Introduction
Lung cancer is a common malignancy with a high
mortality rate and increasing incidence in young people (1). In the last 50 years, many countries
have reported that the incidence and mortality of lung cancer has
significantly increased. The morbidity and mortality of lung cancer
in men rank first among all malignant tumors, and second in women.
The causes of lung cancer include smoking, air pollution, ionizing
radiation, chronic lung infections and occupational and
environmental exposure. A large number of data have shown that
long-term smoking has been closely related to lung cancer. The
incidence of lung cancer among urban residents has been higher than
that in rural areas, which may be related to the presence of
carcinogens in urban air pollution and smoke. Therefore,
non-smoking should be advocated and urban environmental hygiene
should be improved. Studies have demonstrated that long-term
smokers were more likely to develop lung cancer than non-smokers,
and the younger they started smoking, the more likely they were to
develop lung cancer. In addition, smoking not only directly
effected health, but also had a negative effect on the health of
the people around them, which led to the increase of the prevalence
rate of lung cancer in passive smokers (2,3).
Clinical manifestations of lung cancer are complex.
Tumor site, tumor complications, metastasis and pathological type
can determine the severity of lung cancer. However, manifestations
of early lung cancer mainly include cough, chest pain and
hoarseness, but no typical clinical symptoms were observed.
Therefore, the misdiagnosis rate is high and most patients were
diagnosed at an advanced stage, which in turn delays the treatment
(4,5). The symptoms of central lung cancer
appear early and are severe, and the symptoms of peripheral lung
cancer appear late and are mild, even asymptomatic, so it is often
found in physical examination. The symptoms of lung cancer contain
local symptoms, systemic symptoms, extrapulmonary symptoms,
infiltration and metastasis symptoms (6–9).
Lobectomy, as the main treatment of lung cancer, can
improve patients' condition. However, the complication of
lobectomy, such as postoperative pulmonary infection and acute
respiratory distress, can seriously affect patients' lung function,
which in turn reduces the treatment effect (10). Studies have shown that glutamine can
promote nitrogen balance, inhibit the translocation of enterotoxin
and bacterial into blood, maintain intestinal mucosal integrity,
and promote systemic immune and intestinal immune function. In this
study, 78 patients who underwent lobectomy for lung cancer were
selected to investigate the effects of glutamine on cytokines,
including IL-1 and TNF-α, and the prognosis of patients.
Patients and methods
General information
A total of 78 lung cancer patients who underwent
lobectomy were randomly selected from January 2015 to January 2017
in Daqing Oilfield General Hospital. Patients were randomly divided
into two groups, 39 cases in each group. Patients in control group
included 21 males and 18 females, the age ranged from 43 to 76
years with an average age of 59.56±7.89 years, body mass index
(BMI) ranged from 19 to 25 kg/m2 with an average value
of 22.19±2.13 kg/m2, ASA classification: 23 cases of
grade I and 16 cases of grade II. Patients in the observation group
included 23 males and 16 females, the age ranged from 43 to 78
years with an average value of 59.63±7.93 years, and BMI ranged
from 19 to 26 kg/m2 with an average value of 22.21±2.19
kg/m2, ASA classification: 22 cases of grade I and 17
cases of grade II. There were no significant differences in the
basic information (such as sex and age) between the two groups
(p>0.05) (Table I). This study
was approved by the Ethics Committee of Daqing Oilfield General
Hospital (Daqing, China). Signed informed consents were obtained
from the patients or the guardians.
 | Table I.Comparison of basic information
between two groups. |
Table I.
Comparison of basic information
between two groups.
|
|
|
|
| ASA
classification |
|---|
|
|
|
|
|
|
|---|
| Groups | Male/female | Age (years) | BMI
(kg/m2) | I | II |
|---|
| Control (n=39) | 21/18 | 59.56±7.89 | 22.19±2.13 | 23 | 16 |
| Observation
(n=39) | 23/16 | 59.63±7.93 | 22.21±2.19 | 22 | 17 |
| t/χ2 | 0.044 | 0.029 | 0.084 | 0.130 |
| P-value | >0.05 | >0.05 | >0.05 | >0.05 |
Criteria
Inclusion criteria (7)
i) patients diagnosed with lung cancer who underwent
lobectomy; ⅱ) aged between 40 and 65 years; and ⅲ) with ASA grade
of I–II.
