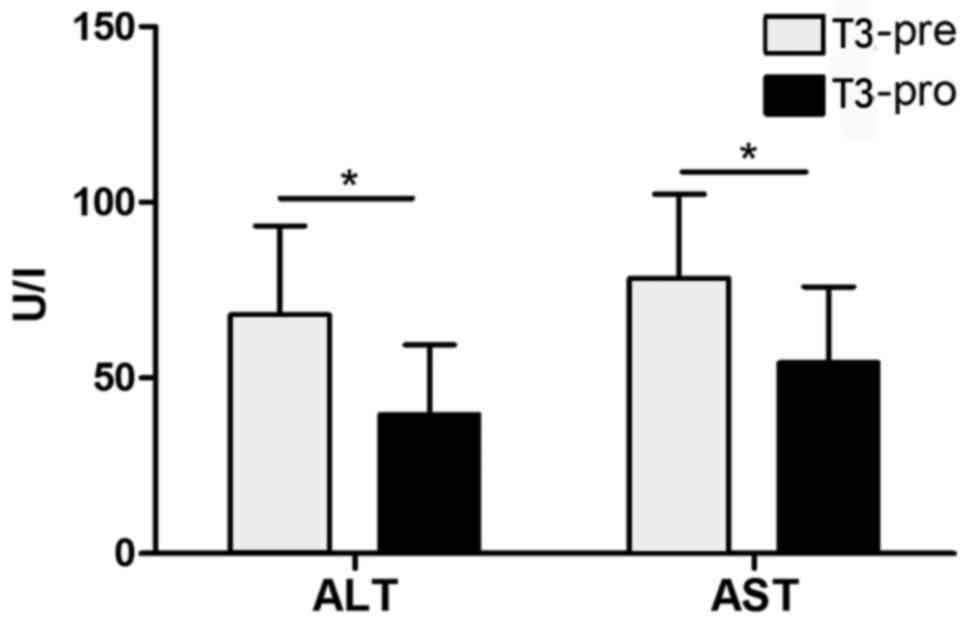
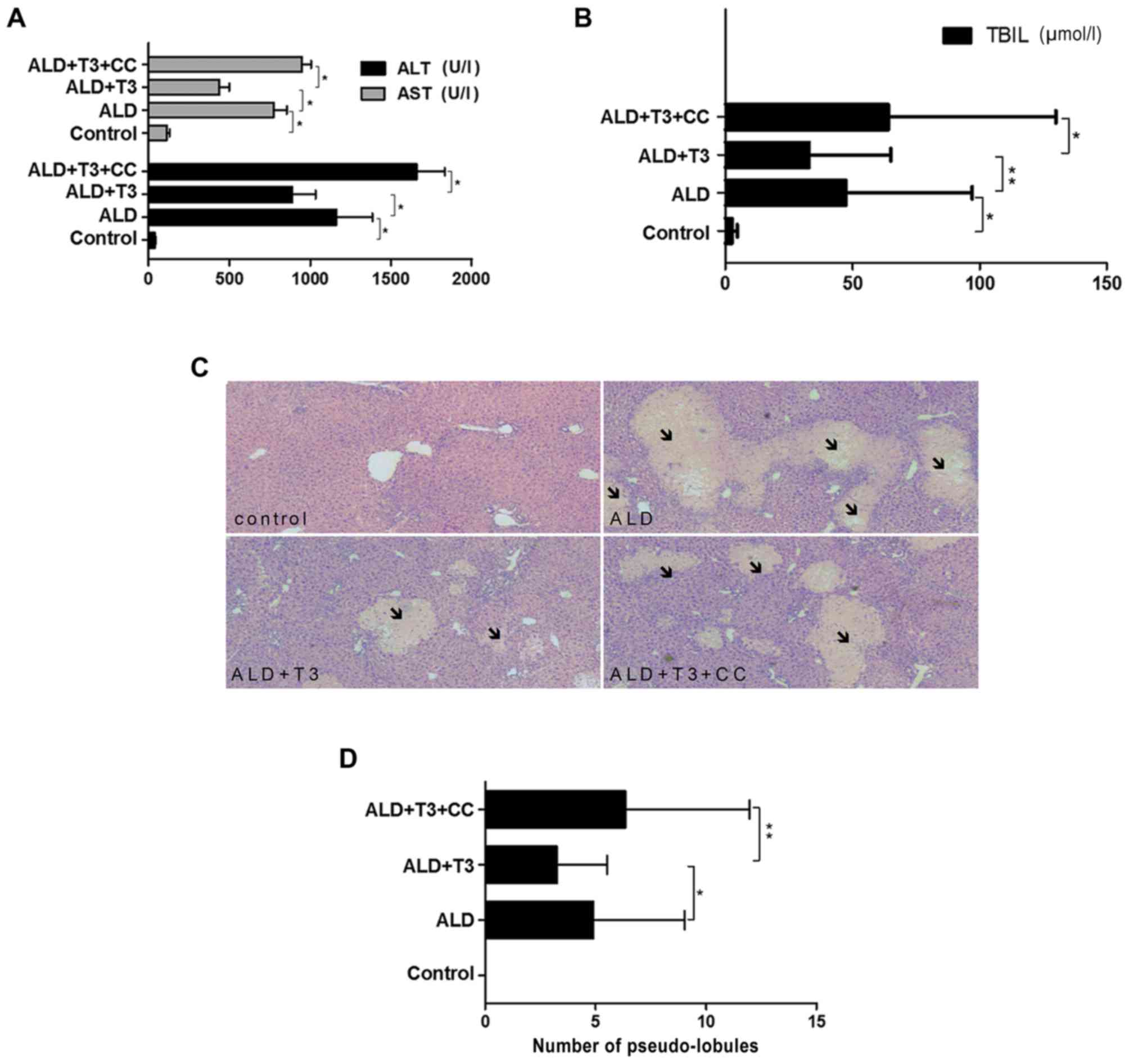
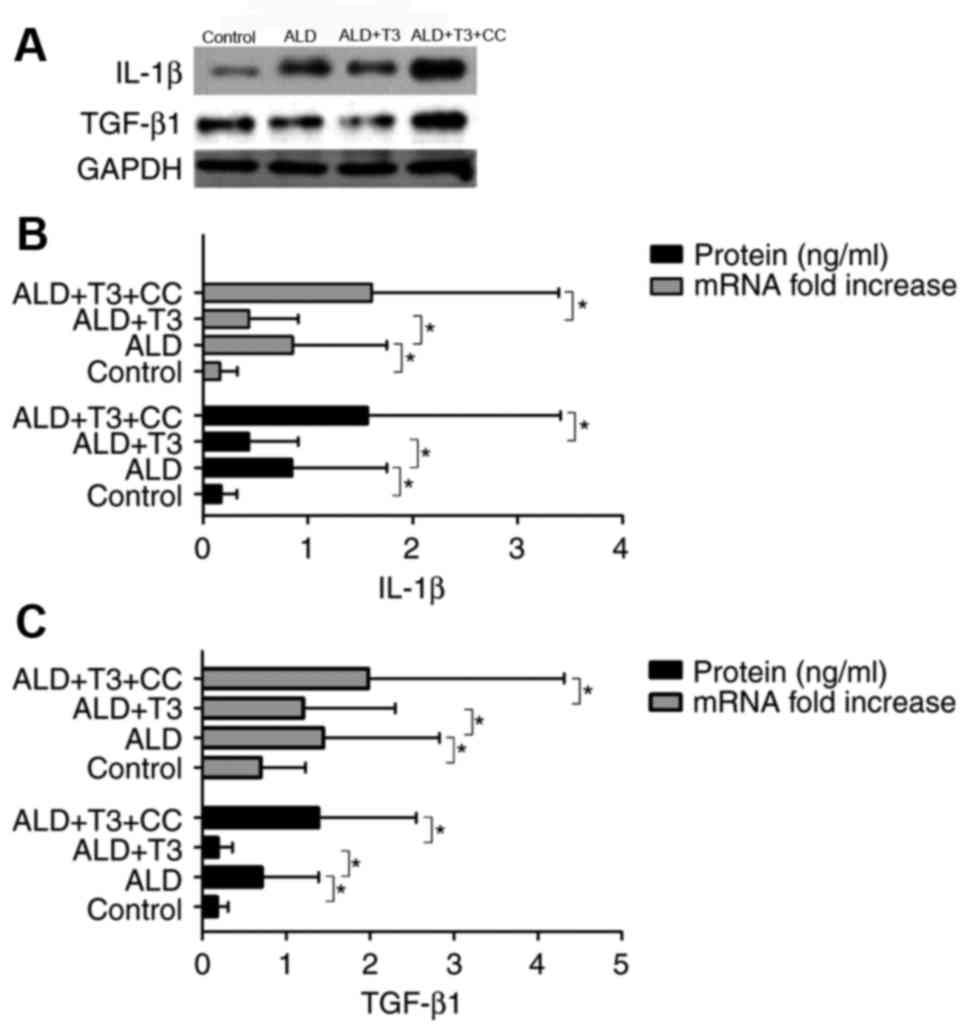
