Introduction
To date, ‘hereditary nonpolyposis colorectal cancer’
(HNPCC) has remained to be fully defined with unified criteria.
However, HNPCCs have various features in common, including an
autosomal pattern of inheritance with 90% penetrance level, early
age of onset, the proximal colon as the most common location,
synchronous and metachronous colorectal cancers (CRS) and an
increased risk for extra-colonic tumors. The most common
pathological features of HNPCC include poor differentiation of
cells, dysplasia, increased signet ring cells, massive
tumor-infiltrating lymphocytes, a high frequency of microsatellite
instability (MSI-H), loss of expression of DNA mismatch repair
(MMR) proteins on immunohistochemistry and a germline mutation in
one of the MMR genes (1).
HNPCC is also known as Lynch syndrome. Basically, it
is a condition with an inherited tendency to develop CRC. The term
‘no polyposis’ indicates that this tumor occurs when no or only few
polyps are present (2,3). In individuals that have already had
colon cancer, but still have a remaining colon, the risk of
developing another colon cancer is up to 60%. HNPCC is considered
the most common hereditary CRC type, accounting for 2–6% of CRC
cases worldwide (4–6). The basic pathology of Lynch syndrome
lies in germline mutations in MMR genes. The basic four genes
involved in the pathology of Lynch syndrome are mutL homolog 1
(MLH1), PMS1 homolog 2, mismatch repair system component (PMS2),
mutS homolog 2(MSH2) and MSH6 (7).
The Amsterdam criteria I and II are recognized by
the majority of researchers as the basic clinical criteria to
identify hereditary Lynch syndrome in affected pedigrees (8–10). A
molecular diagnostic test has been developed on the basis of
knowledge gathered from molecular genetic studies. Furthermore, a
microsatellite I instability test and immunohistochemical analysis
are performed to assess the pathological and genetic aspects of
HNPCC. It has been suggested that wide screening programmes should
be performed to diagnose this syndrome at the early stage (9,10).
High-risk individuals should be screened as soon as possible so
that the development and progression of this tumor type is
prevented.
The Amsterdam I criteria include the following
clinical presentation specifically for Lynch syndrome: i) A minimum
of three cases in one family who have been diagnosed with CRC, and
at least one of them should be a first-degree relative; ii)
occurrence of CRC in at least two consecutive generations; iii)
histopathologically confirmed CRC occurring below the age of 50
years; iv) familial adenomatous polyposis should be excluded
(Table I) (9,10).
 | Table I.AC-I and -II and Bethesda
guidelines. |
Table I.
AC-I and -II and Bethesda
guidelines.
| Guideline | Criteria |
|---|
| AC-I | At least three
relatives with histologically verified colorectal cancer: |
|
| 1. One is a
first-degree relative of the other two; |
|
| 2. At least two
successive generations affected; |
|
| 3. At least one of
the relatives with colorectal cancer diagnosed at <50 years of
age; |
|
| 4. FAP has been
excluded. |
| AC-II | At least three
relatives with a hereditary nonpolyposis colorectal
cancer-associated cancer [colorectal cancer, endometrial, stomach,
ovary, ureter/renal pelvis, brain, small bowel, hepatobiliary tract
and skin (sebaceous tumors)]: |
|
| 1. One is a
first-degree relative of the other two; |
|
| 2. At least two
successive generations affected; |
|
| 3. At least one of
the syndrome-associated cancers should be diagnosed at <50 years
of age; |
|
| 4. FAP should be
excluded in any colorectal cancer cases; |
|
| 5. Tumors should be
verified whenever possible. |
| Bethesda guidelines
for testing of colorectal tumors for MSI | 1. Colorectal cancer
diagnosed in a patient who is <50 years of age. |
|
| 2. Presence of
synchronous or metachronous colorectal, or other
syndrome-associated tumors regard less of age. |
|
| 3. Colorectal cancer
with MSI-H histology diagnosed in a patient who is <60 years of
age. |
|
| 4. Colorectal cancer
or syndrome-associated tumor diagnosed at an age of <50 years in
at least one first-degree relative. |
|
| 5. Colorectal cancer
or syndrome-associated tumor diagnosed at any age in two first- or
second-degree relatives. |
The International Collaborative Group on HNPCC
(ICG-HNPCC) introduced the novel Amsterdam II criteria that include
cancers other than CRC in Lynch syndrome (Table I) (9,10).
HNPCC is inherited in an autosomal dominant manner.
Unlike the diffuse polyposis observed in other hereditary colon
cancer syndromes, patients with Lynch syndrome have a small and
finite number of polyps (n<10), which are usually located in the
right colon, and cannot be endoscopically differentiated from
sporadic colon polyps. Those polyp cases in which pedigree is
affected by lynch syndrome has more probability to progress into
the malignant stage when comparison was done with the normal
individual having the polyp. Recent studies have suggested a median
age of CRC diagnosis of 61.2 years and a lifetime CRC risk of 52.2%
in women and 68.7% in men (9,10). In
HNPCC, metachronous and synchronous tumors are frequently observed.
Histologically, the tumors are characterized by massive lymphocyte
infiltration, a medullary growth pattern and a mucinous or signet
ring cell differentiation. At present, the Bethesda criteria are
used in the clinic, which are considered to be more appropriate
than the Amsterdam criteria by certain clinicians, as those
criteria include the MSI status together with the clinical results
to diagnose HNPCC (Table I)
(9,10).
HNPCC mostly affects the proximal part of the colon
in 70% of cases and in the remaining 30%, its localization is
sporadic. According to our observation, HNPCC occurring in younger
patients (age, <45 years) tends to involve the proximal areas
and sporadic areas in older patients (age >65 years) (11,12). The
average age of Lynch syndrome-associated CRC manifestation is 45
years, which is~20 years lower than in the sporadic counterpart
(11,12). A study by Lindor et al
(13) reported that >40% of
HNPCCs were located on the proximal areas rather than having a
sporadic location.
