Introduction
Myocarditis refers to the inflammatory condition of
the heart caused by various factors (1). Clinical manifestations of myocarditis
range from mild dyspnea to heart failure or even sudden death
(2). Although infection with
parasites, fungi and bacteria, drug-induced hypersensitivity and
autoimmune disorders all induce myocarditis, viruses, including
enteroviruses, influenza viruses, adenoviruses, parvoviruses,
cytomegaloviruses, human immunodeficiency virus and herpes viruses,
are the most common causes of myocarditis (3). High incidence of viral myocarditis
(VMC) is observed in young individuals, and it has been reported
that VMC is responsible for 12% sudden deaths in patients who were
younger than 40 years (4,5). Besides damages of cardiac tissues
directly caused by viruses, reactive inflammatory responses caused
by viral reaction also significantly promote cardiac injury
(6). Although, short-term outcomes
of treatment of VMC are usually satisfactory, patients recovered
from VMC may develop heart failure and recurrent dilated
cardiomyopathy years later (2).
Therefore, it would be of great clinical value to identify novel
drugs to improve the treatment outcomes of VMC.
As a labdane diterpenoid isolated from
Andrographispaniculata, andrographolide has been widely used in
treatment of various human diseases including different types of
cancers (7), obesity (8), oxidative damage (9), and so on. Andrographolide is also a
safe and effective drug for treatment of various inflammatory
diseases (10). A recent study
reported that andrographolide improved cardiac malfunctions in mice
by reducing cell apoptosis and inhibiting phosphorylation of IκB
(11). In another study,
andrographolide was proved to effectively improve infections of
different viruses (12). In view of
the pathogenesis of VMC and the functionality of andrographolide,
it will be reasonable to hypothesize that andrographolide may also
has certain therapeutic effects on VMC.
In this this, mice VMC model was established by
Coxsackie B3m virus infection, which is a main cause of VMC in
human (13). Andrographolide was
used to treat mice VMC model and its effects on cardiac function
was detected. In addition, interactions between andrographolide and
IL-10/STAT3 pathway and phosphoinositide 3-kinase (P13K)/AKT/NF-κβ
pathway were also investigated. The report is as follow:
Materials and methods
Establishment of mice VMC model and
treatments
Thirty-mice (4–6 weeks old) were purchased from
BALB/c Mice Guangdong Medical Laboratory Animal Center (Guangzhou,
China). Mice were randomly divided into three groups including
control group, model group and andrographolide group (n=10). One
week before model construction, mice in Andrographolide group were
intraperitoneally injected with 100 µl PBS containing
Andrographolide at a dose of 1 mg/kg, while mice in control group
and model group were only injected with 100 µl PBS. Mice were
subjected to intraperitoneal injection of 0.2 ml PBS solution
containing 1×106 PFU Coxsackie B3 m virus (CVB3m, Nancy) to induce
VMC. This study has been approved by the ethics committee of Jining
No. 1 People's Hospital.
Measurement of physiological
indexes
One week after model construction, physiological
indexes including heart weight/body weight (HW/BW), heart rate
(HR), mean arterial pressure (MAP), high left ventricular diastolic
end pressure (LVDEP) and ventricular contractility assessment
(dP/dt) were measured using conventional methods. All indexes were
measured 3 times, and the data were expressed as mean ± SD.
Echocardiographic examination
Echocardiographic examination was performed at 1
week after model construction. After anesthesia with isoflurane,
M-model imaging was performed through two-dimensional
echocardiography (Sonos 5500; Philips Medical Systems, Inc.,
Bothell, WA, USA) using a 12-MHz probe to measure left ventricular
systolic dimension (LVDs), left ventricular diastolic dimension
(LVDd), anterior wall thickness (AWT) and posterior wall thickness
(PWT). All indexes were measured 3 times, and the data were
expressed as mean ± SD.