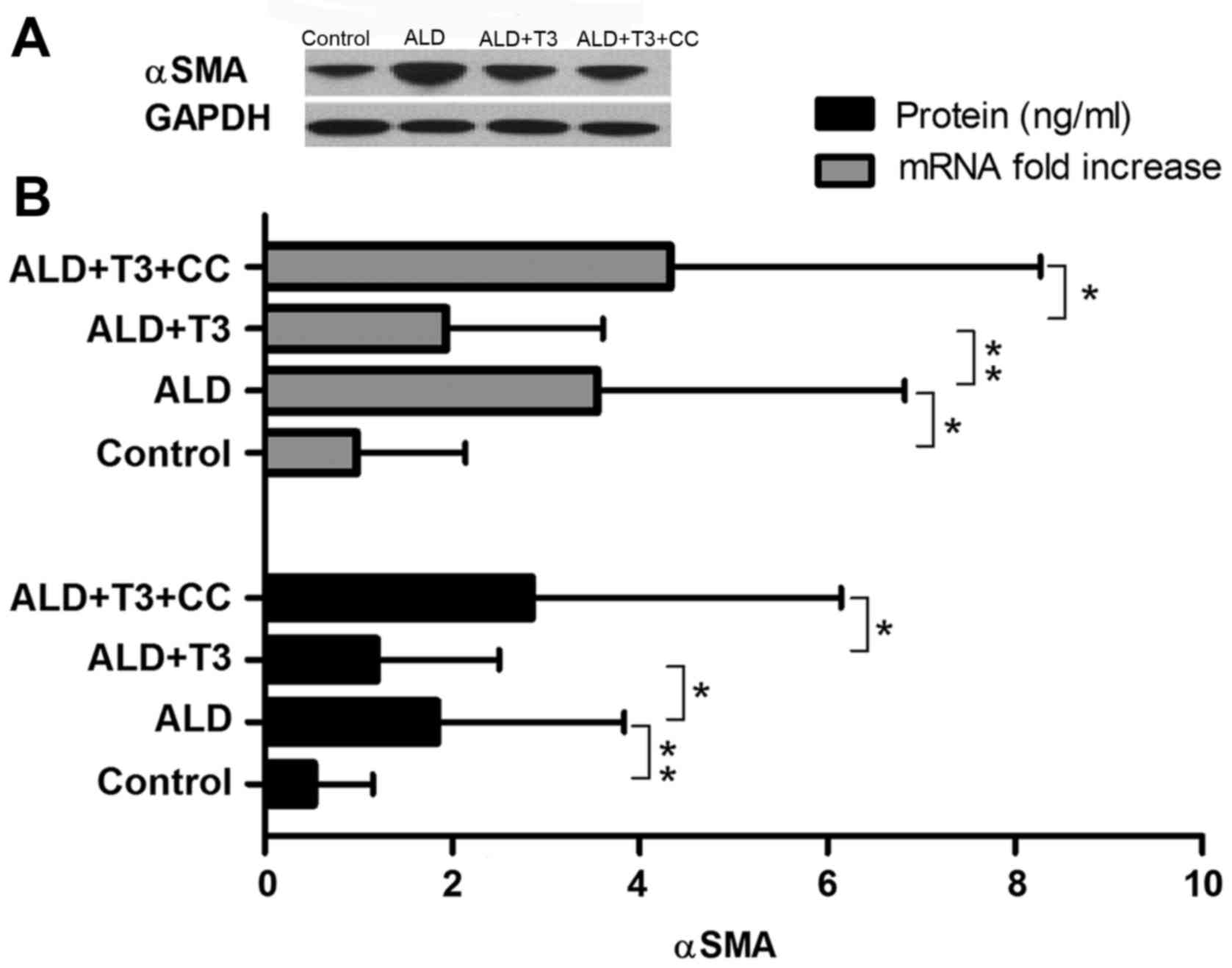
|
1
|
Federico A, Dallio M, Masarone M, Persico
M and Loguercio C: The epidemiology of non-alcoholic fatty liver
disease and its connection with cardiovascular disease: Role of
endothelial dysfunction. Eur Rev Med Pharmacol Sci. 20:4731–4741.
