Introduction
Ozone is an active form of oxygen, consisting of
three oxygen atoms generated from diatomic oxygen by ultraviolet
light and high voltage. Ozone gas is clear, colorless, and smells
slightly like grass (1). Ozone
therapy has been receiving increasing attention in Europe and is
known for its beneficial effects in reperfusion injury, infections,
and cancer (2).
Ozonated water is the liquid form generated when
ozone is dissolved in saline, which is much easier to handle than
the gaseous form. The safety and direct antitumor effect of
ozonated water have been previously described by us (3).
The primary form of ozone therapy is major
autohemotherapy (MAH). In MAH, patient blood is mixed with ozone
gas before being retransfused. It has been known for its beneficial
effects in cardiovascular diseases, infections, cancer, rheumatoid
arthritis, and osteoarthritis (4).
Ozone gas is known to improve peripheral blood
perfusion. Ozone therapy in tumor patients improves intratumoral
oxygen partial pressure (4). Tumor
hypoxia is a serious concern affecting tumor therapy because it
reduces the sensitivity of chemotherapy and radiation therapy
(5–8). Therefore, it is expected to improve the
sensitivity of antitumor therapy. In clinical settings, the
combination of radiation therapy and MAH or rectal administration
of ozone gas/oxygen gas mixture has helped achieve the same effects
as those obtained by using a combination of radiation therapy and
chemotherapy in preventing the progression of head and neck cancer
(9). Reports related to chemotherapy
mostly discuss side effects; few reports discuss therapeutic
effects (10–12). To the best of our knowledge, no other
study has discussed ozone therapy using ozonated water. Therefore,
a new approach using ozonated water was examined to solve concerns
around antitumor treatment. In this report, we decided to study the
effect of ozonated water on tumor hypoxia, alone and in combination
with an antitumor drug.
Materials and methods
Preparation of ozonated water
The device producing ozonated water was provided by
Sakuragawa Pump Co., Ltd., (Osaka, Japan). This device can produce
ozonated water from O2 and water and can regulate the
concentration of dissolved ozone. In all experiments, the
concentration of ozonated water was 208 μM and it was administered
within 10 min of production.
Animals
BALB/c mice (4 to 5-week-old, female) were purchased
from CLEA Japan, Inc. (Osaka, Japan). All mice were maintained
under conventional conditions. The study was performed according to
the rules put down by the Tottori University. The use of these
animals and the procedures undertaken was approved by the Animal
Research Committee of Tottori University. All treatments were
performed under anesthesia, induced by the inhalation of 3–5%
isoflurane; all efforts were made to minimize suffering.
Preparation of the tumor-bearing mouse
model
The mouse cell line derived from the Colon 26
(RCB2657), the mouse colorectal cancer model, was obtained from the
RIKEN BioResource Center (BRC) through the National Bio-Resource
Project of the MEXT (Ibaraki, Japan). The tumor-bearing mouse model
was prepared as described previously, with slight modification
(13). In brief, 1×106
cells (1×107 cells/ml) were subcutaneously injected into
the dorsal region of BALB/c mice. Mice whose tumors grew to a
diameter of 7–10 mm were included in the study.
Measurement of intratumoral oxygen
partial pressure
Mice were randomized into two groups: Sterile saline
(S) group and ozonated water (O3) group (n=5 per group).
Oxygen and indifferent electrodes (Bio Research Co., Nagoya, Japan)
were inserted into the center of the tumor. After intraperitoneal
administration of sterile saline or ozonated water (0.2 ml/head),
intratumoral oxygen partial pressure was measured by polarography
(Bio Research Co.), every 10 min immediately after administration
up to 150 min.
Measurement of blood gas
Blood samples were collected after administration of
sterile saline or ozonated water, and blood gas was measured using
at 0, 10, 30, 60, and 120 min after administration.
Evaluation of intratumoral blood
perfusion
Eighty min after administration of sterile saline or
ozonated water, 30 mg/kg Hoechst 33342 (H33342) dissolved in
phosphate-buffered saline (PBS) was administrated intravenously.
After 4 min, the mice were euthanized by cervical dislocation under
anesthesia induced by the inhalation of 3–5% isoflurane. The tumors
were embedded in an optimal cutting temperature (OCT) compound. The
cryostat sections (10 mm) were fixed in acetone for 5 min at 4°C.
These sections were analyzed by fluorescence microscopy. The
percentage of positive areas in the tumor tissues was calculated by
dividing the total pixel area of the positive areas by the total
pixel area corresponding to the entire tumor tissue in the field of
view. The mean scores for 25 fields were used as the percentages of
positive areas per group.
