Introduction
Malignant ascites are a common and distressing
condition associated with a variety of advanced stage of neoplasms,
particularly breast, ovary, stomach, pancreas, liver, and colon
cancer (1,2). The majority of patients with malignant
ascites experience progressive abdominal swelling and troublesome
symptoms such as pain, nausea, dyspnea, constipation, and edema
(3). With the exception of ovarian
cancer, malignant ascites typically have a poor prognosis and a
median survival time of no more than 4 months (4). In contrast, ovarian cancer patients
with malignant ascites have a superior survival rate, with a median
of ~2 years (4,5).
Treatment of malignant ascites remains challenging.
In the majority of cases, systemic chemotherapy is ineffective.
Furthermore, diuretics and paracentesis are the most common
procedures used; however, intraperitoneal chemotherapy is
potentially one of the promising options for future treatment of
malignant ascites (2).
Intraperitoneal chemotherapy allows for direct exposure of tumor
cells to high peritoneal concentrations of cytotoxic drugs without
increasing systemic toxicity. By destroying tumor cells at the
peritoneal surface, intraperitoneal chemotherapeutic drugs induce a
fibrotic reaction and thus will prevent the formation of peritoneal
fluid (6). The majority of
pharmacological agents with known activity in peritoneal malignant
disease, including 5-fluorouracil (5-Fu), cisplatin, melphalan,
carboplatin, mytomicin, topotecan, etoposide, doxorubicin,
paclitaxel, cytarabine, and methotrexate, have been examined via
the intraperitoneal route (7–9).
5-Fu is a molecule that has a pharmacologic
advantage for first-line clinical treatment of peritoneal malignant
diseases (10,11). In patients who received early
postoperative intraperitoneal treatment, the area under the curve
(AUC) for intraperitoneal 5-Fu was 422 times more than intravenous
5-Fu and the AUC ratio of plasma to tumor tissues was 5.2 (11). However, the serious side effects of
5-Fu, including hepatotoxicity and bone marrow suppression, may
restrict its extensive clinical application (12). Therefore, it is necessary to explore
novel types of pharmacological agents to substitute or combine with
5-Fu.
Synthetic antitumor peptides have received an
increasing amount of worldwide attention. Modern chemotherapy based
on synthetic oligo peptides provides an effective anti-metastasis
medication for patients with tumors, exhibiting high affinity and
low toxicity (13–16). P-5m is an octapeptide derived from
domain 5 of high-molecular weight kininogen; the P-5m peptide has
been indicated to promote the inhibition of metastatic activity
exhibited in human melanoma cells (17). The study indicated that significant
anti-invasion and anti-migration effects were exerted by the
His-Gly-Lys motif of the P-5m peptide in vitro and in
vivo in human melanoma cells (17). However, the results cannot explain
the anti-metastatic molecular mechanisms of P-5m octapeptide. In a
previous study (18), we measured
the anti-metastasis effect of P-5m with HCCLM3 human
hepatocarcinoma cells in vitro and in vivo in order
to attempt to detect the underlying mechanisms of any inhibitory
effects. The data indicated that P-5m treatment significantly
inhibited lung metastasis in nude mice and the expression of
metalloproteinase-2 (MMP-2) in the tumor tissues. These
observations suggest that therapy with P-5m inhibits invasion and
metastasis of hepatocellular carcinoma, at least partially through
modulation of MMP-2 expression. Furthermore, the data indicated
that P-5m may have therapeutic potential in metastatic human
hepatocarcinoma. The present in vivo study aimed to
determine if P-5m peptide is able to increase the sensitivity of
murine ascitic H22 cells to 5-Fu, chemotherapeutically.
In the present study, a significant reduction in
total H22 ascites cell count in mice from the combination group was
observed. Moreover, P-5m plus 5-Fuwas able to induce cell cycle
arrest and inhibit the peritoneal capillary permeability of mice.
P-5m in combination with 5-Fu may be potentially useful when
researching of anti-metastatic agents and cancer intraperitoneal
chemotherapy.
Materials and methods
Peptide synthesis
P-5m peptide (GHGKHKNK) was synthesized in our
laboratory via a standard fluorenylmethoxycarbonyl solid-phase
strategy as described previously (19). The crude peptide was purified by
reverse-phase high performance liquid chromatography (>98%
purity). The molecular weights were identified by electrospray
ionization mass spectrometry (Agilent Technologies GmbH, Waldbronn,
Germany).
Animals and cell lines
A total of 180 Female Kunming mice (weight, 18–22 g;
age, 6–8 weeks) were obtained from Animal Center of Jilin
University (Jilin, China). Mice were bred in-house in the animal
care facility of the University of Beihua under standardized
specific pathogen-free conditions. Mice were housed at an ambient
temperature of 20–23°C, a relative humidity of 30–40% and a 12 h
dark/light cycle). Free access to standard rodent chow and water
was allowed for the duration of the study. All experiments using
laboratory animals were performed according to the guidelines of
the Animal Management Rules of the Ministry of Health of the
People's Republic of China and were approved by the Beihua
University Committee on Laboratory Animals (Changchun, China). The
Mouse hepatoma H22 cell line was obtained from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
Cells were grown in Dulbecco's modified Eagle's medium (cat. no.
SH30249.01; Hyclone; GE Healthcare, Chicago, IL, USA) supplemented
with 10% fetal bovine serum (cat. no. 26400044; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), and maintained at 37°C in
humidified 5% CO2. Cells were passaged when they reached
70–80% confluence. Viable H22 cells in (1×107 cells/0.2
ml 0.9% sodium chloride) were injected into the peritoneal cavity
of mice to trigger ascitic cell growth. Transplantation of H22
cells was performed once weekly, only the fluid transplant
generations were passaged over 10 times, provoking the formation of
regular exudates, were used in the subsequent experiments.
