Introduction
Sex differences are becoming increasingly apparent
in a range of normal physiological processes, as well as
pathological functions in clinical and research settings. These
differences have been determined in cardiovascular structure and
function, lung health and disease, metabolism and cognition
(1,2). Furthermore, there is notable evidence
that sex may affect gastrointestinal (GI) motility. For instance, a
number of studies have demonstrated that females have an increased
probability of GI disturbances, including nausea, vomiting,
bloating and constipation, compared with males (3,4). These
disturbances may vary during a female's lifetime due to the varying
levels of sex hormones during the menstrual cycle, pregnancy and
menopause (5,6). In addition, females have an increased
probability of being affected by gastroparesis, a chronic gastric
motility disorder, in which gastric emptying of solids and liquids
is delayed in the absence of obstruction (7). Although the pathogenesis of the disease
remains to be fully elucidated, the importance of estrogen in the
regulation of gastric motility in females is evident (8). Smooth muscle relaxation is initiated by
targeting the 20-kDa regulatory myosin light chain. Most agents
cause relaxation by stimulating the production of cyclic adenosine
monophosphate (cAMP) and/or cyclic guanosine monophosphate (cGMP).
cAMP-activated protein kinase A and cGMP-activated protein kinase G
are the major enzymes that induce relaxation in the smooth muscle.
Nitric oxide (NO) induces the production of cGMP from guanosine
triphosphate via activating the soluble guanylyl cyclase (sGC).
cGMP is then rapidly degraded by cGMP-specific phosphodiesterases
(9). The elevated levels of
circulating estrogen regulate gastric emptying in healthy females
by elevating NO levels, an important regulator of gastric motility
(10). Furthermore, sex hormones,
particularly estrogen, are known to cause GI motility disorders and
contribute to irritable bowel syndrome (11). In addition, increased ovarian
hormones during pregnancy coincide with a notable increase in
numerous GI symptoms, including gastro-esophageal reflux, nausea,
vomiting, constipation, bloating, delayed gastric emptying and gall
bladder dysfunction (12–15).
The predominant biological effects of estrogen are
mediated through two receptors, estrogen receptor (ER)α and ERβ,
which have distinct tissue expression patterns. These ERs are
expressed at different levels in various regions of the body,
including the female reproductive tract, vasculature and the GI
tract (16). These ERs may influence
each other; therefore, estrogen action in tissues where they are
co-expressed is complex, and if one of the receptors is deleted,
the resulting changes in physiological function may be
unpredictable and difficult to understand (17). Estrogen was also determined to induce
a number of rapid-signaling or non-genomic events in a variety of
cell types, providing significant functional evidence that surface
membrane ERs are also involved in the rapid relaxant effects of
estrogen (18). Estrogen was
demonstrated to cause relaxation in smooth muscles of the gall
bladder (13), trachea (2), urinary bladder (19), blood vessels (20) and colon (21,22). In
addition, estrogen induces relaxation of vascular smooth muscle via
a process involving the activation of the NO/cGMP pathway (23); however, whether this mechanism
underlying estrogen-mediated relaxation occurs in gastric smooth
muscle has remained elusive.
In the present study, the hypothesis that
sex-associated differences in rat gastric smooth muscle cell (GSMC)
contractions exist, which may be a result of differences in the
expression and/or activity of ER subtypes, was investigated. The
effect of estrogen on the NO/cGMP pathway in the GSMCs was also
investigated. Due to motility disorders being major characteristics
of numerous GI disturbances, the present study may be of notable
importance in understanding the cause of their disproportionate
prevalence among females; in addition, it may further pave the way
for understanding the ER-mediated smooth muscle
contraction-relaxation pathways and thereby establishing novel
therapeutic approaches for the treatment of GI disorders.
Materials and methods
Materials
A protein assay kit (cat. no. 500–0119) was obtained
from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). Rat estrogen β
(cat. no. CSB-EL007831RA) and rat 17β-estradiol (E2; cat. no.
CSB-E06848r) ELISA kits were obtained from Cusabio Biotech (Newark,
DE, USA). 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; cat.
no. ab120022), Nω-nitro-L-arginine (L-NNA; cat. no. ab141312) and
anti-calponin antibodies (cat. no. ab46794) were purchased from
Abcam (Cambridge, MA, USA). Diarylpropionitrile (DPN) was purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The 500-µm
Nitex mesh (Sefar Nitex 06–500/38) was from Sefar Inc. (Thal,
Switzerland). All remaining chemicals were from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). A stock solution of E2 was
prepared in 100% ethanol. Stock solutions of
1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT), DPN, L-NNA
and ODQ were prepared in dimethyl sulfoxide (DMSO). The final
concentration of ethanol and DMSO used was 1% (volume/volume).
Animals
Young Sprague Dawley rats [age, 12 weeks; weight,
250–300 g; n=93 (49 males and 44 females)] were supplied by the
animal center of Jordan University of Science and Technology
(Irbid, Jordan). Rats were euthanized by CO2 inhalation
and euthanasia was further confirmed by incising the diaphragm with
a scalpel blade. The present study was approved by the Animal Care
and Use Committee of Jordan University of Science and Technology
(Irbid, Jordan). All experimental procedures followed the NIH's
guidelines.
Preparation of dispersed GSMCs
The stomach was rapidly excised following
euthanasia. GSMCs were isolated from the circular muscle layer of
the rat stomach by sequential enzymatic digestion, filtration and
centrifugation, as previously described (24). Sections of circular muscle from the
stomach were dissected and incubated at 31°C for 30 min in
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) medium
(pH was adjusted to 7.4), containing 120 mM NaCl, 4 mM KCl, 2.0 mM
CaCl2, 2.6 mM KH2PO4, 0.6 mM
MgCl2, 25 mM HEPES, 14 mM glucose, 2.1% Eagle's
essential amino acid mixture (Sigma-Aldrich; Merck KGaA), 0.1%
collagenase (Sigma-Aldrich; Merck KGaA) and 0.01% soybean trypsin
inhibitor. The tissue was continuously exposed to 100% oxygen
during the entire isolation procedure. Subsequently, the partially
digested sections were washed twice with 50 ml enzyme-free HEPES
medium, and the GSMCs were then incubated at room temperature for
spontaneous dispersion for 30 min. The cells were harvested via
filtration through 500-µm Nitex mesh and centrifuged twice at 350 ×
g for 10 min to remove any broken cells and organelles. The cell
isolation procedure consistently yielded spindle-shaped, viable
GSMCs that exhibited significant contraction in response to
contractile stimuli. All the experiments were performed within 2–3
h of cell dispersion.
Identification of GSMCs
The identity of the rat GSMCs was verified by
immunohistochemical staining. Cells were added to adhesive-coated
slides to enhance attachment and air-dried for 15 min. Slides were
then fixed with 4% formaldehyde in PBS solution for 4 min at 4°C,
and then washed twice for 5 min in fresh PBS. A blocking solution
consisting of 5 mM ethylenediaminetetraacetic acid in PBS with 5%
goat serum and 1% bovine serum albumin was applied for 20 min at
room temperature. Following this, the blocking solution was drained
from each slide and the anti-calponin antibody (150 µl per slide;
1:100) was added. The slides were then incubated for 1 h at 4°C.
