Introduction
Diabetes mellitus (DM) is a chronic metabolic
disease characterized by elevated blood glucose levels and an
estimated 415 million people are living with DM worldwide (1). The International Diabetic Federation
has predicted that ~552 million people will be living with diabetes
by 2030 (2). Among patients with DM,
cardiovascular disease is the leading cause of disability and
mortality (3); DM may affect cardiac
structure and function leading to coronary artery disease and the
development of diabetic cardiomyopathy (DCM). DCM is defined as ‘a
distinct entity characterized by abnormal myocardial structure or
function in the absence of epicardial coronary artery disease,
hypertension or significant valvular disease’ (4). It is caused by diastolic dysfunction in
patients with type 1 or type 2 DM, which is followed by systolic
dysfunction induced by various structural lesions and may lead to
heart failure if left untreated (5,6).
Patients with DM are urged to undergo various lifestyle changes and
are prescribed medical interventions to manage their condition;
however, at present there are no specific treatments for DCM
(7).
MicroRNAs (miRs) are endogenous small noncoding RNAs
that are 20–23 nucleotides long and regulate gene expression
(8). Previous studies have
demonstrated the importance of miR-regulated gene expression in
several different diseases, including diabetes, heart disease,
neurological disorders and various types of cancer (9–11). The
dysregulation of certain miRs is associated with diabetic
cardiovascular dysfunction. It has been reported that miR-1 and
miR-206 regulate the expression of heat shock protein 60,
contributing to high levels of glucose-mediated apoptosis in
cardiomyocytes (12). It has also
been revealed that the expression of the insulin-sensitive glucose
transporter glucose transporter type 4 is modulated by miR-133 and
miR-223 in cardiomyocytes (13,14).
miR-1 is specifically expressed in cardiac and skeletal muscle
cells and its increased expression of miR-1 has been detected in
the hearts of patients with DM (15,16).
Furthermore, it has been demonstrated that miR-1 and miR-206
attenuate lipogenesis by targeting liver X receptor α (LXRα) in
hepatocytes (17).
LXRs, including LXRα and LXRβ, are key regulators of
cholesterol homeostasis, and lipid and glucose metabolism, and also
serve immune regulatory and anti-inflammatory functions. It has
been reported that synthetic LXR activation reduces hyperglycaemia
in insulin-resistant diabetes (18,19).
Cannon et al (20) confirmed
that LXRα improves myocardial glucose tolerance and reduces cardiac
hypertrophy in obesity-induced type 2 diabetes. However, the
signaling events that occur upstream of LXRα in cardiomyocytes and
the regulatory effect of miR-1 on LXRα remain unclear. Therefore,
the present study aimed to investigate the potential role of miR-1
in glucose-induced mitochondrial dysfunction and apoptosis in the
rat cardiomyocyte cell line H9C2, as well as identify the
downstream signaling targets of miR-1 in DCM. The present study
also investigated the underlying mechanisms of miR-1 during the
pathogenesis of DCM.
Materials and methods
Cell culture
The rat cardiomyocyte cell line H9C2 was obtained
from ScienCell Research Laboratories, Inc. (San Diego, CA, USA) and
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with 10%
heat-inactivated fetal bovine serum (FBS; Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) and 100 U/ml penicillin and
streptomycin (Gibco; Thermo Fisher Scientific, Inc.). Cells were
maintained at 37°C in a humidified atmosphere containing 5%
CO2. GW3965, an LXR agonist, was purchased from Selleck
Chemicals (Houston, TX, USA) and glucose was purchased from
Sigma-Aldrich (Merck KGaA Darmstadt, Germany).
Experimental groups
All H9C2 cells were initially treated for 48 h with
10% FBS in 5% CO2 at 37°C in 6- and 12-well plates, and
then divided into 5 groups as follows: i) Control H9C2 cells,
treated with PBS for 24 h and then treated with 33 mmol/l mannitol
for a further 24 h; ii) glucose cells, treated with PBS for 24 h
and then treated with 33 mmol/l glucose for a further 24 h; iii)
NC+Glucose cells, miR-1 negative control (NC) plasmids were
transfected into the cells and following 24 h, cells were treated
with 33 mmol/l glucose for a further 24 h; iv) anti-miR-1+Glucose
cells, anti-miR-1 plasmids were transfected into the cells and
following 24 h the cells were treated with 33 mmol/l glucose for a
further 24 h; v) anti-miR-1+Glucose+GW3965 cells, cells were first
transfected with anti-miR-1 plasmids and following 24 h, cells were
then treated with 33 mmol/l glucose and 1 µM GW3965 for a further
24 h. The volumes of PBS, and anti-miR and NC plasmids were 200 µl
and 100 µl/well in 6- and 12-well plates, respectively. The volumes
of mannitol and glucose, were 20 µl and 10 µl/well in 6- and
12-well plates, respectively.
Plasmid construction and cell
transfection
The oligonucleotide sequences of miR-1 knockdown [an
anti-miR-1 short hairpin (sh)RNA] and miR-1 overexpression were
listed as follows: Anti-miR-1 forward,
5′-GATCCCCATACACACTTCTTTACATTCCATTCAAGAGATGGAATAAAGAAGTGTGTATTTTTT-3′
and reverse,
5′-AGCTAAAAAATACACACTTCTTTACATTCCATCTCTTGAATGGAATAAAGAAGTGTGTATGGG-3′;
miR-1, forward,
5′-GATCCCCTGGAATGTAAAGAAGTGTGTATTTCAAGAGAATACACACTTCTTTATTCCATTTTT-3′;
and reverse,
5′-AGCTAAAATGGAATGTAAAGAAGTGTGTATTCTCTTGAAATACACACTTCTTTATTCCAGGG-3′.
For the negative control, the sequences were as follows: Forward,
5′-GATCCCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTT-3′
and reverse,
5′-AGCTAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAGGG-3′.
To construct the anti-miR-1 and miR-1 overexpression plasmids,
these paired oligonucleotides were annealed and 2 µg
double-stranded products were subsequently inserted into pRNA-H1.1
vectors (GenScript, Nanjing, China) between the BamHI and
HindIII sites. Anti-miR-1 or miR-1 plasmids were transfected
into H9C2 cells using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Lipofectamine® 2000 was added to the diluted
miR-1 shRNA or miR-1 and NC plasmids and incubated for 20 min at
room temperature. The complex was added to the H9C2 cells and
incubated for 4 h at 37°C. Cells were subsequently plated in 6-well
plates with DMEM containing 10% FBS for 24 h at 37°C. Following
this incubation the cells were treated with 33 mmol/l glucose or 1
µmol/l GW3965 for a further 24 h depending on which group the cells
were in. The cells were then collected for gene and protein
examination.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using an RNApure High-purity
Total RNA Rapid Extraction kit (BioTeke Corporation, Beijing,
China) according to the manufacturer's protocol. cDNAs were
synthesized using a Super M-MLV reverse transcriptase kit (BioTeke
Corporation). For reverse transcription, the primer of miR-1 was as
follows: 5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACATACAC-3′.
For other genes, cDNA was directly obtained using oligo
(dT)15 and random primers. The reaction solution
contained 2 µl RNA, 2 µl reverse transcription primers [1 µl oligo
(dT)15 and 1 µl random], 4 µl 5× reaction buffer, 0.75
µl dNTP (2.5 mM each), 0.25 µl ribonuclease inhibitor and 0.2 µl
reverse transcriptase M-MLV (200 U). The reverse transcription
reactions were performed at 16°C for 10 min, 42°C for 50 min, and
95°C for 5 min.
The reverse transcription products were subsequently
amplified by qPCR using SYBR® Green 1 (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China) on an Exicycler
96 RTPCR machine (Bioneer Corporation, Daejeon, Korea). The primers
used for qPCR were as follows: miR-1, forward,
5′-CGCGGTGGAATGTAAAGAAG-3′ and reverse, 5′-GTGCAGGGTCCGAGGTATTC-3′;
U6, forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; LXRα, forward,
5′-CTGAAGCGTCAAGAAGAGGA-3′ and reverse, 5′-CCTGTTACACTGTTGCTGGG-3′;
sterol regulatory element-binding transcription factor 1c
(SREBP-1c), forward, 5′-TCCTGGAGCGAGCATTGAA-3′ and reverse,
5′-CCGACAGCGTCAGAACAGC-3′; β-actin, forward,
5′-GGAGATTACTGCCCTGGCTCCTAGC-3′ and reverse,
5′-GGCCGGACTCATCGTACTCCTGCTT-3′. The qPCR thermocycling conditions
were as follows: 94°C for 10 min, 40 cycles of 94°C for 10 sec,
60°C for 20 sec and 72°C for 30 sec, followed by 72°C for 2 min 30
sec, 40°C for 5 min 30 sec, melting from 60°C to 94°C with a 1°C
rise every 1 sec and 25°C for 1 min. qPCR results were verified by
varying the number of qPCR cycles for each cDNA and set of primers.
