 |
| WOMAC score
(points) |
|
|
|
Baseline | 40.95±8.02 | 41.08±6.83 |
| 6
months | 39.02±6.88 | 40.95±7.70 |
|
P-value | 0.129 | 0.915 |
| Flexion motion of
knee (°) |
|
|
|
Baseline | 126.37±6.35 | 125.50±6.31 |
| 6
months | 126.90±4.52 | 126.73±4.29 |
|
P-value | 0.494 | 0.201 |
Functional improvement and WOMAC
score
The mean WOMAC score were markedly decreased in the
HA&CS and HA groups for 6 months following treatment, compared
with baseline (Fig. 3). In terms of
pain, stiffness and physical function, better knee function was
observed in the HA&CS group compared with the HA group during
the first 3 months post-injection (P<0.05). However, no
significant differences in WOMAC score were observed between groups
at month 6. WOMAC scores were improved in both groups
post-injection; however, the mean WOMAC score at month 6 was not
significantly different to the baseline score in either group
(Table III).
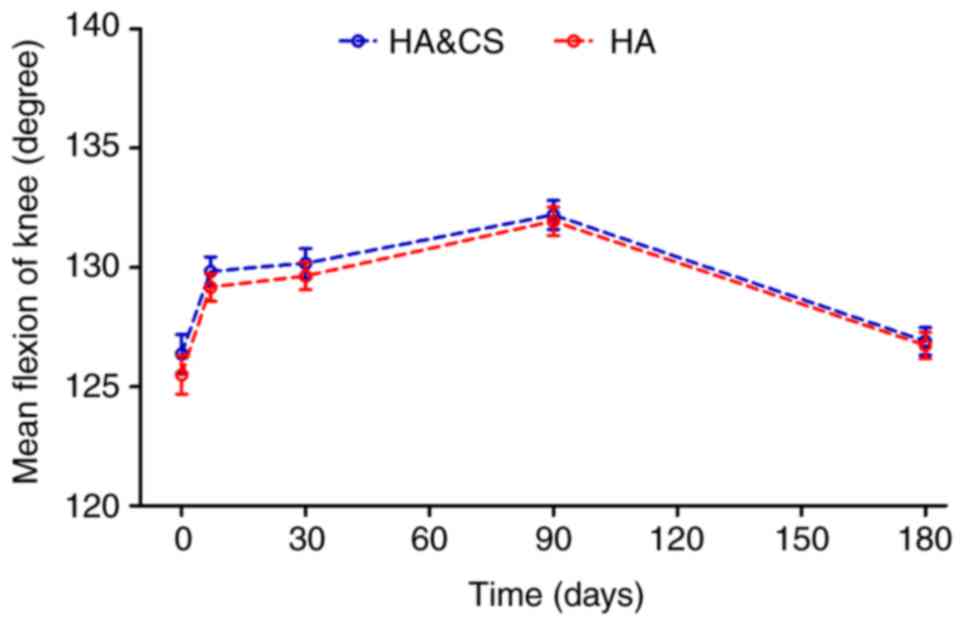
Active flexion motion of the knee
Patients in both groups reported improved flexion
compared with the baseline for the first 3 months post-injection.
No significant difference in mean flexion angle of the knee was
observed between groups at any time point (Fig. 4). There was no significant difference
in the mean flexion angle at month 6 compared with the baseline in
either group (Table III).
Adverse events
No severe systemic or local adverse events were
observed in either group.
Discussion
In the present study, prompt pain relief was clearly
observed in the HA&CS group, whereas the VAS score was
decreased gradually in the HA group. At month 6, the mean change in
VAS score was not significantly different between groups. The fast
action of intra-articular CS injection is well recognized, however
the long-term outcome of repetitive injections remains unknown
(11). CS is able to block the
synthesis and activation of matrix metalloproteinases, slowing down
decomposition of the cartilage matrix (15,20).