Furthermore, tumor transplantation was performed by intraperitoneal
injection of 2.5×106/ml H22 cells suspended in 0.2 ml of
0.9% sodium chloride (20–22).
Treatment protocol
Mice (n=180) were randomly allocated into the
following groups (all n=24 except the control): Negative control
(NC) group, disease group (DG), low-dose P-5m-treated group (LP;
12.5 µg/kg P-5m), medium-dose P-5m group (MP; 50 µg/kg P-5m),
high-dose P-5m group (HP; 200 µg/kg P-5m), low-dose 5-Fu group (LF;
5 mg/kg 5-Fu), high-dose 5-Fu group (HF; 20 mg/kg 5-Fu) and
combination of P-5m (12.5 µg/kg) and 5-Fu (5 mg/kg) group (PF).
Mice in the NC group (n=12) were neither inoculated with H22 cells
nor received any treatment and were only used for body weight
evaluation and survival analysis. For the other groups (n=24), 12
mice in each group were used for body weight evaluation and
survival analysis, another 6 were used for ascitic fluid
evaluation, and the remaining 6 were used for peritoneal capillary
permeability analysis. Mice in the LF and HF groups were
intraperitoneally injected 3 times a week and mice in other groups
were intraperitoneally injected 5 times a week for 30 days. The
physical state characteristics were monitored daily, and body
weight was recorded every 2 days.
Humane endpoints
The endpoint of each experiment was determined
either by spontaneous death or by the elective killing of the
animal when signs of pain or suffering were shown, according to
established criteria (Replication of Animal Models of Human
Diseases) (23). The signs,
depending on severity, duration and response to therapy, were as
follows: rapid or progressive weight loss; sizable abdominal
enlargement or ascites with loss of a righting reflex; possessing a
volume of ascites (g)/body weight (g) of >20%; anorexia or
failure to drink; debilitating diarrhea; dehydration/reduced skin
turgor; edema; progressive dermatitis; rough hair coat/unkempt
appearance; hunched posture; lethargy or persistent recumbency;
coughing; labored breathing; nasal discharge; jaundice; cyanosis;
pallor/anemia; neurological signs (including seizures,
paresis/paralysis, circling or head tilt, blindness); bleeding from
any orifice; and self-induced trauma.
When animals exhibited signs of the aforementioned
humane endpoints, they were immediately sacrificed via compressed
CO2 gas. Animals were placed in a chamber, and the gas
flow rate was 20% vol/min prior to the loss of consciousness. The
flow velocity of CO2 gas was accelerated to 25%
following loss of consciousness. Sacrifice was confirmed by the
absence of a heartbeat.
Survival analysis
Tumor inoculation and treatment were performed and
the mice were observed daily until fatality or 60 days following
the completion of treatments. Results are expressed as percentages
of increased life span (ILS%), calculated according to the formula:
ILS%=(T/C-1) ×100, where T represents mean survival time of treated
mice and C represents mean survival time of the control group.
Ascitic fluid evaluation
On day 13 of the study, mice in the control and
experimental groups used for ascitic fluid evaluation were
sacrificed, ascitic fluid was collected and the volumes were
measured. The total collected H22 cells were washed twice with PBS,
and viable cells were stained with trypan blue and counted using a
hemocytometer (24).
Cell cycle analysis
Flow cytometry (Beckman Coulter, Inc., Brea, CA,
USA) was used to analyze the different stages of cell cycle using a
cell cycle staining kit (cat. no. 04511-1KT-F; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) according to the manufacturers' protocol.
H22 cells (2×106) collected from each ascites-bearing
mouse were fixed using 70% ethanol for 24 h at 4°C. Cells were
washed twice with cold PBS, resuspended in 500 µl PBS and treated
with 10 µl RNase A (10 mg/ml; Sigma-Aldrich; Merck KGaA) for 30 min
at 37°C. For staining, cells were incubated with 10 µl of propidium
iodide/Triton-X 100 (500 µg/ml; Sigma-Aldrich; Merck KGaA) in the
dark for 5 min at 37°C using a previously described method
(24). The fraction of cells in
G0/G1, S, and G2/M phase were
analyzed using a fluorescence activated cell sorting flow cytometer
(Beckman Coulter Epis XL; Beckman Coulter, Inc.) and analyzed using
Expo32 software (Expo32-ADC; Beckman Coulter, Inc.).
Analyzing peritoneal capillary
permeability
Peritoneal capillary permeability was measured as
described by Ujioka et al (25). On day 13, mice in the control and
experimental groups for peritoneal capillary permeability analysis
were injected intravenously with 0.2 ml of 0.8% Evans blue solution
(EB; cat. no. 18909-100ML-F; Sigma-Aldrich; Merck KGaA), and
sacrificed 120 min after the intraperitoneal injection.
Concentrations of EB in the peritoneal fluid were measured via
spectrophotometry at 580 nm wavelength, and expressed as light
absorption × ascitic fluid volume.
Statistical analysis
All data were analyzed with SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA) and expressed as mean ± standard
deviation. Survival of mice was calculated using the Kaplan-Meier
method. For multiple comparisons, one-way analysis of variance was
applied. Least-significant difference (LSD) or Games-Howell was
used according to the homogeneity of variances. P<0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
Effects of P-5m and/or 5-Fu on body
weight gain and survival time of mice bearing H22 hepatocellular
carcinoma
One of our previous studies indicated that P-5m has
therapeutic potential in metastatic human hepatocarcinoma, in which
the efficacy may function, at least partially, through modulation
of MMP-2 expression (18).