Exclusion criteria (11)
i) patients combined with severe hypertension and
cardiovascular and cerebrovascular diseases; ⅱ) patients who cannot
tolerate the drugs used in this study; ⅲ) patients who received
radiotherapy or immunosuppressive therapy; and ⅳ) patients with
severe gastrointestinal ulcers, blood system diseases and liver and
kidney dysfunction.
Methods
The patients in both groups were treated with
lobectomy. After operation, patients in the control group were
treated with conventional methods including nutritional support,
fluid resuscitation, anti-inflammatory treatment, fasting,
gastrointestinal decompression, bed rest and other comprehensive
treatments. Besides conventional treatment, intravenous infusion of
100 ml of glutamine (SFDA approval no. H20153121; Hangzhou Minsheng
Pharmaceutical Group Co., Ltd., Hangzhou, China) was performed
twice a day in the observation group. Treatment was performed for 7
days for both groups.
Evaluation indicators
i) levels of TNF-α and endotoxin (12): peripheral venous blood (5 ml) was
extracted from each patient before and at 7 days after treatment,
after centrifugation at 2,500 × g for 5 min at 4°C, the supernatant
was collected and stored at −20°C in a refrigerator; levels of
TNF-α were measured by enzyme-linked immunosorbent assay and levels
of endotoxin were measured using BET-24A bacterial endotoxin
analyzer, the kit were provided by Beijing Weitonglihua
Experimental Animal Technical Co., Ltd. (Beijing, China), all
operations were performed in strict accordance to the
manufacturer's instructions. ⅱ) Levels of serum IL-1, IL-10, IL-15
and IL-18 (13): levels of IL-1,
IL-10, IL-15 and IL-18 were detected by enzyme-linked immunosorbent
assay. ⅲ) Intercellular adhesion molecule-1 (ICAM-1) expression and
myeloperoxidase (MPO) activity: after treatment, expression of
ICAM-1 in serum was measured by immunohistochemistry, and MPO
activity in 5% lung tissue homogenate was measured by colorimetric
method, all operations were performed in strict accordance with the
manufacturer's instructions. ⅳ) The occurrence of nausea and
vomiting (14). v) Lung function
indicators: the forced expiratory volume in 1 sec (FEV1), forced
vital capacity (FVC) and peak expiratory flow rate (PEFR). ⅵ) Lung
histopathological changes (15):
lung tissue was fixed in 10% formaldehyde solution, followed by
H&E staining. Lung histopathological changes were observed
under microscopy (BX-42; Olympus, Tokyo, Japan).
Statistical analysis
SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was
used to analyze all data in this study. Measurement data were
expressed by mean ± standard deviation (SD), and processed by
t-test. Count data were expressed by the number of cases and
percentages, and processed by χ2 test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Levels of endotoxin and TNF-α
Before treatment, no significant differences in
endotoxin and TNF-α were found between the two groups (p>0.05).
After treatment, the levels of endotoxin and TNF-α were
significantly lower in observation group than those in control
group (p<0.05) (Table II).
 | Table II.Comparison of endotoxin and TNF-α
between two groups (mean ± (SD), ng/l). |
Table II.
Comparison of endotoxin and TNF-α
between two groups (mean ± (SD), ng/l).
| Time-points | Groups | Endotoxin | TNF-α |
|---|
| Before treatment | Control (n=39) | 1.13±0.26 | 78.23±18.71 |
|
| Observation
(n=39) | 1.08±0.31 | 76.29±19.03 |
|
| t value | 0.772 | 0.454 |
|
| P-value | >0.05 | >0.05 |
| After treatment | Control (n=39) | 0.47±0.23 | 49.78±13.54 |
|
| Observation
(n=39) | 0.21±0.15 | 30.16±10.27 |
|
| t value | 5.913 | 7.210 |
|
| P-value | <0.05 | <0.05 |
Levels of IL-1, IL-10, IL-15 and
IL-18
Before treatment, no significant differences in the
levels of IL-1, IL-10, IL-15 and IL-18 were found between the two
groups (p>0.05); after treatment, the levels of IL-1 and IL-10
were significantly higher and the levels of IL-15 and IL-18 were
significantly lower in the observation group than those in the
control group (p<0.05) (Table
III).
 | Table III.Comparison of serum factors between
the two groups (mean ± (SD), pg/ml). |
Table III.