However, a repetitive high dose of CS can hinder the regeneration
of articular cartilage by downregulating proteoglycan and HA
synthesis. Intra-articular CS injection typically reduced pain
associated with OA for 3–4 weeks (21), whereas HA has been reported to have a
greater efficacy beyond week 8 (22). A clinical trial revealed that HA
controls pain better at 12 and 26 weeks compared to CS (23). A similar trend in pain experience and
VAS scores was observed in the present study. However, the duration
of pain relief was less than 6 months regardless of the use of
combined treatment with HA&CS or HA alone. In the present
study, the indication for intra-articular CS and HA injection is
symptomatic knee OA, which is mainly associated with pain,
stiffness, joint warmth and swelling. However, as knee joint
puncture is an invasive operation, patients with severe diabetes or
systemic infection should be excluded due to the risk of skin and
joint capsule infection.
The anti-inflammatory effects of CS may serve an
important role in alleviating the acute symptoms of OA. Patients
with knee OA typically present with joint inflammation, including
morning stiffness, warmth, pain and joint effusions, which occur in
part due to synovial thickening or synovial fluid effusion
(3,24,25). CS
has a powerful anti-inflammatory effect and is able to reduce
edema, capillary expansion and leukocyte infiltration in the early
stages of the inflammatory reaction (26,27). A
patient who dropped out of the study due to unsatisfactory pain
control (lost to follow-up at month 6 following HA injection) later
went on to have a total knee arthroplasty (TKA) in The Affiliated
Zhongda Hospital of Southeast University. Pathological sections and
radiographic images of the joint synovium were obtained, revealing
chronic synovial inflammation and degenerative changes to the knee
joint. For patients with OA and acute synovitis, HA injection alone
is typically insufficient to control the inflammation and alleviate
pain. As such, co-administration of a CS injection may be necessary
to achieve the desired outcome (28).
The results of the present study revealed that
combined treatment with HA and CS resulted in a greater improvement
knee function compared with HA alone for the first 3 months
post-injection. However, at month 6 there was no significant
difference in WOMAC scores between groups. These results suggest
that, in the long-term, combined treatment with HA and CS is not
superior to HA alone. For patients with acute pain, however, the
use of HA and CS together may provide more effective immediate pain
relief. Usually, the use of cross-linked HA results in a
significant reduction in pain and improved knee function from
6->12 months (29,30), as cross-linking is a proven means for
prolonging the intra-articular residence time of HA (31). In the present study, a linear HA was
used, which has a shorter degradation half-life compared with
cross-linked HA. The shorter effect duration may be due to the
intrinsic characteristic of the viscosupplementation injected. In
future studies, researchers should evaluate the efficacy of
co-application of cross-linked HA and CS, which may have better
clinical outcomes.
With regards to the knee range of motion, patients
in both groups reported improved flexion for the first 3 months
post-injection. At month 6, the mean change was greater in the
HA&CS group compared with the HA group, however there was no
statistical significance. For patients with limited knee extension,
a compound betamethasone solution (1 ml compound betamethasone in 4
ml 1% lidocaine hydrochloride) was used for local injection into
the posterior joint capsule and gastrocnemius tendons. The majority
of patients reported a prompt improvement in extension
capabilities, even from the second day following the injection.
However, this procedure may cause unnecessary injury to the
posterior tibial nerve and blood vessels, and patients who
underwent this treatment were not included in the present
study.
