Introduction
Mycoplasma pneumoniae (M. pneumoniae)
is a common respiratory tract pathogen, especially in children
(1–3). Up to 40% of cases of community-acquired
pneumoniae admitted to the hospital are due to M. pneumoniae
infection. The central nervous system (CNS) may be one of the most
susceptible sites of M. pneumoniae besides the respiratory system
(4). M. pneumoniae infection
accounts for 5–10% of pediatric encephalitis patients and up to 60%
of these patients show neurologic sequelae and neuroimaging
discloses normal findings or focal diffuse edema (5). There have been few reports of the
clinical characteristics of adult M. pneumoniae-associated
encephalitis and the use of magnetic resonance imaging (MRI) in the
diagnosis.
Reversible splenial lesion syndrome (RESLES) is a
rare clinico-radiological syndrome that is defined as reversible
lesions that involve the splenium of the corpus callosum (SCC).
RESLES has been reported in patients with a broad spectrum of
diseases and conditions, including infections, hypoglycemia, and
poisoning. However, the pathophysiological mechanism of RESLES is
not well known (6).
We report four cases of adult mycoplasma-associated
encephalitis in which MRI scan revealed reversible lesions in
RESLES. Complete recovery of clinical symptoms and signs were
observed in a few days or weeks after treatment.
Is a common respiratory tract pathogen, especially
in children (1–3). Up to 40% of cases of community-acquired
pneumoniae admitted to the hospital are due to M. pneumoniae
infection. The CNS may be one of the most susceptible sites of
M. pneumoniae besides the respiratory system (4). M. pneumoniae infection accounts
for 5–10% of pediatric encephalitis patients and up to 60% of these