Effect of ozonated water and cisplatin
(cddp) in combination, on colon-26 mice
For this experiment, the mice were randomized into
six groups (n=8 per group): Sterile saline (S) group, ozonated
water (O3) group, sterile saline and 5.0 mg/kg CDDP
group (S-CDDP5.0), ozonated water and 5.0 mg/kg CDDP group
(O3-CDDP5.0), ozonated water and 3.4 mg/kg CDDP group
(O3-CDDP3.4), and ozonated water and 1.7 mg/kg CDDP
group (O3-CDDP1.7). The volume of these tumor tissues
was calculated as follows: (mediastinum × transverse line × depth ×
π)/6 (mm3). Then, CDDP (5.0, 3.4, 1.7 mg/kg) and saline
or ozonated water (0.2 ml/head) were intraperitoneally administered
to the S-CDDP5.0, O3-CDDP5.0, O3-CDDP3.4, and
O3-CDDP1.7 groups. In the S and O3 groups,
only sterile saline or ozonated water was administered. On day 7,
the volumes of the tumor tissues were calculated and the mice were
euthanized by cervical dislocation under anesthesia as described
before. Based on the tumor volumes on days 1 and 7, the tumor
growth rates were calculated as follows: (tumor volume on day
7-tumor volume on day 1)/7 (mm3/day). The tumors were
fixed in 10% buffered formalin.
Ki-67 staining
Tissue sections (3 µm) obtained on glass slides were
deparaffinized, washed with ethanol and water, and soaked in PBS.
The sections were autoclaved with 0.01 M citrate buffer (pH 6.0)
for 15 min (121°C). The sections were then washed with PBS and
incubated with the rabbit polyclonal anti-Ki-67 antibody (1:50;
E0468; Dako, Glostrup, Denmark) for 30 min at room temperature.
After washing with PBS, the sections were incubated with rat