Colonoscopy screenings have been recommended for
mutation carriers to prevent the development and progression of
cancer through early detection. Over a period of 3 years, Järvinen
et al (14) observed that the
screening for HNPCC in high-risk individuals decreased the
incidence of CRC (6% in screened population as compared with 16% in
the controls) and also decreased the mortality rate of HNPCC
patients to 8% in the screened group vs. 22% in the control
subjects.
The treatment of choice for HNPCC is surgical
management, which includes either total abdominal colectomy (TAC),
subtotal colectomy and segmental resection (14,15). At
present, there is controversy over whether total or segmental
colectomy is the best treatment for HNPCC (14,15). It
has been suggested that prophylactic TAC is indicated in cases in
which the frequency of CRC is high, as it is challenging to stop
the spread of this tumor type to the advanced stage due to massive
tumor growth. The decision to perform a prophylactic TAC should be
based on a prior colonoscopy examination to investigate the spread
of the tumor (15).
In patients with Lynch syndrome, the entire colonic
mucosa is unstable and at risk of developing dysplasia and cancer,
and a high incidence of synchronous and metachronous lesions is
encountered (14). Therefore, the
optimal surgical management of CRC in patients with an established
diagnosis of Lynch syndrome usually requires a more extended
approach than that in patients with CRC that do not exhibit lynch
syndrome. It is therefore essential to pre-operatively identify
Lynch syndrome when a patient is newly diagnosed with CRC. The most
important factors that are indicative of the presence of Lynch
syndrome are the patient's age and family history. The suspicion of
Lynch syndrome should be raised when CRC occurs in a young person
(<45 years), in the case of a family history of CRC, or when a
patient develops multiple primary Lynch syndrome-associated cancer
lesions. Internationally recognized guidelines, including the
abovementioned Bethesda guidelines, were established to facilitate
the identification of potential cases of Lynch syndrome during CRC
screening (14). Historically, the
revised Bethesda criteria have been the most frequently used
guidelines, with the major indication for tumor MSI genetic testing
being CRC diagnosis below the age of 50 years. However, it has been
estimated that limiting tumor analysis to patients who fulfill the
Bethesda criteria would lead to a failure to identify 28% of the
cases of Lynch syndrome. Therefore, numerous institutions now
routinely test all CRCs even in the absence of clinical high-risk
features due to the high potential to identify Lynch syndrome
(14). Syngal et al (15) assessed patients with hereditary
gastrointestinal cancer syndromes that had received subtotal
colectomy vs. those that received TAC and concluded that the choice
of surgery was based on numerous factors, including risk due to
family history, patient preferences, ease of screening and
screening guidelines.
The primary objective of the present study was to
compare the choice of colectomy, i.e., TAC vs. segmental colectomy,
in cases of HNPCC in between 2011 and 2013 and between 2014 and
2016. The efficacy and oncological safety of TAC with ileorectal
anastomosis vs. segmental colectomy in these HNPCC patients was
assessed. In addition, patient satisfaction and post-operative
complications were compared between HNPCC patients subjected to TAC
with ileorectal anastomosis vs. those with segmental colectomy.
Materials and methods
Patients
The present study was approved by the Ethical
Research Board of Weihai Second Municipal Hospital of Qingdao
University (Weihai, China; trial registry no. QU/MR2011/CRC5).
Written informed consent was obtained from each of the
participants. A total of 289 patients who fulfilled the Amsterdam I
and II criteria or HNPCC (16) were
included in the study. The sample was selected according to the
ICG-HNPCC rules and regulations (16), and furthermore, a Pedigree analysis
of the 289 patients was performed, with an example presented in
Fig. 1. Those patients for whom the
Amsterdam criteria I and II were not fulfilled but genetic testing
revealed germ cell mutations of MMR genes were also included in the
total sample size. MSI testing was performed in all of the CRC
patients (16). To confirm the
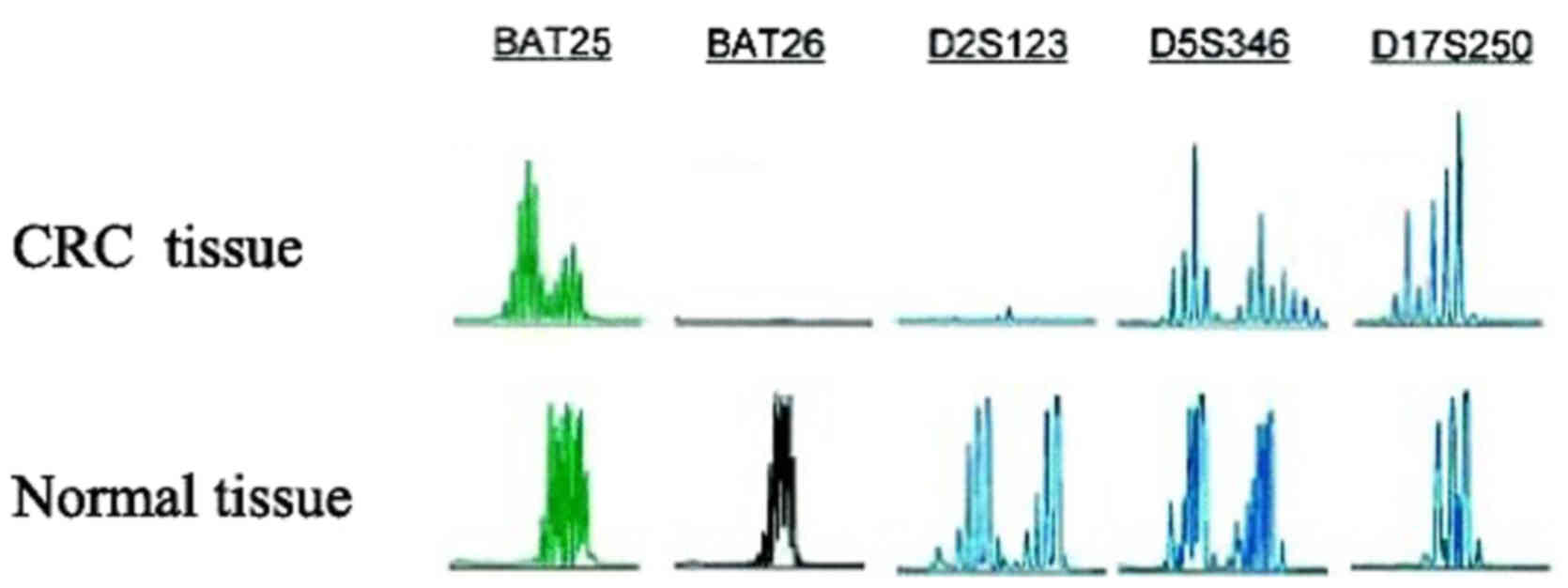
diagnosis, five microsatellite markers, namely BAT25, BAT26,
D2s123, d5S346 and D17S250 (16),
were assessed as additional criteria (Fig. 2).