Comparison of serum factors between
the two groups (mean ± (SD), pg/ml).
| Time-points | Groups | IL-1 | IL-10 | IL-15 | IL-18 |
|---|
| Before treatment | Control (n=39) | 27.23±10.97 | 56.48±16.78 | 51.78±12.31 | 253.08±33.92 |
|
| Observation
(n=39) | 26.86±11.34 | 55.71±17.03 | 52.01±12.09 | 251.83±30.54 |
|
| t value | 0.147 | 0.201 | 0.083 | 0.171 |
|
| P-value | >0.05 | >0.05 | >0.05 | >0.05 |
| After
treatment | Control (n=39) | 31.04±17.69 | 103.76±19.78 | 29.17±7.82 | 172.58±20.59 |
|
| Observation
(n=39) | 39.78±18.37 | 173.27±21.03 | 10.91±6.34 | 110.37±15.76 |
|
| t value | 2.140 | 15.036 | 11.327 | 14.983 |
|
| P-value | <0.05 | <0.05 | <0.05 | <0.05 |
ICAM-1 expression and MPO
activity
Compared with the control group, the expression
level of ICAM-1 and MPO activity in the observation group were
significantly higher than those in the control group (p<0.05)
(Table IV).
 | Table IV.Comparison of the expression level of
ICAM-1 and MPO activity between groups (mean ± (SD). |
Table IV.
Comparison of the expression level of
ICAM-1 and MPO activity between groups (mean ± (SD).
| Groups | ICAM-1 (%) | MPO (U/g) |
|---|
| Control (n=39) | 85.71±10.34 |
4.24±0.61 |
| Observation
(n=39) | 46.03±10.67 | 13.95±1.92 |
| t value | 16.678 | 30.100 |
| P-value | <0.05 | <0.05 |
Survival, postoperational infection
and adverse reactions
All patients survived in the observation and control
groups 1 year after the surgery, except for 2 patients in the
observation group (1 for heart attack and 1 for cerebral
infarction) and 3 patients in the control group (2 for diabetes
mellitus and 1 for heart attack). The survival between the
observation and control groups was not significantly different,
p>0.05. Forty infections were observed in the observation group,
including 5 cases of mycoplasma, 4 cases of Streptococcus
pneumoniae, 2 cases of Haemophilus influenzae, 1 case of
influenza virus and 2 cases of unknown infection. Nine infections
were observed in the control group, including 3 cases of
mycoplasma, 3 cases of Streptococcus pneumoniae, 2 cases of
influenza virus and 1 case of unknown infection. The rate of
postoperational infection in the observation group was slightly
lower than that in the control group. Four cases (10.26%) of nausea
and vomiting were observed in the observation group and 3 cases
(7.69%) were found in the control group, significant differences
were found between the two groups (χ2=0.000,
p>0.05).
Postoperative bed rest and hospital
stay
Postoperative bed rest and hospital stay were
significantly shorter in the observation group than those in the
control group (p<0.05) (Table
V).
 | Table V.Comparison of postoperative bed rest
and hospital stay between the two groups. |
Table V.
Comparison of postoperative bed rest
and hospital stay between the two groups.
| Groups | Bed rest | Hospital stay |
|---|
| Control (n=39) | 2.7±0.7 | 9.4±4.3 |
| Observation
(n=39) | 1.2±0.4 | 6.2±2.8 |
| t value | 14.412 | 4.831 |
| P-value | <0.05 | <0.05 |
Lung function indicator
Compared with the control group, FEV1, FVC and PEFR
were significantly improved in the observation group (p<0.05)
(Table VI).
 | Table VI.Comparison of lung function
indicators between the two groups mean ± (SD). |
Table VI.