anti-IgG antibody (1:100; sc-372; Vector Laboratories, Inc.,
Burlingame, CA, USA) for 30 min at room temperature. The slides
were washed with PBS and stained using the ABC method (PK-4000;
Vector Laboratories, Inc.) for 30 min. Cell counts in 25 random
fields were calculated at a magnification, ×400 by using five mice
from each group.
TUNEL staining
Tissue sections (3 µm) obtained on glass slides were
deparaffinized, washed with ethanol and water, and soaked in
diluted water. TUNEL staining was performed using the
in-situ Apoptosis Detection kit (Takara Bio, Inc., Shiga,
Japan), according to the manufacturer's instructions. Cell counts
were calculated as described in the previous subsection.
Statistical analysis
Data are expressed as the mean ± standard (SD) or
standard error (SE). Statistical analyses were first performed
using F-test or analysis of variance (ANOVA) and compared using the
Student's t-test or Tukey-Kramer test. P<0.05 or 0.01 indicated
statistical significance.
Results
Effect on blood gas and intratumoral
oxygen partial pressure
Before and after the administration of O3
or sterile saline, no apparent change was noted in the blood gas
levels (Table I). No significant
difference was noted between the O3 and S groups.
 | Table I.Effect of ozonated water on blood
gas. |
Table I.
Effect of ozonated water on blood
gas.
| A, Effect in
O3 group |
|---|
|
|---|
| Time (min) | pH | pCO2
(mmHg) | pO2
(mmHg) | HCO3
(mmol/l) | sO2
(%) |
|---|
| 0 | 7.335±0.03 | 38.9±4.5 | 56±4.7 | 20.7±1.2 | 87±4.6 |
| 10 | 7.276±0.04 | 38.6±4.9 | 53±4.3 | 20±0.6 | 85±4.8 |