patients show neurologic sequelae and neuroimaging discloses normal
findings or focal diffuse edema (5).
There have been few reports of the clinical characteristics of
adult M. pneumoniae-associated encephalitis and the use of
MRI in the diagnosis.
RESLES is a rare clinico-radiological syndrome that
is defined as reversible lesions that involve the SCC. RESLES has
been reported in patients with a broad spectrum of diseases and
conditions, including infections, hypoglycemia, and poisoning.
However, the pathophysiological mechanism of RESLES is not well
known (6).
We report four cases of adult mycoplasma-associated
encephalitis in which MRI scan revealed reversible lesions in
RESLES. Complete recovery of clinical symptoms and signs were
observed in a few days or weeks after treatment.
Case report
Between March 2011 and November 2016, four patients
who presented with fever and headache were admitted to Shengjing
Hospital. Examinations of blood and cerebrospinal fluid (CSF), and
MRI scans were completed in the days after admission. The two male
and two female patients in this retrospective study had an average
age of 29.5±11.0 years. None of the patients had suffered from
brain trauma, toxic or metabolic encephalitis/encephalopathy,
cerebral vascular diseases, and cerebral neoplasma.
MRI was performed using 3.0 T units that comprised
axes of T1-and T2-weighted sequences. Fast fluid attenuated
inversion recovery (FLAIR) sequences and diffusion-weighted imaging
(DWI) were performed using an axial multi-section single-shot
echo-planar SE sequence. Enhanced T1-weighted scans obtained after
intravenous administration of 10 ml of gadoteric acid 0.5 mmol/ml.
MRI findings, such as the shape of the splenial lesion, and the DWI
and FLAIR findings were evaluated by two neuroimaging specialists