Prolonging the interval between injections and
decreasing the dosage of CS used may reduce the risk of injury and
drug-related adverse events. In the present study, HA and CS were
used in combination and patients were followed up for 6 months. The
CS used in the present study was a compound betamethasone, which is
primarily composed of betamethasone sodium phosphate and
betamethasone dipropionate. Following administration, the soluble
betamethasone sodium phosphate is rapidly absorbed and often
reaches a peak plasma concentration within 1 h, relieving acute
pain within 6 h. Fat-soluble betamethasone dipropionate was
absorbed slowly in the knee joint capsule, prolonging the duration
of pain management. Betamethasone was injected directly into the OA
knee and the majority of patients experienced rapid pain relief and
improved knee function within 3 days. However, the duration of pain
relief varied significantly among patients, especially in those
diagnosed with advanced knee OA (KL grading III–IV). In a recent
systematic review and meta-analysis, Jüni et al (32) concluded that CS injection had a
negligible effect after 6 months. Due to the potential
cardiovascular side effects and cartilage erosion associated with
CS, single CS injections are not recommended in the Department of
Orthopaedics at The Affiliated Zhongda Hospital of Southeast
University. The results of the present study suggest that, even
when combined with HA, CS is unable to maintain pain relief for
>6 months. As such, co-injection of HA and CS once every 6
months may be an effective treatment for patients with knee OA. The
combined use of HA and CS can provide short-term and comparative
long-term effects in terms of pain relief and knee function
improvement. Meanwhile, HA may serve to protect the cartilage from
CS erosion by adhering to the joint cartilage, thus improving the
safety of CS application.
In the present study, no local anesthetic was used
in the injection spot, as the injection of local anesthetic itself
often causes unnecessary pain that lasts longer than the joint
capsule penetration process. According to clinical observations,
patients who received prompt puncture of the needle into the joint
capsule experienced minor or acceptable pain experience. The use of
a thinner needle also alleviated the pain of penetration.
Therefore, a 22-gauge needle (external diameter, 0.71 mm), instead
of a commonly used 21-gauge needle (external diameter, 0.81 mm)
(19), was used in the present
study. The adverse events and pain experience in this study proved
minimal. However, a patient in the HA&CS group experienced
CS-induced facial flushing and dizziness. This patient recovered
from the symptoms within 24 h without any pharmaceutical
interventions.
No placebo group was used in the present study, as
it has previously been demonstrated that the use of HA and/or CS is
superior compared with a placebo injection (33–35). One
limitation of the present study was that the effect of a combined
use of HA and CS treatment on the cartilage metabolism was not
investigated. Further basic studies are required to determine
whether co-treatment with CS and HA can cause the long-term
cartilage deterioration. The CS injections used in the present
study were able to induce prompt analgesia for acute pain, thus
prolonging the necessity for possible surgical interventions.
However, prior intra-articular injection of CS was reported to be
associated with an increased infection risk for subsequent TKA
(36,37).
In conclusion, patients who received co-treatment
with HA and CS experienced pain relief and improved knee function
faster than those who received HA alone. However, the combined use
of HA and CS was not overall superior to HA in terms of pain
control, knee function and range of motion at month 6
post-injection. By adhering to the joint cartilage, HA may protect
the cartilage from CS erosion, improving the safety of CS
application. However, further in vivo studies are required
to investigate the biological mechanisms underlying this protective
effect.
Acknowledgements
The authors would like to thank Dr Chang Su from the
Department of Medical Database, Dr Li-Wei Huang and Dr Jing-Yuan Xu
from the Department of Intensive Care Unit, and Dr. Jun Lu from the
Department of Orthopaedics of Zhongda Hospital, Medical School of
Southeast University (Nanjing, China) for help with the present
statistical analysis.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 81572188) and Science and
Technology Project of Jiangsu Province (grant no. BK20161439).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZW collected data and drafted the manuscript. DYW
participated in the experimental design and experimentation, and
collected data. QC and YDG participated in designing the study and
interpreting the results. CW took part in the study design,
provided clinical perspectives for the findings of the study and
revised the manuscript. WMF conceived the study and interpreted the
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Affiliated Zhongda Hospital of Southeast
University (Nanjing, China) Ethics Committee approved the present
study prior to patient enrollment (approval no. 2017zdSYLL012-Y01).
All enrolled subjects provided written informed consent prior to
inclusion in the present study.
Patient consent for publication
Informed consent was obtained from all individual
participants to publish the material in the present study. Data of
all individual participants were kept private and confidential.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lawrence RC, Felson DT, Helmick CG, Arnold
LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG,
et al: Estimates of the prevalence of arthritis and other rheumatic
conditions in the United States. Part II. Arthritis Rheum.