| 30 | 7.277±0.03 | 38.1±5.0 | 59±5.2 | 19±1.0 | 87±5.8 |
| 60 | 7.339±0.02 | 36.9±4.3 | 54±4.6 | 21±0.8 | 86±3.8 |
| 120 | 7.323±0.03 | 42.4±4.5 | 64±4.8 | 23±1.3 | 90±5.2 |
|
| B, Effect in S
group |
|
| Time
(min) | pH | pCO2
(mmHg) | pO2
(mmHg) | HCO3
(mmol/l) | sO2
(%) |
|
|
0 | 7.347±0.03 | 39.2±6.7 | 57.3±5.4 | 19.6±1.3 | 83±3.9 |
| 10 | 7.336±0.02 | 39.7±3.7 | 46±6.3 | 19.9±0.5 | 78.6±5.3 |
| 30 | 7.324±0.04 | 39.3±4.3 | 55±4.6 | 20.5±0.7 | 86±4.6 |
| 60 | 7.382±0.03 | 35.2±5.4 | 51±4.2 | 20.5±0.6 | 85±5.3 |
| 120 | 7.229±0.02 | 47.9±4.4 | 58±4.2 | 23.5±0.8 | 87±4.9 |
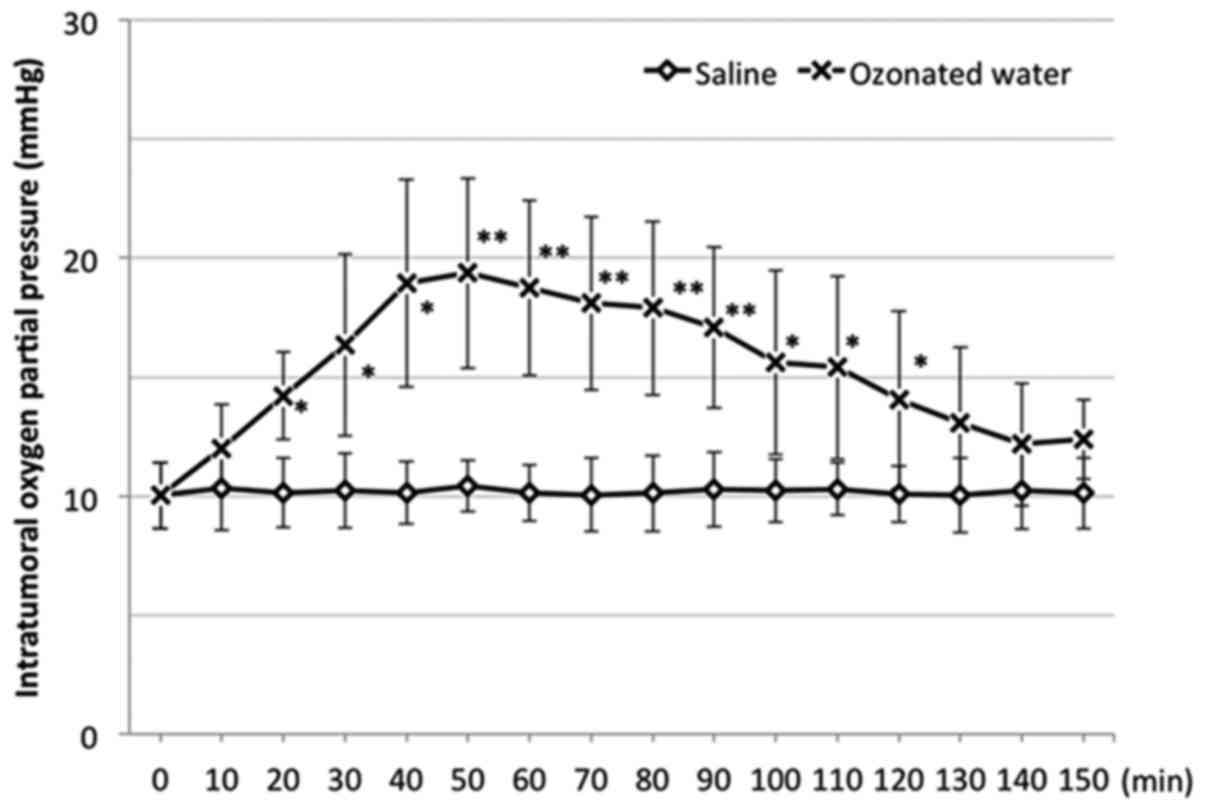
In the S group, there was no apparent change in the
intratumoral oxygen partial pressure. However, the partial pressure
in the O3 group significantly increased compared to that
in the S group at 20 min after administration (Fig. 1). It peaked at 50 min after
administration and gradually decreased afterward. At 130 min after
administration, no significant difference was noted between the
O3 and S groups.
Effect on intratumoral blood
perfusion
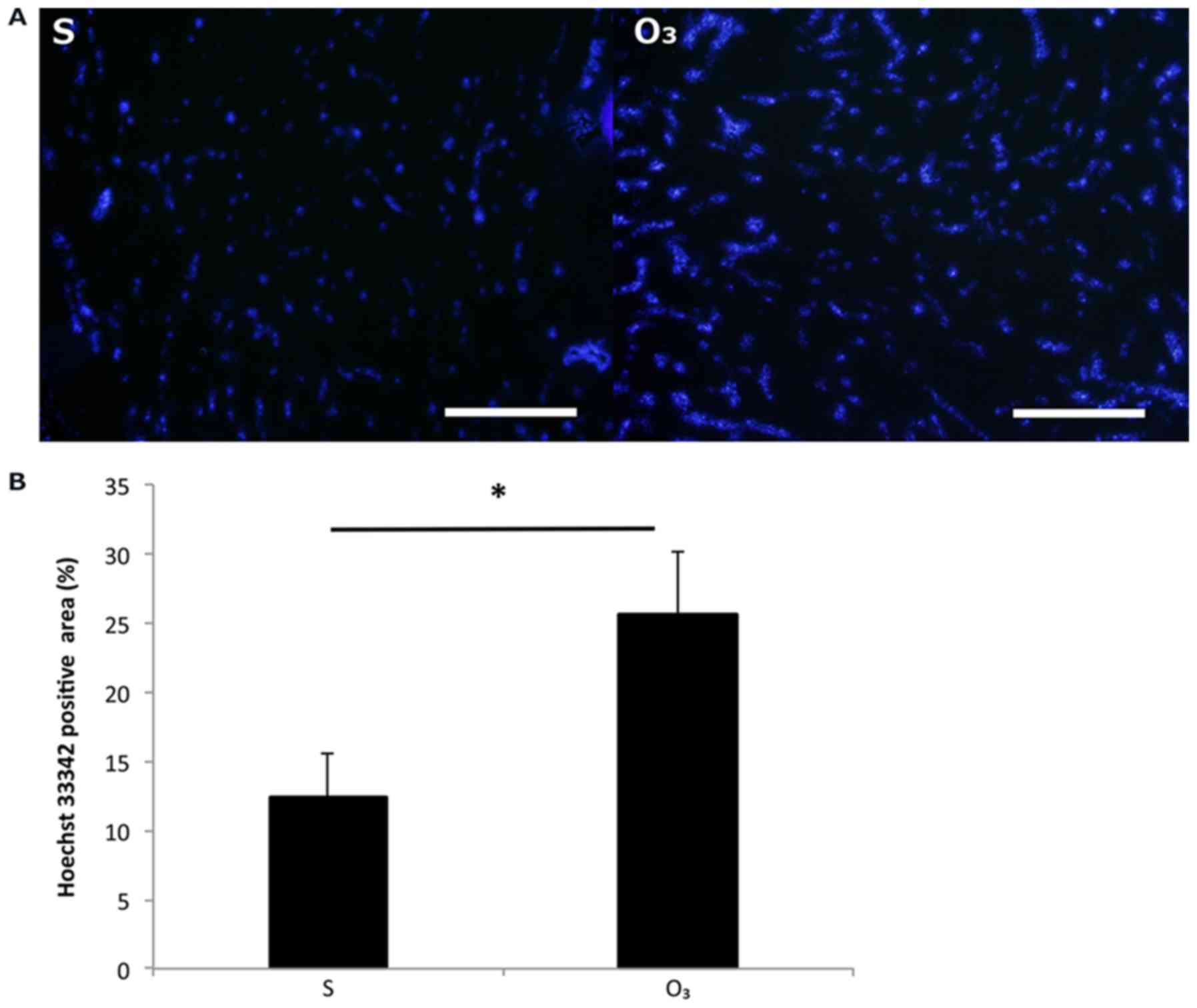
The intratumoral blood perfusion in the
O3 group increased compared to that in the S group
(Fig. 2). The H33342-positive area
was 12.3±3.2% in the S group and 25.6±4.6% in the O3
group. There was a significant difference between the two groups
(P<0.05).
Effect of ozonated water and cddp in
combination
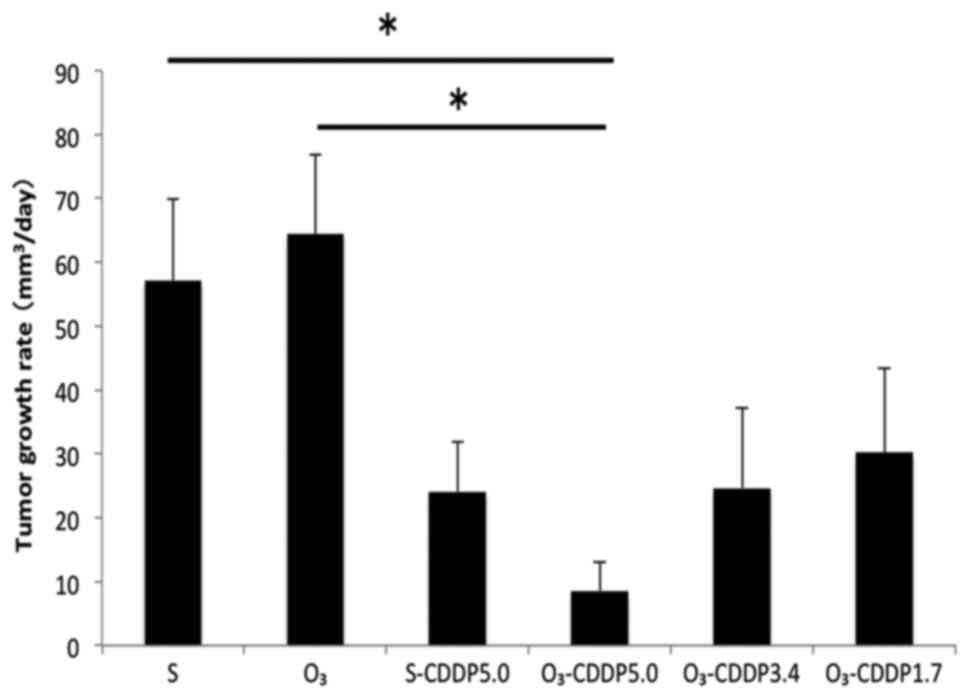
Tumor growth was significantly suppressed in the
O3-CDDP5.0 group compared to that in the S and
O3 groups (P<0.05) (Fig.
