Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
disease which causes pain and dysfunction and leads to the
destruction of joints. Activation and recruitment of immune cells,
especially lymphocytes and monocytes into the joints, are major
characters of RA (1,2). The mechanisms underlying RA are
complex, including genetic and environmental factors, as well as
abnormalities of both innate immunity and adaptive immunity
(3). Although the etiopathology of
RA is not fully understood, it is known that monocytes/macrophages,
neutrophils, T cells and B cells are involved in the mechanisms
that drive the onset of RA (4).
These cells play a key role in the progression of RA through the
production of proinflammatory cytokines, leading to the development
of an inflammatory environment and immune cell recruitment in the
joints.
In humans, monocytes are a heterogeneous cell
population composed of three distinct subsets based on their
expression of CD14 and CD16 (5). The
CD14++ CD16− classical subset is the most
prominent of all circulating monocytes. The second monocyte subset
expresses levels of both CD14 and CD16 (CD14++
CD16+). It is referred to as intermediate monocytes. The
third subset comprises nonclassical monocytes that express low
levels of CD14 and high levels of CD16 (CD14+
CD16++). The CD14++ CD16− monocyte
is the major subset, while the CD14++ CD16+
and CD14+ CD16++ subsets occur in lower
numbers than CD14++ CD16− monocyte (6). The two CD14++ subsets are
thus recognized to expand in various inflammatory diseases and are
suggested to play a significant role in disease processes (7,8). Recent
reports have shown that the proportion of monocyte subsets was
aberrant in RA patients (9,10).
CD64 (FcgRI), a Fc receptor for IgG, is
constitutively expressed on macrophages and monocytes. CD64 is the
high-affinity receptor for monomeric IgG or Ig in immune complexes
that can initiates immunological and inflammatory reactions on
immune competent cells, including monocytes and joint-stationed
macrophages (11–13). Evidences from both human studies and
animal models have demonstrated that CD64 play a important role in
RA pathogenesis (14,15). However, previous monocyte CD64
expression studies in RA have reported conflicting findings,
showing increased, decreased or similar expressions compared with
health volunteers (HV) (16–18). The role of CD64 on monocytes in the
pathogenesis of RA remains to be clarified. And, whether CD64 can
regulate the function of monocyte subsets in RA remains to be
clarified.
In the present study, we detected the expression of
CD64 on monocyte subsets in patients with RA and HV. The
correlation between the expression of CD64 on monocyte subsets and
the activity of RA was also investigated. Moreover, the cytokines
secretion of CD64+ monocyte subsets in patients with RA
was measured.
Patients and methods
Subjects
A total of 46 patients fulfilled the revised
American College of Rheumatology criteria for RA (19) were recruited from the First
Affiliated Hospital of Nanchang University. Among them, 5 patients
were new-onset RA (<6 months disease duration) (20). All patients were administered
disease-modifying anti-rheumatic drugs (DMARDs), including
glucocorticoid and immunosuppressor therapy. Disease activity of RA
was calculated using the disease activity score 28 (DAS28)
(21). The patient characteristics
of this group are shown in Table I.
In addition, the present study included 22 HV (female 81.8%, mean
age 51.2±11.6 years) who were unrelated to the patients and did not
have inflammatory or autoimmune diseases. The study was approved by
the Ethics Committee of the First Affiliated Hospital of Nanchang
University (019) and was carried out in compliance with the
Helsinki Declaration. Written informed consent was obtained from
all participants before they entered the present study.
 | Table I.Clinical characteristics of patients
with RA and HV. |
Table I.