58:26–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y and Jordan JM: Epidemiology of
osteoarthritis. Clin Geriatr Med. 26:355–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robinson WH, Lepus CM, Wang Q, Raghu H,
Mao R, Lindstrom TM and Sokolove J: Low-grade inflammation as a key
mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol.
12:580–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roos EM and Arden NK: Strategies for the
prevention of knee osteoarthritis. Nat Rev Rheumatol. 12:92–101.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McArthur BA, Dy CJ, Fabricant PD and Valle
AG: Long term safety, efficacy, and patient acceptability of
hyaluronic acid injection in patients with painful osteoarthritis
of the knee. Patient Prefer Adherence. 6:905–910. 2012.PubMed/NCBI
|
|
6
|
Harirforoosh S, Asghar W and Jamali F:
Adverse effects of nonsteroidal antiinflammatory drugs: an update
of gastrointestinal, cardiovascular and renal complications. J
Pharm Pharm Sci. 16:821–847. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adams ME, Lussier AJ and Peyron JG: A
risk-benefit assessment of injections of hyaluronan and its
derivatives in the treatment of osteoarthritis of the knee. Drug
Saf. 23:115–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wobig M, Dickhut A, Maier R and Vetter G:
Viscosupplementation with hylan G-F 20: A 26-week controlled trial
of efficacy and safety in the osteoarthritic knee. Clin Ther.
20:410–423. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dougados M, Nguyen M, Listrat V and Amor
B: High molecular weight sodium hyaluronate (hyalectin) in
osteoarthritis of the knee: A 1 year placebo-controlled trial.
Osteoarthritis Cartilage. 1:97–103. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jevsevar DS: Treatment of osteoarthritis
of the knee: Evidence-based guideline, 2nd edition. J Am Acad
Orthop Surg. 21:571–576. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nguyen C, Lefèvre-Colau MM, Poiraudeau S
and Rannou F: Evidence and recommendations for use of
intra-articular injections for knee osteoarthritis. Ann Phys
Rehabil Med. 59:184–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maheu E, Rannou F and Reginster JY:
Efficacy and safety of hyaluronic acid in the management of
osteoarthritis: Evidence from real-life setting trials and surveys.
Semin Arthritis Rheum. 45:S28–S33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koenig KM, Ong KL, Lau EC, Vail TP, Berry
DJ, Rubash HE, Kurtz S and Bozic KJ: The use of hyaluronic acid and
corticosteroid injections among medicare patients with knee
osteoarthritis. J Arthroplasty. 31:351–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Askari A, Gholami T, NaghiZadeh MM, Farjam
M, Kouhpayeh SA and Shahabfard Z: Hyaluronic acid compared with
corticosteroid injections for the treatment of osteoarthritis of
the knee: A randomized control trail. Springerplus. 5:4422016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vandeweerd JM, Zhao Y, Nisolle JF, Zhang
W, Zhihong L, Clegg P and Gustin P: Effect of corticosteroids on
articular cartilage: Have animal studies said everything. Fundam
Clin Pharmacol. 29:427–438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteo-arthrosis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Altman R, Asch E, Bloch D, Bole G,
Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg
M, et al: Development of criteria for the classification and
reporting of osteoarthritis. Classification of osteoarthritis of
the knee. Diagnostic and therapeutic criteria committee of the
American rheumatism association. Arthritis Rheum. 29:1039–1049.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McConnell S, Kolopack P and Davis AM: The
Western Ontario and McMaster Universities Osteoarthritis Index
(WOMAC): A review of its utility and measurement properties.
Arthritis Rheum. 45:453–461. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levin PE: Utilizing Health-Care Resources
Wisely: Understanding the Efficacy of Our Interventions: Commentary
on an article by Nattapol Tammachote, MD, MSc, et al:
Intra-articular, single-shot hylan G-F 20 hyaluronic acid injection
compared with corticosteroid in knee osteoarthritis. A
double-blind, randomized controlled trial. J Bone Joint Surg Am.