3). A statistically significant difference was not observed
between the S-CDDP5.0 and O3-CDDP5.0 groups. However,
tumor growth in the O3-CDDP5.0 group tended to be
suppressed, compared to that in the S-CDDP5.0 group. In the
O3-CDDP3.4 group, despite reducing the concentration of
the antitumor drug, tumor growth suppression was observed, which
was comparable to that observed in the S-CDDP group.
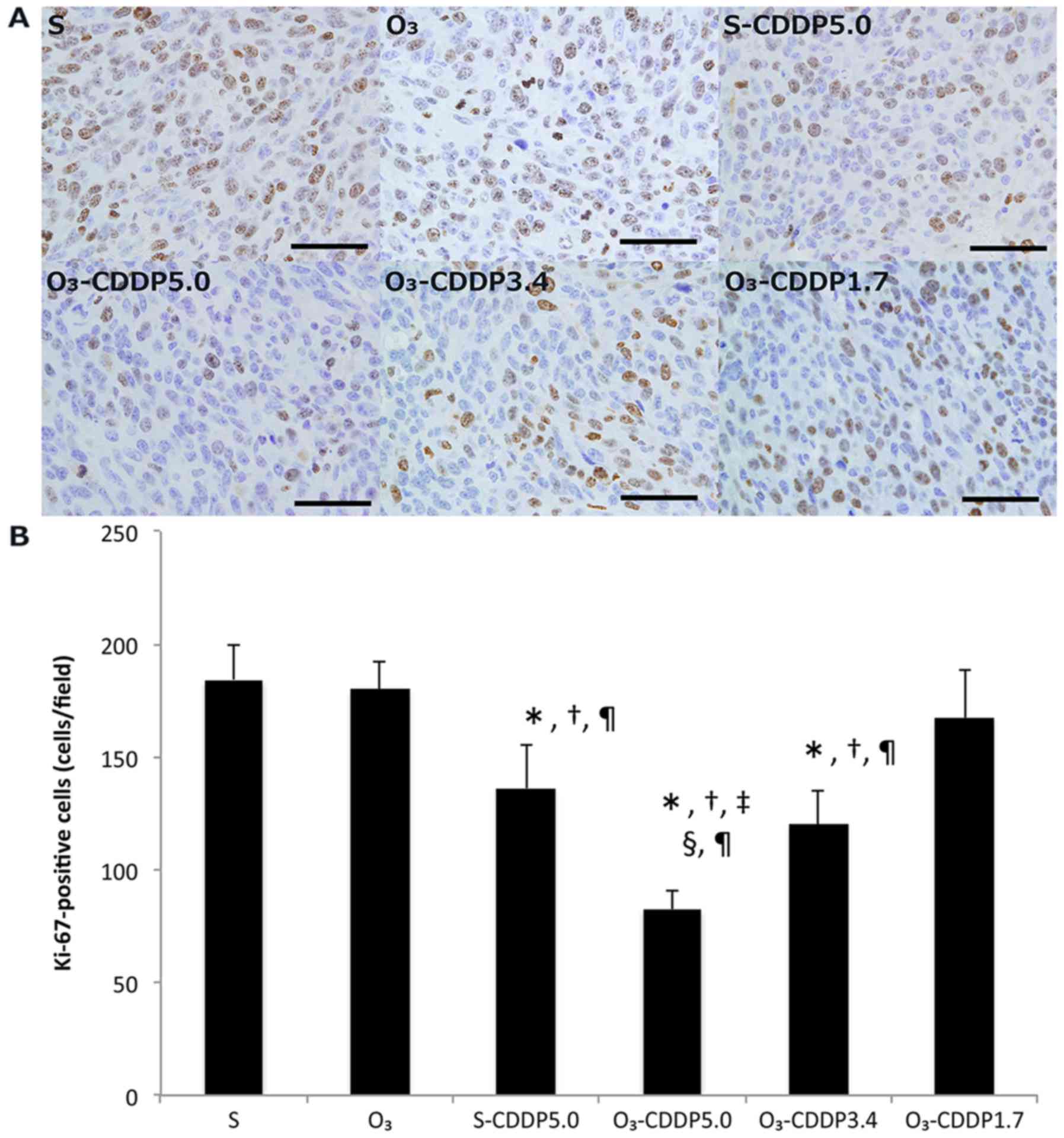
In the O3-CDDP5.0 group, the number of
Ki-67-positive cells significantly decreased, compared to that in
the other groups (Fig. 4). The
number of Ki-67-positive cells in the O3-CDDP3.4 and
S-CDDP groups significantly decreased, compared to that in the S,
O3, and O3-CDDP1.7 groups. There was no
statistically significant difference between the S-CDDP5.0 and
O3-CDDP3.4 groups.