with over 10 years experience each. For M. pneumoniae
serology detection, IgM and IgG antibodies were detected using the
SeroMP IgM and IgG kit (Tecan Group, Ltd., Mannedorf, Switzerland),
which is a semi-quantitative ELISA to determine the antibodies
specific to M. pneumoniae. The antigen used in the SeroMP
kit is the P1 membrane protein, which is the most important
virulence factor of M. pneumoniae (7). Based on the manufacturer's suggestion,
a positive IgM as ≥1.1S/CO, the quantitative IgG was also detected
between 7 to 14 days after the first test, while recording the
multiplier of the increase of IgG (IgG ≥22 RU/ml as positive). An
IgM positive reaction together with an increase of IgG exceeding 4
times in patients indicated the presence of M. pneumoniae
acute infection (8). Other
laboratory tests including biochemical and microbiological
examinations of the CSF were available in all cases. After
treatment with azithromycin, the clinical symptoms and signs
recovered quickly. A follow-up MRI showed all lesions in SCC were
reversible at 10, 24, 12, and 7 days in case 1, 2, 3, and 4,
respectively.
Description of cases
Case 1
A 31-year-old Chinese man was transferred to our
emergency room from a nearby primary hospital. He had developed a
cold and a fever 3 days previously. His body temperature reached
39°C. He had persistent headaches. After 2 days' treatment in the
primary hospital, the fever and headache were not improved. At
admission, we found he had mild somnolence and Kernig sign (+),
with other neurological examinations being negative. Chest computed
tomography (CT) revealed an infective lesion in the right lung
lobe. In the third day following administration, a cranial MRI scan
showed focal hyper-intensities signals on the DWI, T2, and FLAIR