98:e472016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tehranzadeh J, Booya F and Root J:
Cartilage metabolism in osteoarthritis and the influence of
viscosupplementation and steroid: A review. Acta Radiol.
46:288–296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kelly AM: The minimum clinically
significant difference in visual analogue scale pain score does not
differ with severity of pain. Emerg Med J. 18:205–207. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bannuru RR, Natov NS, Obadan IE, Price LL,
Schmid CH and McAlindon TE: Therapeutic trajectory of hyaluronic
acid versus corticosteroids in the treatment of knee
osteoarthritis: A systematic review and meta-analysis. Arthritis
Rheum. 61:1704–1711. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caborn D, Rush J, Lanzer W, Parenti D and
Murray C: A randomized, single-blind comparison of the efficacy and
tolerability of hylan G-F 20 and triamcinolone hexacetonide in
patients with osteoarthritis of the knee. J Rheumatol. 31:333–343.
2004.PubMed/NCBI
|
|
24
|
Maricar N, Callaghan MJ, Parkes MJ, Felson
DT and O'Neill TW: Clinical assessment of effusion in knee
osteoarthritis-A systematic review. Semin Arthritis Rheum.
45:556–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loeser RF, Collins JA and Diekman BO:
Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol.
12:412–420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Richards MM, Maxwell JS, Weng L, Angelos
MG and Golzarian J: Intra-articular treatment of knee
osteoarthritis: From anti-inflammatories to products of
regenerative medicine. Phys Sportsmed. 44:101–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Laev SS and Salakhutdinov NF:
Anti-arthritic agents: Progress and potential. Bioorg Med Chem.
23:3059–3080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ayhan E, Kesmezacar H and Akgun I:
Intraarticular injections (corticosteroid, hyaluronic acid,
platelet rich plasma) for the knee osteoarthritis. World J Orthop.
5:351–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iannitti T, Rottigni V and Palmieri B: A
pilot study to compare two different hyaluronic acid compounds for
treatment of knee osteoarthritis. Int J Immunopathol Pharmacol.
25:1093–1098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee S, Park D and Chmell SJ:
Viscosupplementation with hylan G-F 20 (Synvisc): Pain and mobility
observations from 74 consecutive patients. J Knee Surg. 17:73–77.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Henrotin Y, Raman R, Richette P, Bard H,
Jerosch J, Conrozier T, Chevalier X and Migliore A: Consensus
statement on viscosupplementation with hyaluronic acid for the
management of osteoarthritis. Semin Arthritis Rheum. 45:140–149.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jüni P, Hari R, Rutjes AW, Fischer R,
Silletta MG, Reichenbach S and da Costa BR: Intra-articular
corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev:
CD005328. 2015. View Article : Google Scholar
|
|
33
|
Bellamy N, Campbell J, Robinson V, Gee T,
Bourne R and Wells G: Viscosupplementation for the treatment of
osteoarthritis of the knee. Cochrane Database Syst Rev: CD005321.
2006. View Article : Google Scholar
|
|
34
|
Bellamy N, Campbell J, Robinson V, Gee T,
Bourne R and Wells G: Intraarticular corticosteroid for treatment
of osteoarthritis of the knee. Cochrane Database Syst Rev:
CD005328. 2006. View Article : Google Scholar
|
|
35
|
Godwin M and Dawes M: Intra-articular
steroid injections for painful knees. Systematic review with
meta-analysis. Can Fam Physician. 50:241–248. 2004.PubMed/NCBI
|
|
36
|
Marsland D, Mumith A and Barlow IW:
Systematic review: The safety of intra-articular corticosteroid
injection prior to total knee arthroplasty. Knee. 21:6–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Papavasiliou AV, Isaac DL, Marimuthu R,
Skyrme A and Armitage A: Infection in knee replacements after
previous injection of intra-articular steroid. J Bone Joint Surg
Br. 88:321–323. 2006. View Article : Google Scholar : PubMed/NCBI
|