The patient cohort comprised two groups: Patients in
group 1 had received their diagnosis between 2011 and 2013, and
Group 2 had been diagnosed between 2014 and 2016. The following two
types of operation were applied in the present study: The standard
and extended surgery was TAC with ileal pouch anal anastomosis
(IPAA) and subtotal colectomy, and the second type of surgery was
segmental resection of the colon, including hemicolectomy (right or
left), resection (on the anterior part or on the lower anterior
part) or Hartmann's operation. After the resection, the tumor
sample was sent for histopathological examination
The Amsterdam criteria were used for the inclusion
and exclusion of patients. Only CRC patients with familial
adenomatous polyposis were excluded from the present study
(16).
The age of patients was between 18 to 90 years;
patients with ages of <18 years and >90 years were excluded
from the present study.
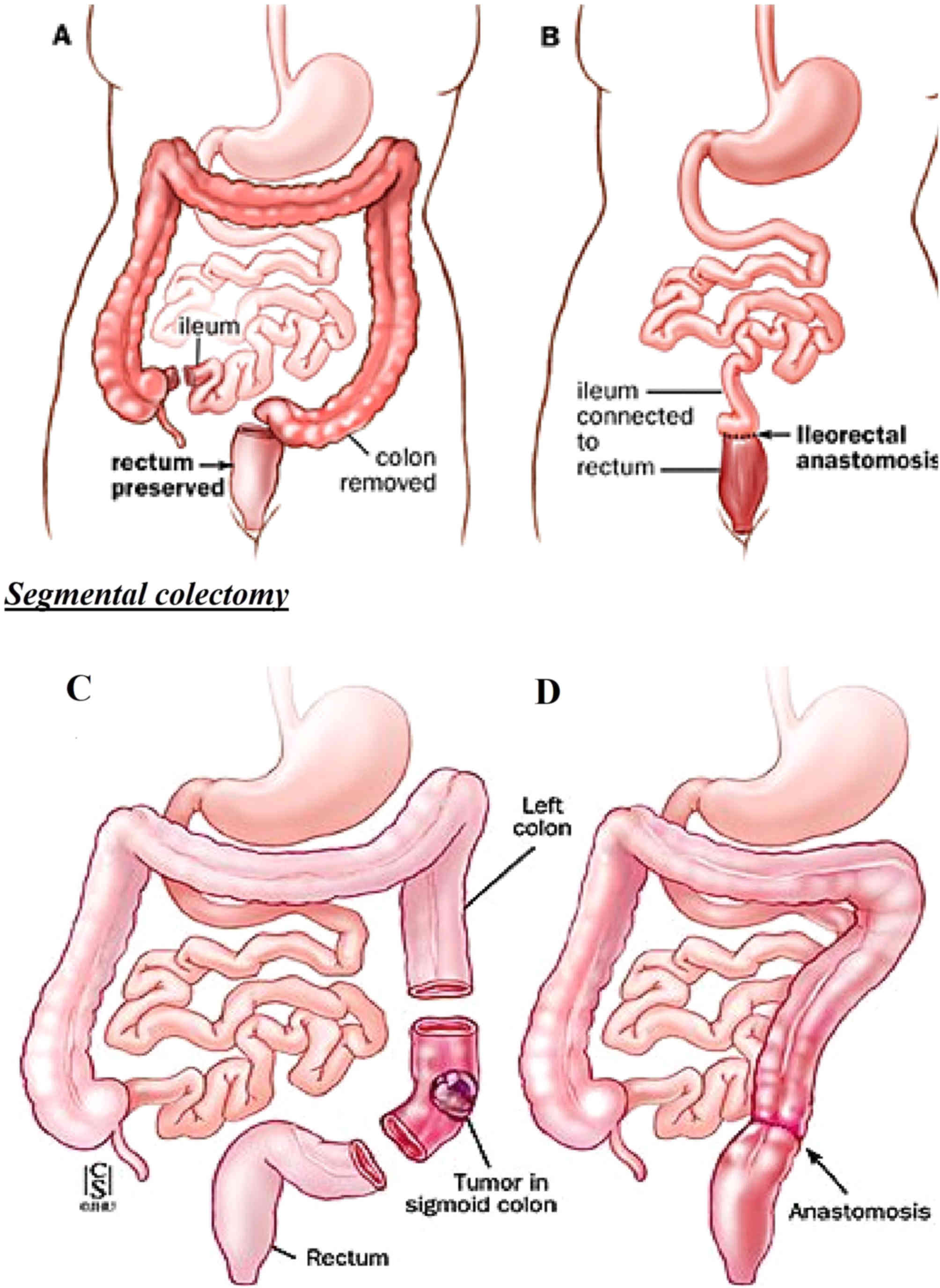
Surgical procedure of TAC with ileal
anastomosis
After the anesthesia, a small cut with the length of
~0.5 inches was made near the navel. The laparoscope was then
inserted in to the abdomen through this incision and the mages
captured by the laparoscope were displayed on the monitor. Once the
laparoscope was in place, a total of 3–5 keyholes were made in the
abdomen. The total number of incisions and their position depended
on the physical build of the patient and difficulty level of the
operation. The sigmoid colon and rectum were exposed, and
subsequently, the colon was exposed and separated in various
sections. The sections comprised the descending colon (left), the
transverse colon, the ascending colon (right), the rectum and the
sigmoid colon. Concurrently, the splenic and hepatic flexure were
removed. Arteries that supplied blood to the colon were carefully
preserved and kept separate. The ileal part was rejoined with the
rectum. Subsequently, the colon was separated from the rectum and
from the ileum to ultimately remove the colon. The surgical
incision was then slightly expanded to pull the colon out of the
abdominal cavity. The next step comprised the rejoining of the
ileum and rectum. This rejoining process is known as Ileorectal
anastomosis. A stapling technique was used for the anastomosis, and
the abdominal cavity was then thoroughly rinsed and the anastomosis
was checked for leakage. In the end, suturing of incisions was
performed. Fig. 3 illustrates how
the segmental resection was performed in the cases of Lynch
syndrome.