Subsequently, slides were washed twice in fresh PBS solution for 5
min at room temperature. Goat anti rabbit Immunoglobulin G
antibodies (cat. no. A0545, 1:0; Sigma-Aldrich, Merck KGaA) were
diluted in PBS and added to sections for 30 min at room
temperature, with two 5 min washes with PBS. Then an
avidin-biotin-horseradish peroxidase complex was added for 30 min
at room temperature. Sections were then washed, covered with
diaminobenzidine chromogen and counter-stained with hematoxylin for
10 min at room temperature. Samples were dehydrated in a graded
series of alcohols and mounted with cover slips and sealed. All
Slides, chemicals and reagents used for immunohistochemical
staining were purchased from Dako (Agilent Technologies, Inc.,
Santa Clara, CA, USA).
Expression of ERα and ERβ via
ELISA
GSMCs collected from 10 ml muscle cell suspension
(3×106 cells/ml) were centrifuged (20,000 × g at 4°C for
1 min) and the pellet was snap-frozen in liquid nitrogen and
homogenized using a Teflon glass pestle in 400 µl ice-cold
distilled water. Following the centrifugation of the lysates at
20,000 × g at 4°C for 10 min, the protein concentration in the
supernatant was determined with a Dc protein assay kit (Bio-Rad
Laboratories, Inc.). Samples containing equal amounts of protein
were used for quantification of ERα and ERβ using the ELISA kits
according to the manufacturer's protocol.
Measurement of smooth muscle NO
The concentration of NO in basal and E2-treated (1
µM for 10 min) smooth muscle samples was indirectly measured by
determining nitrate and nitrite levels utilizing an NO
(NO2−/NO3−) assay kit (cat. no. 23479;
Sigma-Aldrich; Merck KGaA), following the manufacturer's protocol.
The assay determined the NO concentration based on the enzymatic
conversion of nitrate to nitrite by nitrate reductase. The reaction
was followed by colorimetric detection of nitrite as a product of
the Griess reaction, based on the diazotization reaction, in which
acidified NO2− produced a nitrosylating agent that
reacted with sulfanilic acid to yield the diazonium ion. This ion
was then combined with N-(1-naphthyl) ethylenediamine to form a
chromophoric azo derivative, which absorbs light at 540 nm. Protein
interference was avoided by treating samples with zinc sulfate and
centrifugation at 4°C for 10 min at 2,000 × g. Samples were
spectrophotometrically quantified using an ELISA microplate reader
(elx-800; BioTek Instruments, Winooski, VT, USA) at 540 nm.
NaNO2 was used as a standard and a curve of the nitrite
concentration against the optical density was plotted.
Measurement of smooth muscle cGMP
The level of cGMP in control and E2-treated (1 µM
for 10 min) smooth muscle cell samples was measured using an ELISA
kit (cat. no. STA-505; Cell Biolabs, Inc., San Diego, CA, USA)
according to the manufacturer's protocol.
Measurement of contraction of
dispersed GSMCs
Contraction of recently dispersed GSMCs was
determined by scanning micrometry, as previously described
(4). Aliquots (0.4 ml) of cell
suspension containing ~104 cells/ml were prepared. Cells
were pooled from different animals of the same sex to enhance the
cell number. Aliquots were randomly distributed into the control,
E2, PPT [a selective ERα agonist (24)], DPN [a selective ERβ agonist
(25)], ODQ (a guanylyl cyclase
inhibitor), or L-NNA (an NO synthase inhibitor) treatment groups.
Aliquots designated for treatment were incubated with 1 µM E2, PPT,
DPN, L-NNA or ODQ for 10 min. Cells were stimulated with
acetylcholine (ACh; 0.1 µM) for 10 min at room temperature in the
presence or absence of ER modulator treatment, and the reaction was
terminated with 1% acrolein at a final concentration of 0.1%.
Acrolein kills and fixes cells without affecting the cell length.
The cells were viewed using a ×10, ×20 and ×40 magnification with
an inverted Nikon TMS-f microscope (Nikon, Tokyo, Japan), and cell
images were captured using a Canon digital camera (cat. no.
DS126291; Canon, Inc., Tokyo, Japan) and ImageJ software (version
1.45s; National Institutes of Health, Bethesda, MA, USA). The
resting cell length was determined in control experiments, in which
muscle cells were not treated with ACh. The mean length of 50 GSMCs
from each group was measured using ImageJ software. An aliquot of
cells fixed with acrolein was placed on a slide under a coverslip.
Images were captured for each slide with the microscope-connected
camera and the lengths of the first 50 randomly encountered cells
in successive microscopic fields were measured using the ImageJ
software. The contractile response to ACh was defined as a decrease
in the mean length of 50 cells, and expressed as the percentage
change in length relative to the average resting length.
Statistical analysis
The results were expressed as the mean ± standard
error of the mean. Each experiment was performed on cells obtained
from rats of same sex. P-values were determined by an unpaired
Student's t-test when comparing two samples, or by one-way analysis
of variance followed by Tukey's post-hoc test when comparing >2
samples, using Prism 5.0 software (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Verification of the identity of
GSMCs
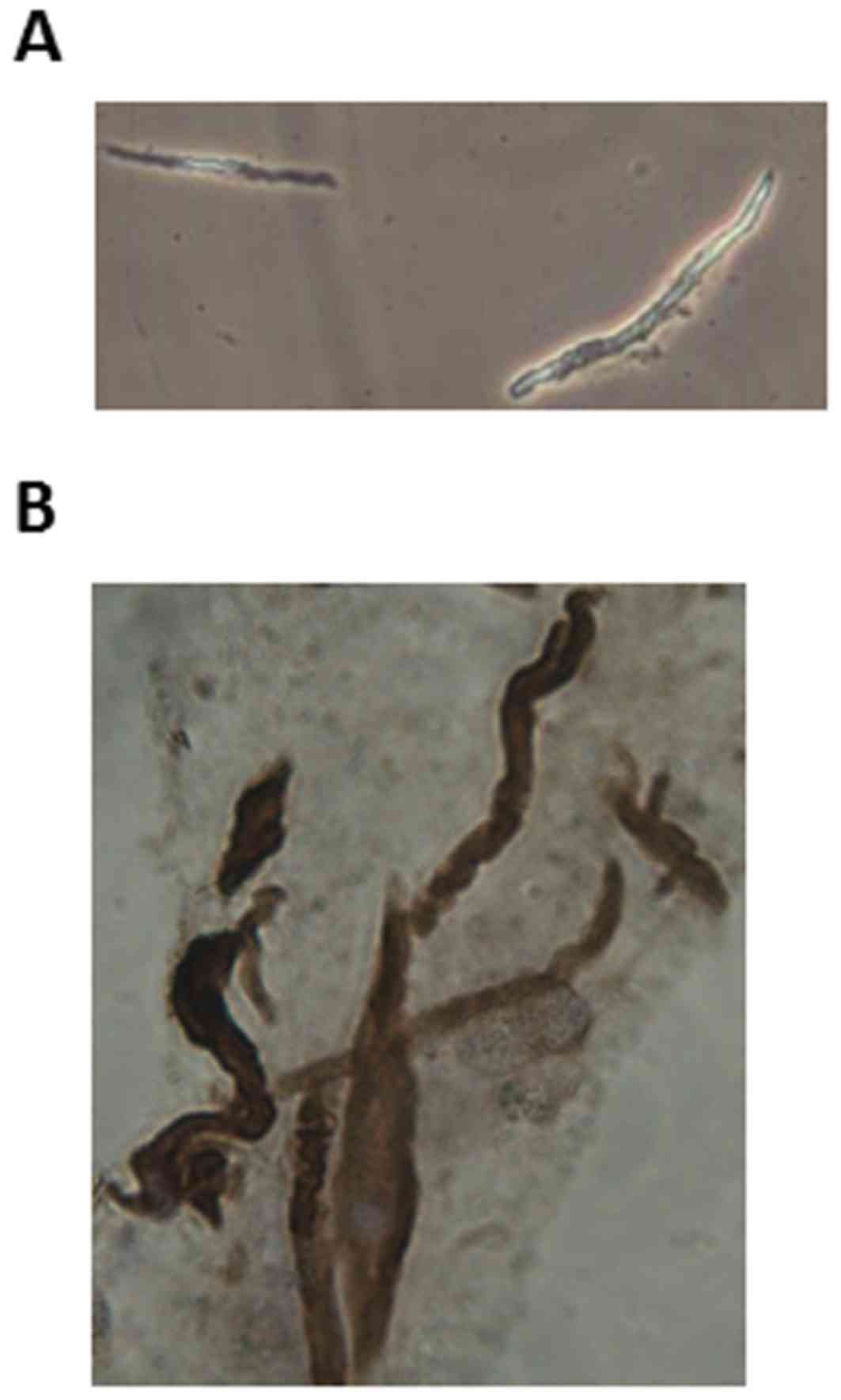
Freshly dispersed and isolated GSMCs appeared to be
spindle-shaped with various lengths, as determined using phase
contrast microscopy; an example of a singular male GSMCs is
depicted in Fig. 1A. The identity of
the rat GSMCs was verified by immunohistostaining with
anti-calponin antibodies (Fig. 1B).