Therefore, it is meaningful to investigate the combined effects of
P-5m and 5-Fu in murine tumor models with hepatocarcinoma H22
malignant ascites. In the present study, a murine H22 hepatoma
ascites model was established using Kunming mice (Fig. 1). In the early few days, there were
no observable differences in the behavior of the mice in every
groups. Up until the sixth day, there was an extensive increase in
the abdominal girth and body-weight of the DG group, and revealed
malaise of spirit, little movement, poor response (Fig. 1A) and abdominal ectasia (Fig. 1B). LP (Fig. 1C) and LF (Fig. 1F) groups exhibited a reduction in
body weight when compared with the DG group. A significant
reduction in body weight was also observed in mice that were
treated with P-5m plus 5-Fu (LP; Fig.
1H), whereas medium-dose of P-5m (MP; Fig. 1D) had a similar effect. There were no
significant differences between the high-dose P-5m (HP) group and
the DG group (Fig. 1E). Although a
high dose of 5-Fu (HF, 20 mg/kg; Fig.
1G) fully suppressed the development of ascites, this treatment
exhibited 5-Fu-associated host toxicities, including a reduction of
body weight, reduced food and water uptake, piloerection, hunched
posture, lethargy and hypoactivity, which resulted in the mortality
of 83.3% of mice.
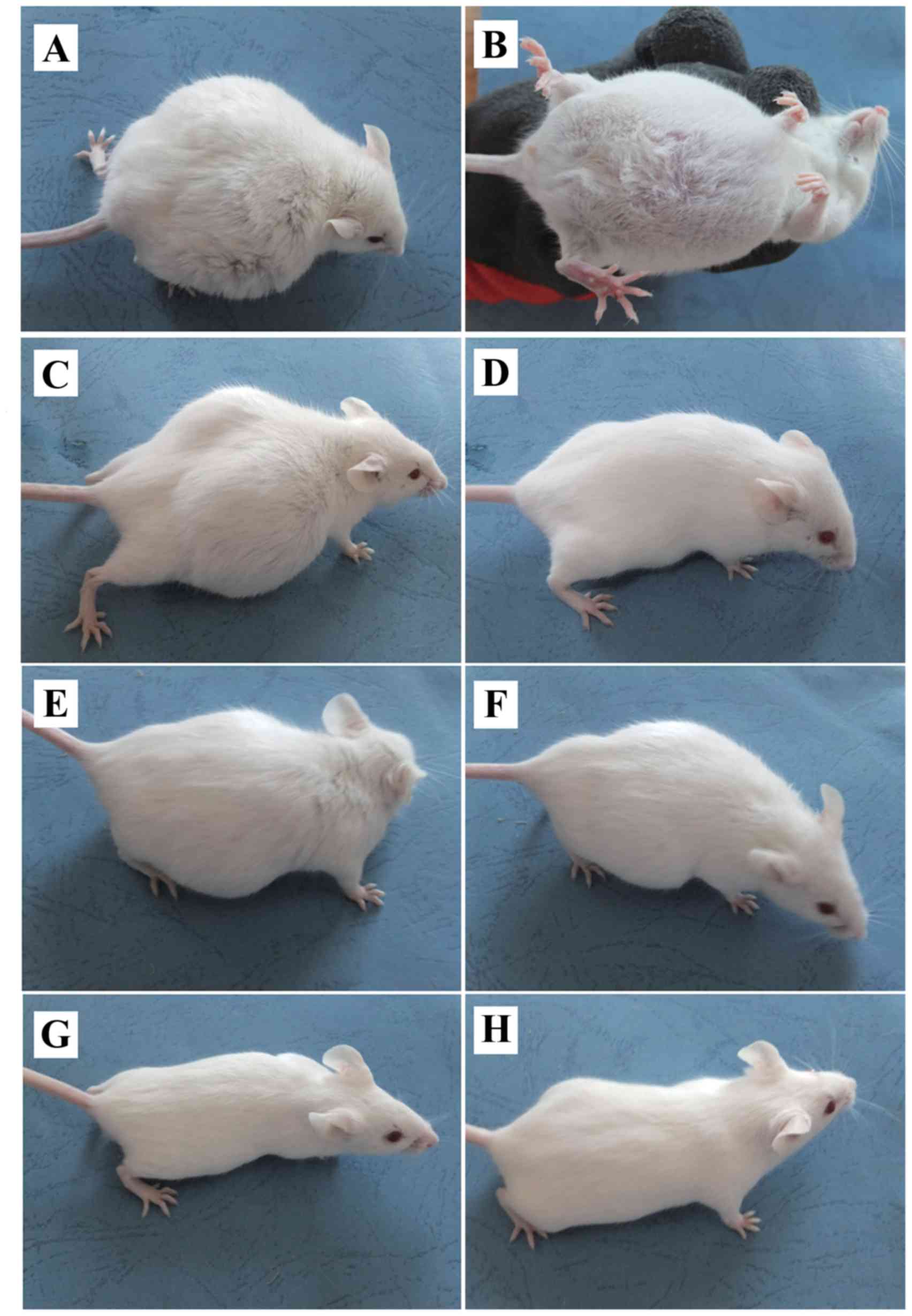 | Figure 1.Effect of P-5m and 5-Fu on the
production of malignant ascites in a mouse model of hepatoma 22
ascites. Images were captured on day 13, following tumor
implantation. (A and B) DG disease group (positive control group
without any drug treatment). (C) HP/high-dose P-5m (200 µg/kg)
group. (D) MP/medium-dose P-5m (50 µg/kg) group. (E) LP/low-dose
P-5m (12.5 µg/kg) group. (F) LF/low-dose 5-Fu (5 mg/kg) group. (G)
HF/high-dose 5-Fu (20 mg/kg) group. (H) PF/combination of P-5m
(12.5 µg/kg) and 5-Fu (5 mg/kg) group. Mice in these groups were
inoculated with H22 cells for 2 days prior to the start of the
various therapies. Mice in the LF and HF groups were
intraperitoneally injected three times a week, other groups were
intraperitoneally injected daily for 30 days. The signs of general
physical state were monitored daily. P-5m, P-5m octapeptide; 5-Fu,
5-fluorouracil; DG, positive control group in which mice with
ascites did not receive therapeutic treatment; HP, high-dose P-5m
(200 µg/kg) group; MP, medium-dose P-5m (50 µg/kg) group; LP,
low-dose P-5m (12.5 µg/kg) group; LF, low-dose 5-Fu (5 mg/kg)
group; HF, high-dose 5-Fu (20 mg/kg) group; PF, combination of P-5m
(12.5 µg/kg) and 5-Fu (5 mg/kg) group. |
LP, MP and LF groups significantly exhibited reduced
body weight and ascites volume (%) in mice compared with the DG
group. However, the combination of P-5m and 5-Fu (PF) further
reduced the tumor load (Fig. 2A and
B). There were no significant differences between the HP group
and the DG group. Although a high dose of 5-Fu (HF, 20 mg/kg) fully
suppressed the development of ascites, this treatment had
5-Fu-associated host toxicities (Fig.