Follow-up
All of the patients were followed up for 2.5 years.
Each patient was reassessed following 3, 6 and 12 months up to 2.5
years.
Assessment of oncological safety
Recurrence of a histopathologically confirmed tumor
at local areas on the ipsilateral side was considered to indicate
poor outcome.
Assessment of patient satisfaction
after the surgery
The Lowery scale was used for evaluation of patient
satisfaction 6 months after surgery using a questionnaire. The
scale ranges from 0 to 8 with 0 indicating poor and 8 excellent
satisfaction.
Analysis of functional outcome
The functional outcome was evaluated 1 year
following surgery and was based on the various aspects of bowel
function. The items taken into account were the number of times the
patients moved their bowels in one day, consistency of the stool,
occurrence of gas, anorexia and incidence of perianal irritations.
The data were collected with a questionnaire.
The gastrointestinal functional outcome (GIFO)
scoring system was used for evaluation of bowel function (17). The effect of the patient age, length
of follow-up, recurrence of CRC and whether the anastomosis
technique was performed on the GIFO score was assessed.
Statistical analysis
SPSS version 20.0 (IBM Corp., Armonk, NY, USA) was
used for statistical analysis. For categorical variables, the
comparison was performed by using the chi square test/F-test
(Fisher's exact test). For the analysis of continuous variables,
Student's t-test was used. P<0.05 was considered to indicate a
statistically significant difference.
Results
Genetic testing
Genetic analysis for Lynch syndrome was performed
during the study period. Genetic evaluation of the genes MLH1,
MSH2, MSH6 and PMS2 was performed in 200 out of 289 patients, and a
germline mutation was detected in a total of 120 patients
Genetic testing also established germline mutations
in the MMR genes in all of the 20 subjects who had a negative
family history but fulfilled the other clinical diagnostic
Amsterdam criteria I and II, and therefore, these subjects were
also included in the population (7).
Patient characteristics
Group 1 was comprised of 156 subjects and group II
was comprised of 133 subjects. The mean age of the patients at the
time of their diagnosis was 48 years (range, 18–90 years) in group
I and 50.2 years in group II (range, 21–87 years). The gender ratio
in the two groups was equal. The clinicopathological
characteristics of the two groups are listed in Table II.
 | Table II.General clinical characteristics of
the patient cohort. |
Table II.
General clinical characteristics of
the patient cohort.
| Variable | Group 1
(n=156) | Group 2
(n=133) | P-value |
|---|
| Median age
(years) | 48 (18–90) | 50.2 (21–87) | 0.023 |
| Sex, no. of
patients (%) |
|
|
|
|
Male | 97 (62.1) | 82
(61.6) | 0.40 |
|
Female | 59 (37.9) | 51 (38.4) |
|
| Location, no. of
patients (%) |
|
|
|
|
Proximal colon | 62 (39.7) | 58 (43.6) | 0.07 |
| Distal
colon | 48 (30.7) | 40 (30.3) |
|
|
Rectum | 38 (24.3) | 32 (24.0) |
|
|
Multiple | 8 (5.1) | 3
(92.2) |
| Well
Differentiated tumor status | 75 | 68 |
|
Tumor location
The most common location of Lynch syndrome lesions
in the population of the present study was the proximal colon in
each of the two groups, occurring in 62 cases (39.7%) of group 1
and 58 (43.6%) in group 2, followed by the distal colon, rectum and
multiple locations. The extent of the disease at diagnosis in the
Lynch syndrome patients did not significantly differ between the
two groups (P=0.186; Table II). The
tumor cell differentiation status in the majority of Lynch syndrome
cases was moderate in each of the two groups, followed by well- and
poorly differentiated status. The number of subjects with
well-differentiated status was 75 in group 1 and 68 in group 2
(Table II).