Enzyme-linked immunosorbent assay
(ELISA)
Blood (about 1 ml) was collected by cutting the tail
at 1 week after model construction. Blood samples were centrifuged
(10,000 rpm) at room temperature for 10 min to separate serum.
Levels of TNF-α, hsCRP and cTnl were measured by ELISA using kits
provided by R&D Systems, Inc., (Minneapolis, MN, USA). Briefly,
standard solutions were made through serial dilution of TNF-α,
hsCRP and cTnl stock solutions with diluent buffer in the kits to
generate standard curves. Biotin-labeled human TNF-α, hsCRP and
cTnl antibodies were used at a dilution of 1:800. Avidin-peroxidase
complex was sued at a dilution of 1:500. OD values at 480 nm were
measured using a microplate reader. ELSIA was performed 3 times,
and the data were expressed as mean ± SD.
Western blot analysis
Mice were sacrificed 1 week after model
construction, and the whole heart was collected. Heart was cut into
pieces and ground in liquid nitrogen. Total protein was extracted
from heart tissues using conventional method, and BCA method was
used to quantify protein concentration. After that, 40 µg of
protein was subjected to 10% SDS-PAGE gel electrophoresis, followed
by transmembrane to PVDF membrane. After blocking with 5% skimmed
milk, membranes were incubated with rabbit anti-NF-κB p65 antibody
(1:1,000, ab16502), rabbit anti-NF-κB p50 antibody (1:1,000,
ab32360), rabbit anti-IL-10 antibody (1:1,000, ab9969), rabbit
anti-STAT3 antibody (1:1,000, ab76315), rabbit anti-p-PI3K antibody
(1:2,000, ab182651), rabbit anti-PI3K antibody (1:2,000, ab5451),
rabbit anti-p-AKT antibody (1:2,000, ab18206), rabbit anti-AKT
antibody (1:2,000, ab126811), rabbit anti-Iκβα antibody (1:1,000,
ab76429), and rabbit anti-GAPDH antibody (1:1,000, ab8245; all
purchased from Abcam, Cambridge, UK) overnight at 4°C. After
washing with TBST 3 times, 15 min for each time, anti-rabbit
IgG-HRP secondary antibody (1:1,000, MBS435036; http://www.mybiosource.com) was used to incubated with
membranes at room temperature for 2 h. After washing with TBST, ECL
detection reagent (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was used to detect the signals. Band intensity within a frame
covers all bands was measured using ImageJ software (National
Institutes of Health, Bethesda, MD, USA). Intensity of target band
was normalized to that of endogenous control. We added this
information. Each experiment was performed 3 times, and the data
were expressed as mean ± SD.
Statistical analysis
Statistical analyses were performed using SPSS
v.19.0 (SPSS, Inc., Chicago, IL, USA). All data were expressed as
mean ± standard deviation (mean ± SD). Comparisons of data among
multiple groups were performed using one-way analysis of variance,
followed by LSD test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of physiological indexes
among groups
Physiological indexes were measured 1 week after
model construction. As shown in Table
I, HR and MAP significantly decreased, and HW/BW, LVDEP and Min
dP/dt significantly increased in model group compared with control
group (P<0.05), indicating the impaired cardiac function caused
by VMC. Compared with model group, HR and MAP significantly
decreased and HW/BW, LVDEP and Min dP/dt significantly decreased in
andrographolide group (P<0.05). Those data suggest that
andrographolide treatment can inhibit the reduction in cardiac
function caused by VMC.
 | Table I.Comparison of physiological parameters
between groups. |
Table I.