Clinical characteristics of patients
with RA and HV.
| Categories | RA (n=46) | HV (n=22) |
|---|
| Females, n (%) | 38 (82.6) | 19 (81.8) |
| Age, mean (SD),
years | 57.3±12.0 | 51.2±11.6 |
| DAS28, mean
(SD) | 4.4±1.9 | – |
| ACPA (+, >25
RU/ml), n (%) (33 patients) | 25 (75.7) | – |
| RF (+, >20
IU/ml), n (%) (33 patients) | 22 (66.7) | – |
| C3, mean (SD), g/l
(30 patients) | 0.9±0.2 | – |
| C4, mean (SD), g/l
(30 patients) | 0.2±0.06 | – |
| IgG, mean (SD), g/l
(30 patients) | 12.9±6.1 | – |
| WBC, mean (SD) | 6.9±3.0 | – |
| Neutrophil count,
mean (SD) | 4.8±2.5 | – |
| The percent of
neutrophil, mean (SD), % | 68.0±8.9 | – |
| ESR, mean (SD),
mm/h | 47.2±34.6 | – |
| CRP, mean (SD),
mg/l (37 patients) | 19.7±22.4 | – |
Flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) were
isolated from the fresh peripheral blood of RA patients and HV on
Ficoll-Paque gradient (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). The membrane molecules of monocytes were analyzed
immediately using flow cytometry. The following antibodies were
used: ECD-conjugated anti-CD14, PC5-conjugated anti-CD16 (BD
Biosciences, San Diego CA, USA), PE-conjugated anti-CD163,
anti-CD206, and anti-CD86, FITC-conjugated anti-CD80, anti-CD40,
anti-CD64, anti-HLA-DR (MIH clones; eBioscience; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Monocyte subsets identified as
detailed above based on their expression of CD14 and CD16 (5). Briefly, 5×105 PBMCs was
incubated simultaneously with 10 µl of ECD-conjugated anti-CD14, 10
µl of PC5-conjugated anti-CD16, and PE-conjugated anti-CD64 on ice
in the dark for 30 min. Cells incubated with PE-conjugated mouse
IgG were used as isotype controls. Expression of CD64 was analyzed
on each monocyte subset using a CYTOMICS FC 500 flow cytometer
(Beckman Coulter, Inc., Brea, CA, USA) and data analyzed with the
associated software programs (CXP).
Serum CRP, IgG, C3 and C4
measurement
The concentrations of serum C-reactive Protein
(CRP), Immunoglobulin G (IgG), Complement 3 (C3) and Complement 4
(C4) were determined by nephelometry methods according to the
instructions described by the manufacturer (IMMUNE800; Beckman
Coulter, Inc.).
Erythrocyte sedimentation rate (ESR)
blood routine measurement
ESR and blood routine were determined according to
the instructions described by the manufacturer.
Autoantibody measurement
Level of rheumatoid factor (RF) was determined using
nephelometry methods according to the instructions described by the
manufacturer (IMMUNE800; Beckman Coulter, Inc.). Anti-citrullinated
protein antibodies (ACPA) from serum IgG were measured using
commercially ELISA kits (Kexin, Shanghai, China).
Cytokine measurement
Human IL-10, IL-6 and IL-8 (Signalway Antibody LLC,
College Park, MD, USA) were measured using commercially available
enzyme-linked immunosorbent assays according to the manufacturers'
instructions.
Statistical analysis
Statistical analysis and graphic presentation were
carried out with GraphPad Prism v.5.0 (GraphPad Software, Inc., La
Jolla, CA, USA). In addition, Student's t-test was used where the
normality test passed; otherwise, the nonparametric Mann-Whitney
test was used to analyze the data. Likewise, the Pearson method or
the nonparametric Spearman method was used for correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Result
Increased expression of CD64 in the
monocytes of RA patients
The monocytes in PBMCs were analyzed for the
expression of membrane molecules including CD40, CD64, CD163,
CD206, HLA-DR, CD80 and CD86 by flow cytometry. Representative dot
plots of population gating and CD64 expressing cells from RA
patients and HV were showed in Fig.
1A. Results showed that the expression of CD64 on monocytes was
significantly elevated in RA patients compared to HV (P=0.0103;
Fig. 1B). No significant difference
was observed in the expression of CD40, CD163, CD206, HLA-DR, CD80,
CD86 on monocytes between RA patients and HV (Fig. 1).