The relative expression values of miR-1 and LXRα and SREBP-1c mRNA
were calculated using the 2−ΔΔCq method (21) with the housekeeping genes U6 and
β-actin used as the internal controls. RT-qPCR was performed ≥3
times for each individual experiment.
Apoptosis detection
Apoptosis was determined using an
Annexin-fluorescein isothiocyanate (FITC) apoptosis detection kit
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) by
fluorescence-activated cell sorting flow cytometry. All of the
reagents were part of this kit. Cells were harvested, collected and
washed twice in PBS. Cells were then resuspended in 500 µl binding
buffer and stained with 5 µl Annexin V-FITC and 5 µl propidium
iodide at room temperature for 15 min in the dark. Following this
the cells were transferred into flow cytometry tubes for detection
using a BD Accuri™ C6 flow cytometer and BD Accuri C6 Plus software
(version C6; BD Biosciences, Franklin Lakes, NJ, USA) for 10,000
events.
Measurement of the mitochondrial
membrane potential (ΔΨ)
To determine the ΔΨ, cells were collected and washed
in PBS and subsequently incubated with rhodamine 123 (Molecular
Probes; Thermo Fisher Scientific, Inc.) at 37°C for 30 min. Cells
were then washed twice with PBS and resuspended in fresh DMEM
medium for incubation for 60 min at 37°C. Then the cells were
detected with a BD Accuri™ C6 flow cytometer and BD Accuri C6 Plus
software for 10,000 events.
Western blot analysis
The cells were harvested and lysed using
radioimmunoprecipitation assay buffer and phenylmethylsulfonyl
fluoride (Beyotime Institute of Biotechnology, Haimen, China) for
30 min on ice. Protein content was determined using a BCA Protein
assay kit (Beyotime Institute of Biotechnology, Haimen, China)
following the manufacturer's protocol. Proteins (40 µg/lane) were
separated by 10–15% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes
were incubated with primary antibodies overnight at 4°C and
subsequently incubated with horseradish peroxidase-conjugated goat
anti-rabbit/mouse immunoglobulin G secondary antibodies (1:5,000;
Beyotime Institute of Biotechnology) at room temperature for 45
min. Anti-cleaved-poly (adenosine diphosphate-ribose) polymerase
(PARP; cat. no. ab32561; 1:1,000), anti-cleaved-caspase-3 (cat. no.
ab2302; 1:1,000), anti-cleaved-caspase-9 (cat. no. ab25758;
1:1,000) and anti-LXRα (cat. no. ab3585; 1:300) primary antibodies
were obtained from Abcam (Cambridge, MA, USA). Anti-PARP (cat. no.
9532; 1:5,000), anti-caspase-3 (cat. no. 9662; 1:5,000) and
anti-caspase-9 (cat. no. 9508; 1:5,000) primary antibodies were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Anti-B-cell lymphoma-2 (Bcl-2; cat. no. BA0412) and anti-Bax (cat.
no. BA0315) primary antibodies were obtained from Wuhan Boster
Biological Technology, Ltd. (Wuhan, China) and used at a 1:400
dilution. Anti-SREBP-1c (cat. no. 14088-1-AP) primary antibodies
were obtained from ProteinTech Group, Inc. (Chicago, IL, USA) and
used at a 1:1,000 dilution. Anti-β-actin (cat. no. sc-47778)
primary antibodies were obtained from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA) and used at a 1:1,000 dilution. Goat
anti-rabbit (cat. no. A0208) and goat anti-mouse horseradish
peroxidase-conjugated immunoglobulin G (cat. no. A0216) secondary
antibodies were obtained from Beyotime Institute of Biotechnology
and used at a 1:5,000 dilution. β-actin was utilized as the loading
control for total protein expression. The amounts of transferred
protein were quantified by the Gel Imaging System (WD-9413B;
Beijing Liuyi Biotechnology, Co., Ltd., Beijing, China). Western
blot quantitative analysis was performed using the Gel-Pro Analyzer
software (version 4.0; Media Cybernetics, Inc., Rockville, MD,
USA), and the experiments were repeated a minimum of three
times.
Statistical analysis
Data are presented as the mean + standard deviation
of 3 independent experiments. Multiple comparisons were performed
by one-way analysis of variance and Bonferroni's post-hoc test.
Data analysis and plotting were conducted using GraphPad Prism 4.0
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Silencing miR-1 expression suppresses
apoptosis in glucose-induced H9C2 cells
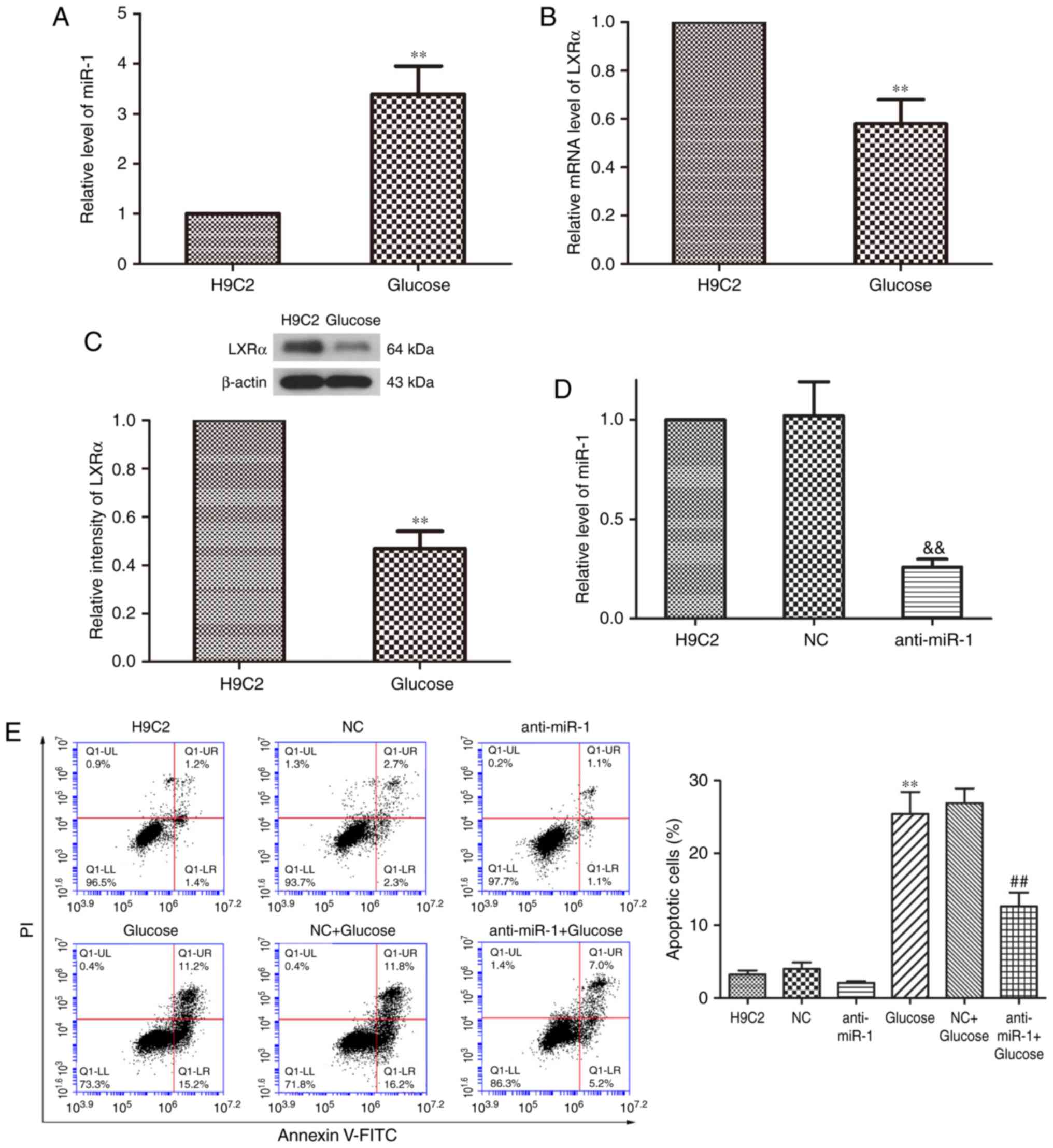
To examine the expression of miR-1 in
glucose-induced cardiomyocytes, the expression of miR-1 in H9C2
cells treated with or without glucose was determined by RT-qPCR
analysis when the cells were in the logarithmic growth phase. It
was revealed that the expression of miR-1 was significantly
increased in glucose-treated H9C2 cells compared with
non-glucose-treated control cells (P<0.01; Fig. 1A). LXRα is involved in the
pathogenesis of DCM and serves a key role in diabetic heart disease
(20). The results of RT-qPCR
revealed that the expression of LXRα mRNA was significantly
decreased in glucose-induced H9C2 cells compared with
non-glucose-treated control cells (P<0.01; Fig. 1B). The expression of LXRα protein was
also significantly downregulated in cells treated with glucose
compared with the control cells (P<0.01; Fig. 1C). These results indicate that miR-1
expression is significantly increased in H9C2 cells following
treatment with glucose, whereas the expression of LXRα is
significantly decreased.