2C). However, LP treatment significantly prolonged survival
time of mice. Although mice in MP and PF groups gained weight at
every time point from day 2 to 16, these changes may reflect health
status rather than the development of ascites, since these mice
exhibited flat abdomens. Therefore, the order of body
weight/ascites volume (%) reduction of mice compared with DG group
was: HF>PF>MP>LP>LF>HP.
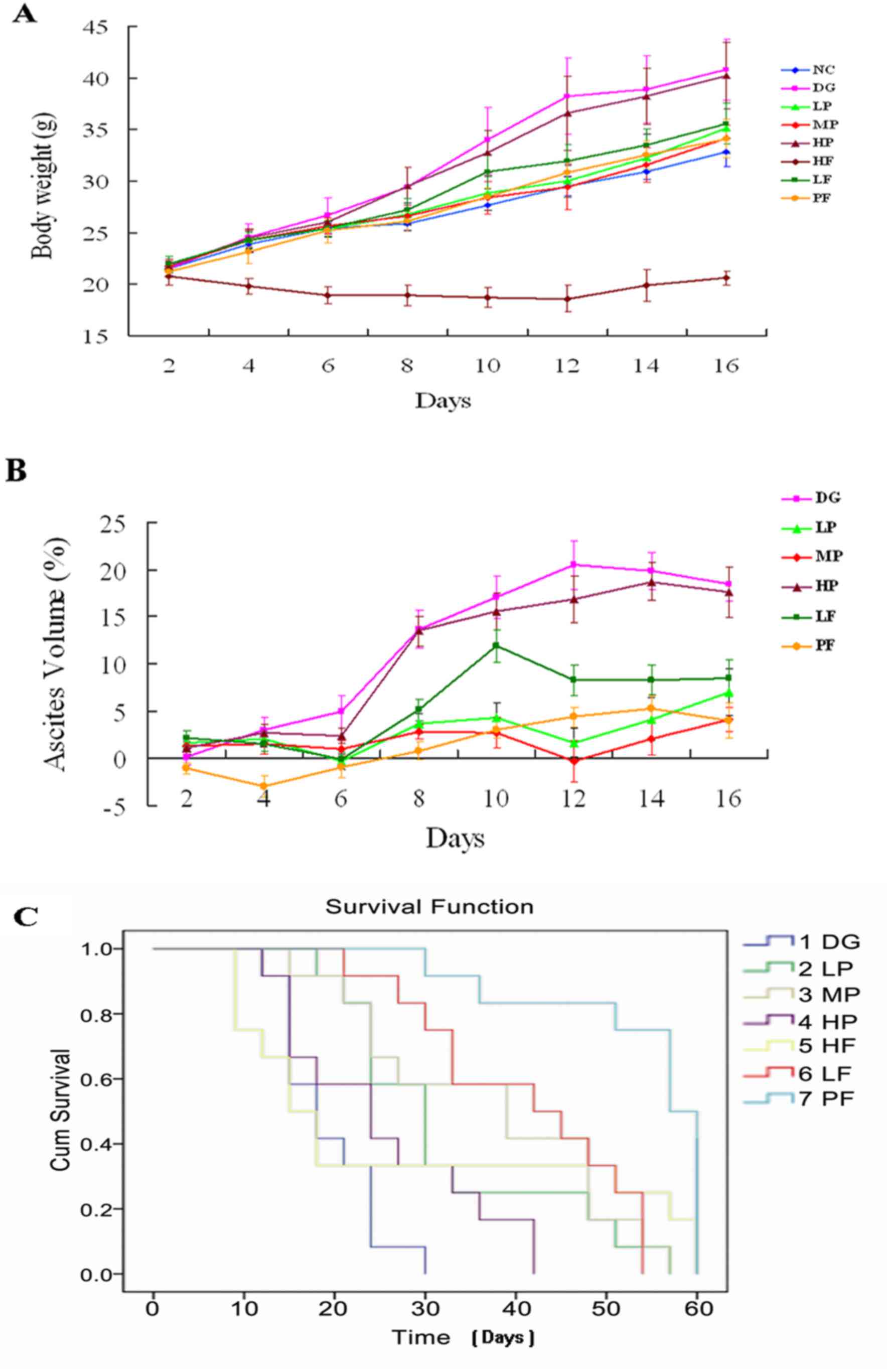 | Figure 2.Effects of P-5m and/or 5-Fu on (A)
body weight gain, (B) ascites volume (%) and (C) survival time of
hepatoma 22-bearing mice. P-5m, P-5m octapeptide; 5-Fu,
5-fluorouracil; NC, negative control group in which mice were not
treated; DG, positive control group in which mice with ascites did
not receive therapeutic treatment; HP, high-dose P-5m (200 µg/kg)
group; MP, medium-dose P-5m (50 µg/kg) group; LP, low-dose P-5m
(12.5 µg/kg) group; LF, low-dose 5-Fu (5 mg/kg) group; HF,
high-dose 5-Fu (20 mg/kg) group; PF, combination of P-5m (12.5
µg/kg) and 5-Fu (5 mg/kg) group; Cum, cumulative. Ascitic volume
are estimate according to the formula: Ascitic volume (%)=(X-N)/X
×100, where X represents the body weight of an individual mouse and
N represents mean body weight of the Negative control (NC) group.