Comparison of physiological parameters
between groups.
| Items | Control | Model | Andrographolide |
|---|
| HR (bpm) | 391.2±5.1 |
334.1±3.9a |
377.4±9.6b |
| MAP (mmHg) | 98.4±7.7 | 71.3±4.8a | 87.5±4.6b |
| HW/BW (g/kg) | 2.4±0.1 | 3.9±0.1a | 2.7±0.1b |
| LVDEP (mmHg) | 4.4±0.7 | 6.3±0.4a | 5.0±0.5b |
| Min dP/dt
(mmHg/s) | −7998.6±345.2 |
−4675.8±312.5a |
−6671.7±341.9b |
Comparison of echocardiographic
examination results among groups
As shown in Table
II, echocardiographic examination results showed that LVDd,
LVDs, PWT diastole and AWT diastole were significantly increased in
model group than in control group (P<0.05). Compared with model
group, LVDd, LVDs, PWT diastole and AWT diastole significantly
reduced in andrographolide group. Those data suggest that
andrographolide treatment can significantly reduce the adverse
effects of VMC on cardiac function.
 | Table II.Comparison of echocardiographic
examination results between groups. |
Table II.
Comparison of echocardiographic
examination results between groups.
| Items | Control | Model | Andrographolide |
|---|
| LVDd (mm) | 7.0±0.1 | 7.4±0.1a | 7.0±0.1b |
| LVDs (mm) | 3.5±0.1 | 4.7±0.2a | 3.9±0.2b |
| PWT diastole
(mm) | 1.6±0.2 | 2.8±0.3a | 1.9±0.1b |
| AWT diastole
(mm) | 1.5±0.1 | 2.9±0.2a | 1.8±0.2b |
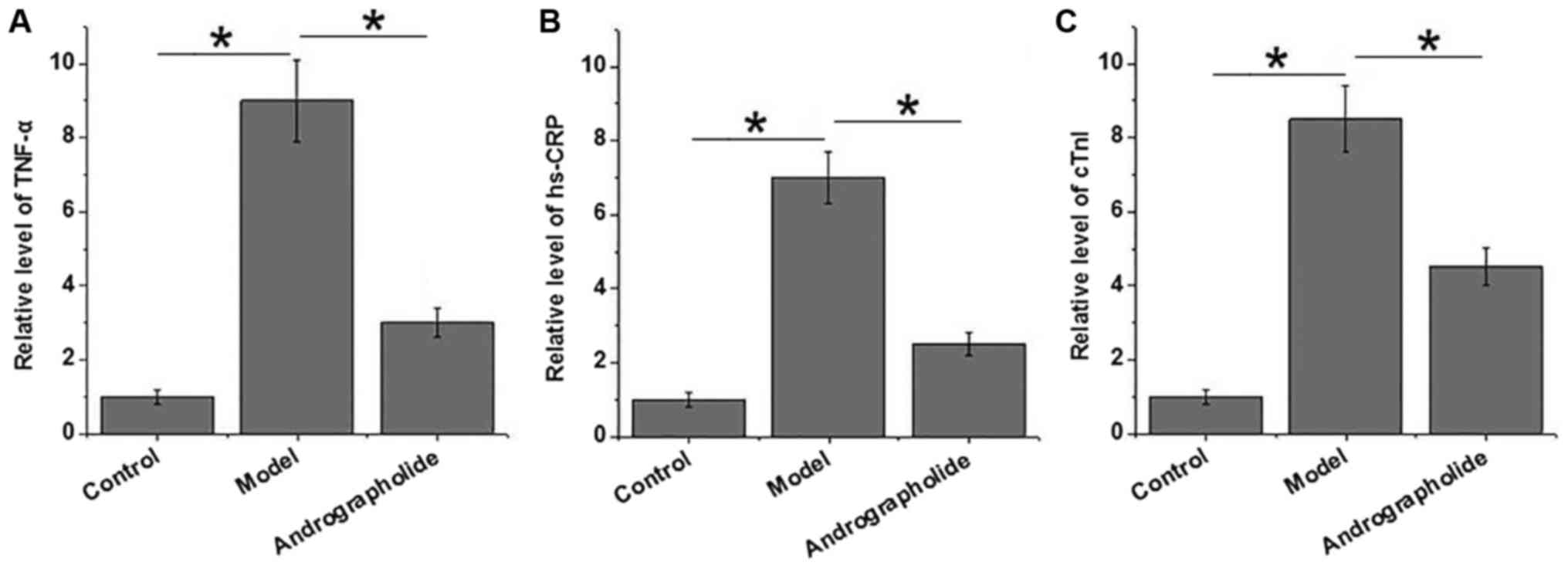
Comparison of serum levels of TNF-α,
hs-CRP and cTnl between groups
TNF-α, hs-CRP and cTnl are closely related to the
development of VMC. As shown in Fig.