miR-1 expression was knocked down by transfecting
anti-miR-1 plasmids into H9C2 cells (Fig. 1D) and RT-qPCR revealed that miR-1
expression was significantly inhibited in the anti-miR-1 group
compared with the NC group (P<0.01). Fluorescence-activated cell
sorting analysis was performed to determine the rate of apoptosis
of cells within the different treatment groups. Transfection with
anti-miR-1 alone did not significantly affect the rate of apoptosis
in the cells (Fig. 1E). Treatment of
untransfected cells with glucose significantly increased the rate
of apoptosis compared with the control group (P<0.01); however
miR-1 silencing significantly inhibited glucose-induced apoptosis
compared with the NC+Glucose group (P<0.01). These results
indicate that silencing miR-1 inhibits apoptosis in glucose-induced
H9C2 cells.
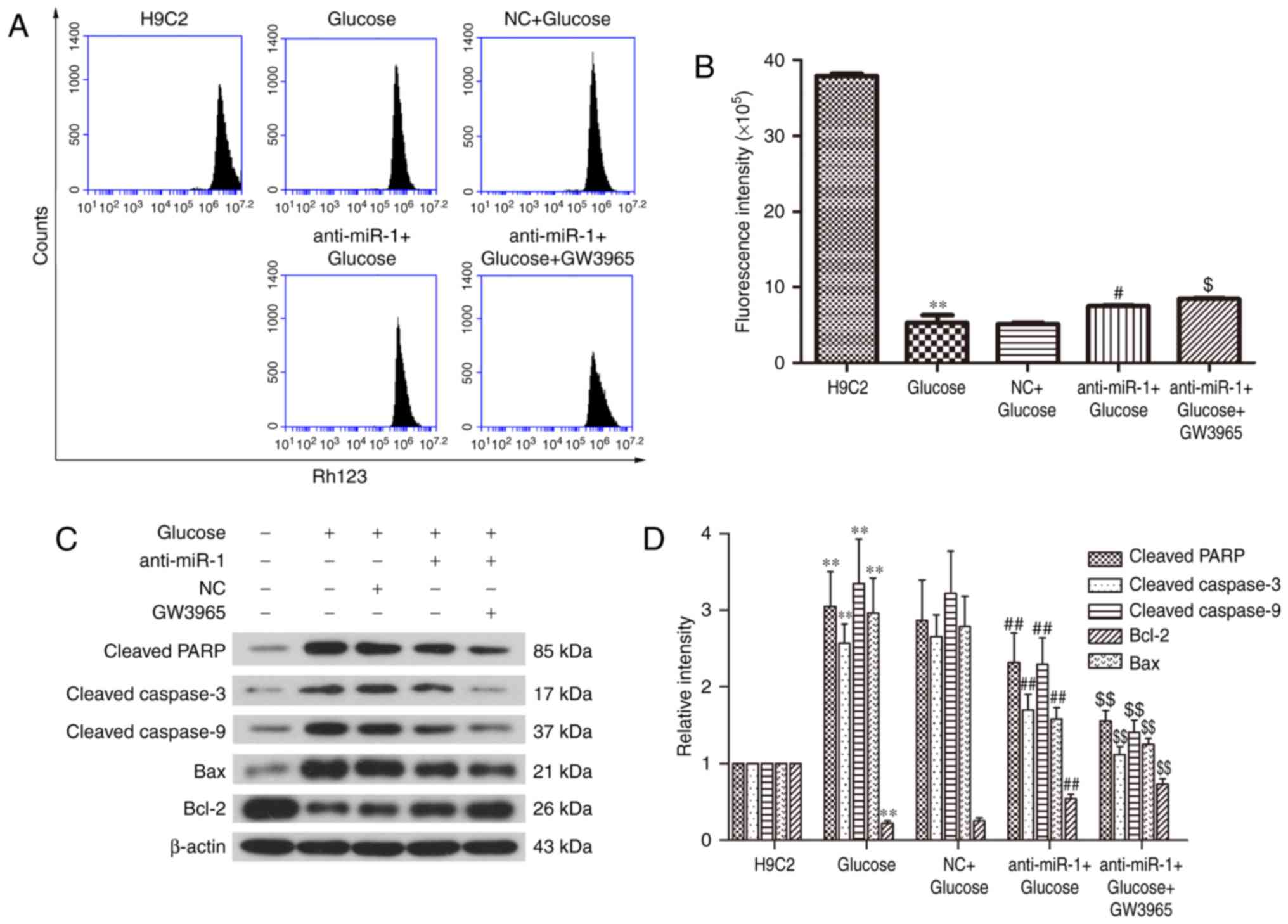
miR-1 silencing attenuates the
glucose-induced decrease of the ΔΨ in H9C2 cells
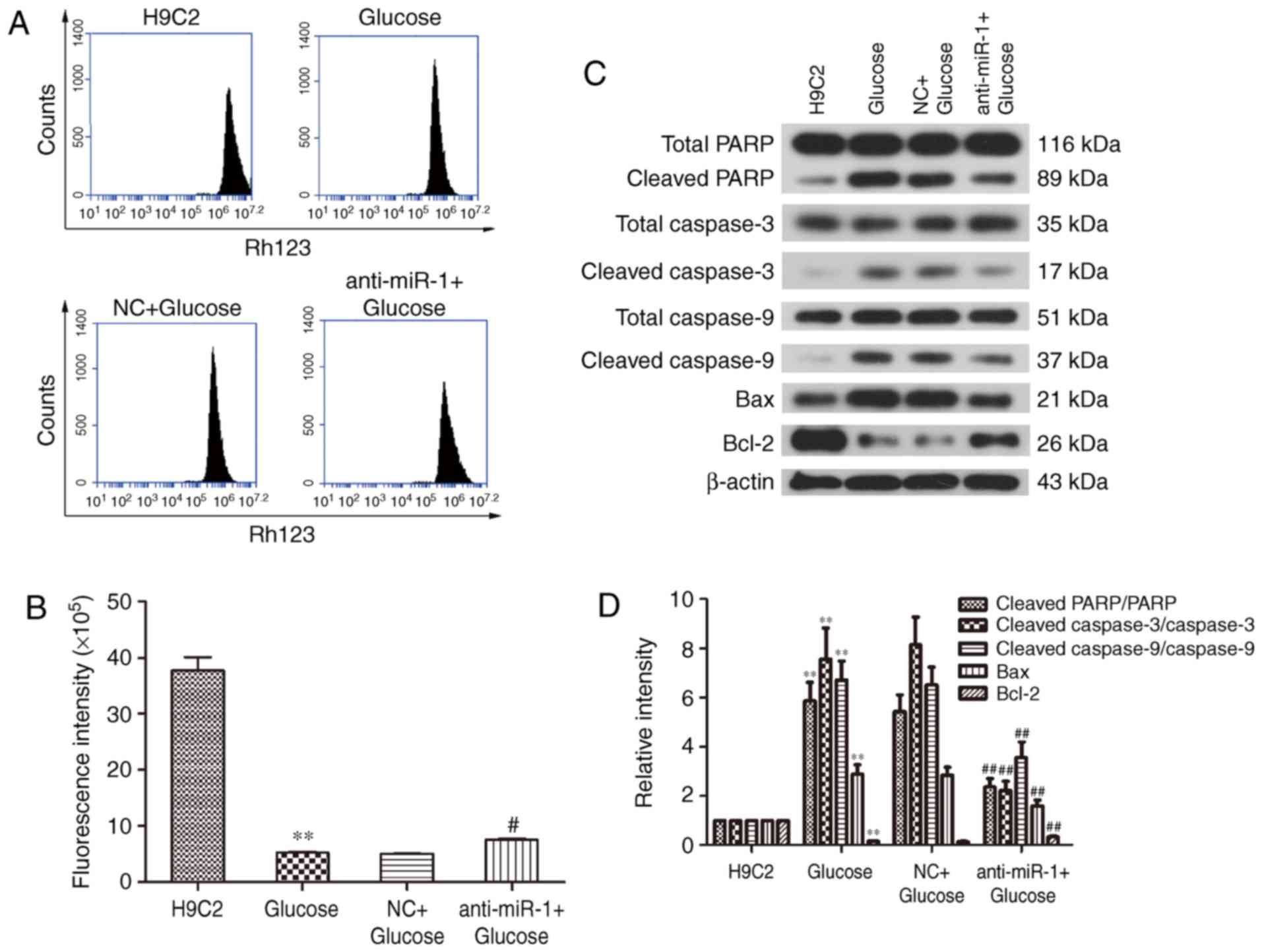
Mitochondrial dysfunction is a key characteristic of
apoptosis (22,23). To elucidate the potential mechanism
underlying the silencing of miR-1 in glucose-induced H9C2 cells,
the ΔΨ was measured to determine mitochondrial function in H9C2
cells (Fig. 2A). Treatment with
glucose significantly reduced the fluorescence intensity of the ΔΨ
compared with the control cells (P<0.01; Fig. 2B), however, miR-1 knockdown
significantly reversed the glucose-induced ΔΨ decrease in H9C2
cells (P<0.05; Fig. 2B). These
results reveal that silencing miR-1 attenuates glucose-induced
apoptosis via the mitochondrial pathway.