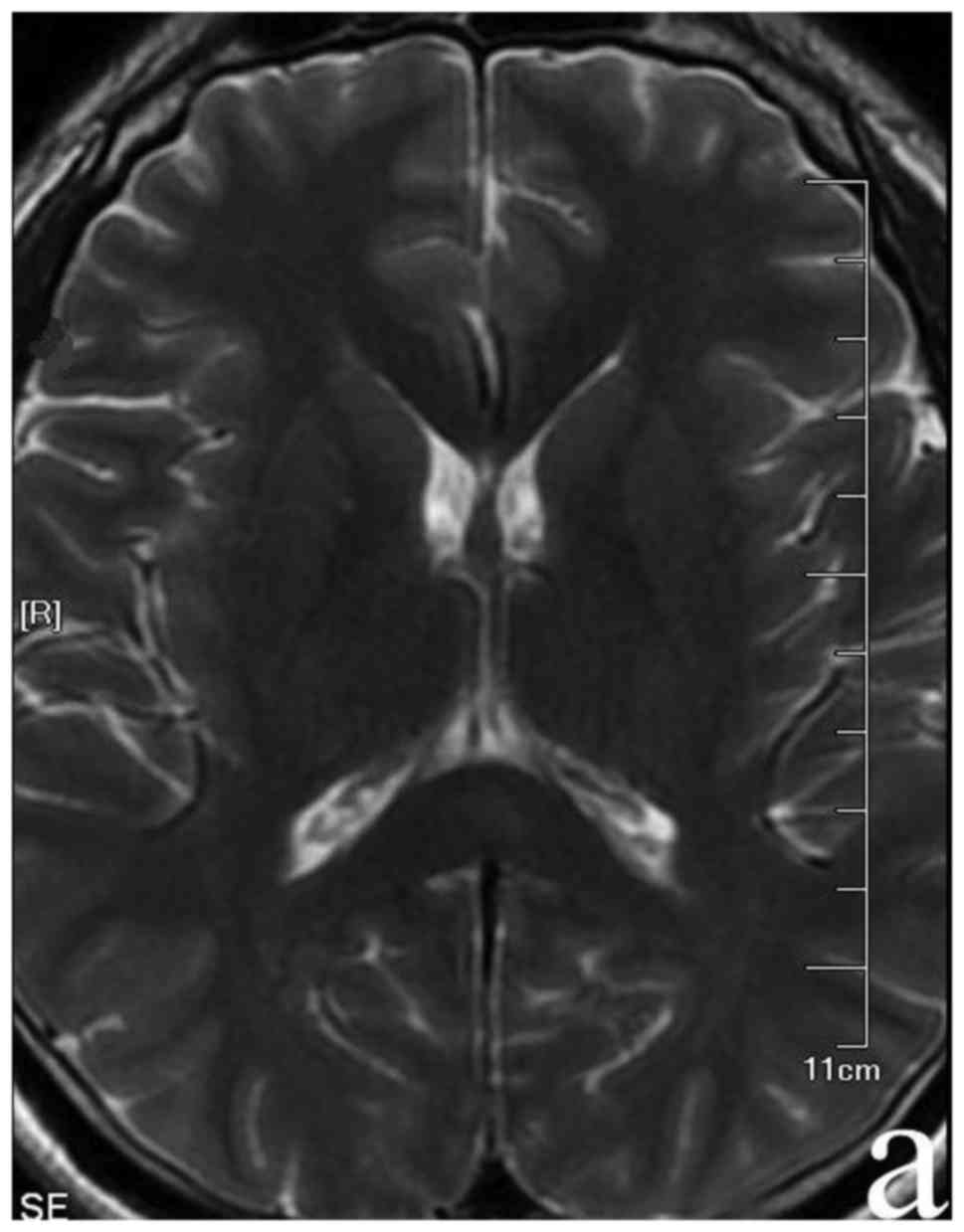
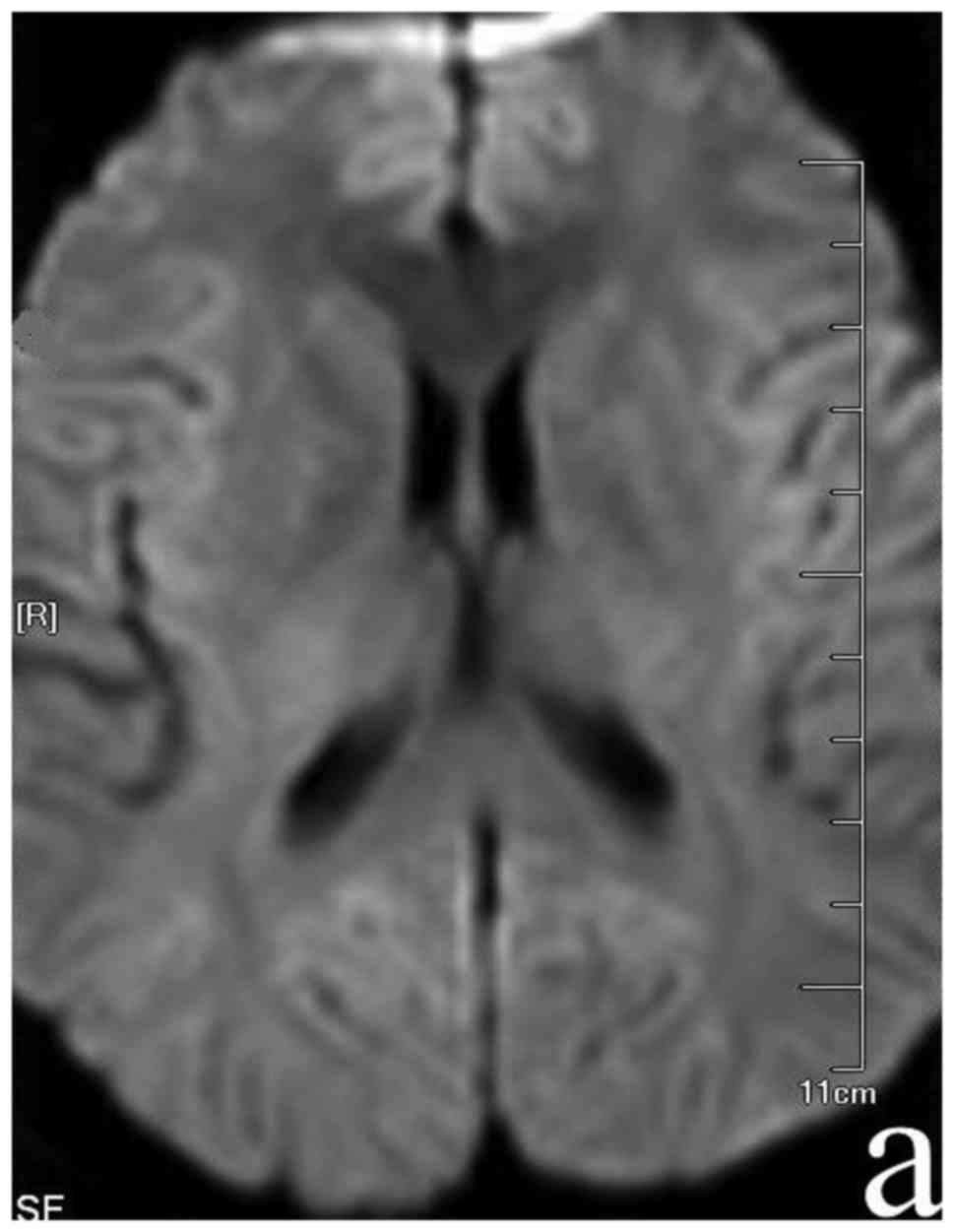
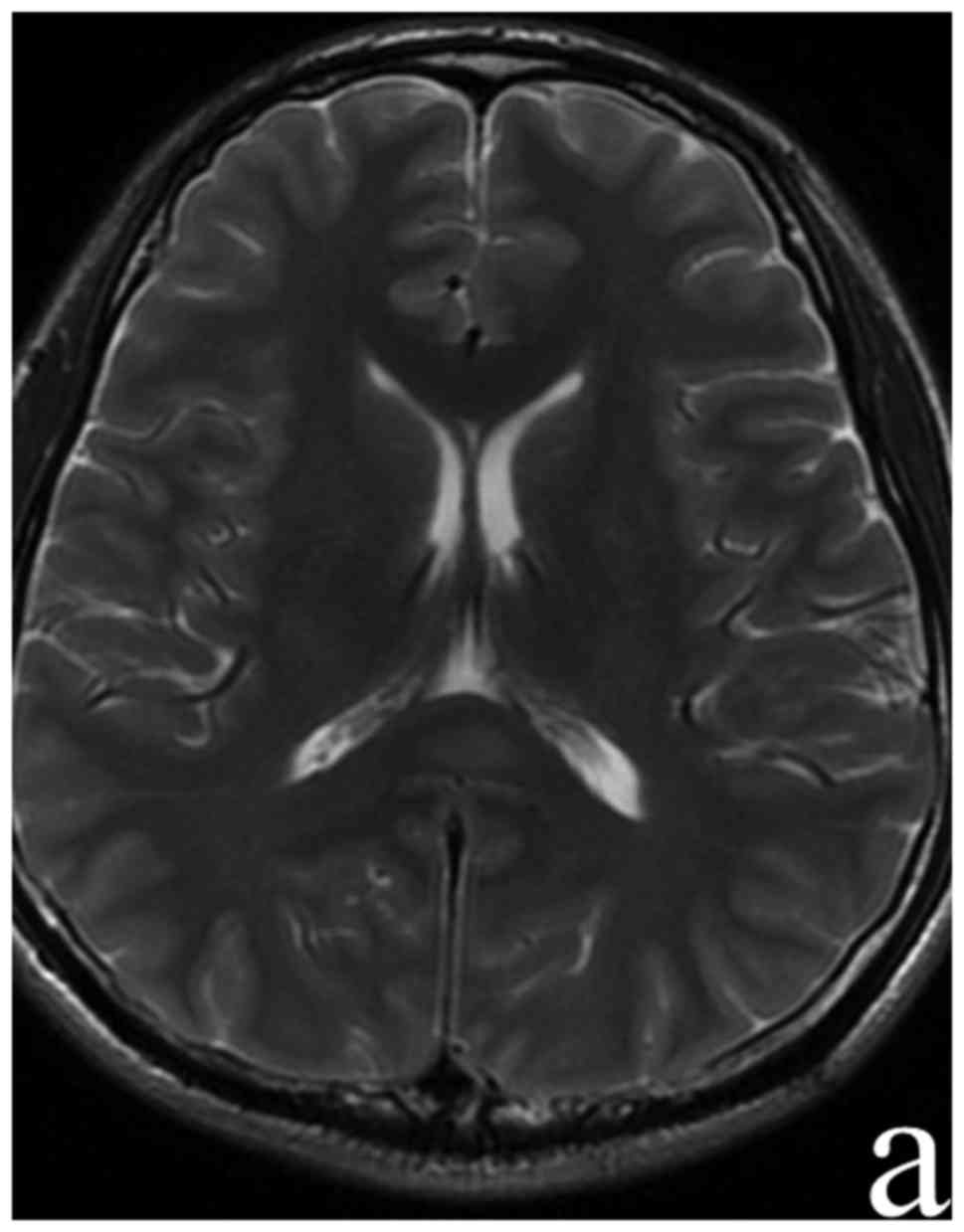
sequences and hypo-intensities on the T1 sequence in SCC (Fig. 1). Routine blood laboratory
examination revealed no obvious abnormalities. Serum M. pneumoniae
antibody showed IgM positive, with an IgG titer elevated to 1:80.
Serologic and pathogenic detection for other pathogens including
Chlamydia pneumoniae, varicella zoster virus (VZV), mumps virus,
measles virus, herpes simplex virus (HSV) 1 and 2, cytomegalovirus
(CMV) and Japanese encephalitis virus (JEV) were negative. CSF
analysis showed a high pressure (200 mmH2O), a normal
total white cell count of 6×106/l (reference range
0–5×106/l), CSF protein of 0.25 g/l (reference range
0.15–0.45 g/l), glucose of 4.14 mmol/l (reference range 2.5–4.5
mmol), and chloride of 120.6 mmol/l (reference range 120–132
mmol/l). General bacteria, tubercle bacilli, and cryptococcus were
not detected. Considering the clinical and serological results
together with the radiological findings, acute encephalitis due to
M. pneumoniae infection was diagnosed. Patient was treated with
intravenous azithromycin (10 mg/kg/day) for 2 weeks. The body
temperature returned to normal 3 days later during hospitalization.
The headache improved significantly after 6 days. Cranial MRI scan
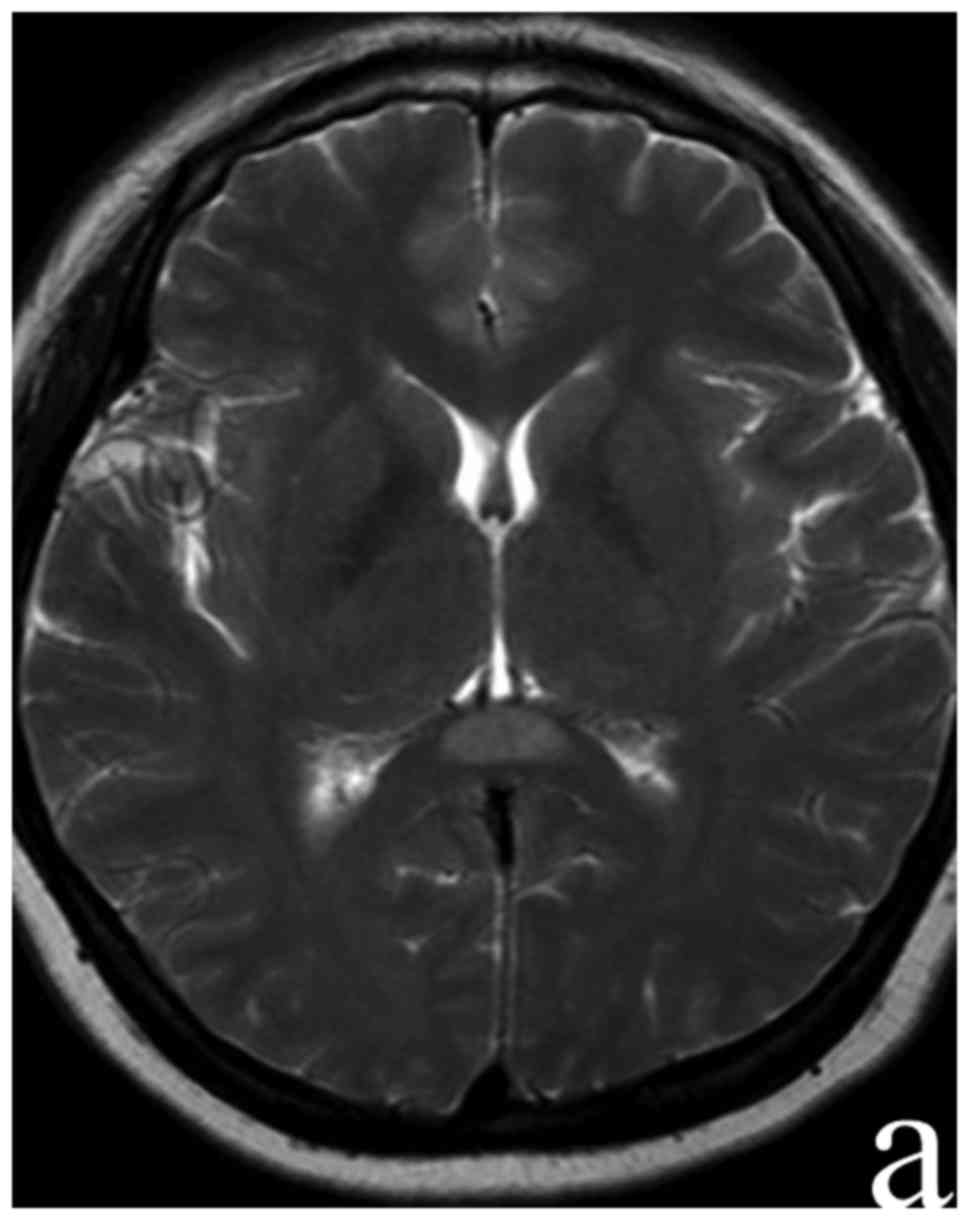
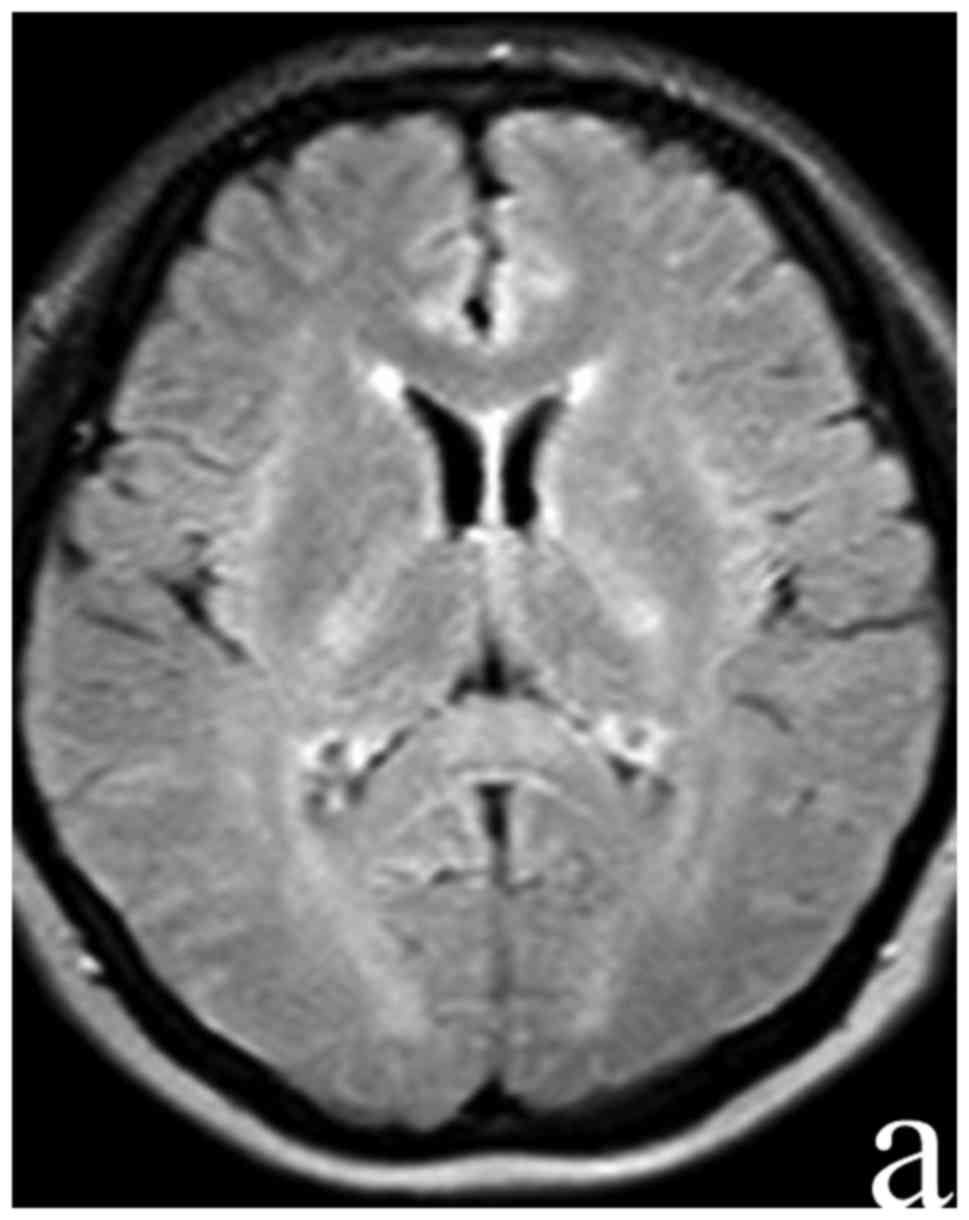
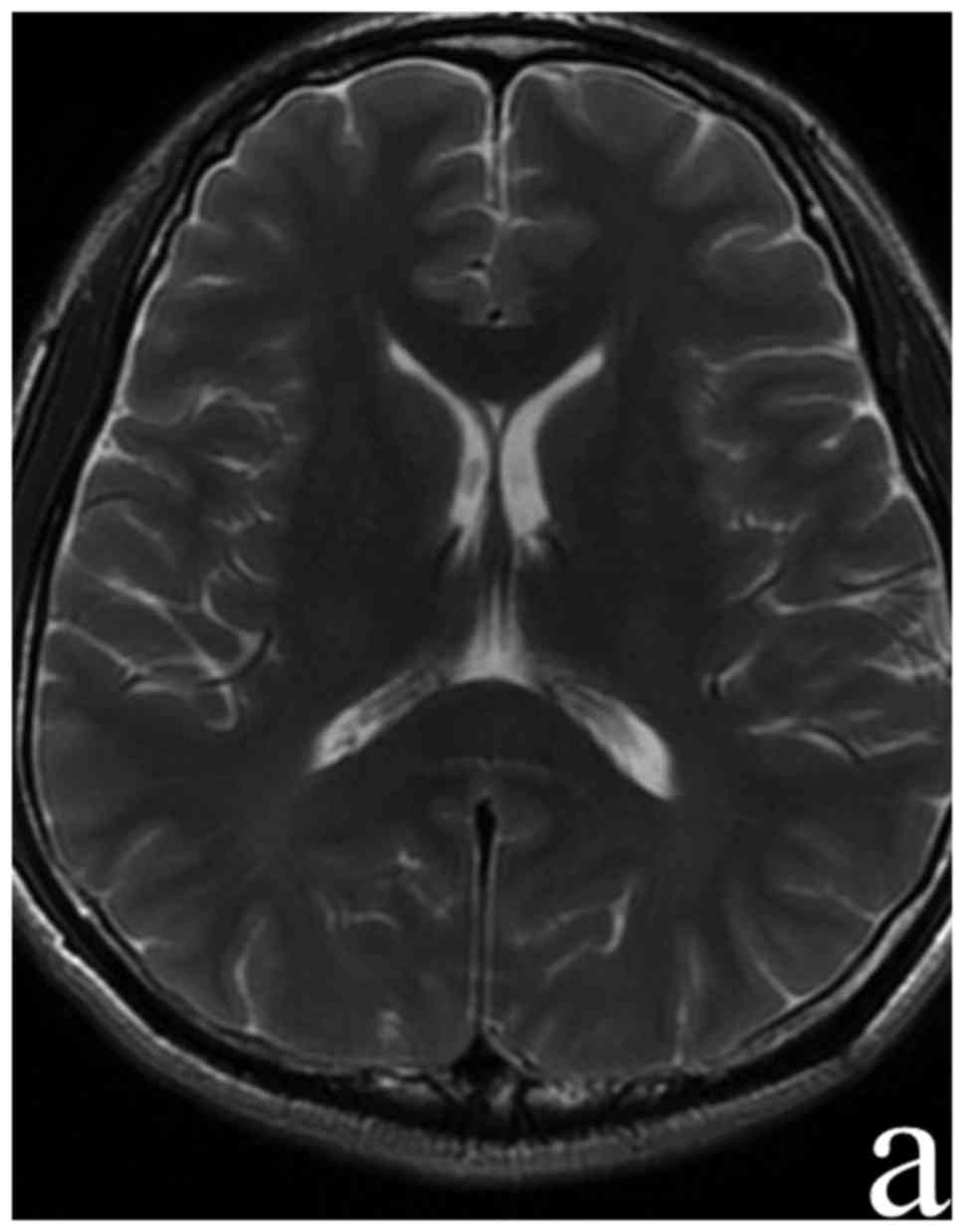
performed at day 10 following admission showed the complete
disappearance of focal hyperintersities in SCC (Fig. 2). The serum M. pneumoniae antibody
IgG was retested at day 12; an elevated titer of 1:1,280 was
evident. The results confirmed the diagnosis of M. pneumoniae
encephalitis.