Comparison of lung function
indicators between the two groups mean ± (SD).
| Groups | FEV1 (l) | FVC (l) | PEFR (l/sec) |
|---|
| Control (n=39) | 1.26±0.24 | 1.41±0.63 | 2.34±1.33 |
| Observation
(n=39) | 1.79±0.32 | 2.21±0.77 | 3.38±1.52 |
| t value | 3.928 | 2.538 | 2.084 |
| P-value | <0.05 | <0.05 | <0.05 |
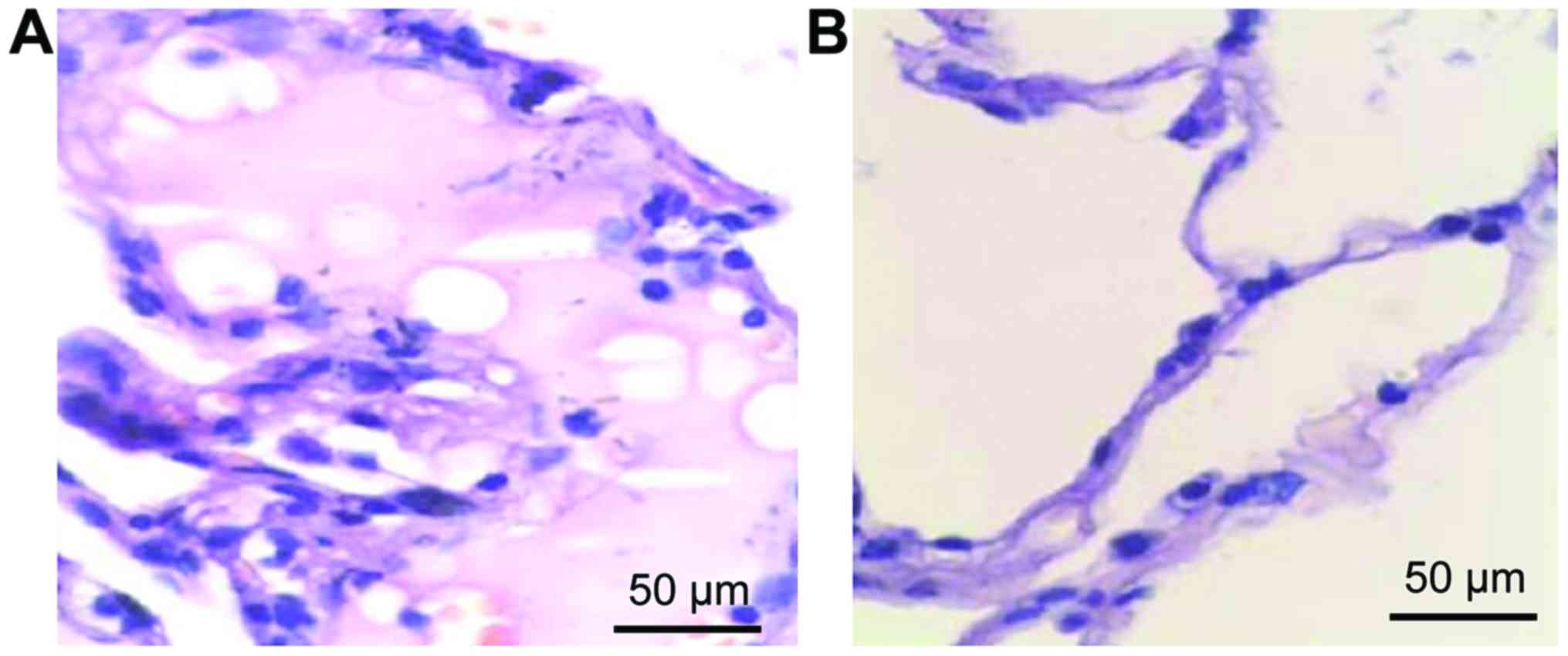
Lung histopathological changes
Normal lung tissue structure was observed in the
control group although a small amount of inflammatory cell
infiltration appeared, but edema was not found. Finally,
inflammatory cell infiltration and alveolar hemorrhage were
significantly improved in the observation group (Fig. 1).
Discussion
Overview of glutamine. Glutamine is a precursor
material for glutathione (GSH), pyrimidine, purine and amino acids.
It is also a major source of energy for immune, lymph and
fibroblasts in the body. Glutamine provides not only ATP but also
materials for the synthesis of proteins and nucleic acids (16,17).