Of the cells collected, >95% stained positive for h1-calponin, a
protein whose expression is specific for differentiated SMCs
(26).
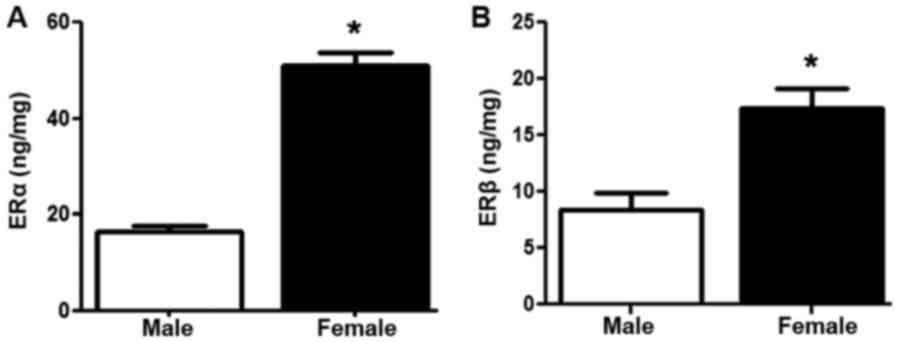
ER expression
The ELISAs revealed that the protein expression of
ERα and ERβ (P<0.05) was significantly increased in the GSMCs
from females compared with those from males (Fig. 2A and B, respectively).
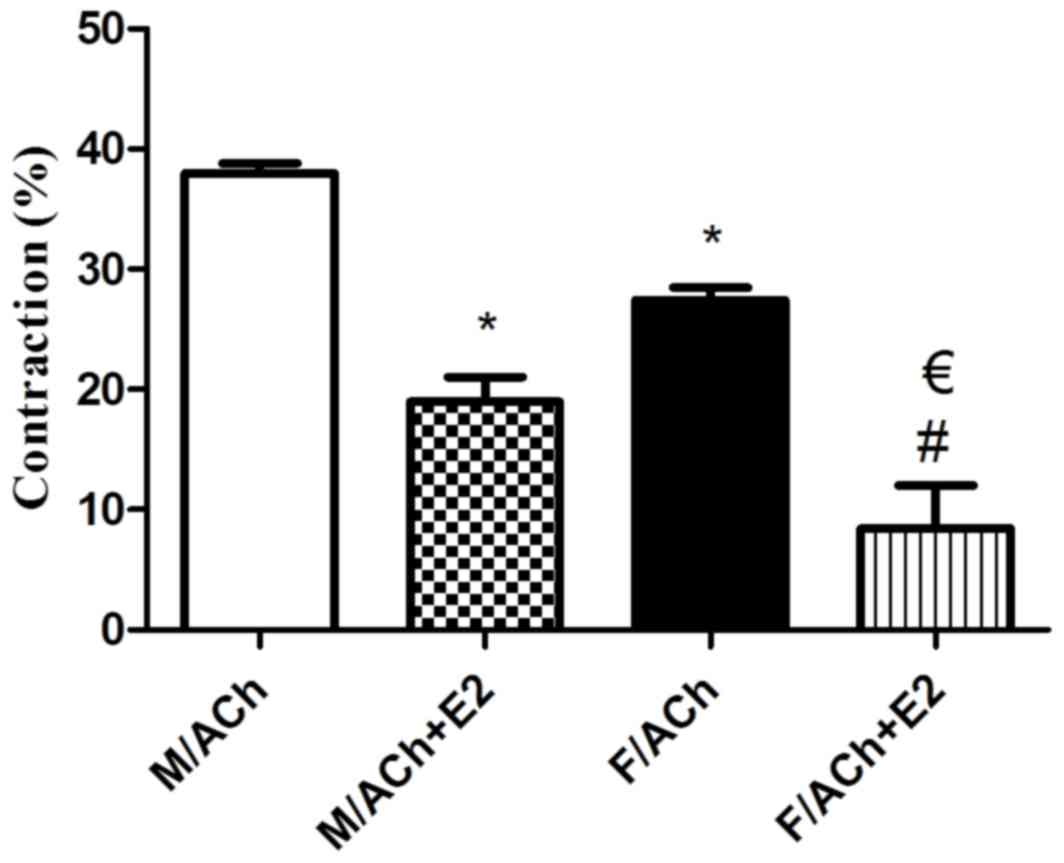
Effect of estrogen on muscle cell
contraction
Recently isolated and dispersed GSMCs from both
sexes were treated with ACh, and scanning micrometry was performed
to measure the decrease in muscle cell length. Resting muscle
length was identical in male and female cells. ACh caused muscle
cell contraction in both sex groups. Contraction in response to ACh
was significantly reduced in the female cells compared with that in
male cells (P<0.05). Of note, pre-incubation of GSMCs from males
and females with E2 significantly decreased the ACh-induced
contraction (P<0.05). Furthermore, the estrogen-induced
relaxation was greater in female cells compared with that in male
cells (50 vs. 70% reduction in contraction of males and females,
respectively) (P<0.05; Fig. 3).
Due to the increased effect of estrogen in female GSMCs, an
investigation into the effect of various ER agonists on the muscle
contraction of female GSMCs was then pursued. The ERα agonist PPT
and the ERβ agonist DPN reduced ACh-induced contraction. DPN
induced relaxation to a greater extent than PPT, although this
result was not statistically significant (Fig. 4).