Case 2
A week prior to admission, a 33-year-old Chinese
woman developed headache and fever without obvious predisposing
causes. The highest body temperature was 37.8°C. Four days later,
the patient developed blurred vision and a paroxysmal amaurosis. A
brain CT at that time performed at the fourth hospital affiliated
to China medical university revealed no abnormalities. Specific
serum M. pneumoniae antibody was markedly elevated to 1:320 on day
6 after onset. CSF analysis was abnormal with a pressure of 290
mmH2O, 316×106/l white cells, and 1.32 g/l
protein. Other laboratory tests of CFS, including general bacteria,
tubercle bacilli, and cryptococcus were all negative. At that time,
the patient was treated with mannitol (250 ml every 8 h),
ganciclovir (50 mg/kg/day with treatment every 12 h) and
azithromycin (10 mg/kg/day). Symptoms did not improve. The patient
displayed nausea and vomiting, and body temperature reached 39.5°C.
The patient was transferred to our hospital on day 9. The patient
had no past history of other related diseases except gastric ulcer
and hypertension. Neurological examination on administration showed
poor mental status and drowsiness, limb muscle strength grade 4,
and neck rigidity (+). Other neurological signs were negative.
Laboratory examination showed specific serum M. pneumoniae antibody
(IgM) was positive and IgG antibody titer was elevated to 1:640.
Serology for other pathogens including C. pneumoniae, VZV, mumps
virus, measles virus, HSV 1 and 2, CMV, and JEV were negative. CSF
analysis showed a pressure of 350 mmH2O, Pandy reaction
(+), an abnormal white cell count of 286×106/l, monocyte
percentage 96.9%, protein 0.96 g/l, 2.46 mmol/l glucose, and 119.7
mmol/l chloride. Chest CT scan showed infective lesions in the
middle lobe of the right lung and the upper lobe of the left lung.
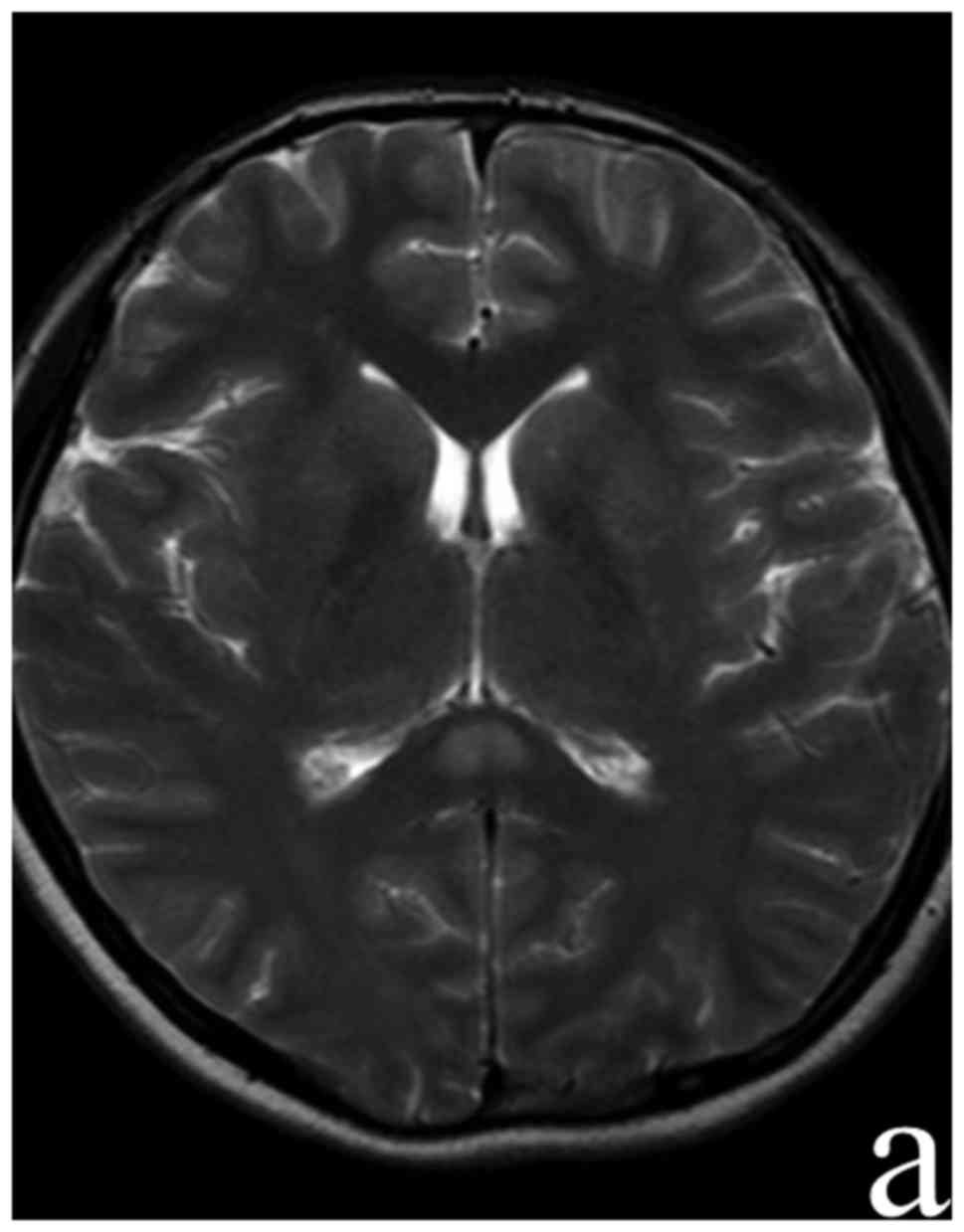
Brain enhanced MRI showed focal high-signal lesion in the SCC on
DWI, indicating a cytotoxic edema (Fig.
3). The clinical and serological results together with the
radiological findings lead to a diagnosis of acute encephalitis due
to M. pneumoniae infection and intrapulmonary infection.
Azithromycin (10 mg/kg/day) was administered for 10 days. Mannitol
(250 ml every 8 h) was given to reduce intracranial pressure.