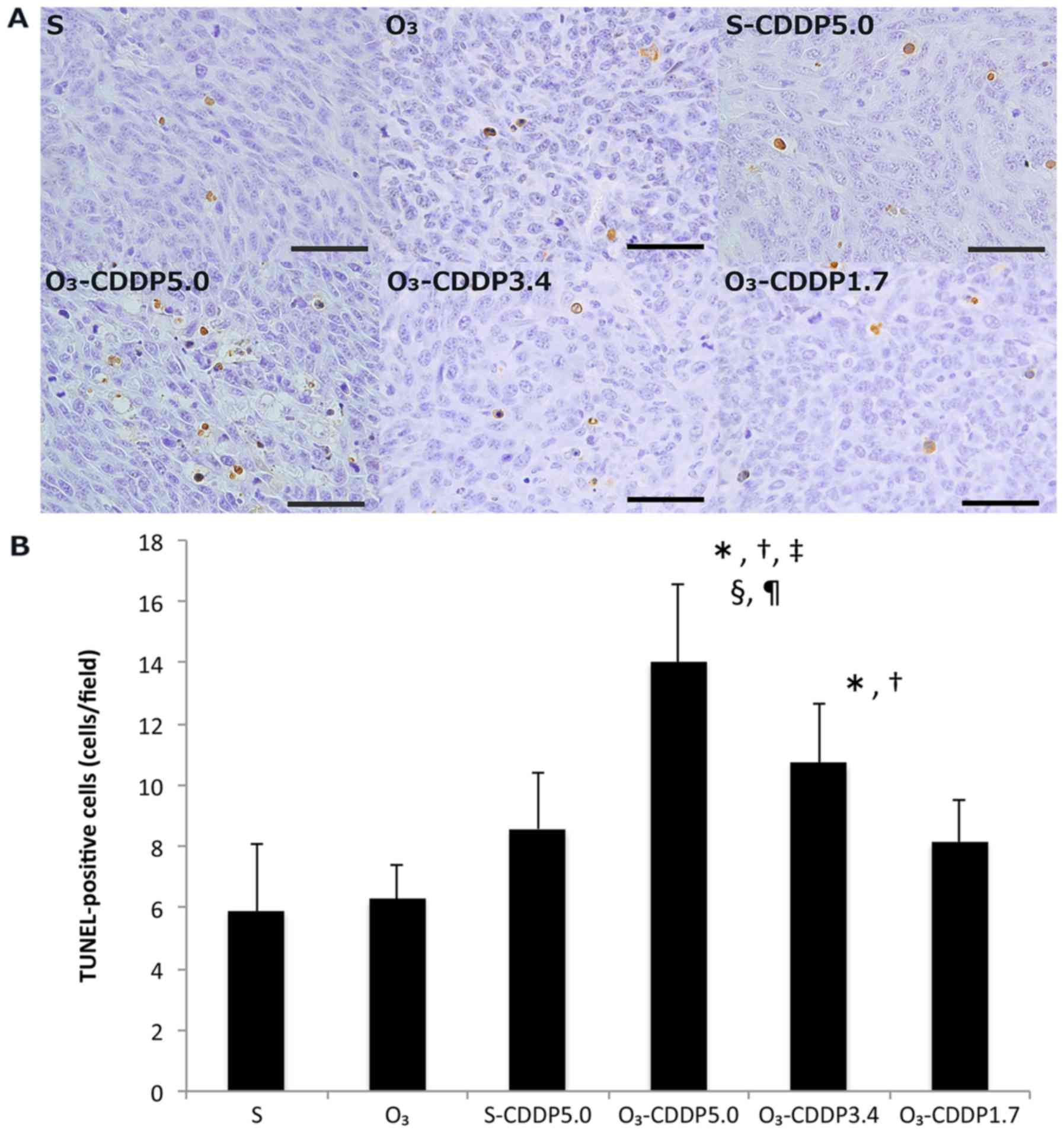
In the O3-CDDP5.0 group, the number of
TUNEL-positive cells significantly increased, compared to that of
the other groups (Fig. 5). The
number of TUNEL-positive cells in the O3-CDDP3.4 group
significantly increased, compared to that in the S and
O3 groups. No statistically significant difference was
observed among the S-CDDP5.0, O3-CDDP3.4, and
O3-CDDP1.7 groups.
Discussion
In the present study, the intratumoral blood
perfusion was found to have increased and the intratumoral oxygen
partial pressure was found to have improved after intraperitoneal
administration of ozonated water. To the best of our knowledge,
such effects of ozonated water have not been reported thus far.
Further, when ozonated water was used in combination with CDDP, the
antitumor effect of CDDP was enhanced. Most studies related to
chemotherapy discuss side effects; very few reports discuss
therapeutic effects (10–12). The findings of this study can be
considered extremely important evidence with respect to the use of
ozonated water.
When the ozonated water was intraperitoneally
administered, no apparent change was noted in the blood gas levels.