The expression of apoptosis-associated proteins was
measured by western blot analysis (Fig.
2C). There was a significant increase in the cleavage of PARP
and caspase-3 in glucose treated cells (P<0.01; Fig. 2D); however inhibition of miR-1
significantly suppressed the cleavage of PARP and caspase-3 in the
anti-miR-1+Glucose cells (P<0.01). This suggests that
anti-miR-1-inhibited apoptosis is mediated via a caspase-associated
signaling pathway. In addition, the level of cleaved caspase-9 was
significantly increased by glucose (P<0.01) and also
significantly inhibited in the anti-miR-1+Glucose cells
(P<0.01), suggesting that the endogenous apoptotic signaling
pathway is also involved in anti-miR-1-inhibited apoptosis.
The endogenous apoptotic signaling pathway is
regulated by the balance between pro-apoptotic proteins, such as
Bax and anti-apoptotic proteins, such as Bcl-2 (24). Therefore, the expression of Bcl-2 and
Bax in the different groups of cells were measured. The significant
downregulation of anti-apoptotic protein Bcl-2 induced by glucose
(P<0.01), was significantly reversed in the anti-miR-1+Glucose
cells (P<0.01) and the upregulation of pro-apoptotic protein Bax
was significantly decreased following miR-1 silencing (P<0.01).
There were no significant differences between the expression of
proteins in the Glucose and NC+Glucose groups. These results
suggest that the downregulation of Bcl-2 and the upregulation of
Bax are responsible for the activation of the endogenous apoptotic
signaling pathway in glucose-induced apoptosis and that this is
significantly suppressed by miR-1 knockdown in H9C2 cells.
miR-1 regulates the expression of LXRα
in glucose-induced H9C2 cells
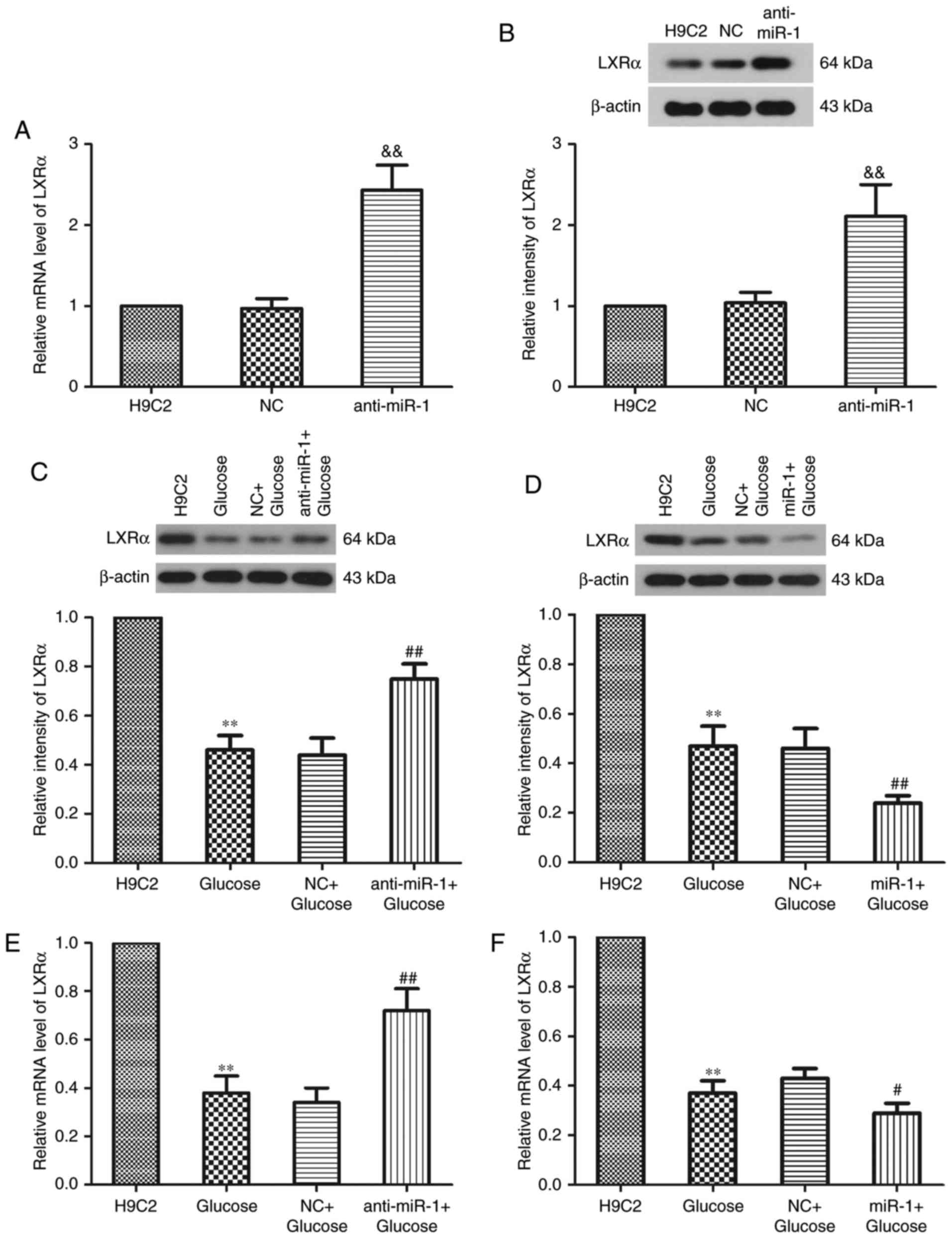
It has been reported that miR-1 functions via the
miR-1/LXRα signaling pathway in lipogenesis-associated diseases
(17). LXRα serves a key role in the
normal and DCM hearts of db/db mice with type 2 diabetes and
serves a role in the development of DCM (25). Western blot analysis was performed to
investigate whether miR-1 silencing alters the expression of LXRa
in H9C2 cells (Fig. 3). The results
demonstrated that miR-1 silencing significantly increased the
expression of LXRα compared with the NC group (P<0.01; Fig. 3A and B). Following treatment with
glucose, LXRα expression was significantly decreased (P<0.01;
Fig. 3C and D). However,
transfection of H9C2 cells with anti-miR-1 and treatment with
glucose caused a significant increase in the relative intensity of
LXRa compared with the NC+Glucose group (P<0.01; Fig. 3C). By contrast, the relative
intensity of LXRa significantly decreased further following the
transfection of cells with miR-1 and treatment with glucose
compared with the NC+Glucose group (P<0.01; Fig. 3D). RT-qPCR was performed to determine
the expression of LXRα mRNA following transfection with anti-miR-1
or miR-1 plasmids in glucose-induced H9C2 cells. miR-1 knockdown
significantly increased the expression of LXRα mRNA compared with
the NC+Glucose group (P<0.01; Fig.