2016.PubMed/NCBI
|
|
2
|
Arsene D, Farooq O and Bataller R: New
therapeutic targets in alcoholic hepatitis. Hepatol Int.
10:538–552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwartz JM and Reinus JF: Prevalence and
natural history of alcoholic liver disease. Clin Liver Dis.
16:659–666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrasek J, Bala S, Csak T, Lippai D,
Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA and Szabo G:
IL-1 receptor antagonist ameliorates inflammasome-dependent
alcoholic steatohepatitis in mice. J Clin Invest. 122:3476–3489.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim EJ, Park SY, Baek SE, Jang MA, Lee WS,
Bae SS, Kim K and Kim CD: HMGB1 increases IL-1β production in
vascular smooth muscle cells via NLRP3 inflammasome. Front Physiol.
9:3132018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim HY, Kim SJ and Lee SM: Activation of
NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic
ischemia/reperfusion. Febs J. 282:259–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watanabe A, Sohail MA, Gomes DA, Hashmi A,
Nagata J, Sutterwala FS, Mahmood S, Jhandier MN, Shi Y, Flavell RA
and Mehal WZ: Inflammasome-mediated regulation of hepatic stellate
cells. Am J Physiol Gastrointest Liver Physiol. 296:G1248–G1257.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esser N, Legrand-Poels S, Piette J, Scheen
AJ and Paquot N: Inflammation as a link between obesity, metabolic
syndrome and type 2 diabetes. Diabetes Res Clin Pract. 105:141–150.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dinarello CA: Immunological and
inflammatory functions of the interleukin-1 family. Annu Rev
Immunol. 27:519–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vargas R and Videla LA: Triiodothyronine
suppresses ischemia-reperfusion-induced liver NLRP3 inflammasome
activation: Role of AMP-activated protein kinase. Immunol Lett.
184:92–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao J, Zhu Y, Liu Y, Tipoe GL, Xing F and
So KF: Lycium barbarum polysaccharide attenuates alcoholic cellular
injury through TXNIP-NLRP3 inflammasome pathway. Int J Biol
Macromol. 69:73–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cable EE, Finn PD, Stebbins JW, Hou J, Ito
BR, van Poelje PD, Linemeyer DL and Erion MD: Reduction of hepatic
steatosis in rats and mice after treatment with a liver-targeted
receptor agonist. Hepatology. 49:407–417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Shea RS, Dasarathy S and McCullough AJ:
Alcoholic liver disease. AM J Gastroenterol. 105(14–32):
332010.
|
|
14
|
Rej R: Aspartate aminotransferase activity
and isoenzyme proportions in human liver tissues. Clin Chem.
24:1971–1979. 1978.PubMed/NCBI
|
|
15
|
Guesdon JL, Ternynck T and Avrameas S: The
use of avidin-biotin interaction in immunoenzymatic techniques. J
Histochem Cytochem. 27:1131–1139. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kozutsumi Y, Segal M, Normington K,
Gething MJ and Sambrook J: The presence of malfolded proteins in
the endoplasmic reticulum signals the induction of
glucose-regulated proteins. Nature. 332:462–464. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian X, Zhao C, Guo J, Xie S, Yin F, Huo X
and Zhang X: Carvedilol attenuates the progression of hepatic
fibrosis induced by bile duct ligation. Biomed Res Int.