However, the intratumoral oxygen partial pressure significantly
increased. These results indicate that ozonated water increases
intratumoral oxygen partial pressure without affecting blood gas
levels. Ozone gas is known to improve peripheral blood perfusion
owing to its vasodilating effect (14,15).
When the extent of intratumoral blood perfusion was evaluated by
H33342, a significant increase was noted in the O3
group. Tumor hypoxia generally progresses because of reduction in
the intratumoral blood perfusion caused by an imbalance between the
tumor growth rate and tumor blood vessel formation rate (16,17).
This result indicates that ozonated water as well as ozone gas
increase peripheral blood perfusion. In addition, it indicated that
the increase in the intratumoral oxygen partial pressure might be
due to the increase in intratumoral blood perfusion. Oxidation as
well as activation of antioxidant enzymes are caused by the
administration of ozone. When active oxygen is removed by
superoxide dismutase (SOD), hydrogen peroxide
(H2O2) is produced.
H2O2 is involved in vasodilation as an
endothelium-derived hyperpolarizing factor (EDHF), one of the
endothelium-derived vasorelaxation factors (18). Therefore, it has been thought that
ozone extends the peripheral vessels by means of
H2O2 and increases peripheral blood perfusion
(14). Ozonated water is likely to
increase blood perfusion by a similar mechanism. However, the
underlying mechanism needs to be studied further. In addition, the
changes in peripheral blood perfusion in normal tissues warrant
further study.
Tumor hypoxia is a serious problem for tumor
treatment because it reduces the sensitivity to chemical,
radiation, and photodynamic therapy (5–8,16). The reasons for resistance to
chemotherapy include cell cycle arrest (19,20),
acquisition of antiapoptotic activity by inhibition of
apoptosis-inducing proteins such as Bid and Bax (21,22), and
reduction in the amount of drug reaching the tumor because of
decreased blood flow (8).
The rate of tumor growth in the
O3-CDDP5.0 group tended to decrease compared to that in
the S-CDDP5.0 group. In addition, the number of Ki-67-positive
cells significantly decreased and the number of TUNEL-positive
cells significantly increased in the O3-CDDP5.0 group
compared to that in the S-CDDP5.0 group. CDDP exerts an antitumor
effect by inducing apoptosis and suppressing tumor growth by
inhibiting deoxyribonucleic acid (DNA) synthesis (23). Therefore, the results of Ki-67 and
TUNEL staining suggested that the effect of CDDP was enhanced by
the administration of ozonated water. The antitumor effect of CDDP
depends on the amount of CDDP reaching the site of tumor rather
than the proliferation activity of tumor cells (24,25).
These findings as well as previous reports suggest that tumor
growth is suppressed on treatment with ozonated water because the
amount of CDDP reaching the tumor is increased when the
intratumoral blood perfusion is increased because of ozonated
water. We plan to measure the concentration of intratumoral
antitumor drug in the future and investigate in detail.
In recent years, it has been reported that the
inhibition of apoptosis-inducing protein is associated with
resistance to platinum-based drug preparations (21). However, it is unknown whether those
proteins are involved in the resistance mechanism. It might not
affect protein expression because the increase in oxygen partial
pressure observed in this study occurred only 2 h after the
administration of ozonated water. In order to clarify the
mechanism, the genes and proteins related to hypoxia and apoptosis
need to be studied further.
The O3-CDDP5.0 group showed tumor growth
suppression, to almost the same extent as that observed in the
S-CDDP5.0 group. This suggests that it is possible to reduce the
required drug concentration, while maintaining the antitumor
effect, by using ozonated water and general chemotherapy in
combination. We intend to conduct detailed studies on other drugs
and tumor types in the future.
The present study showed that ozonated water
increases intratumoral blood perfusion and improves intratumoral
oxygen partial pressure. In addition, tumor growth was more
suppressed when ozonated water and CDDP therapy were combined.
Thus, the administration of ozonated water could be a new approach
to solve current concerns around antitumor treatment, such as tumor
hypoxia and drug resistance of tumors.
References
|
1
|
Viebahn-Haensler R and Lee A: The Use of
Ozone in Medicine. 5th edition. ODREI-Publishers; Iffezheim: pp.
1482007
|
|
2
|
Nogales CG, Ferrari PH, Kantorovich EO and
Lage-Marques JL: Ozone therapy in medicine and dentistry. J Contemp
Dent Pract. 9:75–84. 2008.PubMed/NCBI
|
|
3
|
Kuroda K, Azuma K, Mori T, Kawamoto K,
Murahata Y, Tsuka T, Osaki T, Ito N, Imagawa T, Itoh F and Okamoto
Y: The safety and anti-tumor effects of ozonated water in vivo. Int
J Mol Sci. 16:25108–25120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clavo B, Pérez JL, López L, Suárez G,
Lloret M, Rodríguez V, Macías D, Santana M, Hernández MA,
Martín-Oliva R and Robaina F: Ozone therapy for tumor oxygenation:
A pilot study. Evid Based Complement Alternat Med. 1:93–98. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Graeber TG, Osmanian C, Jacks T, Housman
DE, Koch CJ, Lowe SW and Giaccia AJ: Hypoxia-mediated selection of
cells with diminished apoptotic potential in solid tumours. Nature.