Some scholars believe that glutamine can play a two-way immune
regulation role. It can inhibit the expression of inflammatory
factors, but also improve immune function, and the protective role
of glutamine is different in different organs (18). At present, the pharmacological
effects of glutamine are mainly reflected in the following points:
i) glutamine can promote protein synthesis, inhibit excessive
muscle tissue decomposition, and prevent multiple organ dysfunction
(19,20); ⅱ) glutamine can be converted to
glutamate, and further promote liver synthesis of GSH, which is the
most important antioxidant in the body, thereby enhancing the
body's antioxidant capacity (21);
ⅲ) glutamine can participate in the energy and material metabolism
of immune cells to reduce infection (22); ⅳ) glutamine can reduce intestinal
damage, prevent endotoxin and bacteria migration, protect the
mucosal barrier, and reduce the risk of intestinal infection
(17,23); and v) glutamine can reduce protein
decomposition under stress conditions, reduce complications, and
improve the overall condition (24).
Glutamine, TNF-α and endotoxin. Lobectomy, as the
main treatment of advanced lung disease, can effectively remove the
tumor lesions. However, systemic inflammatory response and other
serious complications caused by lobectomy can seriously aggravate
the patients' condition (25).
Therefore, the identification of an effective measure to reduce the
occurrence of multiple complications after surgery as well as
improving the prognosis of patients is particularly important.
TNF-α can directly kill human tumor cells, but has no significant
toxicity to normal cells. As the most active factor in the process
of killing tumor cells, TNF-α can reflect the conditions of
patients (26). Endotoxin is
composed of a non-specific core polysaccharide, cell-specific
polysaccharide and lipid A. In this study, endotoxin and TNF-α
levels in the observation group were significantly improved
compared with those in the control group, indicating that glutamine
can reduce the postoperative inflammatory response and
side-effects. This result is consistent with the finding of
Cetinbas et al (27).
Glutamine is the most abundant amino acid in cells and human body.
Under normal circumstances, glutamine has the characteristics of
non-essential amino acids. Glutamine, which is important for the
catabolic acceleration process, can improve metabolism of the
intestinal mucosal cells; glutamine can promote the rapid synthesis
of GSH substances, and further strengthen the body's antioxidant
capacity (27). At the same time, as
raw materials and nitrogen donor of nucleotide precursor, glutamine
can greatly promote the differentiation and proliferation of
macrophages and lymphocyte, increase the synthesis of phospholipid
mRNA, and inhibit TNF-α over-production, and thus effectively
improve immune function. In addition, glutamine can protect the
mucosal barrier, prevent endotoxin and bacterial migration, reduce
endotoxin levels and tissue damage (28).
Glutamine, IL-1, IL-10, IL-15, IL-18, MPO and
ICAM-1. IL-1 can promote the secretion of IL-2 substances from T
cells by stimulating platelet growth factor to repair the body
immune response system, thus effectively improve patients'
condition. As a neutrophil-specific reductase, MPO level is stable
in cells and MPO can be used to reflect neutrophil aggregation and
activity. As a surface protein of endothelial cells, ICAM-1 can
promote inflammatory response, control tumor tissue deterioration
and metastasis, so as to regulate immune response. The expression
level of ICAM-1 is low under normal conditions, and the increased
level of ICAM-1 can lead to the binding of ICAM-1 to its specific
receptor, which in turn enhances the adhesion of white blood cells,
inflammatory cells and tumor cells to endothelial cells, so as to
promote endothelial cell activation, resulting in excessive
inflammatory response. In this study, the levels of IL-1 and IL-10,
the expression level of ICAM-1 and MPO activity were significantly
higher, and the levels of IL-15 and IL-18 were significantly lower
in the observation group than those in the control group,
indicating that glutamine could effectively reduce the inflammatory
response, and improve body immunity. The possible explanation is
that, as an important antioxidant, glutamine can increase the level
of GSH under stress conditions, reduce endothelial cell damage,
stabilize cell membrane and protein structure, promote antioxidant
capacity, and protect cell function. At the same time, glutamine
can enhance immune function, promote cell metabolism, prevent
muscle over-decomposition, and reduce damage to tissues and organs.
Glutamine can also enhance the immune function, reduce the
production of inflammatory factors, and effectively regulate the
levels of cytokines (29).