When the animal observed sizable abdominal enlargement or ascites
with loss of a righting reflex, or [Volume of ascites (g)/body
weight (g)] was >20%, euthanasia would be prompted. |
Survival analysis indicated that while all mice in
the disease control group succumbed to disease within 30 days, a
significantly prolonged survival time and delayed development of
malignant ascites were demonstrated in mice treated with low- or
medium-doses of P-5m, low doses of 5-Fu, and particularly in the
groups of mice treated with a combination of P-5m and 5-Fu
(Fig. 2C; Table I). However, this was not exhibited in
mice treated with high-doses of P-5m or 5-Fu. The median survival
time in mice in the group treated with a combination of P-5m and
5-Fu was 52.8 days, which corresponds to an increase of 204.8% when
compared with the disease control group (Table I). Taking the results of 5 and 20
mg/kg 5-Fu into account in the ascites model, the cure rate (no
ascites symptom 30 days after stopping of intraperitoneal
chemotherapy) of 5-Fu alone was 16.67%. Conversely, the combination
of 5-Fu with P-5m increased the cure rate up to 50% (Table I). Therefore, the order of life span
prolongation of mice with ascites was:
PF>LF>MP>LP>HF>HP.
 | Table I.Effects of various treatments on the
survival time of hepatoma 22 ascites mice. |
Table I.
Effects of various treatments on the
survival time of hepatoma 22 ascites mice.
| Group | N | Survival time
(days) | Median survival
time ± standard deviation (days) | Increased life span
(%)a | Long-term
survivorsb | Cure rate (%) |
|---|
| DG | 12 | 13–30 | 17.33±5.65 | – | 0 | 0 |
| LP | 12 | 18–57 |
30.50±13.21c |
76.0 | 0 | 0 |
| MP | 12 | 15–57 |
35.00±14.06d | 102.0 | 0 | 0 |
| HP | 12 | 13–42 | 23.50±10.56 |
35.6 | 0 | 0 |
| LF | 12 | 21–54 |
39.00±11.932d | 125.0 | 0 | 0 |
| HF | 12 | 10–60 | 26.00±22.39 |
50.0 | 2 | 16.67 |
| PF | 12 | 30–60 |
52.83±10.667d | 204.8 | 6 | 50.00 |
Effects of P-5m and/or 5-Fu on
accumulation of the ascites, number of red blood cells and tumor
cells, and the cell cycle of H22 hepatocellular carcinoma
cells
On day 13 of the present study, the transplanted H22
cells and fluid from ascites were harvested from the peritoneal
cavity of the mice in all experimental groups. Results indicated
that low- and medium-doses of P-5m alone (12.5 and 50 µg/kg,
respectively) significantly decreased tumor growth, whereas the
combination of 12.5 µg/kg P-5m and 5 mg/kg 5-Fu further reduced the
tumor load (Table II). Furthermore,
5-Fu-associated host toxicities were observed, although 20 mg/kg of
5-Fu produced a significant inhibitory effect on the growth of
ascites. Additionally, H22 cell viability was decreased when
compared with untreated controls. Among the groups, the lowest
viability of malignant cells was observed in mice in the
combination treatment group (Table
II).
 | Table II.Effects of various treatments on the
accumulation of ascites, numbers of tumor cells and red blood
cells. |
Table II.
Effects of various treatments on the
accumulation of ascites, numbers of tumor cells and red blood
cells.
| Group | N | Volume of ascites
(ml) | Tumor cells
(107/ml) | Red blood cells
(106/ml) |
|---|
| DG | 5 | 7.9±1.91 | 8.06±1.48 | 13.52±7.47 |
| LP | 6 |
4.13±2.80a | 5.47±2.03 |
2.57±2.85a |
| MP | 6 |
1.6±1.55a |
2.43±2.3a |
1.6±1.74a |
| HP | 4 | 5.4±3.91 | 7.6±3.58 | 8.98±5.89 |
| LF | 6 |
4.38±2.65b | 5.38±2.95 |
2.77±2.17a |
| HF | 4 |
0.1±0.14a |
0.00±0.00a |
0.00±0.00a |
| PF | 6 |
0.38±0.48a |
0.58±0.94a |
0.00±0.00a |
These results suggested that P-5m increased the
antitumor effect of 5-Fu in vivo without triggering mice to
succumb to disease early. Furthermore, P-5m acted as a biochemical
modulator of 5-Fu, as P-5m may neutralize the toxicity caused by
5-Fu. However, high-dose P-5m administration did not exert any
antitumor effects in the present study. One potential explanation
is that the high doses of P-5m may induce internalization of its
receptor. It has been shown that most biologically active peptides
have receptors on the plasma membrane of cells and binding to
ligands will trigger receptor internalization and downregulation,
although the fate of these receptors within cells differ (26,27).
Optimal doses of P-5m used in the present study were chosen
according to our preliminary experiments in which a medium dose of
P-5m (50 µg/kg) had the most effective antitumor activities (data
not shown).