379:88–91. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bertout JA, Patel SA and Simon MC: The
impact of O2 availability on human cancer. Nat Rev
Cancer. 8:967–975. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teicher BA: Hypoxia and drug resistance.
Cancer Metastasis Rev. 13:139–168. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clavo B, Ruiz A, Lloret M, López L, Suárez
G, Macías D, Rodríguez V, Hernández MA, Martín-Oliva R, Quintero S,
et al: Adjuvant ozonetherapy in advanced head and neck tumors: A
comparative study. Evid Based Complement Alternat Med. 1:321–325.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borrego A, Zamora ZB, González R, Romay C,
Menéndez S, Hernández F, Montero T and Rojas E: Protection by ozone
preconditioning is mediated by the antioxidant system in
cisplatin-induced nephrotoxicity in rats. Mediators Inflamm.
13:13–19. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borrego A, Zamora ZB, González R, Romay C,
Menéndez S, Hernández F, Berlanga J and Montero T: Ozone/oxygen
mixture modifies the subcellular redistribution of bax protein in
renal tissue from rats treated with cisplatin. Arch Med Res.
37:717–722. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
González R, Borrego A, Zamora Z, Romay C,
Hernández F, Menéndez S, Montero T and Rojas E: Reversion by ozone
treatment of acute nephrotoxicity induced by cisplatin in rats.
Mediators Inflamm. 13:307–312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nitta M, Azuma K, Hata K, Takahashi S,
Ogiwara K, Tsuka T, Imagawa T, Yokoe I, Osaki T, Minami S and
Okamoto Y: Systemic and local injections of lupeol inhibit tumor
growth in a melanoma-bearing mouse model. Biomed Rep. 1:641–645.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mukhina IV, Dudina EV, Yakovleva EI,
Zhemarina NV, Prodanecs NN, Evdokimova OS, Manuhina EB and
Dvornikov AV: The dose-dependent effect of ozonated physiological
solution on arterial vasodilation. IOA 17th World Ozone
Congress-Strasbourg 2005. III:3.9–1. 2005.
|
|
15
|
Dutka M, Adamczak M, Kopieczna-Grzebieniak
E, Grabowska-Bochenek R and Wesolowski W: Vasorelaxant activity of
ozone-in vitro studies. Adv Clin Exp Med. 4:391–398. 1998.
|
|
16
|
Brown JM and Wilson WR: Exploiting tumour
hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lunt SJ, Chaudary N and Hill RP: The tumor
microenvironment and metastatic disease. Clin Exp Metastasis.
26:19–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takaki A, Morikawa K, Tsutsui M, Murayama
Y, Tekes E, Yamagishi H, Ohashi J, Yada T, Yanagihara N and
Shimokawa H: Crucial role of nitric oxide synthases system in
endothelium-dependent hyperpolarization in mice. J Exp Med.
205:2053–2063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amellem O, Löffler M and Pettersen EO:
Regulation of cell proliferation under extreme and moderate
hypoxia: The role of pyrimidine (deoxy)nucleotides. Br J Cancer.
70:857–866. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gardner LB, Li Q, Park MS, Flanagan WM,
Semenza GL and Dang CV: Hypoxia inhibits G1/S transition through
regulation of p27 expression. J Biol Chem. 276:7919–7926. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Erler JT, Cawthorne CJ, Williams KJ,
Koritzinsky M, Wouters BG, Wilson C, Miller C, Demonacos C,
Stratford IJ and Dive C: Hypoxia-mediated down-regulation of Bid
and Bax in tumors occurs via hypoxia-inducible factor 1-dependent
and -independent mechanisms and contributes to drug resistance. Mol
Cell Biol. 24:2875–2889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mayes PA, Dolloff NG, Daniel CJ, Liu JJ,
Hart LS, Kuribayashi K, Allen JE, Jee DI, Dorsey JF, Liu YY, et al:
Overcoming hypoxia-induced apoptotic resistance through
combinatorial inhibition of GSK-3β and CDK1. Cancer Res.
71:5265–5275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kartalou M and Essigmann JM: Mechanisms of
resistance to cisplatin. Mutat Res. 478:23–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takahashi K: The effect of
cis-Dichlorodiammineplatinum (II) on tumor growth and progress in
the cell cycle. Japanese Journal of Cancer and Chemotherapy.
9:624–631. 1982.(In Japanese). PubMed/NCBI
|
|
25
|
Drewinko B, Brown BW and Gottlieb JA: The
effect of cis-diamminedichloroplatinum (II) on cultured human
lymphoma cells and its therapeutic implications. Cancer Res.
33:3091–3095. 1973.PubMed/NCBI
|