3E), whereas the overexpression of miR-1 significantly
amplified the decrease in LXRα mRNA expression (P<0.05; Fig. 3F). These results indicate that the
inhibition of miR-1 suppresses glucose-induced apoptosis,
potentially by regulating the expression of LXRα in H9C2 cells.
GW3965 regulates apoptosis in
anti-miR-1 treated cells
LXRα may be involved in the pathogenesis of various
cardiovascular and metabolic diseases and serve a key role in miR-1
knockdown in H9C2 cells. Therefore, the synthetic LXRα agonist
GW3965 was used to investigate whether LXRα was associated with the
inhibition of apoptosis, which is regulated by miR-1 knockdown in
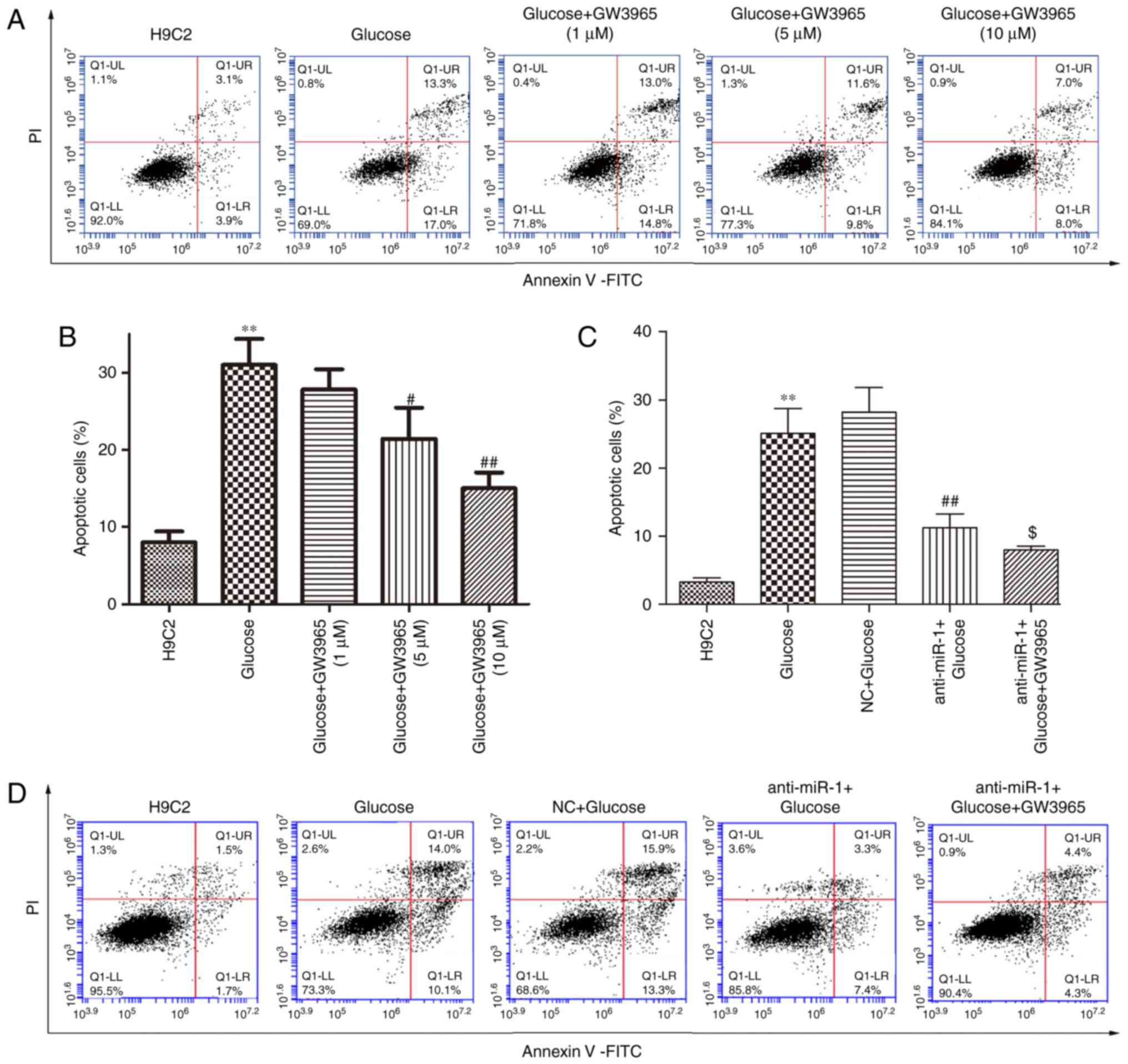
glucose-induced H9C2 cells. The effect of GW3965 alone on
glucose-induced apoptosis was determined (Fig. 4A). GW3965 significantly inhibited
glucose-induced apoptosis in a dose-dependent manner (P<0.05 and
P<0.01; Fig. 4B), however
treatment with 1 µM GW3965 alone did not significantly inhibit the
level of apoptosis induced by glucose in H9C2 cells. Therefore, 1
µM GW3965 was used to assess whether LXRa was involved in the
underlying mechanism of miR-1 on glucose-induced apoptosis in H9C2
cells. Treatment with 1 µM GW3965 significantly enhanced the
inhibitory effect of anti-miR-1 on apoptosis compared with the
anti-miR-1+Glucose cells (P<0.05; Fig. 4C and D). In addition, the increase in
the ΔΨ following transfection with anti-miR-1 was significantly
enhanced by treatment with 1 µM GW3965 compared with
anti-miR-1+Glucose cells (P<0.05; Fig. 5A and B).
Western blot analysis indicated that the expression
of cleaved PARP, cleaved caspases-3 and −9, and Bax in
anti-miR-1+Glucose+GW3965 cells were significantly decreased
compared with the anti-miR-1+Glucose cells (P<0.01), whereas the
expression of Bcl-2 was significantly upregulated (P<0.01;
Fig. 5C and D). These results
indicate that the inhibition of apoptosis following miR-1 silencing
on glucose-induced H9C2 cells is significantly enhanced following
the activation of LXRα by GW3965 in glucose-induced H9C2 cells.
Therefore, miR-1 silencing may suppress the apoptosis induced by
glucose by regulating LXRα in H9C2 cells.