Methylprednisolone (20 mg/kg/day) for a week, and immunoglobulin
(0.4 g/kg/day) were also given for 5 days for immunoregulation and
to inhibit the inflammatory response. The symptoms improved
significantly. Ten days after admission, the patient no longer had
headache and fever. Neurological examination showed fully-recovered
right limb muscle strength and neck rigidity (−). Serum M.
pneumoniae antibody IgG titer was markedly elevated to 1:1280. CSF
analysis showed a pressure of 200 mmH2O, Pandy reaction
(−), an abnormal white cell count (86×106/l), monocyte
percentage of 96.5%, protein 0.69 g/l, glucose 2.91 mmol/l, and
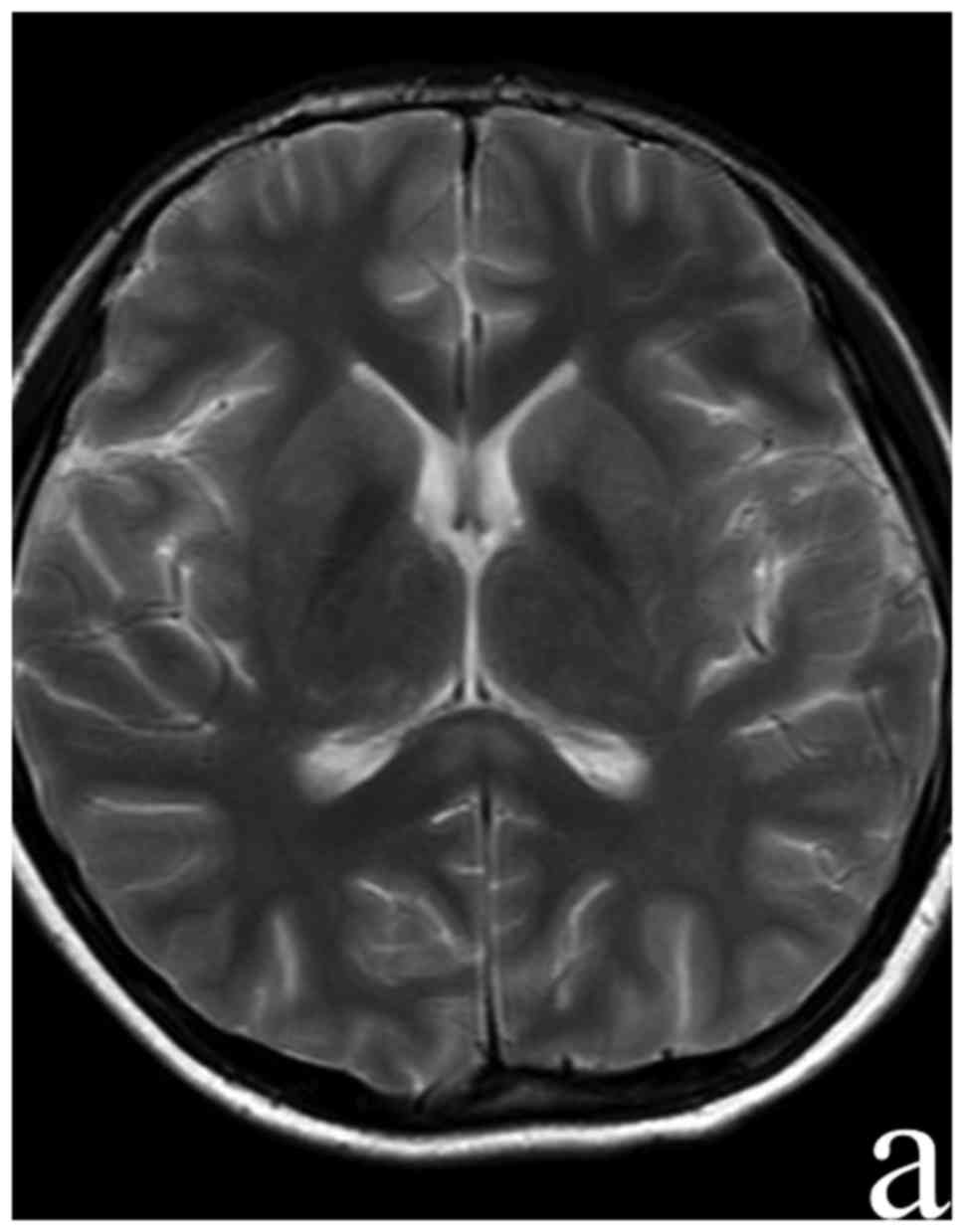
chloride 121.1 mmol/l. A follow-up brain MRI on day 24 of the
disease revealed no abnormalities (Fig.
4).
Case 3
A 40-year-old man was admitted to the hospital from
a nearby primary hospital with headache and fever for more than 10
days that occurred after overworking and becoming chilled. The
highest body temperature of the patient was 38.9°C. The patient
went to a nearby hospital when his vision blurred. At that time,
mannitol, ganciclovir, and ceftriaxone were given for treatment
(the detailed dosages are unknown). The headache did not improve
and he became disoriented. The patient was transferred to our
hospital. He had no past history of other related disease, except
acute hepatitis A and chronic cholecystitis. Neurological
examination on admission revealed drowsiness, limb muscle strength
grade 4+, Kernig sign (+), and neck rigidity (+). Other
neurological examinations were negative. Cranial MRI scan done on
2015-5-14 at the local hospital revealed no obviously
abnormalities. Chest CT scan done at Shengjing Hospital on
2015-05-20 revealed double infective lesions. Brain enhanced MR
scan done at Shengjing Hospital on 2015-05-22 showed focal signal
hyper-intensities on the T2, DWI, FLAIR sequence, and
hypo-intensities on the T1 sequence in SCC (Fig. 5). Laboratory examination revealed
markedly elevated serum M. pneumoniae antibody titer (1:1,280).
Serology for other pathogens including C. pneumoniae, VZV, mumps
virus, measles virus, HSV 1 and 2, CMV, and JEV were negative. CSF
analysis revealed a pressure of 310 mmH2O, Pandy
reaction (++), an abnormal white cell count (86×106/l),
monocyte percentage of 98.7%, protein 2.83 g/l, glucose 2.24
mmol/l, and chloride 122.6 mmol/l. General bacteria, tubercle
bacilli, and cryptococcus were not detected. The clinical and
serological results (especially elevated serum M. pneumoniae
antibody titer) together with the radiological findings supported a
diagnosis of acute Mycoplasma encephalitis. Azithromycin (10
mg/kg/day) was administrated for treatment, while mannitol (250 ml
every 8 h) was given to reduce intracranial pressure. The treatment
was effective. On the seventh day after admission, the headache and
blurred vision had significantly improved. Fever remained
intermittent and the body temperature reached 37.5°C. On day 14
after admission, body temperature had returned to normal and M.
pneumoniae antibody titer determined on 2015-05-31 had markedly
declined to 1:160. A follow-up brain MRI scan on 2015-06-02 did not
show obvious anomalies (Fig. 6).
Case 4
A 14-year-old female patient was transferred to our
hospital due to fever and headache for one week, accompanied with
vomiting twice. Physical examination showed a body temperature of
38.8°C. Neurological examination found neck rigidity (+). Other
neurological examinations were normal. Chest CT scan done on
2016-11-21 revealed some inflammation in the upper segment of the
inferior lobe of the right lung and frontier segment of the
superior lobe of the left lung. Cranial MRI done on 2016-11-22
showed focal hyper-intensities signal on the T2, DWI, FLAIR
sequence, and hypo-intensities on the T1 sequence in SCC (Fig. 7). Laboratory examination showed M.
pneumoniae IgM antibody positive, and the IgG titer was 1:160.
Serology for other pathogens including C. pneumoniae, VZV, mumps
virus, measles virus, HSV 1 and 2, CMV and JEV were negative. CSF
analysis showed a pressure of 200 mmH2O, an abnormal
white cell count (341×106/l), 96.7% monocytes, Pandy
reaction (+), protein 1.42 g/l, with normal glucose and chloride.