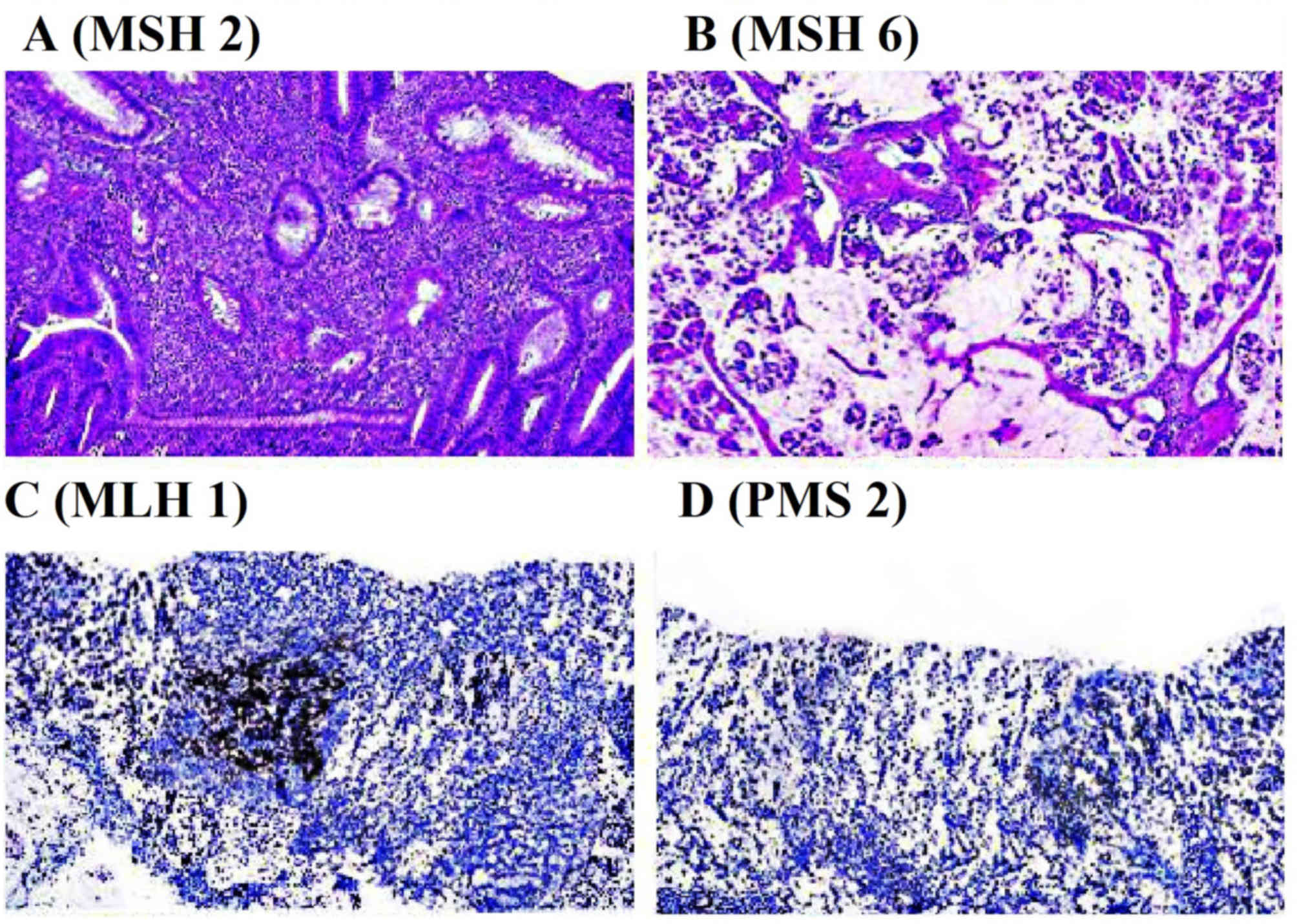
Histopathological examination
Lynch syndrome tumors demonstrated heterogeneity
among poorly and well-differentiated carcinoma on histology.
Immunohistochemical staining for the mismatch repair proteins MLH1,
MSH2, MSH6 and PMS2 was performed. The results indicated that the
expression of MSH2 and MSH6 mismatch repair proteins was retained
in cancerous compared with tissues from patients without lynch
syndrome. However, the expression of MLH1 and PMS2 was lost in
cancerous compared with normal tissue (Fig. 4) (18).
The tumor-nodes-metastasis (TNM) stage in the
majority of the patients was T2N2M0 (n=37 and 32 in group 1 and 2,
respectively), followed by the T2N0M0 (n=32 and 31 in group 1 and
2, respectively).
Surgeries
In group 1, the extended resection was performed in
67.9% of cases; however, in group 2, only in 34% of cases received
this surgery. Segmental resection was performed in 64.5% of cases
in group 2 as compared with 32% in group 1; there was a significant
difference in the rate at which each of the two types of surgery
was performed between the two groups (P<0.001; Table III). It was clearly demonstrated
that previously (group 1), total abdominal colectomy was the
surgery of choice in Lynch syndrome patients, while segmental
resection was significantly preferred in group 2. (Table IV). The sites of the cancer
occurrence were similar between the two groups, i.e. the proximal
colon and distal colon. The present study indicated that the age of
the patient is a major consideration for the choice of the surgery
as, if the age of the patient is >60 years, the segmental
resection was selected, and at an age of ≤60 years, total resection
with ileorectal anastomosis was performed (Tables IV and V).
 | Table III.TNM staging and oncological
safety. |
Table III.
TNM staging and oncological
safety.
| TNM stage | Group 1
(n=156) | Type of
surgery | Local
recurrence | Group 2
(n=133) | Type of
surgery | Local
recurrence |
|---|
| Tis | 15 |
| 0 | 13 |
| 0 |
| TisN1 | 4 |
| 0 | 2 |
| 0 |
| T1N0 M0 | 28 |
| 0 | 24 | Segmental
correction | 4 |
| T1N1 M0 | 4 |
| 0 | 4 |
| 0 |
| T2N0 M0 | 32 | Segmental
correction | 1 | 31 | Segmental
correction | 3 |
| T2N1 M0 | 31 | TAC | 2 | 23 | TAC | 1 |
| T3N1 M0 | 2 |
| 0 | 2 | TAC | 1 |
| T2N2M0 | 37 | Segmental
correction | 5 | 32 | Segmental
correction | 6 |
| T2N2 M1 | 3 |
| 0 | 2 |
| 0 |
| Total | 156 |
| 8 | 133 |
| 15 |
 | Table IV.Comparison of surgical methods
between period 1 group and period 2 group. |
Table IV.