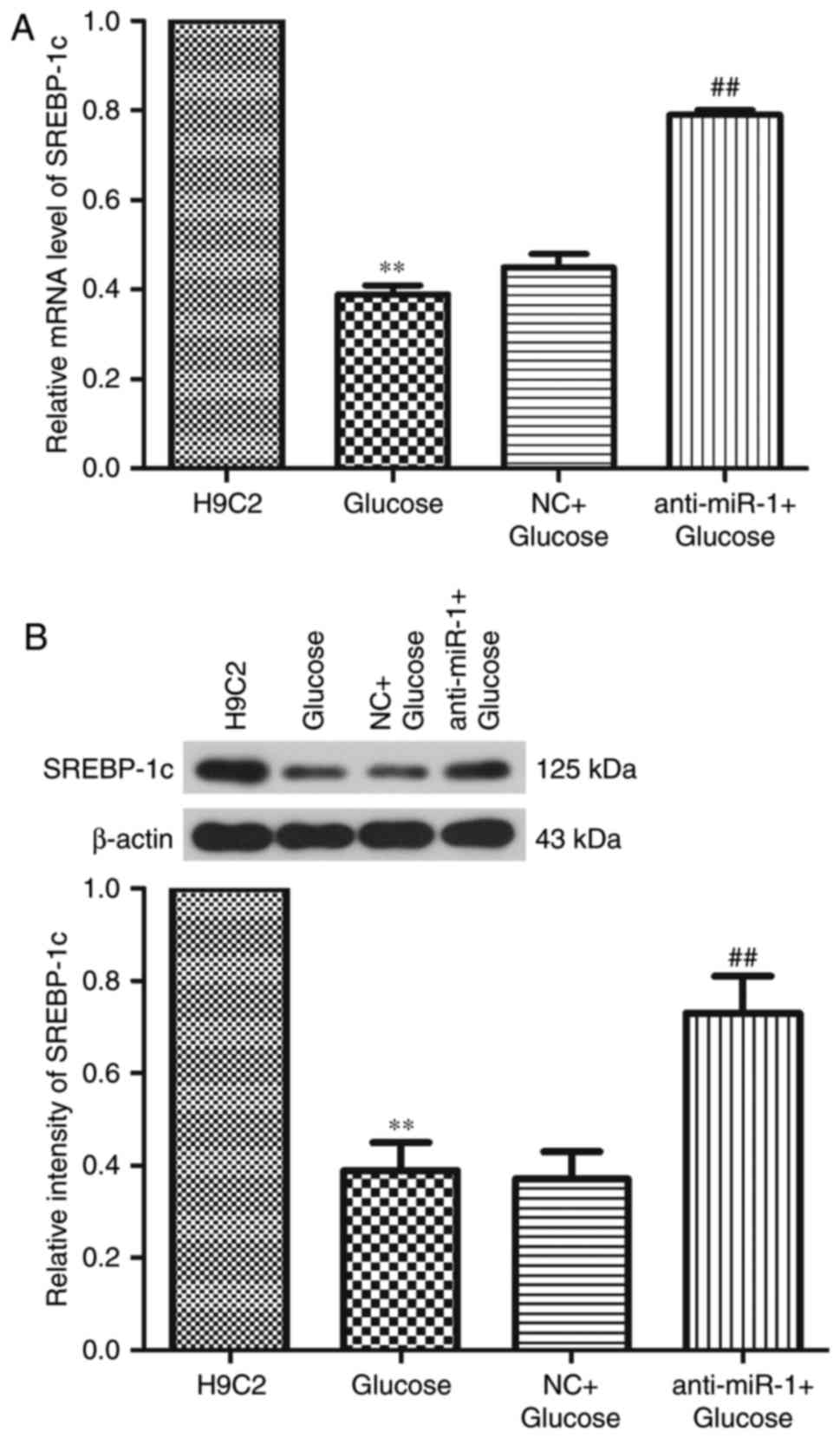
To investigate the regulatory effect of miR-1 on
glucose-induced apoptosis, the expression of SREBP-1c, a known LXR
target gene, was determined by RT-qPCR and western blot analysis.
Levels of SREBP-1c mRNA and protein were significantly
downregulated following treatment with glucose (P<0.01), however
miR-1 silencing significantly reversed the decrease in SREBP-1c
expression (P<0.01; Fig. 6).
These results suggest that silencing miR-1 inhibits glucose-induced
apoptosis via the miR-1/LXRα signaling pathway in H9C2 cells.
Discussion
DCM is characterized by left ventricular
hypertrophy, diastolic dysfunction and impaired myocardial
substrate metabolism (26,27). However, the effect of DM on cardiac
function is not always clear, which may lead to a delayed
diagnosis. The role of miRs in various diseases has attracted
increased attention and previous studies have investigated the role
miR-1 serves in lipid metabolic disorders as well as in diabetic
complications. It has been demonstrated that circulating miR-1 is
involved in the onset of myocardial infarction (28). In addition, miR-1 is a mediator of
non-diabetic cardiac hypertrophy (29). However, the precise mechanism of
miR-1 during the development of DCM remains unclear. In the present
study, the role of miR-1 in the glucose-induced apoptosis of H9C2
cells was investigated and the association between miR-1 and LXRα
was assessed. The results revealed that the expression of miR-1 was
significantly increased in glucose induced H9C2 cells and silencing
this miR-1 expression significantly inhibited glucose-induced
apoptosis. This reduction in apoptosis occurred via the
mitochondrial apoptotic pathway by upregulating Bcl-2 and
downregulating Bax in H9C2 cells. The expression of LXRα mRNA was
significantly decreased in glucose-treated H9C2 cells, whereas its
expression was significantly increased by anti-miR-1. In addition,
the inhibitory effect of anti-miR-1 on apoptosis was amplified by
GW3965.
There is an association between the expression and
certain regulatory functions of miR-1 and its homologue miR-206;
however they are transcribed from different chromosomes and may
undergo individual transcriptional activation (30). Therefore, the present study focused
on the regulatory effect of miR-1 on glucose-induced H9C2
cells.
miR-1 contributes to glucose-mediated apoptosis in
cardiomyocytes (12). It has been
demonstrated that the levels of >125 miRs, including miR-1, are
altered in glucose-induced endothelial cells and endothelin-1 has
been identified as a potential target of miR-1 (31). Yu et al (32) reported that high glucose levels
induce the apoptosis of cardiomyocytes via the miR-1 regulated
insulin-like growth factor-1 signaling pathway. It has also been
determined that the inhibition of miR-1 confers a protective effect
via the p38/mitogen-activated protein kinase signaling pathway in
high glucose induced neonatal rat cardiomyocytes (33). However, the underlying mechanisms
associated with glucose and miR-1 remains unknown and further
studies are required to confirm this.
Zhai et al (34) revealed that the inhibition of miR-1
attenuates the hypoxia/re-oxygenation-induced apoptosis of
cardiomyocytes. These results suggest that miR-1 acts as a key
factor during the apoptosis of cardiomyocytes. In the present
study, miR-1 silencing significantly inhibited the glucose-induced
apoptosis of H9C2 cells by attenuating the decrease in ΔΨ and
regulating the expression of Bcl-2 and Bax. Apoptosis is mediated
by the caspase cascade (35) and the
interaction between anti-apoptotic proteins, such as Bcl-2 and
pro-apoptotic proteins, such as Bax (36), determines whether cells undergo
apoptosis. The ratio of anti-apoptotic Bcl-2 and pro-apoptotic Bax
determines the response to an apoptotic signal (37). It has been reported that miR-1
participates in the H2S protection of cardiomyocytes
against ischemia/reperfusion injury-induced apoptosis by regulating
Bcl-2 (34). In the present study,
levels of cleaved PARP, cleaved caspase-3 and cleaved caspase-9
induced by glucose were significantly suppressed following miR-1
inhibition, suggesting that the activation of the endogenous
apoptotic signaling pathway was inhibited by miR-1 knockdown. In
addition, the upregulation of Bax and the downregulation of Bcl-2
induced by glucose in H9C2 cells were reversed by miR-1 silencing.
Bcl-2 binds to Bax and inhibits its activation (38,39).
In the present study, treatment with glucose caused
a significant reduction in Bcl-2 expression, thus releasing Bax
from the Bax/Bcl-2 heterodimeric complex, leading to the
upregulation of Bax. This released Bax may accelerate the formation
of outer-mitochondrial membrane spanning pores and decrease the ΔΨ,
triggering the activation of the caspase cascade by caspase-3 to
initiate apoptosis. However, miR-1 silencing may have enhanced the
expression of Bcl-2 and suppressed the expression of Bax, thereby
inhibiting the release of Bax and increasing the ΔΨ. The
inactivation of the caspase cascade was attributed to transfection
with anti-miR-1 in glucose-induced H9C2 cells. The results of the
present study suggest that silencing miR-1 by anti-miR-1 inhibits
apoptosis via the mitochondrial signaling pathway in
glucose-induced H9C2 cells.
LXRα may serve a role in regulating metabolism
(40) and inflammation (41). It has been demonstrated that changes
in LXRs are involved in the pathogenesis of various diseases,
including cardiovascular (42) and
metabolic diseases (43),
Alzheimer's disease (44) and
various types of cancer (45). LXRα
is highly expressed in the majority of metabolically active
tissues, including the adipose tissue, the kidney, liver and
intestines, where it regulates metabolic and inflammatory signaling
pathways (46). LXR is therefore
considered to be a potential therapeutic target in the treatment of
cardiovascular and metabolic diseases (47).