Glutamine and prognosis. In this study, no
significant differences in the incidence of nausea and vomiting
were found between the two groups. But observation under microscope
revealed that postoperative recovery of the observation group was
significantly better than that of the control group, indicating
that glutamine plays an important role in energy metabolism and
immunity. In addition, average postoperative bed rest and hospital
stay in the observation group were significantly shorter than those
in the control group (p<0.05). FEV1, FVC and PEFR in the
observation group were also significantly better than those in the
control group (p<0.05), indicating that glutamine can promote
the recovery of lung cancer patients after lobectomy.
In conclusion, glutamine can regulate the levels of
cytokines IL-1, IL-10, IL-15 and IL-18 in patients with lobectomy
to improve immune function and promote postoperative
rehabilitation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW, LH and XH conceived and designed the study. XW,
YQ and HL were responsible for the collection and analysis of the
patient data. LH and XH interpreted the data and drafted the
manuscript. XW revised the manuscript critically for important
intellectual content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Daqing Oilfield General Hospital (Daqing, China). Signed informed
consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vlahos I: Dilemmas in lung cancer staging.
Radiol Clin North Am. 56:419–435. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Jiang M, Du C, Yu Y, Liu Y, Li M
and Luo F: Utilization of lung cancer cell lines for the study of
lung cancer stem cells. Oncol Lett. 15:6791–6798. 2018.PubMed/NCBI
|
|
3
|
de Sousa VML and Carvalho L: Heterogeneity
in lung cancer. Pathobiology. 85:96–107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma A and Shepard JO: Lung cancer
biopsies. Radiol Clin North Am. 56:377–390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shroff GS, Viswanathan C, Carter BW,
Benveniste MF, Truong MT and Sabloff BS: Staging lung cancer:
Metastasis. Radiol Clin North Am. 56:411–418. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brand T and Haithcock B: Lung cancer and
lung transplantation. Thorac Surg Clin. 28:15–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayashi Y, Sawa Y, Fukuyama N, Nakazawa H
and Matsuda H: Preoperative glutamine administration induces
heat-shock protein 70 expression and attenuates cardiopulmonary
bypass-induced inflammatory response by regulating nitric oxide
synthase activity. Circulation. 106:2601–2607. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fahrner R, Trochsler M, Corazza N,
Graubardt N, Keogh A, Candinas D, Brunner T, Stroka D and Beldi G:
Tumor necrosis factor-related apoptosis-inducing ligand on NK cells
protects from hepatic ischemia-reperfusion injury. Transplantation.
97:1102–1109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Souba WW, Klimberg VS, Plumley DA, Salloum
RM, Flynn TC, Bland KI and Copeland EM III: The role of glutamine
in maintaining a healthy gut and supporting the metabolic response
to injury and infection. J Surg Res. 48:383–391. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou XP, Chen M, Wei W, Cao J, Chen L and
Tian M: Effects of enteral immunonutrition on the maintenance of
gut barrier function and immune function in pigs with severe acute
pancreatitis. JPEN J Parenter Enteral Nutr. 34:554–566. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng ZY, Hamiel CR, Banerjee A, Wischmeyer
PE, Friese RS and Wischmeyer P: Glutamine attenuation of cell death
and inducible nitric oxide synthase expression following
inflammatory cytokine-induced injury is dependent on heat shock
factor-1 expression. JPEN J Parenter Enteral Nutr. 30:400–406;
discussion 406-7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Von Bültzingslöwen I, Adlerberth I, Wold
AE, Dahlén G and Jontell M: Oral and intestinal microflora in
5-fluorouracil treated rats, translocation to cervical and
mesenteric lymph nodes and effects of probiotic bacteria. Oral
Microbiol Immunol. 18:278–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singleton KD, Serkova N, Banerjee A, Meng
X, Gamboni-Robertson F and Wischmeyer PE: Glutamine attenuates
endotoxin-induced lung metabolic dysfunction: Potential role of
enhanced heat shock protein 70. Nutrition. 21:214–223. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Butler EB, Zhao Y, Muñoz-Pinedo C, Lu J
and Tan M: Stalling the engine of resistance: Targeting cancer
metabolism to overcome therapeutic resistance. Cancer Res.