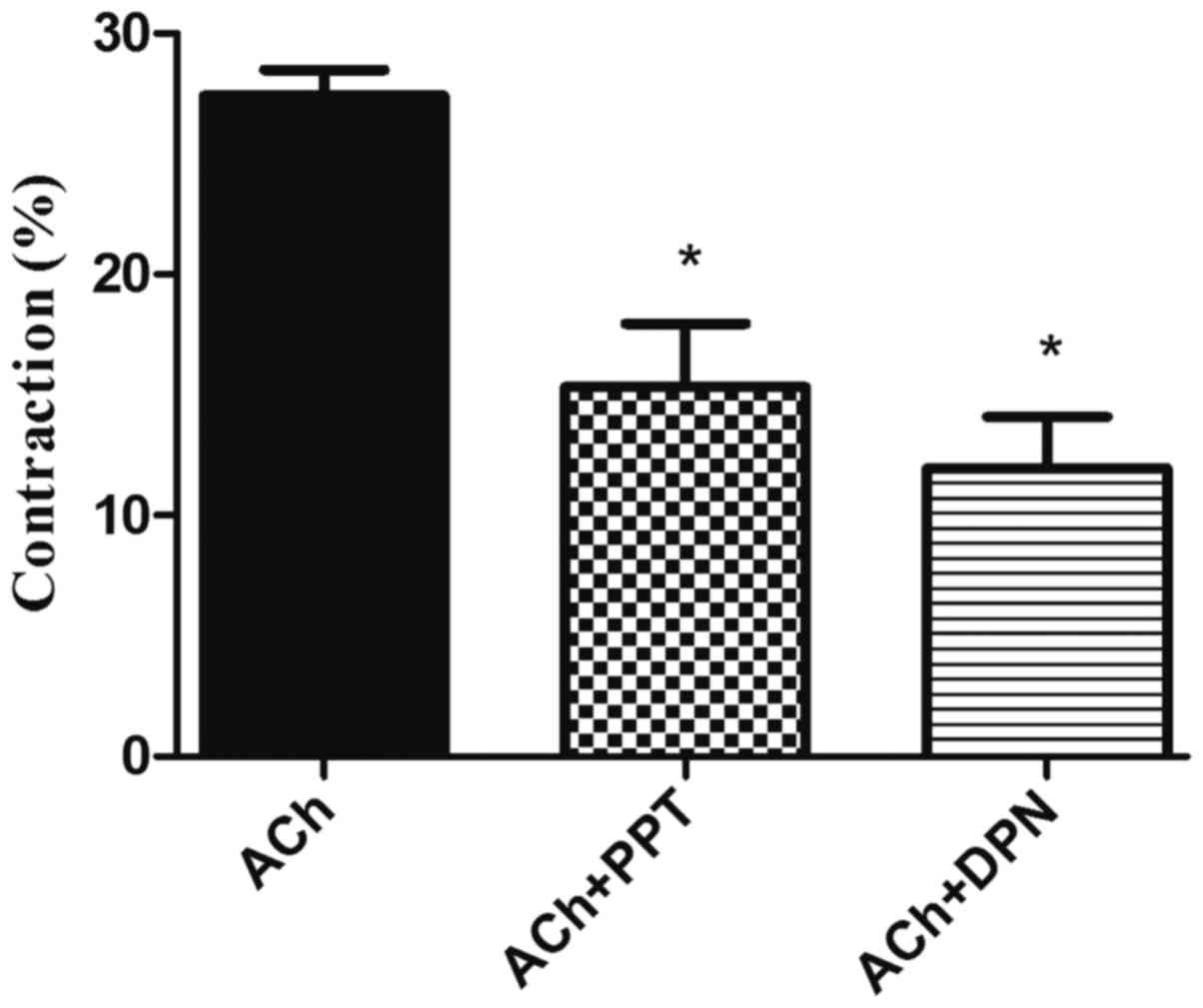 | Figure 4.Effect of ER modulators on
ACh-induced contraction in GSMCs from female rats. GSMCs of female
rats were stimulated with ACh in the presence or absence of PPT, an
ERα agonist, or DPN, an ERβ agonist. Pre-incubation with PPT or DPN
significantly reduced ACh-induced contraction in the GSMCs. Values
are expressed as the mean ± standard error of the mean (n=50 cells
from 10 female rats). *P<0.05 vs. ACh. ER, estrogen receptor;
ACh, acetylcholine; GSMCs, gastric smooth muscle cells; PPT,
1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole; DPN,
diarylpropionitrile. |
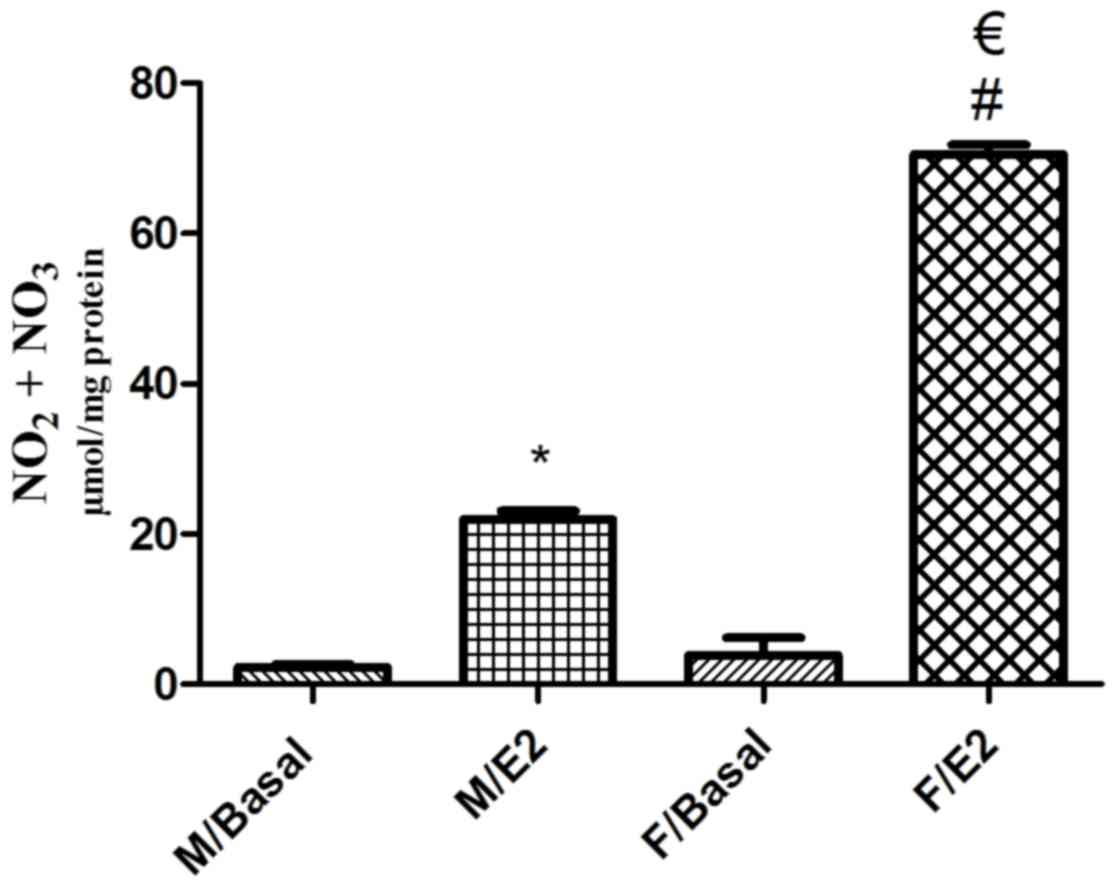
Effect of estrogen on NO formation in
singular GSMCs
Basal NO levels were similar in male and female
singular GSMCs (P>0.05), with mean values of 2.27±0.40 and
3.89±2.33 µmol/mg protein, respectively. Treatment of the GSMCs
with E2 significantly increased the NO levels in male and female
cells (P<0.05). Of note, the E2-induced increase in NO levels in
female cells was significantly greater than that in male cells
(>3-fold; P<0.05; Fig. 5).