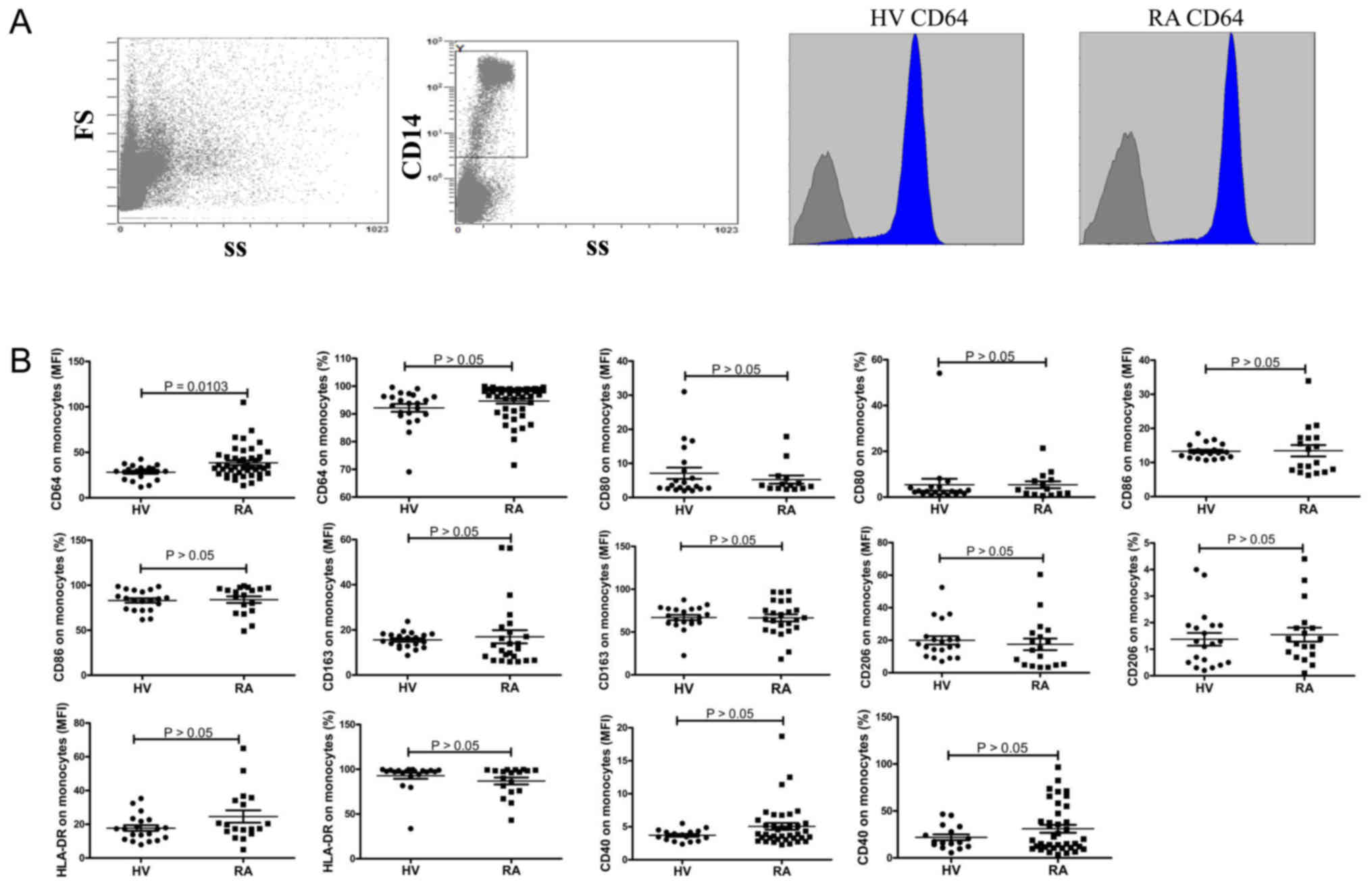 | Figure 1.CD40, CD64, CD163, CD206, HLA-DR,
CD80 and CD86 expression on monocytes. (A) Representative dot plots
of population gating and CD64 expressing cells from RA patients and
HV. (B) Summary data of the expression of CD40, CD64, CD163, CD206,
HLA-DR, CD80 and CD86 on monocytes. RA, rheumatoid arthritis; HV,
health volunteers. |
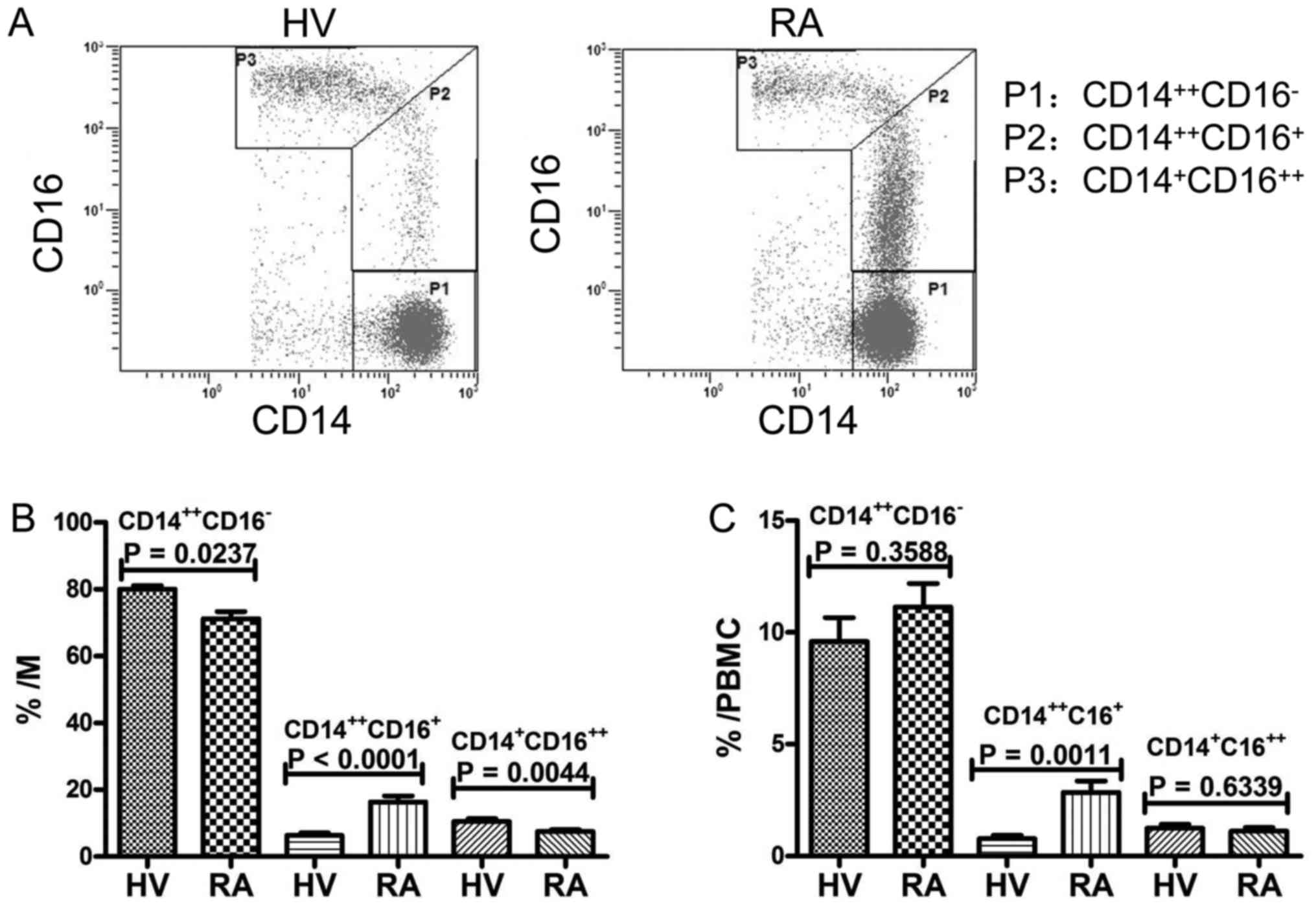
Proportions of each monocyte
subset
Representative dot plots of each monocyte subset
from flow cytometry analysis of CD14++CD16−
(P1), CD14++ CD16+ (P2) and CD14+
CD16++ (P3) blood monocytes from HV and RA patients are
shown in Fig. 2A. The three monocyte
subsets in total monocytes of peripheral blood cells from patients
with RA and healthy volunteers are shown in Fig. 2B. The proportion of
CD14++CD16+ monocytes in patients with RA was
significantly higher than that in HV (P<0.0001), while the