General bacteria, tubercle bacilli, and cryptococcus were not
detected. The combined clinical and serological results together
with the radiological findings supported a diagnosed as acute
Mycoplasma encephalitis. The patient was treated with intravenous
azithromycin (10 mg/kg/day) for 2 weeks. The symptoms improved
significantly at the sixth day after admission. At that time the
headache and vomiting had ended, and she had normal body
temperature. Two weeks after treatment, neurological examination
showed Kernig sign (−), neck rigidity (−), and other neurological
examinations were all negative. Re-examination of the cranial MRI
on 2016-11-28 showed that the high signal of SCC had decreased
(Fig. 8). Re-examination of CSF
analysis on 2016-12-06 showed a pressure of 150 mmH2O,
abnormal white cell count (50×106/l), 98% monocytes,
Pandy reaction (+), protein 0.78 g/l, and normal glucose and
chloride.
Discussion
Adult CNS involvement associated with M.
pneumoniae infection is being reported with increasing
frequency (9,10), but the diagnostic criteria, clinical
manifestations, and cranial imaging changes of M. pneumoniae
encephalitis are still not elucidated. In this case series, we
observed four clinically confirmed M. pneumoniae
encephalitis patients, all of whom had transient SCC lesions in
cranial MRI. Through the review of this series of cases, we hope to
help clinicians to get a better understanding of M. pneumoniae
encephalitis.
Despite advances in diagnostic techniques, the
diagnosis of M. pneumoniae encephalitis is still
challenging. Although depending on the serological testing for
diagnosis of M. pneumoniae encephalitis may be problematic,
it is undeniable that serological testing is the mainstay for
diagnosis of M. pneumoniae infection, especially in
developing countries where PCR technology and Western blot are not
readily available. Additionally, serological test saves more time
than Mycoplasma culture, and may reach a similar sensitivity as PCR
for diagnosis of acute infection of M. pneumoniae (11,12). In
our study, one of our diagnostic staples was the detection of serum
antibody. Specific IgM antibody to M. pneumoniae in an acute
infectious phase responses usually in the first 7–10 days of
illness and precedes IgG response by a few days (13). A four-fold increase in titer between
acute and convalescent sera of IgG is also important to patients'
diagnosis. In our cases, all four adult patients have 4-fold
increase in IgG, confirming the M. pneumoniae infection
(14).
CNS disorders, such as encephalitis, meningitis,
cerebellitis, and myelitis, are common extrapulmonary
manifestations of M. pneumoniae infection (15). One of the most common clinical
manifestations of M. pneumoniae encephalitis in adults is
encephalopathy. Symptoms may range from a subtle effect on mental
status with personality and behavioral changes to stupor and coma
(16). Other frequently reported
manifestations include epileptic activity, meningeal signs (i.e.,
headache, neck stiffness, vomiting) and ataxia. Focal neurological
deficits may be observed as well (8,17,18). In
the present study, all patients had fever and headache, three of
four had neck stiffness, two had disturbance of consciousness, and
one patient had visual disturbance, which was consistent with
previous reports.
The MRI findings of M. pneumoniae-associated
encephalitis are quite variable and haphazard. It is more sensitive
than CT in the detection of subtle lesions even in the early stage
of infection (19). However, the
proportion of imaging abnormalities is still unclear (20). The involved regions include the
cerebral cortex, hemisphere, basal ganglia, hippocampus,
cerebellum, and corpus callosum (21). We observed reversible focal lesions
in the SCC in all four patients, which has been reported and
defined as RESLES in hypoglycemia, hypernatremia, high-altitude
cerebral edema or antiepileptic drug toxicity or withdrawal
(6), but has rarely been reported in
acute M. pneumoniae-associated encephalitis. We reported the
largest number of acute M. pneumoniae-associated
encephalitis with RESLES to date. The pathophysiological mechanisms
of M. pneumoniae-associated encephalitis, especially
selective splenial lesions in the SCC, are not very clear. It has
been proposed that the specific affinity of
infective/auto-immunological antigens or induced antibodies to the
splenial axonal receptors are responsible for the splenial
involvement in infective/auto-immunological encephalitis (22,23).
Moreover, it has been postulated that the SCC results in specific
vulnerability to excitotoxicity injury in metabolic diseases, which
makes this area selectively involved in different pathological
events (24). Besides these two
possible mechanisms, direct infection of the brain could be another
underlying mechanism for post infectious complications of M.
pneumoniae infection, but it is incapable of explaining the
solitary involvement of the SCC (16). Therefore, we speculate that
autoimmune-mediated brain injury may be a possible reason. In this
scenario, M. pneumoniae-derived antigenic elements could
bound to blood macrophages or monocytes may initiate complex immune
reactions (25). The CSF analyses of
our cases showed the clearly increased percentage of monocytes,
which might be related with immune complex formation and could
further lead to subsequent neurological damage (16). Other disorders that could lead to
RESLES include viral encephalitis, antiepileptic drug toxicity or
withdrawal, hypoglycemia, and autoimmune
encephalopathy/encephalitis (6). All
of the aforementioned disorders could be excluded in the four
patients described in this study. The role of antibiotic in the
treatment of M. pneumoniae associated encephalitis remains
unresolved because of the lack of controlled trials (2,26).
Several therapeutic measures including antibiotics,
corticosteroids, intravenous immunoglobulin and plasmapheresis have
been reported for treatment of M. pneumoniae-associated
encephalitis (27–29). The macrolides are considered the
first-line agents for M. pneumoniae infections, although
such antibiotics may poorly traverse the blood-brain barrier
(30). Despite lack of the available
literature data that may help to provide guidance regarding the
efficacy of antibiotic therapies, azithromycin was administrated to
treat our patients and good curative effects were obtained. As the
possible immunological origin of M. pneumoniae-associated
encephalitis, immunomodulatory treatment may help (31). One of our patients received
corticosteroids combined with intravenous immunoglobulin; good
prognosis was obtained in both the respiratory and nervous
systems.
In this case series, four cases with the involvement
of splenium due to M. pneumoniae infection are described.
All cases received azithromycin treatment alone or combined with
immunomodulatory treatment. All of the patients made a full
recovery and we found reversible signal changes in MRI findings in
the corpus callosum lesions during the follow-up periods. Further
studies should be performed to clarify the possible mechanisms
underlying CNS involvement due to M. pneumoniae infection
and controlled trials should be conducted to assess the
effectiveness of therapeutic methods.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (no. 81371271), and was also sponsored
by ‘Liaoning BaiQianWan Talents Program’.
Funding
The present study received funding from the National
Natural Science Foundation of China (grant no. 81371271).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XD was responsible for collecting the cases and
writing articles and SC was responsible for the design of the
present study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in the studies involving
human participants were in accordance with the ethical standards of
the institutional and/or national research committee and performed
following the guidelines of the 1964 Helsinki Declaration and its
later amendments or comparable ethical standards.
Patient consent for publication
Consent for publication was obtained from the
patients of this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lind K, Benzon MW, Jensen JS and Clyde WA
Jr: A seroepidemiological study of Mycoplasma pneumoniae infections
in Denmark over the 50-year period 1946–1995. Eur J Epidemiol.