1, serum levels of TNF-α, hs-CRP and cTnl significantly
increased in model group than in control group (P<0.05),
indicating the progression of VMC in mice of model group. Compared
with model group, serum levels of TNF-α, hs-CRP and cTnl were
significantly reduced in andrographolide group (P<0.05). Those
data suggest that andrographolide treatment can inhibit the
development of VMC by reducing serum levels of TNF-α, hs-CRP and
cTnl.
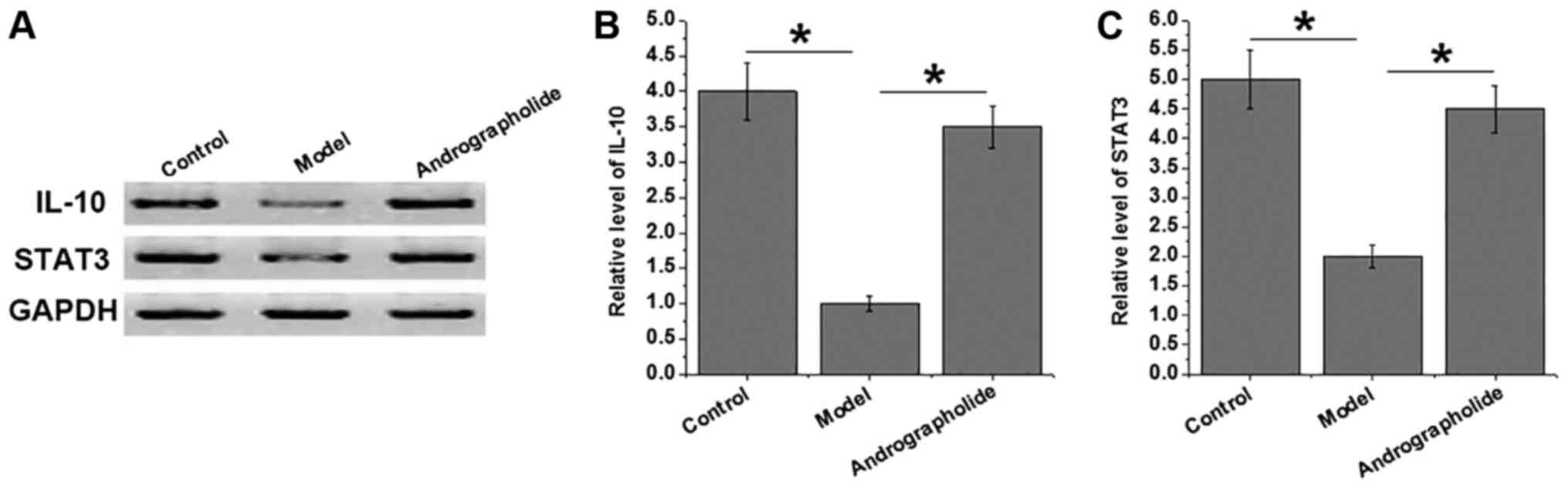
Effects of andrographolide on
IL-10/STAT3 pathway
IL-10/STAT3 pathway mediates the anti-inflammatory
signal transduction in various pathological processes. As shown in
Fig. 2, expression levels of IL-10
protein and STAT3 protein in heart tissues were significantly
reduced in model group than in control group (P<0.05),
indicating that the inhibition of IL-10/STAT3 pathway is involved
in the development of VMC. Compared with model group, expression
levels of IL-10 protein and STAT3 protein in heart tissue
significantly increased in andrographolide group (P<0.05). Those
data suggest that andrographolide treatment can inhibit the
development of VMC by activating IL-10/STAT3 anti-inflammatory
pathway.