In the present study, it was observed that the
expression of LXRα was significantly decreased in glucose-induced
H9C2 cells, which was consistent with the results of Lee et
al (48). Furthermore, the
expression of LXRα was regulated by miR-1 in glucose-induced H9C2
cells. These results indicate that miR-1 may be important in the
regulation of LXRα. Zhong et al (17) reported that LXRα may be a target of
miR-1 and miR-206. Ou et al (49) demonstrated that miR-613 targets the
human LXR gene and mediates a feedback loop of LXR autoregulation
in human hepatocytes. It has been previously reported that the
activation of LXRα exhibits a cardioprotective effect against DCM
by attenuating insulin resistance, reducing oxidative/nitrative
stress and inhibiting the inflammatory response (25). To further explore the interaction
between miR-1 and LXRα in the present study, the synthetic LXR
agonist GW3965 was used. It was observed that the activation of
LXRα by GW3965 significantly enhanced the inhibitory effect of
anti-miR-1 on apoptosis in glucose-treated H9C2 cells. These
results suggest that the activation of LXRα enhances the regulatory
effect of miR-1 in H9C2 cells.
At present, more than a dozen LXR target genes have
been identified. The transcription of several genes concerning the
cellular cholesterol efflux, including adenosine
triphosphate-binding cassette (ABC) A1, ABCG1 and apolipoprotein E
are controlled by LXRs (50–52). LXRs serve key roles in the reverse
cholesterol transport pathway in macrophages and other peripheral
cells (53–55). In the liver, LXRs regulate the
expression of proteins involved in cholesterol and fatty acid
metabolism, including cholesterol 7α-hydroxylase (56,57) and
SREBP-1c (58). Harasiuk et
al (59) reported that
administration of the LXRα agonist TO901317 increases the
expression of LXR isoforms and their target gene SREBP-1c in the
hearts of streptozotocin-diabetic rats. In the present study, the
expression of SREBP-1c mRNA and protein were significantly
downregulated following the administration of glucose, whereas the
miR-1 silencing significantly inhibited this decrease. These
results suggest that miR-1 silencing inhibits glucose-induced
apoptosis via the miR-1/LXRα signaling pathway in H9C2 cells.
The present study had certain limitations, including
the fact that experiments were only performed in vitro.
Previous studies have reported that the activation of LXRα may
attenuate cardiac dysfunction and improve glucose tolerance in
diabetic db/db mice (25,60);
therefore the regulatory effect of miR-1 on the activation of LXRα
in vivo requires further investigation.
In conclusion, the present study demonstrated that
silencing miR-1 significantly inhibits apoptosis via the
mitochondrial signaling pathway by regulating LXRα, the activation
of which attenuates the apoptosis of cardiac H9C2 cells. These
results suggest that miR-1 serves an important role in the
development of DCM and provide novel insights into understanding
the complex underlying mechanisms involved in the pathogenesis of
DCM.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81500629
and 81371362), The Assisted Project by Heilongjiang Postdoctoral
Funds for Scientific Research Initiation (grant nos. LBH-Q16223 and
LBH-Q16222) and the Heilongjiang Province Science Funds for
Distinguished Young Scientists (grant no. JC2015019).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YXC designed the study and performed the
experiments. WZ contributed to the western blot and reverse
transcription-quantitative polymerase chain reaction analysis. XDZ
performed the transfections and fluorescence-activated cell sorting
flow cytometry experiments. LXS and YC were involved in the
statistical analysis, and HRY critically reviewed the manuscript
and contributed to statistical analysis; YW performed the cell
culture and transfections. YHC managed the experimental design and
reviewed the manuscript. GBL reviewed the manuscript and provided
funding support. YXC and WZ drafted the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman
M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C,
et al: Heart disease and stroke statistics-2017 update: A report
from the american heart association. Circulation. 135:e146–e603.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mishra PK, Ying W, Nandi SS, Bandyopadhyay
GK, Patel KK and Mahata SK: Diabetic cardiomyopathy: An
immunometabolic perspective. Front Endocrinol (Lausanne). 8:722017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gersh BJ, Sliwa K, Mayosi BM and Yusuf S:
Novel therapeutic concepts: The epidemic of cardiovascular disease
in the developing world: Global implications. Eur Heart J.
31:642–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aneja A, Tang WH, Bansilal S, Garcia MJ
and Farkouh ME: Diabetic cardiomyopathy: Insights into
pathogenesis, diagnostic challenges, and therapeutic options. Am J
Med. 121:748–757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shivalkar B, Dhondt D, Goovaerts I, Van
Gaal L, Bartunek J, Van Crombrugge P and Vrints C: Flow mediated
dilatation and cardiac function in type 1 diabetes mellitus. Am J
Cardiol. 97:77–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carugo S, Giannattasio C, Calchera I,
Paleari F, Gorgoglione MG, Grappiolo A, Gamba P, Rovaris G, Failla
M and Mancia G: Progression of functional and structural cardiac
alterations in young normotensive uncomplicated patients with type
1 diabetes mellitus. J Hypertens. 19:1675–1680. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pappachan JM, Varughese GI, Sriraman R and
Arunagirinathan G: Diabetic cardiomyopathy: Pathophysiology,
diagnostic evaluation and management. World J Diabetes. 4:177–189.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karp X and Ambros V: Developmental
biology. Encountering microRNAs in cell fate signaling. Science.
310:1288–1289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allegra A, Alonci A, Campo S, Penna G,
Petrungaro A, Gerace D and Musolino C: Circulating microRNAs: New
biomarkers in diagnosis, prognosis and treatment of cancer
(review). Int J Oncol. 41:1897–1912. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sayed D and Abdellatif M: MicroRNAs in
development and disease. Physiol Rev. 91:827–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shan ZX, Lin QX, Deng CY, Zhu JN, Mai LP,
Liu JL, Fu YH, Liu XY, Li YX, Zhang YY, et al: miR-1/miR-206
regulate Hsp60 expression contributing to glucose-mediated
apoptosis in cardiomyocytes. FEBS Lett. 584:3592–3600. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu H, Buchan RJ and Cook SA: Microrna-223
regulates glut4 expression and cardiomyocyte glucose metabolism.
Cardiovasc Res. 86:410–420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horie T, Ono K, Nishi H, Iwanaga Y, Nagao
K, Kinoshita M, Kuwabara Y, Takanabe R, Hasegawa K, Kita T and
Kimura T: MicroRNA-133 regulates the expression of GLUT4 by
targeting KLF15 and is involved in metabolic control in cardiac
myocytes. Biochem Biophys Res Commun. 389:315–320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B,
Zhang Y, Xu C, Bai Y, Wang H, et al: The muscle-specific microRNA
miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1
and KCNJ2. Nat Med. 13:486–491. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen JF, Mandel EM, Thomson JM, Wu Q,
Callis TE, Hammond SM, Conlon FL and Wang DZ: The role of
microRNA-1 and microRNA-133 in skeletal muscle proliferation and
differentiation. Nat Genet. 38:228–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong D, Huang G, Zhang Y, Zeng Y, Xu Z,
Zhao Y, He X and He F: MicroRNA-1 and microRNA-206 suppress
LXRalpha-induced lipogenesis in hepatocytes. Cell Signal.
25:1429–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao G, Liang Y, Broderick CL, Oldham BA,
Beyer TP, Schmidt RJ, Zhang Y, Stayrook KR, Suen C, Otto KA, et al:
Antidiabetic action of a liver × receptor agonist mediated by
inhibition of hepatic gluconeogenesis. J Biol Chem. 278:1131–1136.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Yan C, Wang Y, Nakagawa Y, Nerio N,
Anghel A, Lutfy K and Friedman TC: Liver X receptor agonist
T0901317 inhibition of glucocorticoid receptor expression in
hepatocytes may contribute to the amelioration of diabetic syndrome
in db/db mice. Endocrinology. 147:5061–5068. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cannon MV, Silljé HHW, Sijbesma JWA, Khan
MAF, Steffensen KR, van Gilst WH and de Boer RA: LXRα improves
myocardial glucose tolerance and reduces cardiac hypertrophy in a
mouse model of obesity-induced type 2 diabetes. Diabetologia.
59:634–643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi D and Young LH: AMPK: Energy sensor and
survival mechanism in the ischemic heart. Trends Endocrinol Metab.
26:422–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumarapeli AR and Wang X: Genetic
modification of the heart: Chaperones and the cytoskeleton. J Mol
Cell Cardiol. 37:1097–1109. 2004.PubMed/NCBI
|
|
24
|
Renault TT, Teijido O, Antonsson B, Dejean
LM and Manon S: Regulation of Bax mitochondrial localization by
Bcl-2 and Bcl-x(L): Keep your friends close but your enemies
closer. Int J Biochem Cell Biol. 45:64–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He Q, Pu J, Yuan A, Yao T, Ying X, Zhao Y,
Xu L, Tong H and He B: Liver X receptor agonist treatment
attenuates cardiac dysfunction in type 2 diabetic db/db mice.