13:581–586. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bébéar C, Dupon M, Renaudin H and de
Barbeyrac B: Potential improvements in therapeutic options for
mycoplasmal respiratory infections. Clin Infect Dis. 17 Suppl
1:S202–S207. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Foy HM: Infections caused by Mycoplasma
pneumoniae and possible carrier state in different populations of
patients. Clin Infect Dis. 17 Suppl 1:S37–S46. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Narita M: Pathogenesis of neurologic
manifestations of Mycoplasma pneumoniae infection. Pediatr Neurol.
41:159–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuwahara M, Samukawa M, Ikeda T, Morikawa
M, Ueno R, Hamada Y and Kusunoki S: Characterization of the
neurological diseases associated with Mycoplasma pneumoniae
infection and anti-glycolipid antibodies. J Neurol. 264:467–475.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang S, Ma Y and Feng J:
Clinicoradiological spectrum of reversible splenial lesion syndrome
(RESLES) in adults: A retrospective study of a rare entity.
Medicine (Baltimore). 94:e5122015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rastawicki W, Rokosz N, Gierczyński R and
Jagielski M: Use of recombinant P1 protein of Mycoplasma pneumoniae
for the serodiagnosis of mycoplasmosis. Med Dosw Mikrobiol.
64:229–237. 2012.(In Polish). PubMed/NCBI
|
|
8
|
Jacobs E: Serological diagnosis of
Mycoplasma pneumoniae infections: A critical review of current
procedures. Clin Infect Dis. 17 Suppl 1:S79–S82. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo ZN, Zhang HL, Bai J, Wu J and Yang Y:
Meningitis associated with bilateral optic papillitis following
Mycoplasma pneumoniae infection. Neurol Sci. 33:355–358. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Narita M: Acute necrotizing encephalopathy
by Mycoplasma pneumoniae infection? Arch Intern Med. 162:16472002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li SL, Sun HM, Zhao HQ, Cao L, Yuan Y,
Feng YL and Xue GH: A single tube modified allele-specific-PCR for
rapid detection of erythromycin-resistant Mycoplasma pneumoniae in
Beijin. Chin Med J (Engl). 125:2671–2676. 2012.PubMed/NCBI
|
|
12
|
Waris ME, Toikka P, Saarinen T, Nikkari S,
Meurman O, Vainionpää R, Mertsola J and Ruuskanen O: Diagnosis of
Mycoplasma pneumoniae pneumonia in children. J Clin Microbiol.
36:3155–3159. 1998.PubMed/NCBI
|
|
13
|
Li S, Zhao H, Sun H, Feng Y, Xue G and Yan
C: Comparison of culture method, polymerase chain reaction and
serological test for the detection of Mycoplasma pneumoniae
infection in children with pneumoniae. Chin J Microbiol Immunol.
37:73–77. 2017.
|
|
14
|
Sauteur Meyer PM, Jacobs BC, Spuesens EB,
Jacobs E, Nadal D, Vink C and van Rossum AM: Antibody responses to
Mycoplasma pneumoniae: Role in pathogenesis and diagnosis of
encephalitis? PLoS Pathog. 10:e10039832014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al-Zaidy SA, MacGregor D, Mahant S,
Richardson SE and Bitnun A: Neurological complications of
PCR-Proven M. pneumoniae infections in children: Prodromal Illness
duration may reflect pathogenetic mechanism. Clin Infect Dis.
61:1092–1098. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsiodras S, Kelesidis I, Kelesidis T,
Stamboulis E and Giamarellou H: Central nervous system
manifestations of Mycoplasma pneumoniae infections. J Infect.
51:343–354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shibuya H, Osamura K, Hara K and Hisada T:
Clinically mild encephalitis/encephalopathy with a reversible
splenial lesion due to Mycoplasma pneumoniae infection. Intern Med.
51:1647–1648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sugeno N, Kawaguchi N, Hasegawa T, Kuroda
T, Nakashima I, Kanbayashi T, Kusunoki S and Aoki M: A case with
anti-galactocerebroside antibody-positive Mycoplasma pneumoniae
meningoencephalitis presenting secondary hypersomnia. Neurol Sci.
33:1473–1476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bitnun A, Ford-Jones EL, Petric M,
MacGregor D, Heurter H, Nelson S, Johnson G and Richardson S: Acute
childhood encephalitis and Mycoplasma pneumoniae. Clin Infect Dis.
32:1674–1684. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Socan M, Ravnik I, Bencina D, Dovc P,
Zalotnik B and Jazbec J: Neurological symptoms in patients whose
cerebrospinal fluid is culture- and/or polymerase chain
reaction-positive for Mycoplasma pneumoniae. Clin Infect Dis.
32:E31–E35. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lanczik O, Lecei O, Schwarz S and
Hennerici M: Mycoplasma pneumoniae infection as a treatable cause
of brainstem encephalitis. Arch Neurol. 60:1813–1814. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kizilkilic O and Karaca S:
Influenza-associated encephalitis-encephalopathy with a reversible
lesion in the splenium of the corpus callosum: Case report and
literature review. AJNR Am J Neuroradiol. 25:1863–1864.
2004.PubMed/NCBI
|
|
23
|
Tada H, Takanashi J, Barkovich AJ, Oba H,
Maeda M, Tsukahara H, Suzuki M, Yamamoto T, Shimono T, Ichiyama T,
et al: Clinically mild encephalitis/encephalopathy with a
reversible splenial lesion. Neurology. 63:1854–1858. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamashita S, Kawakita K, Hosomi N, Naya T,
Ohkita H, Kuroda Y and Tamiya T: Reversible magnetic resonance
imaging changes associated with hypoglycemia. Case report. Neurol
Med Chir (Tokyo). 50:651–654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishimura M, Saida T, Kuroki S, Kawabata
T, Obayashi H, Saida K and Uchiyama T: Post-infectious encephalitis
with anti-galactocerebroside antibody subsequent to Mycoplasma
pneumoniae infection. J Neurol Sci. 140:91–95. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koskiniemi M: CNS manifestations
associated with Mycoplasma pneumoniae infections: Summary of cases
at the University of Helsinki and review. Clin Infect Dis. 17 Suppl
1:S52–S57. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Candler PM and Dale RC: Three cases of
central nervous system complications associated with Mycoplasma
pneumoniae. Pediatr Neurol. 31:133–138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Daxboeck F, Blacky A, Seidl R, Krause R
and Assadian O: Diagnosis, treatment, and prognosis of Mycoplasma
pneumoniae childhood encephalitis: systematic review of 58 cases. J
Child Neurol. 19:865–871. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakoulas G: Brainstem and striatal
encephalitis complicating Mycoplasma pneumoniae pneumonia: Possible
benefit of intravenous immunoglobulin. Pediatr Infect Dis J.
20:543–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guleria R, Nisar N, Chawla TC and Biswas
NR: Mycoplasma pneumoniae and central nervous system complications:
A review. J Lab Clin Med. 146:55–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gucuyener K, Simsek F, Yilmaz O and
Serdaroğlu A: Methyl-prednisolone in neurologic complications of
Mycoplasma pneumonia. Indian J Pediatr. 67:467–469. 2000.
View Article : Google Scholar : PubMed/NCBI
|