Comparison of surgical methods
between period 1 group and period 2 group.
| Operation type | Group 1
(n=156) | Group 2
(n=133) | P-value |
|---|
| Standard
operation | 106 (67.5) | 46 (32.5) | <0.001 |
| Subtotal
colectomy | 20 | 23 |
|
| Total
colectomy | 86 | 23 |
|
| Segmental
resection | 50
(32.0) | 87 (65.4) | <0.001 |
| Right
hemicolectomy | 15 | 18 |
|
| Transverse
colectomy | 3 | 7 |
|
| Left
hemicolectomy | 2 | 8 |
|
| Anterior
resection | 1 | 27 |
|
| Low anterior
resection | 26 | 23 |
|
| Ultralow anterior
resection | 0 | 0 |
|
| Hartmann's
operation | 0 | 1 |
|
| Abdominoperineal
resection | 0 | 1 |
|
| Segmental resection
of colon | 3 | 1 |
|
 | Table V.Factors affecting the surgical
method. |
Table V.
Factors affecting the surgical
method.
| Variable | Standard
operation | Segmental
resection | P-value |
|---|
| Period |
|
|
|
| Group
1 | 106 (67.9) | 50 (32) | 0.001 |
| Group
2 | 46
(34.5) | 87
(65.5) |
|
| Location of
CRC |
|
|
|
|
Proximal colon | 62 | 58 | – |
| Distal
colon | 48 | 40 |
|
| Age at diagnosis,
years |
|
|
|
|
≤60 | 130 (85.5) | 58 (42.3) | 0.002 |
|
>60 | 12
(143.5) | 79 (57.6) |
|
Oncological safety
In the patients subjected to TAC, a lower frequency
of recurrence was recorded 1 year following surgery when compared
with those that received segmental corrections; however, the
difference was not statistically significant P>0.05 (Table III).
Post-operative complications
In the cohort of the present study, no mortalities
occurred following 1 year of surgery, indicating oncological safety
in each of the two groups and for each surgical method. However,
the patients who received TAC had more complications than those
subjected to segmental resection. Complications after TAC were
noted in 27 patients in group 1 and in 13 patients in group 2.
However, less complications were noted in each of the two the
groups after the segmental resection (3 patients in group 1 and 5
patientsin group 2). The most common complication noted was
intestinal obstruction, followed by intra-abdominal abscess. Out of
the 34 patients with intestinal obstruction, this complication was
managed with a conservative approach in 30 patients and with a
surgical approach in only 4 patients (Table VI).
 | Table VI.Post-operative complications. |
Table VI.
Post-operative complications.
|
|
| Occurrence |
|---|
|
|
|
|
|---|
| Complication | Management | Total abdominal
correction group | Segmental
correction group |
|---|
| Intestinal
obstruction | Conservative
(n=30) | Group 1 (n=20) | Group 1 (n=1) |
|
| Surgical (n=4) | Group 2 (n=10) | Group 2 (n=3) |
| Intraabdominal
abscess | Conservative | Group 1 (n=3) | Group 1 (n=1) |
|
|
| Group 2 (n=2) | Group 2 (n=1) |
| Wound
infection | Conservative | Group 1 (n=1) | Group 1 (n=1) |
|
|
| Group 2 (n=1) | Group 2 (n=1) |
| Small bowel stump
leakage | Conservative | Group 1 (n=1) | Group 1 (n=0) |
|
|
| Group 2 (n=0) | Group 2 (n=0) |
|
Microperforation | Conservative | Group 1 (n=1) | Group 1 (n=0) |
|
|
| Group 2 (n=0) | Group 2 (n=0) |
| Anastomotic
leakage | Conservative | Group 1 (n=1) | Group 1 (n=0) |
|
|
| Group 2 (n=0) | Group 2 (n=0) |
The long-term follow-up period was up to 12 months
to monitor patients for post-operative complications. The most
common complication was intestinal obstruction. In the present
cohort, one patient presented with small-bowel leakage as a
post-operative complication, which was further diagnosed as
enterocutaneous fistula, and one patient presented with anastomosis
stricture and micro-perforation; a surgical approach was used for
the management of complications in these two patients (Table VI).
Patients' satisfaction level following
surgery
Out of the 152 patients subjected to TAC, the
cosmetic outcome was rated by 54 (35.5%), 60 (39.4%), 20 (25.0%)
and 18 patients (11.2%), respectively. Of the 137 patients who had
received segmental corrections, the above ratings of 4–1 were given
by 70 (51.1%), 40 (29.2%), 27 (19.7%) and 0 patients (0.0%),
respectively (Table VII).
 | Table VII.Aesthetic outcome rated by the
patients. |
Table VII.
Aesthetic outcome rated by the
patients.
| Lowery score | TAC (%) | Segmental
correction (%) |
|---|
| Excellent (score
7–8) | 35.5 | 51.1 |
| Good (score
6–6.9) | 39.5 | 29.1 |
| Fair (score
5–5.9) | 20.8 | 19.8 |
| Poor (score
<5) | 4.2 | 0.0 |
| Total | 152 (100) | 137 (100) |
Functional outcome
The functional outcome in the patients after the
segmental approach revealed better results when compared with the
total abdominal colectomy. The number of bowel movements per day in
cases who had received the segmental approach was 4 vs. 6 in the
TAC group (P<0.001). Regarding the items assessing rectal
incontinence (e.g., soiling, particularly at night, incidental
passive incontinence, perianal skin irritation, ability to
distinguish between flatus and feces) (17), patients who had received segmental
resection had significantly better results when compared with those
who had received TAC. Between the two groups, no differences
regarding anorexia and episodes of bowel discomfort were observed.