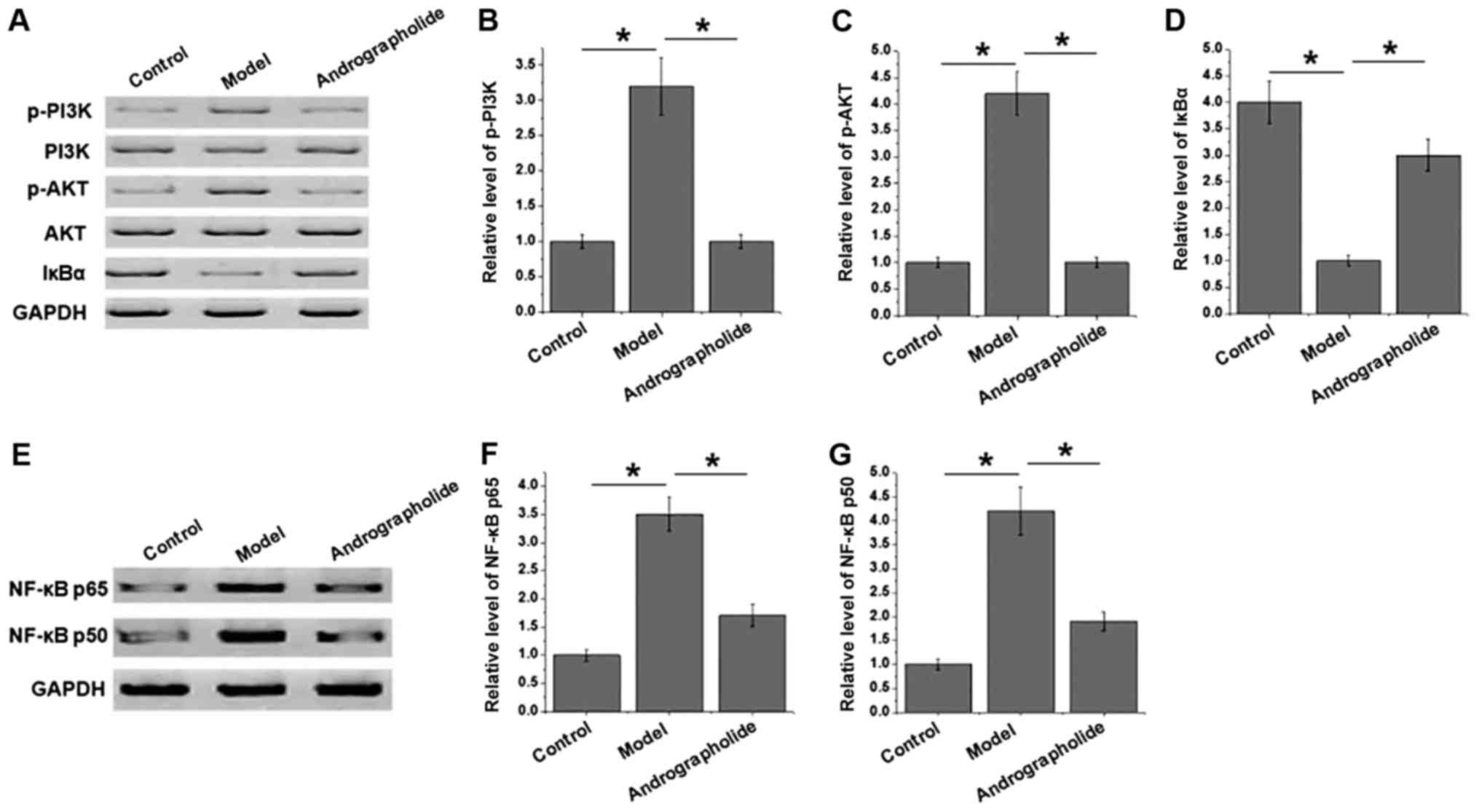
Effects of andrographolide on NF-κβ
pathway
NF-κβ pathway plays pivotal roles in development of
various human diseases, and PI3K/Akt signaling pathway is involved
in the activation of NF-κβ pathway by regulating the degradation of
Iκβα. As shown in Fig. 3A-d, no
significantly differences in levels of total PI3K and Akt in heart
tissue were found between groups, while phosphorylation of PI3K and
Akt significantly increased in model group compared with control
group (P<0.05), indicating the activation of PI3K/Akt signaling
pathway. Compared with model group, phosphorylation of PI3K and Akt
significantly decreased in andrographolide group. In contrast,
level of Iκβα significantly decreased in model group compared with
control group (P<0.05). Compared with model group, level of Iκβα
significantly increased in andrographolide group. In addition, as
shown in Fig. 3E-G, levels of NF-κβ
p65 and NF-κβ p50 significantly increased in model group than in
control group (P<0.05), indicating the involvement of NF-κβ
pathway in progression of VMC. Compared with model group, levels of
NF-κβ p65 and NF-κβ p50 significantly decreased in andrographolide
group (P<0.05). Those data suggest that andrographolide
treatment may inhibit the development of VMC by inhibiting
PI3K/Akt/NF-κβ pathway.
Discussion
Andrographolide is the main active ingredient of
Andrographis paniculata, which is traditional herbal medicine that
has been widely used in Scandinavia and Asia to treat sore throat,
upper respiratory tract infections and flu (10,14).
Recently, effects of andrographolide on virus infection have also
been reported. Wintachai et al (15), reported that andrographolide could
significantly alleviate chikungunya virus infection by inhibiting
or stimulating a variety of targets. In the present study of
cervical carcinoma, Ekalaksananan et al (16), found that andrographolide and its
derivatives inhibited HPV16 pseudovirus infection by inhibiting the
expression of viral oncogene. In another study, andrographolide was
proved to inhibit the activity of hepatitis C virus by increasing
the expression level of haeme oxygenase-1 through interactions with
p38 MAPK/Nrf2 pathway in hepatoma cells of human (17). As a cardiac disease caused by virus