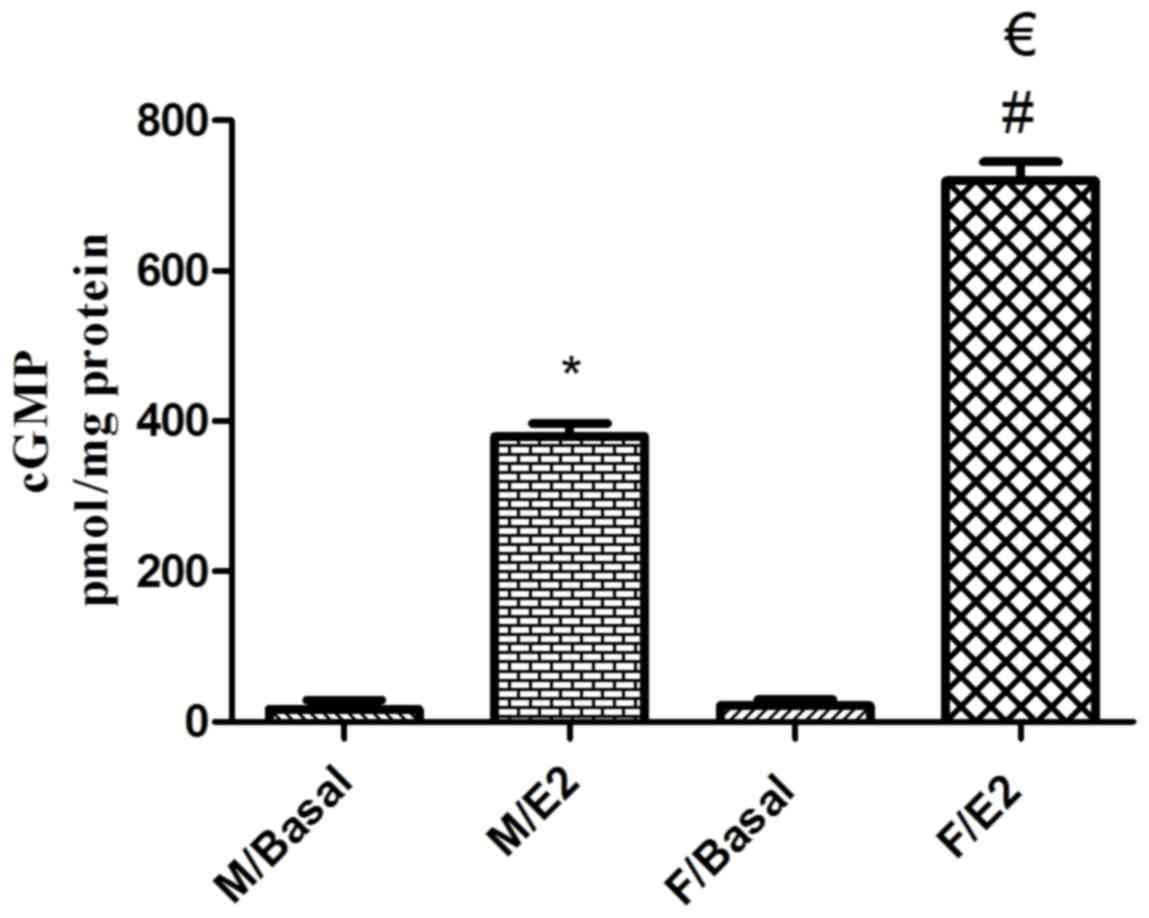
Effect of estrogen on cGMP formation
in singular GSMCs
The mean basal cGMP levels in singular male and
female GSMCs were 16.75±12.33 and 21.36±7.97 pmol/mg protein,
respectively. Treatment of the male and female GSMCs with E2
significantly increased the cGMP levels (P<0.05). Of note, the
E2-induced increase in cGMP levels in female cells was
significantly greater than that in male cells (~1.9-fold;
P<0.05; Fig. 6).
Effect of the blockade of NO synthase
and sGC on E2-induced relaxation
As the production of NO and cGMP stimulated by
estrogen was greater in female cells, the focus was on
investigating the effect of the NO synthase blocker L-NNA and the
sGC blocker ODQ on the E2-induced inhibition of muscle contraction
in female cells. L-NNA and ODQ significantly reduced the E2-induced
inhibition of GSMC contraction (P<0.05; Fig. 7).
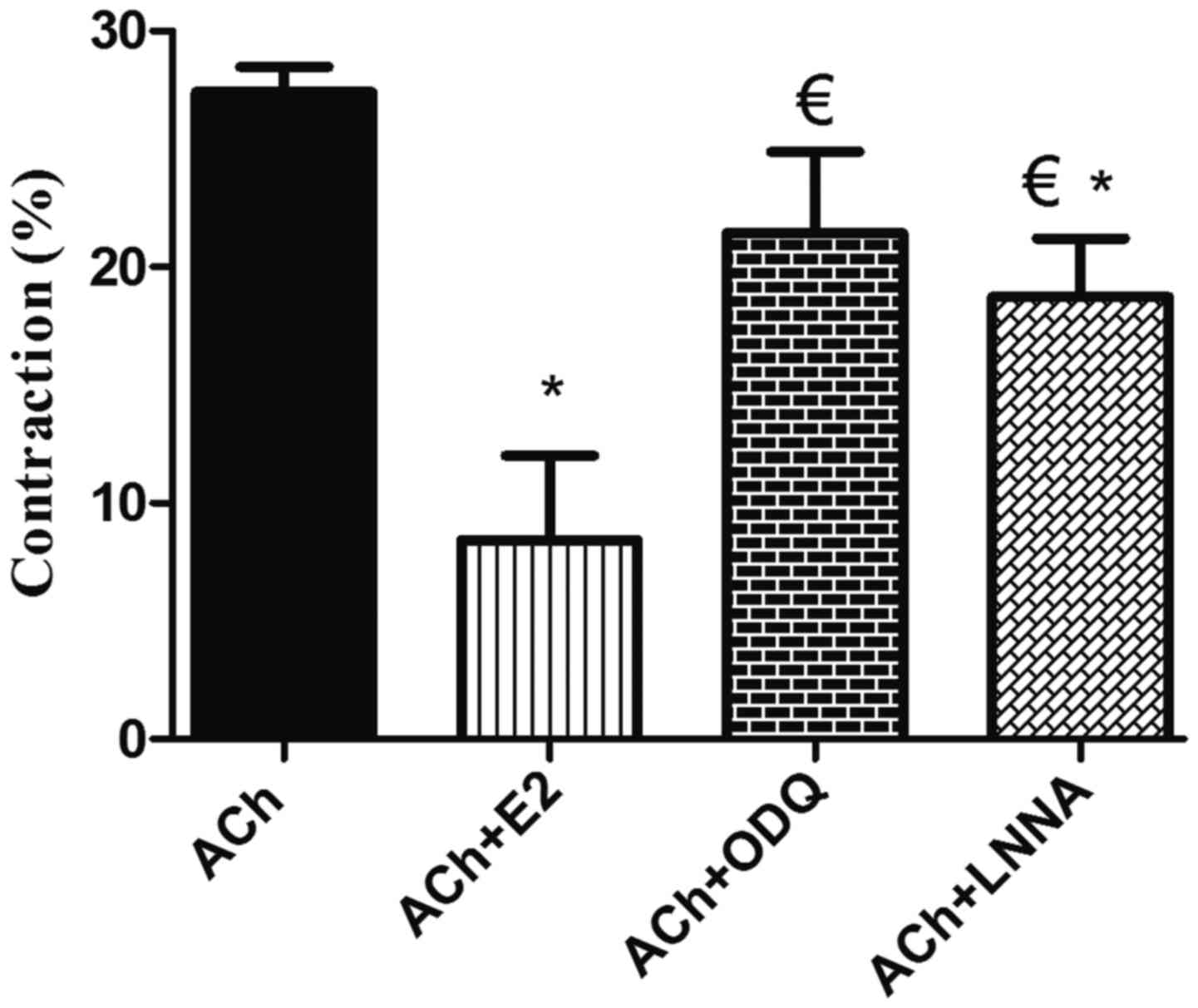 | Figure 7.Effect of blockade of NO synthase and
sGC on estrogen-induced relaxation in GSMCs from female rats. Cells
were treated with ACh and contraction was expressed relative to
control-cell contraction. ACh induced GSMC contraction, whereas
pre-treatment of the GSMCs with E2 significantly reduced
ACh-induced contraction. Relaxation induced by E2 was significantly
inhibited in muscle cells pre-incubated with ODQ or L-NNA.