Effects of P-5m and/or 5-Fu on the
cell cycle of H22 hepatocellular carcinoma cells
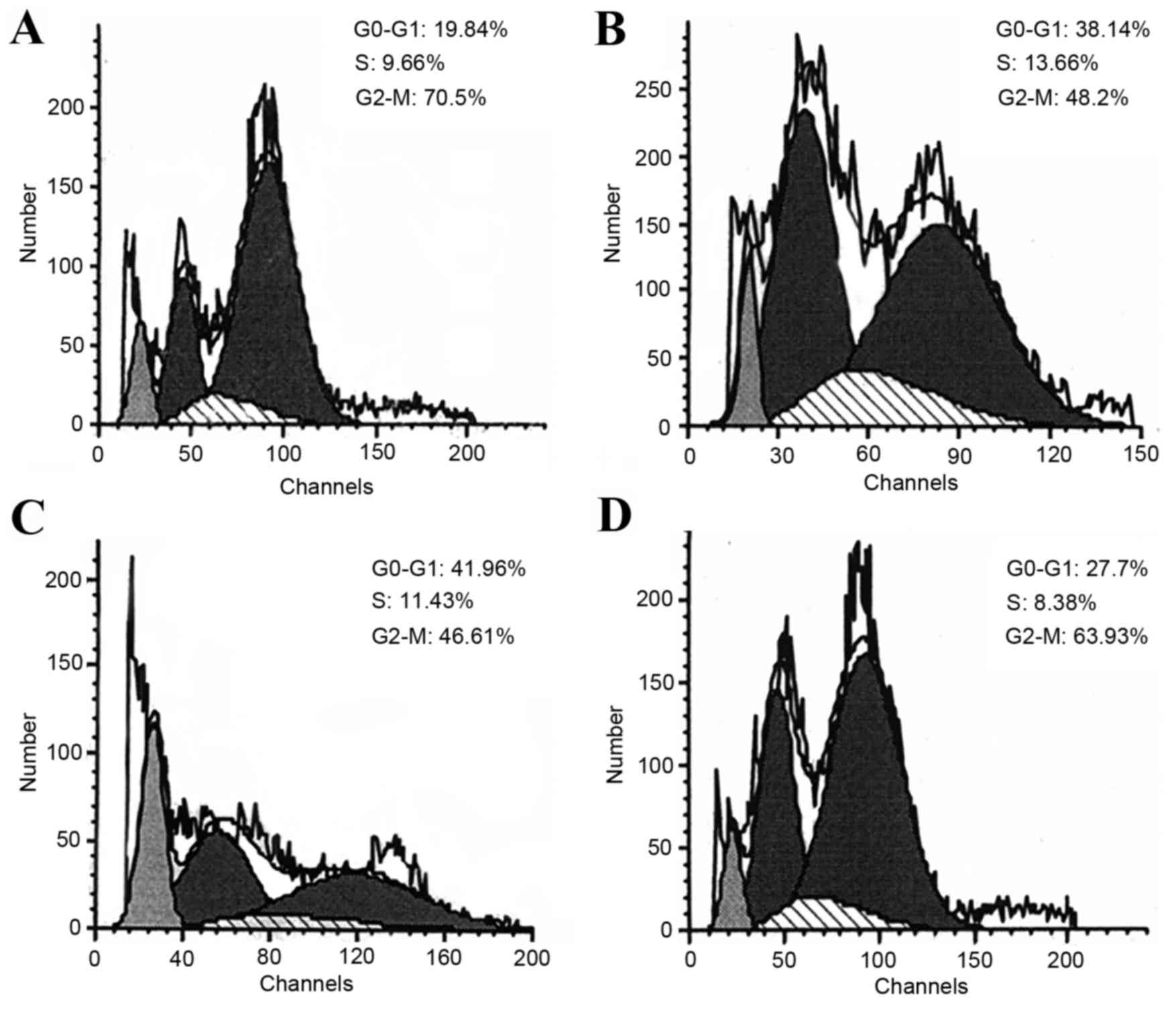
Cell cycle analysis revealed that the proportions of
H22 cells in G0-G1 phase significantly
increased from 20.04±3.74% (DG group; Fig. 3A) to 32.46±8.75% (LP group; Fig. 3B) and 42.44±6.74% (MP group; Fig. 3C), respectively, while the proportion
of cells in G2-M phase significantly decreased from
68.06±3.06% to 52.73±10.47 and 45.63±2.97% (Table III), suggesting that P-5m may
induce a G1-cell cycle arrest in the ascites model.
However, high doses of P-5m (200 mg/kg; Fig. 3D; Table
III) did not exhibit a significant difference when compared
with the disease control group, suggesting this dose did not affect
the cell cycle distributions. In contrast to P-5m, low-doses of
5-Fu significantly increased the proportion of cells in S-phase
(disease group vs. LF group, 11.90±3.60 vs. 28.34±5.27%); however,
a significantly decreased proportion of cells was exhibited in G2-M
phase (68.06±3.06 vs. 48.66±4.16%) (Table III), suggesting that 5-Fu induced
S-cell cycle arrest. The later observations are consistent with
previous studies, which demonstrated that 5-Fu exerts antitumor
effects by inducing S-phase arrest (28–30).
Therefore, it is likely that both P-5m and 5-Fu have important and
varying roles in regulating cell cycle distributions in combined
treatments. Although in the present study it was not possible to
statistically analyze cycle distributions in HF and PF groups due
to insufficient amounts of cell sample numbers, the data of one
sample in PF group indicated that the proportion of cells in
G2-M phase was merely 14.66%, implying the decline of
meiosis competence (Fig. 3; Table III).
 | Table III.Cell cycles of hepatoma 22 ascites
mice in different groups. |
Table III.