2017:46127692017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ke B, Shen XD, Zhang Y, Ji H, Gao F, Yue
S, Kamo N, Zhai Y, Yamamoto M, Busuttil RW and Kupiec-Weglinski JW:
KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of
mouse liver transplants. J Hepatol. 59:1200–1207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee YA, Wallace MC and Friedman SL:
Pathobiology of liver fibrosis: A translational success story. Gut.
64:830–841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Liu S, Wang Y, Chang B and Wang B:
Nod-like receptor protein 3 inflammasome activation by Escherichia
coli RNA induces transforming growth factor beta 1 secretion in
hepatic stellate cells. Bosn J Basic Med Sci. 16:126–131.
2016.PubMed/NCBI
|
|
21
|
Li J, Li J, Li S, He B, Mi Y, Cao H, Zhang
C and Li L: Ameliorative effect of grape seed proanthocyanidin
extract on thioacetamide-induced mouse hepatic fibrosis. Toxicol
Lett. 213:353–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiao J, Friedman SL and Aloman C: Hepatic
fibrosis. Curr Opin Gastroenterol. 25:223–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dara L, Ji C and Kaplowitz N: The
contribution of endoplasmic reticulum stress to liver diseases.
Hepatology. 53:1752–1763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao S, Li N, Zhen Y, Ge M, Li Y, Yu B, He
H and Shao RG: Protective effect of gastrodin on bile duct
ligation-induced hepatic fibrosis in rats. Food Chem Toxicol.
86:202–207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu JT, Yang GW, Qi CH, Zhou L, Hu JG and
Wang MS: Anti-inflammatory activity of platycodin d on
alcohol-induced fatty liver rats via tlr4-myd88-nf-kappab signal
path. Afr J Tradit Complement Altern Med. 13:176–183. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang W, Zhong W, Sun Q, Sun X and Zhou Z:
Adipose-specific lipin1 overexpression in mice protects against
alcohol-induced liver injury. Sci Rep. 8:4082018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen X, Bian M, Zhang C, Kai J, Yao Z, Jin
H, Lu C, Shao J, Chen A, Zhang F and Zheng S: Dihydroartemisinin
inhibits ER stress-mediated mitochondrial pathway to attenuate
hepatocyte lipoapoptosis via blocking the activation of the
PI3K/Akt pathway. Biomed Pharmacother. 97:975–984. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vargas R and Videla LA: Triiodothyronine
suppresses ischemia-reperfusion-induced liver NLRP3 inflammasome
activation: Role of AMP-activated protein kinase. Immunol Lett.
184:92–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cannito S, Morello E, Bocca C, Foglia B,
Benetti E, Novo E, Chiazza F, Rogazzo M, Fantozzi R, Povero D, et
al: Microvesicles released from fat-laden cells promote activation
of hepatocellular NLRP3 inflammasome: A pro-inflammatory link
between lipotoxicity and non-alcoholic steatohepatitis. PLoS One.
12:e1725752017. View Article : Google Scholar
|
|
30
|
Kawada N: Evolution of hepatic fibrosis
research. Hepatol Res. 41:199–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Artlett CM: Inflammasomes in wound healing
and fibrosis. J Pathol. 229:157–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gasse P, Mary C, Guenon I, Noulin N,
Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF,
Lagente V, et al: IL-1R1/MyD88 signaling and the inflammasome are
essential in pulmonary inflammation and fibrosis in mice. J Clin
Invest. 117:3786–3799. 2007.PubMed/NCBI
|
|
33
|
Shinde AV and Frangogiannis NG:
Fibroblasts in myocardial infarction: A role in inflammation and
repair. J Mol Cell Cardiol. 70:74–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wree A, Eguchi A, McGeough MD, Pena CA,
Johnson CD, Canbay A, Hoffman HM and Feldstein AE: NLRP3
inflammasome activation results in hepatocyte pyroptosis, liver
inflammation, and fibrosis in mice. Hepatology. 59:898–910. 2014.
View Article : Google Scholar : PubMed/NCBI
|