Cumulative data (n=50 cells from 10 female rats) are presented as
the mean ± standard error of the mean. *P<0.05 vs. ACh;
€P<0.05 vs. ACh+E2. NO, nitric oxide; sGC, soluble
guanylyl cyclase; GSMC, gastric smooth muscle cells; ACh,
acetylcholine; E2, 17β-estradiol; ODQ,
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; L-NNA,
Nω-nitro-L-arginine. |
Discussion
In the present study, an increased expression of ERα
and ERβ, and a decreased contraction of GSMCs from females compared
with those from males was demonstrated. Estrogen induced a greater
extent of relaxation in the GSMCs from females compared with those
from males, probably via the increased production of NO and cGMP.
Previous studies demonstrated sex-specific differences in smooth
muscle, which has functions in a number of different organs and in
various species (27,28). It was recently determined that the
extent of activation of the small G protein Ras homolog gene family
(Rho), member A and its downstream effector, Rho-associated protein
kinase, members of an important pathway in developing smooth muscle
tone, is elevated in response to the muscarinic agonist ACh, and
thus, the contraction of male GSMCs is greater compared with that
of female GSMCs (29). Numerous
studies have investigated the effect of sex steroids on the
function of the GI tract, indicating that sex differences may be
due to differences in the expression/activity of estrogen and its
receptors (30–32). For instance, previous studies
demonstrated that circulating levels of estrogen, which fluctuate
during the various stages of the ovarian cycle, may serve a role in
gastric motility, GI transit times and GSMC reactivity (10,33).
Previous studies have also indicated that estrogen affects gastric
motility at the tissue level, with an evident effect on the
neuronal NO synthase of non-adrenergic non-cholinergic neurons
(33). Taking into consideration
that the multi-cellular composition of the stomach makes it
difficult to differentiate between the specific roles of cells,
recently dispersed GSMC were used and the contraction of singular
cells in response to ACh, the major contractile agonist in the GI
tract, was determined. Cells were isolated from different animals
of the same sex to enhance the number of cells collected. However,
improving the isolation procedure in the future may enhance the
amount of cells collected, even from a single animal. Of note, a
reduced ACh-induced contraction was observed in GSMCs of female
rats compared with that in GSMCs from male rats, which is
consistent with the results of previous studies by our group
(29,34) and with observations made in non-GI
smooth muscle regions (20).
A number of the effects of estrogen on muscles are
mediated via its classical receptors. ER subtypes have been
identified in the female reproductive tract, mammary glands and
blood vessels, and throughout the GI tract of humans and
experimental animals (16). The
present study provided evidence for sex-associated differences in
the amount of gastric ERα and ERβ in GSMCs, with a greater amount
in female compared with that in male GSMCs; however, a number of
studies have reported that ERs are only present in the gastric
mucosa and not in the muscular layer (35). This variation may be due to
differences in sensitivity of the techniques applied, as in
situ hybridization was used, as well as differences in species,
due to the studies being performed on human tissue samples.
Functionally, estrogen has been demonstrated to have
an inhibitory effect on the contractility of smooth muscle.
Consistent with previous studies on other parts of the GI tract
(13,15,21,13),
it was determined that estrogen, an agonist for both ER subtypes,
inhibited muscle contraction in both sexes. Of note, the extent of
the relaxation effect induced by estrogen was greater in female
GSMCs compared with that in male GSMCs, in parallel with the ER
expression pattern in males and females. The next aim was to
examine the contribution of each specific ER subtype to the effect
of E2 using ER type-specific agonists. As ER expression and the
effect of estrogen were greater in female GSMCs compared with those
in male GSMCs, the effect of ER agonists on female GSMCs was
further investigated. PPT and DPN inhibited the ACh-induced
contraction of the GSMCs. DPN induced a greater extent of
relaxation compared with PPT, although this difference was not
statistically significant. This may be due to differences in the
expression of ER subtypes in GSMCs. The results of the present
study are in accordance with a previous study, which indicated that
ERβ serves a predominant role in inhibiting colonic contractility
(37). Future contraction studies
examining the effect of various ER agonists on muscle contraction
to test the sensitivity of each receptor may be required. The rapid
time-course (≤10 min) of the muscle relaxant action of estrogen in
the present study indicated the non-genomic effect of the hormone,
as a characteristic genomic effect involves time-consuming
transcription and translation processes (38). This is supported by the fact that
membrane ERs are implicated in the rapid vasodilation effects of
estrogen (18).
It is notable that the concentrations of E2 used in
the present experiments are far greater than the
picomolar-to-nanomolar levels of free hormone present in the plasma
under normal physiological conditions (i.e., in the absence of
pregnancy). As estrogen is lipophilic, its plasma levels may not
reflect its gastric tissue levels, and prolonged exposure to small
estrogen concentrations in vivo may result in gradual tissue
accumulation, eventually reaching levels similar to those used in
acute studies; thus, in vitro studies may require higher E2
concentrations than those usually encountered in vivo to
bind plasma membrane lipids and ERs (39). After reviewing the E2 dose-response
curve from a previous study (40),
it was determined that a concentration of 1 µM, which lies in the
middle of the curve, is adequate. Furthermore, the concentrations
of the various agonists used in this study were found to properly
affect muscle contraction in previous experiments. In addition,
previous research reported that ethanol and DMSO had no effect on
muscle tone at a final concentration of 1% (volume/volume)
(20,41,42).
However, including vehicles of the dissolved reagents would further
strengthen these results.
Based on estrogen-associated studies in the muscle
of other body regions, it was hypothesized that the mechanism
underlying the effect of estrogen on muscle contraction may result
from activation of the NO/cGMP pathway. Numerous studies have
demonstrated the stimulatory effect of estrogen on the production
of NO, an important regulatory neurotransmitter that controls
gastric motility, and the downstream Cgmp (23,33). In
addition, the present study determined that estrogen enhanced the
production of NO in male and female GSMCs, and that the effect was
greater in female cells. NO is a potent relaxant due to its
stimulatory effect on smooth muscle guanylate cyclase and the
production of cGMP (43), and is
produced by the enzyme NO synthase. As the present study used
singular GSMCs, the elevated NO production was primarily due to the
activation of the constitutive NO synthase isoform in SMCs
(44). This estrogen-induced NO
production was paralleled by an increased production of cGMP in
GSMCs from both sexes, which was increased in females compared with
that in males. To prove the contribution of the NO/cGMP pathway in
the estrogen-mediated relaxation of GSMCs, female GSMCs were
treated with inhibitors of NO synthase and guanylyl cyclase.
Consistent with other studies, the blockade of NO synthase by L-NNA
or guanylyl cyclase by ODQ significantly inhibited estrogen-induced
relaxation (23,45–47).