infection, VMC significantly affects cardiac function. Even worse,
cardiac function may be impaired after the recovery from VMC
(18). Consistent with previous
studies, cardiac function was significantly reduced in mice VMC
model compared with control mice. However, VMC mice pretreated with
andrographolide showed significantly better cardiac function
compared with VMC mice without andrographolide treatment. Those
data suggest that andrographolide can improve impaired cardiac
function after VMC. Cardiac TNF-α, hs-CRP and cTnl play pivotal
roles in development of VMC, and detection of levels of TNF-α,
hs-CRP and cTnl have been proved to be an effective and accurate
method to predict VMC (19). In our
study, serum levels of TNF-α, hs-CRP and cTnl were significantly
increased in mice VMC model, while andrographolide pretreatment
significantly inhibited the increase in serum levels of TNF-α,
hs-CRP and cTnl in mice with VMC. Those data suggest that
andrographolide treatment can promote VMC by reducing serum levels
of TNF-α, hs-CRP and cTnl.
To investigate the molecular mechanism of the
therapeutic effects of andrographolide on VMC, the possible
involvement of IL-10/STAT3 pathway was investigated. Cardiac injury
caused by reactive inflammatory responses after viral reaction has
stronger promotion effects on development of VMC compared with the
cell damages directly caused by virus (6). As an anti-inflammatory factor, IL-10
inhibits various human diseases by mediating anti-inflammatory
response (20). Animal studies have
showed that increased levels of IL-10 protected heart cells from
damages caused by acute myocarditis (21). IL-10 achieves it biological functions
by interacting with its downstream STAT3, which is a transcription
factor and regulates the expression of various targets (20). In our study, expression levels of
IL-10 and STAT3 were significantly lower in mice with VMC than in
control mice, indicating the involvement of IL-10/STAT3 in
development of VMC. Compared with model group, andrographolide
pretreatment significantly inhibited the decrease in expression
levels of IL-10 and STAT3. Those data suggest that andrographolide
may improve VMC by activating IL-10/STAT3 pathway.