Cell cycles of hepatoma 22 ascites
mice in different groups.
|
|
| Cell cycle
stage |
|---|
|
|
|
|
|---|
| Group | N |
G0-G1 (%) | S (%) | G2-M
(%) |
|---|
| DG | 5 | 20.04±3.74 | 11.90±3.60 | 68.06±3.06 |
| LP | 5 |
32.46±8.75a | 15.03±3.76 |
52.73±10.47a |
| MP | 5 |
42.44±6.74a | 11.94±3.87 |
45.63±2.97a |
| HP | 4 | 27.3±3.35 | 10.27±4.31 | 62.43±7.52 |
| LF | 5 | 23.00±3.69 |
28.34±5.27a |
48.66±4.16a |
Effects of P-5m and/or 5-Fu on the
permeability peritoneal capillaries of mice bearing H22
hepatocellular carcinoma
EB dye was used to evaluate the peritoneal capillary
permeability of mice. Concentrations of EB in the peritoneal fluid
were measured via spectrophotometry at 580 nm wavelength, and
expressed as light absorption × ascitic fluid volume. The
peritoneal concentrations of EB in fluid from ascites of MP
(11.53±3.44), PF (11.37±2.67), LF (10.3±2.49) and HF (9.32±3.29)
groups were significantly lower than the disease control group
(16.58±3.85; P<0.05; Fig. 4). The
data suggested that P-5m enhances the anti-tumor effect of 5-Fu on
H22 bearing mice and antagonizes its toxicity, markedly.
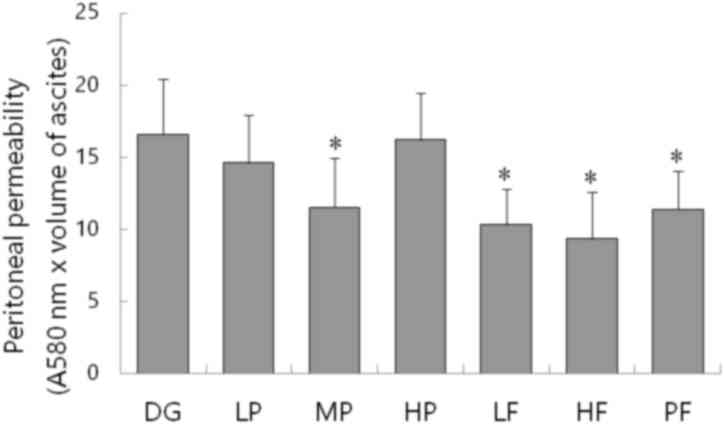 | Figure 4.Effect of different treatments on the
peritoneal permeability of mice (n=4-6). Evans blue dye was used to
evaluate the peritoneal capillary permeability of mice in DG, LP,
MP, HP, LF, HF and PF groups. Mice were injected via the caudal
vein with 8% Evans blue, ascites were collected and the light
absorption at the wavelength of 580 nm was analyzed using a
spectrophotometer. Data are presented as the mean + standard
deviation. *P<0.05 vs. DG. P-5m, P-5m octapeptide; 5-Fu,
5-fluorouracil; DG, positive control group in which mice with
ascites did not receive therapeutic treatment; HP, high-dose P-5m
(200 µg/kg) group; MP, medium-dose P-5m (50 µg/kg) group; LP,
low-dose P-5m (12.5 µg/kg) group; LF, low-dose 5-Fu (5 mg/kg)
group; HF, high-dose 5-Fu (20 mg/kg) group; PF, combination of P-5m
(12.5 ug/kg) and 5-Fu (5 mg/kg) group. |
Collectively, the findings of the present study
suggest that the combination of P-5m octapeptide with 5-Fu may
provide an alternative therapeutic strategy in the treatment of
tumors, specifically ascites caused by H22 hepatocellular
carcinoma. Further studies are required to investigate the
intensive mechanisms of this combined therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 31201061); Jilin
Province Sci-tech Department, China (grant nos. 20110729 and
20130522051JH); Jilin Province Development and Reform Commission,
China (grant no. 2013G019); Jilin Province Health Bureau
Development (grant no. 2017J082); and Jilin City Sci-tech Bureau,
China (grant no. 2013625030).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH, XZ and PD designed the study. XH, LA, DY, MZ
performed the experiments. XH, LA, MW, NZ collected and analyzed
the data. GX, GY and JS contributed to sample collection and
intellectual input. XH and LA drafted and wrote the manuscript. MH,
WD and YS gave advice on the experimental design, interpreted the
results and critically revised the manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All experiments using laboratory animals were
performed according to the guidelines of the Animal Management
Rules of the Ministry of Health of the People's Republic of China
and were approved by the Beihua University Committee on Laboratory
Animals (Changchun, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ayantunde AA and Parsons SL: Pattern and
prognostic factors in patients with malignant ascites: A
retrospective study. Ann Oncol. 18:945–949. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barni S, Cabiddu M, Ghilardi M and
Petrelli F: A novel perspective for an orphan problem: Old and new
drugs for the medical management of malignant ascites. Crit Rev
Oncol Hematol. 79:144–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coupe NA, Cox K, Clark K, Boyer M and
Stockler M: Outcomes of permanent peritoneal ports for the
management of recurrent malignant ascites. J Palliat Med.
16:938–940. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Woopen H and Sehouli J: Current and future
options in the treatment of malignant ascites in ovarian cancer.
Anticancer Res. 29:3353–3359. 2009.PubMed/NCBI
|
|
5
|
Chung M and Kozuch P: Treatment of
malignant ascites. Curr Treat Options Oncol. 9:215–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akgol G, Yildiz C, Karakus S, Koc M, Dogan
M, Turan M and Karadayi K: The effects of intraperitoneal
chemotherapeutic agents on adhesion formation. European J Gynaecol
Oncol. 37:781–785. 2016.
|
|
7
|
Cashin PH, Mahteme H, Graf W, Karlsson H,
Larsson R and Nygren P: Activity ex vivo of cytotoxic drugs in
patient samples of peritoneal carcinomatosis with special focus on
colorectal cancer. BMC Cancer. 13:4352013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parson EN, Lentz S, Russell G, Shen P,
Levine EA and Stewart JH IV: Outcomes after cytoreductive surgery
and hyperthermic intraperitoneal chemotherapy for peritoneal
surface dissemination from ovarian neoplasms. Am J Surg.