These results provide evidence for the involvement of NO and cGMP
in gastric estrogen action. cGMP may also induce relaxation through
its well-established ability to reduce the cytosolic
Ca2+ concentration (48)
and modulate the activity of potassium channels (49). Taking into consideration the possible
effect of estrogen on these and other possible muscle targets may
explain the disproportionate difference in E2-induced relaxation
and the E2-induced activation of NO and GC activity between female
and male GSMCs. Further studies are required to investigate the
signaling pathway mediation of estrogen-induced SMC relaxation
downstream of NO/cGMP.
A novel transmembrane ER known as G-protein-coupled
ER (GPR30) is implicated in various physiological processes in the
reproductive, nervous, endocrine, immune and cardiovascular
systems, as well as pathological processes in a diverse array of
disorders (50–52). This third ER type may serve an
important role in the regulation of muscle tone, works solely
through non-genomic pathways, and also stimulates NO and cGMP
production in various cell types, including SMCs (51,53,54). In
addition to the activation of ERα, PPT has also been demonstrated
to activate GPR30 in a range of contexts, particularly when used in
a high dose range (55). Whether
GPR30 is expressed in GSMCs and whether it displays any
sex-associated differences remains elusive; therefore, further
studies are required to investigate its contribution.
In conclusion, the present study demonstrated an
increased expression of ERα and ERβ in GSMCs from females compared
with those from males. The greater reduction in contraction of
female GSMCs following estrogen treatment may be due to the
sex-associated increases in the expression of ERα and ERβ,
resulting in a greater activation of the NO/cGMP pathway. ERs may
potentially exert non-genomic effects as well as genomic effects on
the contraction-relaxation pathway in SMCs. The exact mechanisms by
which ERs may affect smooth muscle contraction should be further
investigated. Sex-associated differences are present in the GI
system, with ER expression and sensitivity serving a pivotal role
in GI function. An improved understanding of the role of sex
hormones and their receptors in modulating the normal and
pathophysiological GI tract function may provide the possibility
for more effective and sex-specific therapeutic approaches for
various GI diseases.
Acknowledgements
Not applicable.
Funding
The present work was supported by Jordan University
of Science and Technology (Irbid, Jordan; grant no. 20140234).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
Conception and design of the study were performed by
OAA. Acquisition of data and drafting of the manuscript were
performed by OAA, MSN and AAO. Analysis and interpretation of data
and critical revision of the manuscript for important intellectual
content were performed by OAA, MSN, AGM, ANA, MoAA1, AAO, MaAA2 and
MIA. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The current study protocols were approved by and
followed the guidelines of the Animal Care and Use Committee of
Jordan University of Science and Technology (Irbid, Jordan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACh
|
acetylcholine
|
|
cGMP
|
cyclic guanosine monophosphate
|
|
DPN
|
diarylpropionitrile
|
|
E2
|
17β-estradiol
|
|
ER
|
estrogen receptor
|
|
GI
|
gastrointestinal
|
|
GSMC
|
gastric smooth muscle cells
|
|
L-NNA
|
Nω-nitro-L-arginine
|
|
NO
|
nitric oxide
|
|
ODQ
|
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
|
|
PPT
|
1,3,5-tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole
|
References
|
1
|
Farhat MY, Lavigne MC and Ramwell PW: The
vascular protective effects of estrogen. FASEB J. 10:615–624. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Townsend EA, Miller VM and Prakash YS: Sex
differences and sex steroids in lung health and disease. Endocr
Rev. 33:1–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gonenne J, Esfandyari T, Camilleri M,
Burton DD, Stephens DA, Baxter KL, Zinsmeister AR and Bharucha AE:
Effect of female sex hormone supplementation and withdrawal on
gastrointestinal and colonic transit in postmenopausal women.
Neurogastroenterol Motil. 18:911–918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matchock RL, Levine ME, Gianaros PJ and
Stern RM: Susceptibility to nausea and motion sickness as a
function of the menstrual cycle. Womens Health Issues. 18:328–335.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Turnbull GK, Thompson DG, Day S, Martin J,
Walker E and Lennard-Jones JE: Relationships between symptoms,
menstrual cycle and orocaecal transit in normal and constipated
women. Gut. 30:30–34. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang X, Liu R and Dong Y: Regulative
effects of ovarian steroids on rat gastric motility and
sensitivity. Sheng Li Xue Bao. 58:275–280. 2006.PubMed/NCBI
|
|
7
|
Rao JN: Estrogens and gastroparesis: A
clinical relevance. Dig Dis Sci. 58:1449–1451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ravella K, Al-Hendy A, Sharan C, Hale AB,
Channon KM, Srinivasan S and Gangula PR: Chronic estrogen
deficiency causes gastroparesis by altering neuronal nitric oxide
synthase function. Dig Dis Sci. 58:1507–1515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murthy KS: Signaling for contraction and
relaxation in smooth muscle of the gut. Annu Rev Physiol.
68:345–374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gangula PR, Sekhar KR and Mukhopadhyay S:
Gender bias in gastroparesis: Is nitric oxide the answer? Dig Dis
Sci. 56:2520–2527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mulak A, Taché Y and Larauche M: Sex
hormones in the modulation of irritable bowel syndrome. World J
Gastroenterol. 20:2433–2448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gill SK, Maltepe C and Koren G: The effect
of heartburn and acid reflux on the severity of nausea and vomiting
of pregnancy. Can J Gastroenterol. 23:270–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riezzo G, Pezzolla F, Darconza G and
Giorgio I: Gastric myoelectrical activity in the first trimester of
pregnancy: A cutaneous electrogastrographic study. Am J
Gastroenterol. 87:702–707. 1992.PubMed/NCBI
|
|
14
|
Everson GT: Gastrointestinal motility in
pregnancy. Gastroenterol Clin North Am. 21:751–776. 1992.PubMed/NCBI
|
|
15
|
Kline L and Karpinski E: A comparison of
the effects of various sex steroids on cholecystokinin- and
KCl-induced tension in female guinea pig gallbladder strips. Gen
Comp Endocrinol. 185:37–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuiper GG, Carlsson B, Grandien K, Enmark
E, Häggblad J, Nilsson S and Gustafsson JA: Comparison of the
ligand binding specificity and transcript tissue distribution of
estrogen receptors alpha and beta. Endocrinology. 138:863–870.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nilsson S, Mäkelä S, Treuter E, Tujague M,
Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M and
Gustafsson JA: Mechanisms of estrogen action. Physiol Rev.
81:1535–1565. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mendelsohn ME: Genomic and nongenomic
effects of estrogen in the vasculature. Am J Cardiol. 90:3F–6F.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dambros M, van Koeveringe GA, Bast A and
van Kerrebroeck PE: Relaxant effects of estradiol through
non-genomic pathways in male and female pig bladder smooth muscle.
Pharmacology. 72:121–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma Y, Qiao X, Falone AE, Reslan OM,
Sheppard SJ and Khalil RA: Gender-specific reduction in contraction
is associated with increased estrogen receptor expression in single
vascular smooth muscle cells of female rat. Cell Physiol Biochem.
26:457–470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hogan AM, Kennelly R, Collins D, Baird AW
and Winter DC: Oestrogen inhibits human colonic motility by a
non-genomic cell membrane receptor-dependent mechanism. Br J Surg.
96:817–822. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zielińska M, Fichna J, Bashashati M,
Habibi S, Sibaev A, Timmermans JP and Storr M: G protein-coupled
estrogen receptor and estrogen receptor ligands regulate colonic
motility and visceral pain. Neurogastroenterol Motil.