The functional outcome measured with the GIFO score was
significantly better for patients who had received segmental
resection than for those subjected to TAC.
Discussion
At the time of surgical treatment of CRC, HNPCC
patients frequently remain undiagnosed. Therefore, it is necessary
to assess the detailed family history, hereditary factors and the
past medical history. If Lynch syndrome is suspected, MSI analysis,
immunohistochemistry and germline mutation analysis should be
performed.
CRC has been considered the most common cancer type
in numerous countries, including China. The rate has been
continuously on the rise since the last 10 years. As CRC is known
to have a familial predominance in the majority of instances, there
is a high change and also a requirement to diagnose hereditary
colorectal syndrome at the early stage (19,20). The
diagnosis of Lynch syndrome at the early stages currently relies on
standard screens for CRC in the majority of cases. Various studies
and clinical trials suggested that registry-based screening is
required to reduce the mortality rate of CRC in patients with Lynch
syndrome (20,21).
Regarding best treatment option for HNPCC, extensive
resection, including TAC and subtotal colectomy, has been the
method of choice as compared with segmental resection, as the
latter does not abrogate the high risk for synchronous CRCs.
A retrospective study by Win et al (21) from 2014 concluded that in patients
who had received segmental correction of Lynch syndrome, the risk
of metachronous colon cancer was 19 at 10 years, 47 at 20 years and
69% at 30 years. Various other studies also indicated that
segmental resection was only performed in those cases in which
total colectomy is not recommended. However, the choice of surgery
in those cases varied from patient to patient, which was also
reported by Rodriguez-Bigas and Möeslein (22). However, to date, no research study or
clinical trial has concluded that extensive resection is a better
treatment option than segmental resection. A study performed by
Haanstra et al (23) revealed
that in early stages of HNPCC, segmental resection or less extended
surgery is better than TAC, as the 5-year survival rate was higher
in the former group.
de Vos tot Nederveen Cappel et al (24) indicated that for young patients (age
≤27 years) with CRC and an MMR gene defect, TAC conferred a
survival benefit of 2.3 years relative to segmental resection.
However, the model used in the above study was severely limited by
a failure to account for patient utility and quality-adjusted life
years; their results only considered absolute survival.
Furthermore, in the above study, total proctocolectomy and IPAA was
a treatment strategy of choice due to the reduced occurrence rates
of metachronous cancer, but it failed to consider the impact of
IPAA on the quality of life (QOL).
You et al (25) examined the long-term bowel function
and QOL after segmental resection, subtotal colectomy and TAC. They
used validated survey methods, including a questionarre, to
evaluate the QOL. Their results indicated that subtotal colectomy
appears to represent a midpoint between TAC and segmental resection
in terms of impairment of bowel function and QOL. However, the
above study was retrospective and the patients who underwent each
type of procedure exhibited a wide variation in terms of age,
operative indication and pre-operative function. Subtotal colectomy
may represent a compromise between the relative advantages of
segmental resection and TAC, but the outcomes of this procedure in
terms of QOL and metachronous occurrences of CRC require further
study.
The present study also revealed that after segmental
resection, the patients encountered less post-operative
complications when compared with those that had received TAC,
although this was not statistically significant. In addition, an
improved overall QOL and an excellent patient satisfaction level
was achieved for cosmetic and functional in the patients who
received segmental resection when we compared with the patients
with TAC and ileorectal anastomosis. However, with regard to
oncological safety, the rate of recurrence in the patients
subjected to TAC with ileorectal anastomosis was lower than that in
the patients who had received segmental corrections; however, the
difference was not significant. In order to assess if TAC is the
best and safest procedure, a larger sample size with a longitudinal
approach will be required for future study.
Natarajan et al (26) compared surgical management of Lynch
syndrome using TAC with segmental resection and revealed that there
was no significant difference in 5-year survival rate and overall
survival between the patients that received the two different
surgeries. Therefore, the present study recommends subtotal
colectomy in patients with HNPCC.
Regarding the functional outcome the present study
concluded that after TAC, the patients revealed more frequent bowel
movements and rectal incontinence when compared with the segmental
approach. However, a similar study performed in the Netherlands
indicated a higher QOL and less post-operative complications in
Lynch syndrome patients who underwent segmental resection when
compared with those that had received TAC, with statistical
significance (26).
In conclusion, the present study indicated that, the
surgical method of choice in cases of Lynch syndrome is segmental
resection. The study also suggested that the functional outcome of
the segmental approach was better than that of TAC. However, the
results of our study reveal that the oncological safety of TAC was
higher than that of segmental resection. As a strategy for reducing
the occurrence of HNPCC, a prompt nationwide effort to raise public
awareness of hereditary CRC and an increased support for registries
are required in China.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JS proposed the project, aims and objectives, and
submitted the manuscript; MD calculated the results and XX
collected the data, conducted statistical analysis and wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Research Board of Weihai Second Municipal Hospital of Qingdao
University (Weihai, China; trial registry no. QU/MR2011/CRC5).
Written informed consent was obtained from each of the
participants. All methods were performed in accordance with the
relevant guidelines and regulations as per the instructions of the
Ethical Research Board.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Steinke V, Engel C, Büttner R, Schackert
HK, Schmiegel WH and Propping P: Hereditary nonpolyposis colorectal
cancer (HNPCC)/Lynch syndrome. Dtsch Arztebl Int. 110:32–38.
2013.PubMed/NCBI
|
|
2
|
Kalady MF: Surgical management of
hereditary nonpolyposis colorectal cancer. Adv Surg. 45:265–274.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rebeccah B and Wise PE: Endoscopic and
surgical management of hereditary nonpolyposis colorectal cancer.