202:481–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guarneri V, Piacentini F, Barbieri E and
Conte PF: Achievements and unmet needs in the management of
advanced ovarian cancer. Gynecol Oncol. 117:152–158. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oh SY, Kwon HC, Lee S, Lee DM, Yoo HS, Kim
SH, Jang JS, Kim MC, Jeong JS and Kim HJ: A Phase II study of
oxaliplatin with low-dose leucovorin and bolus and continuous
infusion 5-fluorouracil (modified FOLFOX-4) for gastric cancer
patients with malignant ascites. Jpn J Clin Oncol. 37:930–935.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van der Speeten K, Stuart OA, Mahteme H
and Sugarbaker PH: Pharmacology of perioperative 5-fluorouracil. J
Surg Oncol. 102:730–735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wyllie E and Wyllie R: Routine laboratory
monitoring for serious adverse effects of antiepileptic
medications: The controversy. Epilepsia. 32 Suppl 5:S74–S79.
1991.PubMed/NCBI
|
|
13
|
Jang JH, Kim MY, Lee JW, Kim SC and Cho
JH: Enhancement of the cancer targeting specificity of buforin IIb
by fusion with an anionic peptide via a matrix
metalloproteinases-cleavable linker. Peptides. 32:895–899. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lo A, Lin CT and Wu HC: Hepatocellular
carcinoma cell-specific peptide ligand for targeted drug delivery.
Mol Cancer Ther. 7:579–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao W, Wang Y, Lau EY, Luo J, Yao N, Shi
C, Meza L, Tseng H, Maeda Y, Kumaresan P, et al: The use of
one-bead one-compound combinatorial library technology to discover
high-affinity αvβ3 integrin and cancer targeting
arginine-glycine-aspartic acid ligands with a built-in handle. Mol
Cancer Ther. 9:2714–2723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao Z, Lu R, Jia J, Zhao P, Yang J, Zheng
M, Lu J, Jin M, Yang H and Gao W: The effect of tripeptide
tyroserleutide (YSL) on animal models of hepatocarcinoma. Peptides.
27:1167–1172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawasaki M, Maeda T, Hanasawa K, Ohkubo I
and Tani T: Effect of His-Gly-Lys motif derived from domain 5 of
high molecular weight kininogen on suppression of cancer metastasis
both in vitro and in vivo. J Biol Chem. 278:49301–49307. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han X, Yan DM, Zhao XF, Matsuura H, Ding
WG, Li P, Jiang S, Du BR, Du PG and Zhu X: GHGKHKNK octapeptide
(P-5m) inhibits metastasis of HCCLM3 cell lines via regulation of
MMP-2 expression in in vitro and in vivo studies. Molecules.
17:1357–1372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamaguchi N and Kiick KL:
Polysaccharide-poly (ethylene glycol) star copolymer as a scaffold
for the production of bioactive hydrogels. Biomacromolecules.
6:1921–1930. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Wang X and Lu H: Amifostine
increases cure rate of cisplatin on ascites hepatoma 22 via
selectively protecting renal thioredoxin reductase. Cancer Lett.
260:127–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Santos FM, Latorre AO, Hueza IM, Sanches
DS, Lippi LL, Gardner DR and Spinosa HS: Increased antitumor
efficacy by the combined administration of swainsonine and
cisplatin in vivo. Phytomedicine. 18:1096–1101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghosh P, Sur P and Bag SP: Enhancement of
antitumor effects of a new boron compound combined with ultrasound
on the mouse ascites tumor. Med Chem. 8:1026–1031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li C and Ren LQ: Replication of Animal
Models of Human Diseases. People's Medical Publishing House;
Beijing: pp. 612008, (In Chinese).
|
|
24
|
Su ZQ, Liu YH, Guo HZ, Sun CY, Xie JH, Li
YC, Chen JN, Lai XP, Su ZR and Chen HM: Effect-enhancing and
toxicity-reducing activity of usnic acid in ascitic tumor-bearing
mice treated with bleomycin. Int Immunopharmacol. 46:146–155. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ujioka T, Matsuura K, Tanaka N and Okamura
H: Involvement of ovarian kinin-kallikrein system in the
pathophysiology of ovarian hyperstimulation syndrome: Studies in a
rat model. Hum Reprod. 13:3009–3015. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morel G: Internalization and nuclear
localization of peptide hormones. Biochem Pharmacol. 47:63–76.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Finch AR, Caunt CJ, Armstrong SP and
McArdle CA: Agonist-induced internalization and downregulation of
gonadotropin-releasing hormone receptors. Am J Physiol Cell
Physiol. 297:C591–C600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim GD, Rhee GS, Chung HM, Chee KM and Kim
GJ: Cytotoxicity of 5-fluorouracil: Effect on endothelial
differentiation via cell cycle inhibition in mouse embryonic stem
cells. Toxicol In Vitro. 23:719–727. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen XX, Lai MD, Zhang YL and Huang Q:
Less cytotoxicity to combination therapy of 5-fluorouracil and
cisplatin than 5-fluorouracil alone in human colon cancer cell
lines. World J Gastroenterol. 8:841–846. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang SR, Hu GR, Fang LJ, Huang SJ, Li JS,
Zhao MY and Meng MJ: CpG oligodeoxynucleotides enhance
chemosensitivity of 5-fluorouracil in HepG2 human hepatoma cells
via downregulation of the antiapoptotic factors survivin and livin.
Cancer Cell Int. 13:1062013. View Article : Google Scholar : PubMed/NCBI
|