29:e130252017. View Article : Google Scholar
|
|
23
|
Darkow DJ, Lu L and White RE: Estrogen
relaxation of coronary artery smooth muscle is mediated by nitric
oxide and cGMP. Am J Physiol. 272:H2765–H2773. 1997.PubMed/NCBI
|
|
24
|
Stauffer SR, Coletta CJ, Tedesco R,
Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS and
Katzenellenbogen JA: Pyrazole ligands: Structure-affinity/activity
relationships and estrogen receptor-alpha-selective agonists. J Med
Chem. 43:4934–4947. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harrington WR, Sheng S, Barnett DH, Petz
LN, Katzenellenbogen JA and Katzenellenbogen BS: Activities of
estrogen receptor alpha- and beta-selective ligands at diverse
estrogen responsive gene sites mediating transactivation or
transrepression. Mol Cell Endocrinol. 206:13–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hossain MM, Hwang DY, Huang QQ, Sasaki Y
and Jin JP: Developmentally regulated expression of calponin
isoforms and the effect of h2-calponin on cell proliferation. Am J
Physiol Cell Physiol. 284:C156–C167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hogg ME, Vavra AK, Banerjee MN, Martinez
J, Jiang Q, Keefer LK, Chambon P and Kibbe MR: The role of estrogen
receptor α and β in regulating vascular smooth muscle cell
proliferation is based on sex. J Surg Res. 173:e1–e10. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wierman ME: Sex steroid effects at target
tissues: Mechanisms of action. Adv Physiol Educ. 31:26–33. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Al-Shboul O: The role of the RhoA/ROCK
pathway in gender-dependent differences in gastric smooth muscle
contraction. J Physiol Sci. 66:85–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Shboul O, Mustafa A and Al-hashimi F:
Non-genomic effects of progesterone on Rho kinase II in rat gastric
smooth muscle cells. J Smooth Muscle Res. 49:55–62. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen TS, Doong ML, Chang FY, Lee SD and
Wang PS: Effects of sex steroid hormones on gastric emptying and
gastrointestinal transit in rats. Am J Physiol. 268:G171–G176.
1995.PubMed/NCBI
|
|
32
|
Meleine M and Matricon J: Gender-related
differences in irritable bowel syndrome: Potential mechanisms of
sex hormones. World J Gastroenterol. 20:6725–6743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shah S, Nathan L, Singh R, Fu YS and
Chaudhuri G: E2 and not P4 increases NO release from NANC nerves of
the gastrointestinal tract: Implications in pregnancy. Am J Physiol
Regul Integr Comp Physiol. 280:R1546–R1554. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Al-Shboul OA, Al-Dwairi AN, Alqudah MA and
Mustafa AG: Gender differences in the regulation of MLC20
phosphorylation and smooth muscle contraction in rat stomach.
Biomed Rep. 8:283–288. 2018.PubMed/NCBI
|
|
35
|
Enmark E, Pelto-Huikko M, Grandien K,
Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M and
Gustafsson JA: Human estrogen receptor beta-gene structure,
chromosomal localization, and expression pattern. J Clin Endocrinol
Metab. 82:4258–4265. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kline LW and Karpinski E: 17β-Estradiol
relaxes cholecystokinin- and KCl-induced tension in male guinea pig
gallbladder strips. Steroids. 76:553–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hogan AM, Collins D, Sheehan K, Zierau O,
Baird AW and Winter DC: Rapid effects of phytoestrogens on human
colonic smooth muscle are mediated by oestrogen receptor beta. Mol
Cell Endocrinol. 320:106–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bayard F, Clamens S, Meggetto F, Blaes N,
Delsol G and Faye JC: Estrogen synthesis, estrogen metabolism, and
functional estrogen receptors in rat arterial smooth muscle cells
in culture. Endocrinology. 136:1523–1529. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
White RE, Darkow DJ and Lang JL: Estrogen
relaxes coronary arteries by opening BKCa channels through a
cGMP-dependent mechanism. Circ Res. 77:936–942. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Andersen HL, Weis JU, Fjalland B and
Korsgaard N: Effect of acute and long-term treatment with
17-beta-estradiol on the vasomotor responses in the rat aorta. Br J
Pharmacol. 126:159–168. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Patkar S, Farr TD, Cooper E, Dowell FJ and
Carswell HV: Differential vasoactive effects of oestrogen,
oestrogen receptor agonists and selective oestrogen receptor
modulators in rat middle cerebral artery. Neurosci Res. 71:78–84.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Raffetto JD, Qiao X, Beauregard KG and
Khalil RA: Estrogen receptor-mediated enhancement of venous
relaxation in female rat: Implications in sex-related differences
in varicose veins. J Vasc Surg. 51:972–981. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nathan C: Nitric oxide as a secretory
product of mammalian cells. FASEB J. 6:3051–3064. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Teng B, Murthy KS, Kuemmerle JF, Grider
JR, Sase K, Michel T and Makhlouf GM: Expression of endothelial
nitric oxide synthase in human and rabbit gastrointestinal smooth
muscle cells. Am J Physiol. 275:G342–G351. 1998.PubMed/NCBI
|
|
45
|
Rosenfeld CR, Cox BE, Roy T and Magness
RR: Nitric oxide contributes to estrogen-induced vasodilation of
the ovine uterine circulation. J Clin Invest. 98:2158–2166. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wong CM, Au CL, Tsang SY, Lau CW, Yao X,
Cai Z and Chung AC: Role of inducible nitric oxide synthase in
endothelium-independent relaxation to raloxifene in rat aorta. Br J
Pharmacol. 174:718–733. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pang JJ, Xu XB, Li HF, Zhang XY, Zheng TZ
and Qu SY: Inhibition of beta-estradiol on trachea smooth muscle
contraction in vitro and in vivo. Acta Pharmacol Sin.
23:273–277. 2002.PubMed/NCBI
|
|
48
|
Twort CH and van Breemen C: Cyclic
guanosine monophosphate-enhanced sequestration of Ca2+ by
sarcoplasmic reticulum in vascular smooth muscle. Circ Res.
62:961–964. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Archer SL, Huang JM, Hampl V, Nelson DP,
Shultz PJ and Weir EK: Nitric oxide and cGMP cause vasorelaxation
by activation of a charybdotoxin-sensitive K channel by
cGMP-dependent protein kinase. Proc Natl Acad Sci USA.
91:7583–7587. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Prossnitz ER and Barton M: The
G-protein-coupled estrogen receptor GPER in health and disease. Nat
Rev Endocrinol. 7:715–726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tropea T, De Francesco EM, Rigiracciolo D,
Maggiolini M, Wareing M, Osol G and Mandalà M: Pregnancy augments G
protein estrogen receptor (GPER) induced vasodilation in rat
uterine arteries via the nitric oxide-cGMP signaling pathway. PloS
One. 10:e01419972015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Prossnitz ER and Hathaway HJ: What have we
learned about GPER function in physiology and disease from knockout
mice? J Steroid Biochem Mol Biol. 153:114–126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lindsey SH, Liu L and Chappell MC:
Vasodilation by GPER in mesenteric arteries involves both
endothelial nitric oxide and smooth muscle cAMP signaling.
Steroids. 81:99–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lee HJ, Suh JK, Song HH, Jeong MA, Yeom JH
and Kim DW: Antioxidant effects of methylprednisolone and
hydrocortisone on the impairment of endothelium dependent
relaxation induced by reactive oxygen species in rabbit abdominal
aorta. Korean J Anesthesiol. 64:54–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Petrie WK, Dennis MK, Hu C, Dai D,
Arterburn JB, Smith HO, Hathaway HJ and Prossnitz ER: G
protein-coupled estrogen receptor-selective ligands modulate
endometrial tumor growth. Obstet Gynecol Int. 2013:4727202013.
View Article : Google Scholar : PubMed/NCBI
|