Cardiovasc Diabetol. 13:1492014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tarquini R, Lazzeri C, Pala L, Rotella CM
and Gensini GF: The diabetic cardiomyopathy. Acta Diabetol.
48:173–181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rubler S, Dlugash J, Yuceoglu YZ, Kumral
T, Branwood AW and Grishman A: New type of cardiomyopathy
associated with diabetic glomerulosclerosis. Am J Cardiol.
30:595–602. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ai J, Zhang R, Li Y, Pu J, Lu Y, Jiao J,
Li K, Yu B, Li Z, Wang R, et al: Circulating microRNA-1 as a
potential novel biomarker for acute myocardial infarction. Biochem
Biophys Res Commun. 391:73–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fichtlscherer S, Zeiher AM and Dimmeler S:
Circulating microRNAs: Biomarkers or mediators of cardiovascular
diseases? Arterioscler Thromb Vasc Biol. 31:2383–2390. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Townley-Tilson WHD, Callis TE and Wang D:
MicroRNAs 1, 133, and 206: Critical factors of skeletal and cardiac
muscle development, function, and disease. Int J Biochem Cell Biol.
42:1252–1255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng B, Cao Y, Chen S, Ruiz M and
Chakrabarti S: Reprint of: miRNA-1 regulates endothelin-1 in
diabetes. Life Sci. 118:275–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu XY, Song YH, Geng YJ, Lin QX, Shan ZX,
Lin SG and Li Y: Glucose induces apoptosis of cardiomyocytes via
microRNA-1 and IGF-1. Biochem Biophys Res Commun. 376:548–552.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu L, Yu H, Li X, Jin C, Zhao Y, Xu S and
Sheng X: P38 MAPK/miR-1 are involved in the protective effect of
EGCG in high glucose-induced C×43 downregulation in neonatal rat
cardiomyocytes. Cell Biol Int. 40:934–942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhai C, Tang G, Peng L, Hu H, Qian G, Wang
S, Yao J and Zhang X, Fang Y, Yang S and Zhang X: Inhibition of
microRNA-1 attenuates hypoxia/re-oxygenation-induced apoptosis of
cardiomyocytes by directly targeting Bcl-2 but not GADD45Beta. Am J
Transl Res. 7:1952–1962. 2015.PubMed/NCBI
|
|
35
|
Adams JM: Ways of dying: Multiple pathways
to apoptosis. Genes Dev. 17:2481–2495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pawlowski J and Kraft AS: Bax-induced
apoptotic cell death. Proc Natl Acad Sci USA. 97:529–531. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pena-Blanco A and Garcia-Saez AJ: Bax, bak
and beyond-mitochondrial performance in apoptosis. FEBS J.
285:416–431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brown LM, Hanna DT, Khaw SL and Ekert PG:
Dysregulation of BCL-2 family proteins by leukemia fusion genes. J
Biol Chem. 292:14325–14333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma Z, Deng C, Hu W, Zhou J, Fan C, Di S,
Liu D, Yang Y and Wang D: Liver × receptors and their agonists:
Targeting for cholesterol homeostasis and cardiovascular diseases.
Curr Issues Mol Biol. 22:41–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fessler MB: The challenges and promise of
targeting the liver × receptors for treatment of inflammatory
disease. Pharmacol Ther. 181:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Parikh M, Patel K, Soni S and Gandhi T:
Liver X receptor: A cardinal target for atherosclerosis and beyond.
J Atheroscler Thromb. 21:519–531. 2014.PubMed/NCBI
|
|
43
|
Ceroi A, Masson D, Roggy A, Roumier C,
Chague C, Gauthier T, Philippe L, Lamarthee B, Angelot-Delettre F,
Bonnefoy F, et al: LXR agonist treatment of blastic plasmacytoid
dendritic cell neoplasm restores cholesterol efflux and triggers
apoptosis. Blood. 128:2694–2707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cao G, Bales KR, DeMattos RB and Paul SM:
Liver X receptor-mediated gene regulation and cholesterol
homeostasis in brain: Relevance to Alzheimer's disease
therapeutics. Curr Alzheimer Res. 4:179–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Traversari C, Sozzani S, Steffensen KR and
Russo V: LXR-dependent and -independent effects of oxysterols on
immunity and tumor growth. Eur J Immunol. 44:1896–1903. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Calkin AC and Tontonoz P: Transcriptional
integration of metabolism by the nuclear sterol-activated receptors
LXR and FXR. Nat Rev Mol Cell Biol. 13:213–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Im SS and Osborne TF: Liver × receptors in
atherosclerosis and inflammation. Circ Res. 108:996–1001. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee HJ, Ryu JM, Jung YH, Lee SJ, Kim JY,
Lee SH, Hwang IK, Seong JK and Han HJ: High glucose upregulates
BACE1-mediated Aβ production through ROS-dependent HIF-1α and
LXRα/ABCA1-regulated lipid raft reorganization in SK-N-MC cells.
Sci Rep. 6:367462016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ou Z, Wada T, Gramignoli R, Li S, Strom
SC, Huang M and Xie W: MicroRNA hsa-miR-613 targets the human
LXRalpha gene and mediates a feedback loop of LXRalpha
autoregulation. Mol Endocrinol. 25:584–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Laffitte BA, Repa JJ, Joseph SB, Wilpitz
DC, Kast HR, Mangelsdorf DJ and Tontonoz P: LXRs control
lipid-inducible expression of the apolipoprotein E gene in
macrophages and adipocytes. Proc Natl Acad Sci USA. 98:507–512.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Venkateswaran A, Repa JJ, Lobaccaro JM,
Bronson A, Mangelsdorf DJ and Edwards PA: Human white/murine ABC8
mRNA levels are highly induced in lipid-loaded macrophages. A
transcriptional role for specific oxysterols. J Biol Chem.
275:14700–14707. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Repa JJ, Turley SD, Lobaccaro JA, Medina
J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM and Mangelsdorf
DJ: Regulation of absorption and ABC1-mediated efflux of
cholesterol by RXR heterodimers. Science. 289:1524–1529. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Laffitte BA, Joseph SB, Chen M, Castrillo
A, Repa J, Wilpitz D, Mangelsdorf D and Tontonoz P: The
phospholipid transfer protein gene is a liver X receptor target
expressed by macrophages in atherosclerotic lesions. Mol Cell Biol.
23:2182–2191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiang XC, Beyer TP, Li Z, Liu J, Quan W,
Schmidt RJ, Zhang Y, Bensch WR, Eacho PI and Cao G: Enlargement of
high density lipoprotein in mice via liver × receptor activation
requires apolipoprotein e and is abolished by cholesteryl ester
transfer protein expression. J Biol Chem. 278:49072–49078. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mak PA, Kast-Woelbern HR, Anisfeld AM and
Edwards PA: Identification of PLTP as an LXR target gene and apoE
as an FXR target gene reveals overlapping targets for the two
nuclear receptors. J Lipid Res. 43:2037–2041. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Javitt NB: Cholesterol,
hydroxycholesterols, and bile acids. Biochem Biophys Res Commun.
292:1147–1153. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Niesor EJ, Flach J, Lopes-Antoni I, Perez
A and Bentzen CL: The nuclear receptors FXR and LXRalpha: Potential
targets for the development of drugs affecting lipid metabolism and
neoplastic diseases. Curr Pharm Des. 7:231–259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jianhua L, Xueqin M and Jifen H:
Expression and clinical significance of LXRα and SREBP-1c in
placentas of preeclampsia. Open Med (Wars). 11:292–296.
2016.PubMed/NCBI
|
|
59
|
Harasiuk D, Baranowski M, Zabielski P,
Chabowski A and Górski J: Liver × receptor agonist to901317
prevents diacylglycerols accumulation in the heart of
streptozotocin-diabetic rats. Cell Physiol Biochem. 39:350–359.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Laffitte BA, Chao LC, Li J, Walczak R,
Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ,
Collins JL, et al: Activation of liver X receptor improves glucose
tolerance through coordinate regulation of glucose metabolism in
liver and adipose tissue. Proc Natl Acad Sci USA. 100:5419–5424.
2003. View Article : Google Scholar : PubMed/NCBI
|