73:2709–2717. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barquissau V, Beuzelin D, Pisani DF,
Beranger GE, Mairal A, Montagner A, Roussel B, Tavernier G, Marques
MA, Moro C, et al: White-to-brite conversion in human adipocytes
promotes metabolic reprogramming towards fatty acid anabolic and
catabolic pathways. Mol Metab. 5:352–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kou Y, Zheng WT and Zhang YR: Inhibition
of miR-23 protects myocardial function from ischemia-reperfusion
injury through restoration of glutamine metabolism. Eur Rev Med
Pharmacol Sci. 20:4286–4293. 2016.PubMed/NCBI
|
|
17
|
Tanaka K, Sasayama T, Irino Y, Takata K,
Nagashima H, Satoh N, Kyotani K, Mizowaki T, Imahori T, Ejima Y, et
al: Compensatory glutamine metabolism promotes glioblastoma
resistance to mTOR inhibitor treatment. J Clin Invest.
125:1591–1602. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jacque N, Ronchetti AM, Larrue C, Meunier
G, Birsen R, Willems L, Saland E, Decroocq J, Maciel TT, Lambert M,
et al: Targeting glutaminolysis has antileukemic activity in acute
myeloid leukemia and synergizes with BCL-2 inhibition. Blood.
126:1346–1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McRae MP: Therapeutic benefits of
glutamine: An umbrella review of meta-analyses. Biomed Rep.
6:576–584. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuen CA, Asuthkar S, Guda MR, Tsung AJ and
Velpula KK: Cancer stem cell molecular reprogramming of the Warburg
effect in glioblastomas: A new target gleaned from an old concept.
CNS Oncol. 5:101–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pusapati RV, Daemen A, Wilson C, Sandoval
W, Gao M, Haley B, Baudy AR, Hatzivassiliou G, Evangelista M and
Settleman J: MTORC1-dependent metabolic reprogramming underlies
escape from glycolysis addiction in cancer cells. Cancer Cell.
29:548–562. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Csibi A, Lee G, Yoon SO, Tong H, Ilter D,
Elia I, Fendt SM, Roberts TM and Blenis J: The mTORC1/S6K1 pathway
regulates glutamine metabolism through the eIF4B-dependent control
of c-Myc translation. Curr Biol. 24:2274–2280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kamata S, Kishimoto T, Kobayashi S,
Miyazaki M and Ishikura H: Possible involvement of persistent
activity of the mammalian target of rapamycin pathway in the
cisplatin resistance of AFP-producing gastric cancer cells. Cancer
Biol Ther. 6:1036–1043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gini B, Zanca C, Guo D, Matsutani T, Masui
K, Ikegami S, Yang H, Nathanson D, Villa GR, Shackelford D, et al:
The mTOR kinase inhibitors, CC214-1 and CC214-2, preferentially
block the growth of EGFRvIII-activated glioblastomas. Clin Cancer
Res. 19:5722–5732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pochini L, Scalise M, Galluccio M and
Indiveri C: Membrane transporters for the special amino acid
glutamine: Structure/function relationships and relevance to human
health. Front Chem. 2:612014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu W, Le A, Hancock C, Lane AN, Dang CV,
Fan TW and Phang JM: Reprogramming of proline and glutamine
metabolism contributes to the proliferative and metabolic responses
regulated by oncogenic transcription factor c-MYC. Proc Natl Acad
Sci USA. 109:8983–8988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cetinbas F, Yelken B and Gulbas Z: Role of
glutamine administration on cellular immunity after total
parenteral nutrition enriched with glutamine in patients with
systemic inflammatory response syndrome. J Crit Care.
25:661.e1–661.e6. 2010. View Article : Google Scholar
|
|
28
|
Mousavi SN, Samini F, Nematy M, Philippou
E, Safarian M, Tavallaiee S and Norouzy A: Hyperglycemia and
antibody titres against heat shock protein 27 in traumatic brain
injury patients on parenteral nutrition. Iran J Basic Med Sci.
17:119–122. 2014.PubMed/NCBI
|
|
29
|
Chen H, Chen D, Michiels J and De Smet S:
Dietary fiber affects intestinal mucosal barrier function by
regulating intestinal bacteria in weaning piglets. Commun Agric
Appl Biol Sci. 78:71–78. 2013.PubMed/NCBI
|