NF-κβ pathway is involved in the pathogenesis of VMC
through the regulation of immune responses (22), and the excessive inflammation caused
by NF-κβ pathway in VMC is the main cause chronic heart failure and
cardiac hypertrophy. Previous studies have shown that drug that
targets NF-κβ pathway can be used to treat VMC (23). It's well accepted that activation of
NF-κβ can be mediated by PI3K/Akt signaling pathway via the
regulation of Iκβα degradation (24,25). In
our study, phosphorylation levels of PI3K and Akt increased, while
level of Iκβα decreased by VMC. In addition, expression levels of
NF-κβ p65 and NF-κβ p50 also increased in VMC. Those results
suggest that PI3K/Akt/NF-κβ pathway is activated in the progression
of VMC. Compared with model group, andrographolide pretreatment
significantly inhibited the phosphorylation of PI3K and Akt,
decreased the level of Iκβα in heart tissue and downregulated the
expression levels of NF-κβ p65 and NF-κβ p50. Those results suggest
that andrographolide treatment may improve VMC by inhibiting
PI3K/Akt/NF-κβ pathway.
In conclusion, andrographolide may improve VMC by
inhibiting the increase in serum levels of TNF-α, hs-CRP and cTnl
caused by VMC, activating IL-10/STAT3 anti-inflammatory pathway and
inactivating PI3K/Akt/NF-κβ pathway. Further clinical studies are
needed to confirm our conclusions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, YL and WD conceived and designed the study. YZ
and MW performed experiments and analyzed data. YZ and YL
interpreted the data. YL drafted the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jining No. 1 People's Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Corsten MF, Schroen B and Heymans S:
Inflammation in viral myocarditis: Friend or foe? Trends Mol Med.
18:426–437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sagar S, Liu PP and Cooper LT Jr:
Myocarditis. Lancet. 379:738–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dennert R, Crijns HJ and Heymans S: Acute
viral myocarditis. Eur Heart J. 29:2073–2082. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blauwet LA and Cooper LT: Myocarditis.
Prog Cardiovasc Dis. 52:274–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Esfandiarei M and McManus BM: Molecular
biology and pathogenesis of viral myocarditis. Annu Rev Pathol.
3:127–155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shauer A, Gotsman I, Keren A, Zwas DR,
Hellman Y, Durst R and Admon D: Acute viral myocarditis: Current
concepts in diagnosis and treatment. Isr Med Assoc J. 15:180–185.
2013.PubMed/NCBI
|
|
7
|
Liu Y, Liang RM, Ma QP, Xu K, Liang XY,
Huang W, Sutton R, Ding J, O'Neil PM and Cheng CR: Synthesis of
thioether andrographolide derivatives and their inhibitory effect
against cancer cells. MedChemComm. 8:1268–1274. 2017. View Article : Google Scholar
|
|
8
|
Ding L, Li J, Song B, Xiao X, Huang W,
Zhang B, Tang X, Qi M, Yang Q, Yang Q, et al: Andrographolide
prevents high-fat diet-induced obesity in C57BL/6 mice by
suppressing the sterol regulatory element-binding protein pathway.
J Pharmacol Exp Ther. 351:474–483. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen HW, Huang CS, Li CC, Lin AH, Huang
YJ, Wang TS, Yao HT and Lii CK: Bioavailability of andrographolide
and protection against carbon tetrachloride-induced oxidative
damage in rats. Toxicol Appl Pharmacol. 280:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan WSD, Liao W, Zhou S and Wong WSF: Is
there a future for andrographolide to be an anti-inflammatory drug?
Deciphering its major mechanisms of action. Biochem Pharmacol.