proportion of CD14++CD16− and
CD14+CD16++ monocytes in patients with RA was
significantly lower than that in HV (P=0.0237; P=0.0044). Moreover,
as showed in Fig. 2C, the proportion
of CD14++CD16+ monocytes in PBMCs was
significantly increased in patients with RA than that in HV
(P=0.0011), when that of CD14++CD16− and
CD14+CD16++ monocytes did not differ between
the two groups.
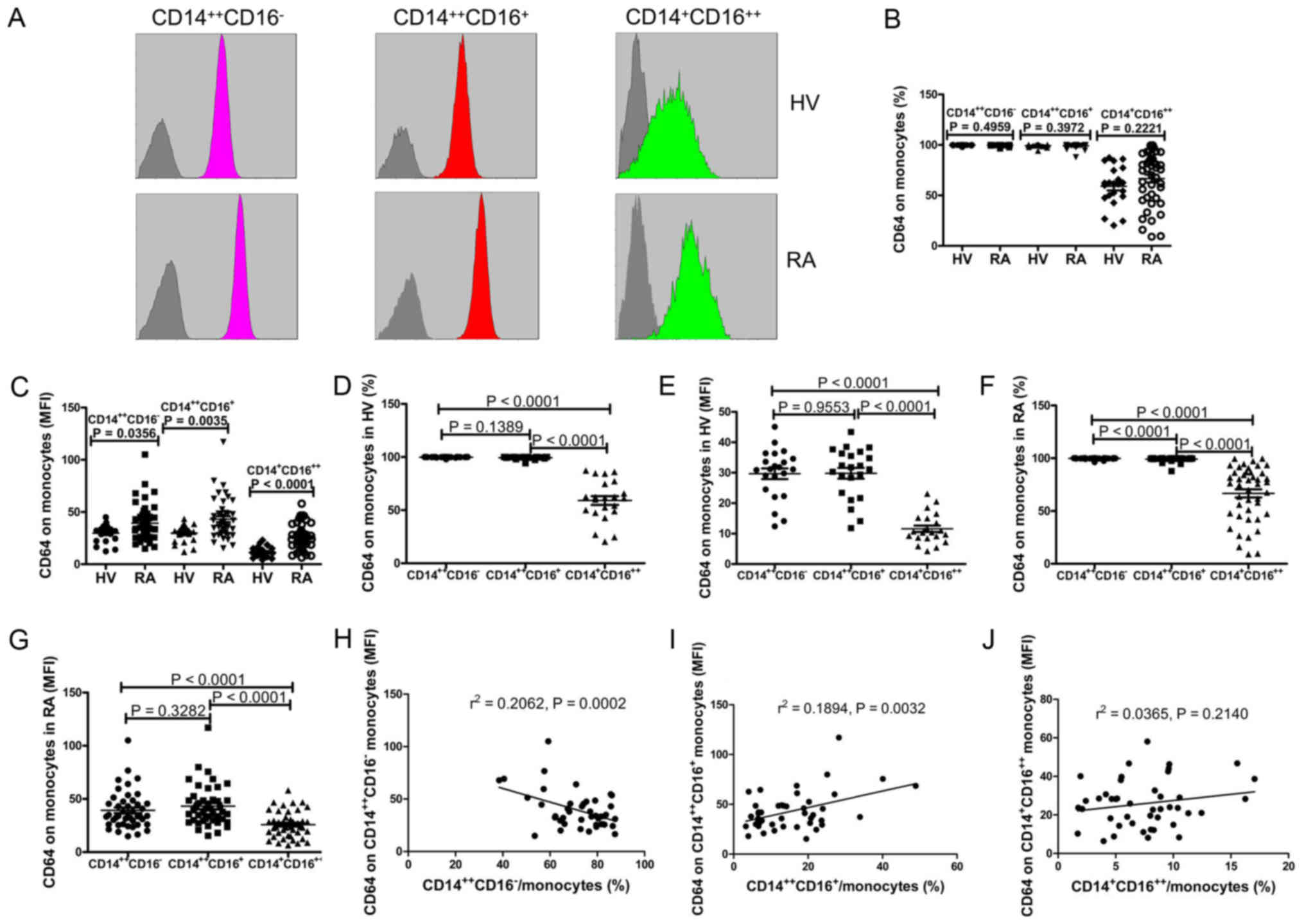
CD64 expression on monocyte subsets in
RA patients and HV
To determine the expression profile of CD64 on
monocyte subsets in RA patients and HV, we used flow cytometry to
assess the expression of CD64 on monocyte subsets including
CD14++CD16− monocytes,
CD14++CD16+ monocytes, and
CD14+CD16++ monocytes (Fig. 3). Data showed that although the
frequency of CD64-expressing CD14++CD16−
monocytes, CD64-expressing CD14++CD16+
monocytes, and CD64-expressing CD14+CD16++