Clin Colon Rectal Surg. 25:90–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lynch HT and de la Chapelle A: Hereditary
colorectal cancer. N Engl J Med. 348:919–932. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodriguez-Bigas MA, Chang GJ and Skibber
JM: Surgical implications of colorectal cancer genetics. Surg Oncol
Clin N Am. 15(51–66): vi2006.
|
|
6
|
Brandão C and Lage J: Management of
patients with hereditary colorectal cancer syndromes. GE Port J
Gastroenterol. 22:204–212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vasen HF, Möslein G, Alonso A, Bernstein
I, Bertario L, Blanco I, Burn J, Capella G, Engel C, Frayling I, et
al: Guidelines for the clinical management of Lynch syndrome
(hereditary non-polyposis cancer). J Med Genet. 44:353–362. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Son IT, Kim DW, Jeong SY, Shin YK, Ihn MH,
Oh HK, Kang SB, Park KJ, Oh JH, Ku JL and Park JG:
Clinicopathological features and type of surgery for lynch
syndrome: Changes during the past two decades. Cancer Res Treat.
48:605–611. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vasen HF, Watson P, Mecklin JP and Lynch
HT: New clinical criteria for hereditary nonpolyposis colorectal
cancer (HNPCC, Lynch syndrome) proposed by the International
Collaborative group on HNPCC. Gastroenterology. 116:1453–1456.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Umar A, Boland CR, Terdiman JP, Syngal S,
de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ,
Hamelin R, et al: Revised Bethesda Guidelines for hereditary
nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite
instability. J Natl Cancer Inst. 96:261–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schneider R, Schneider C, Jakobeit C,
Fürst A and Möslein G: Gender-specific aspects of lynch syndrome
and familial adenomatous polyposis. Viszeralmedizin. 30:82–88.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dunlop MG, Farrington SM, Carothers AD,
Wyllie AH, Sharp L, Burn J, Liu B, Kinzler KW and Vogelstein B:
Cancer risk associated with germline DNA mismatch repair gene
mutations. Hum Mol Genet. 6:105–110. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindor NM, Petersen GM, Hadley DW, Kinney
AY, Miesfeldt S, Lu KH, Lynch P, Burke W and Press N:
Recommendations for the care of individuals with an inherited
predisposition to Lynch syndrome: A systematic review. JAMA.
296:1507–1517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Järvinen HJ, Mecklin JP and Sistonen P:
Screening reduces colorectal cancer rate in families with
hereditary nonpolyposis colorectal cancer. Gastroenterology.
108:1405–1411. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Syngal S, Brand RE, Church JM, Giardiello
FM, Hampel HL and Burt RW: American College of Gastroenterology:
ACG clinical guideline: Genetic testing and management of
hereditary gastrointestinal cancer syndromes. Am J Gastroenterol.
110:223–263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samadder NJ, Smith KR, Wong J, Thomas A,
Hanson H, Boucher K, Kopituch C, Cannon-Albright LA, Burt RW and
Curtin K: Cancer risk in families fulfilling the Amsterdam criteria
for lynch syndrome. JAMA Oncol. 3:1697–1701. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Duijvendijk P, Slors JF, Taat CW,
Oosterveld P and Vasen HF: Functional outcome after colectomy and
ileorectal anastomosis compared with proctocolectomy and ileal
pouch-anal anastomosis in familial adenomatous polyposis. Ann Surg.
230:648–654. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karahan B, Argon A, Yıldırım M and Vardar
E: Relationship between MLH-1, MSH-2, PMS-2, MSH-6 expression and
clinicopathological features in colorectal cancer. Int J Clin Exp
Pathol. 8:4044–4053. 2015.PubMed/NCBI
|
|
19
|
Barrison AF, Smith C, Oviedo J, Heeren T
and Schroy PC III: Colorectal cancer screening and familial risk: A
survey of internal medicine residents' knowledge and practice
patterns. Am J Gastroenterol. 98:1410–1416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park J, Lee SY, Kim DW, Kang SB, Jeong SY
and Park KJ: Knowledge of and practice patterns for hereditary
colorectal cancer syndromes in korean surgical residents. Ann
Coloproctol. 29:186–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Win AK, Parry S, Parry B, Kalady MF,
Macrae FA, Ahnen DJ, Young GP, Lipton L, Winship I, Boussioutas A,
et al: Risk of metachronous colon cancer following surgery for
rectal cancer in mismatch repair gene mutation carriers. Ann Surg
Oncol. 20:1829–1836. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodriguez-Bigas MA and Möeslein G:
Surgical treatment of hereditary nonpolyposis colorectal cancer
(HNPCC, Lynch syndrome). Fam Cancer. 12:295–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haanstra JF, de Vos Tot Nederveen Cappel
WH, Gopie JP, Vecht J, Vanhoutvin SA, Cats A, van der Zaag-Loonen
HJ, Langers AM, Bergmann JH, van de Meeberg PC, et al: Quality of
life after surgery for colon cancer in patients with Lynch
syndrome: Partial versus subtotal colectomy. Dis Colon Rectum.
55:653–659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Vos tot Nederveen Cappel WH, Buskens E,
van Duijvendijk P, Cats A, Menko FH, Griffioen G, Slors JF,
Nagengast FM, Kleibeuker JH and Vasen HF: Decision analysis in the
surgical treatment of colorectal cancer due to a mismatch repair
gene defect. Gut. 52:1752–1755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
You YN, Chua HK, Nelson H, Hassan I,
Barnes SA and Harrington J: Segmental vs. extended colectomy:
Measurable differences in morbidity, function, and quality of life.
Dis Colon Rectum. 51:1036–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Natarajan N, Watson P, Silva-Lopez E and
Lynch HT: Comparison of extended colectomy and limited resection in
patients with Lynch syndrome. Dis Colon Rectum. 53:77–78. 2010.
View Article : Google Scholar : PubMed/NCBI
|