139:71–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Zhu D, Wang Y and Ju Y:
Andrographolide attenuates LPS-induced cardiac malfunctions through
inhibition of IκB phosphorylation and apoptosis in mice. Cell
Physiol Biochem. 37:1619–1628. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu RH, Jacob JR and Tennant B:
Andrographolide derivatives to treat viral infections: U.S. Patent
8445,533 (P). 2013.
|
|
13
|
Weller AH, Simpson K, Herzum M, Van Houten
N and Huber SA: Coxsackievirus-B3-induced myocarditis: Virus
receptor antibodies modulate myocarditis. J Immunol. 143:1843–1850.
1989.PubMed/NCBI
|
|
14
|
Jayakumar T, Hsieh CY, Lee JJ and Sheu JR:
Experimental and clinical pharmacology of andrographis paniculata
and its major bioactive phytoconstituent andrographolide. Evid
Based Complement Alternat Med. 2013:8467402013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wintachai P, Kaur P, Lee RC, Ramphan S,
Kuadkitkan A, Wikan N, Ubol S, Roytrakul S, Chu JJ and Smith DR:
Activity of andrographolide against chikungunya virus infection.
Sci Rep. 5:141792015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ekalaksananan T, Sookmai W, Fangkham S,
Pientong C, Aromdee C, Seubsasana S and Kongyingyoes B: Activity of
andrographolide and its derivatives on HPV16 pseudovirus infection
and viral oncogene expression in cervical carcinoma cells. Nutr
Cancer. 67:687–696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JC, Tseng CK, Young KC, Sun HY, Wang
SW, Chen WC, Lin CK and Wu YH: Andrographolide exerts
anti-hepatitis C virus activity by up-regulating haeme oxygenase-1
via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br J
Pharmacol. 171:237–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Becher PM, Gotzhein F, Klingel K, Escher
F, Blankenberg S, Westermann D and Lindner D: Cardiac function
remains impaired despite reversible cardiac remodeling after acute
experimental viral myocarditis. J Immunol Res. 2017:65906092017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang D, Li T, Cui H and Zhang Y: Analysis
of the indicating value of cardiac troponin i, tumor necrosis
factor-α, interleukin-18, Mir-1 and Mir-146b for viral myocarditis
among children. Cell Physiol Biochem. 40:1325–1333. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hutchins AP, Diez D and Miranda-Saavedra
D: The IL-10/STAT3-mediated anti-inflammatory response: Recent
developments and future challenges. Brief Funct Genomics.
12:489–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roffê E, Rothfuchs AG, Santiago HC, Marino
AP, Ribeiro-Gomes FL, Eckhaus M, Antonelli LR and Murphy PM: IL-10
limits parasite burden and protects against fatal myocarditis in a
mouse model of Trypanosoma cruzi infection. J Immunol. 188:649–660.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maier HJ, Schips TG, Wietelmann A, Krüger
M, Brunner C, Sauter M, Klingel K, Böttger T, Braun T and Wirth T:
Cardiomyocyte-specific IκB kinase (IKK)/NF-κB activation induces
reversible inflammatory cardiomyopathy and heart failure. Proc Natl
Acad Sci USA. 109:11794–11799. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valaperti A: Drugs targeting the canonical
NF-κB pathway to treat viral and autoimmune myocarditis. Curr Pharm
Des. 22:440–449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hyam SR, Lee IA, Gu W, Kim KA, Jeong JJ,
Jang SE, Han MJ and Kim DH: Arctigenin ameliorates inflammation in
vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing
M1 macrophages to M2-like macrophages. Eur J Pharmacol. 708:21–29.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han W, Xiong Y, Li Y, Fang W, Ma Y, Liu L,
Li F and Zhu X: Anti-arthritic effects of clematichinenoside (AR-6)
on PI3K/Akt signaling pathway and TNF-α associated with
collagen-induced arthritis. Pharm Biol. 51:13–22. 2013. View Article : Google Scholar : PubMed/NCBI
|