monocytes did not differ between the two groups (Fig. 3B), the mean fluorescence intensity
(MFI) of CD64 on CD14++CD16− monocytes,
CD14++CD16+ monocytes and
CD14+CD16++ monocytes were significantly
elevated in patients with RA compared to HV (P<0.0001; Fig. 3C). Further, results showed that the
frequency of CD64-expressing CD14++CD16−
monocytes and CD64-expressing CD14++CD16+
monocytes were significantly elevated compared to CD64-expressing
CD14+CD16++ monocytes in both HV
(P<0.0001; Fig. 3D) and RA
patients (P<0.0001; Fig. 3F).
And, the frequency of CD64-expressing
CD14++CD16− monocytes was significantly
elevated compared to CD64-expressing
CD14++CD16+ monocytes in RA patients
(P<0.0001; Fig. 3F), but no
differences was found in HV (P=0.1389; Fig. 3D). As showed in Fig. 3E and G, the expression of CD64 on
CD14++CD16− monocytes and
CD14++CD16+ monocytes were significantly
elevated compared to CD14+CD16++ monocytes in
both RA patients (P<0.0001) and HV (P<0.0001). Moreover, we
investigated the correlation between the expression of CD64 on
monocyte subsets and the proportions of each monocyte subset. Data
showed that the proportion of CD14++CD16−
monocytes negatively correlated with the expression of CD64 on
CD14++CD16− monocytes (r=0.4541, P=0.0002;
Fig. 3H), whereas the proportion of
CD14++CD16+ monocytes positively correlated
with the expression of CD64 on CD14++CD16+
monocytes in RA patients (r=0.4352, P=0.0032; Fig. 3I). But no obvious correlation was
observed between the expression of CD64 on
CD14+CD16++ monocytes and the proportions of
CD14+CD16++ monocytes (r=0.1910, P=0.2140;
Fig. 3J). No obvious correlation was
observed between the expression of CD64 on monocyte subsets and the
proportions of each monocyte subset in HV (data no show).
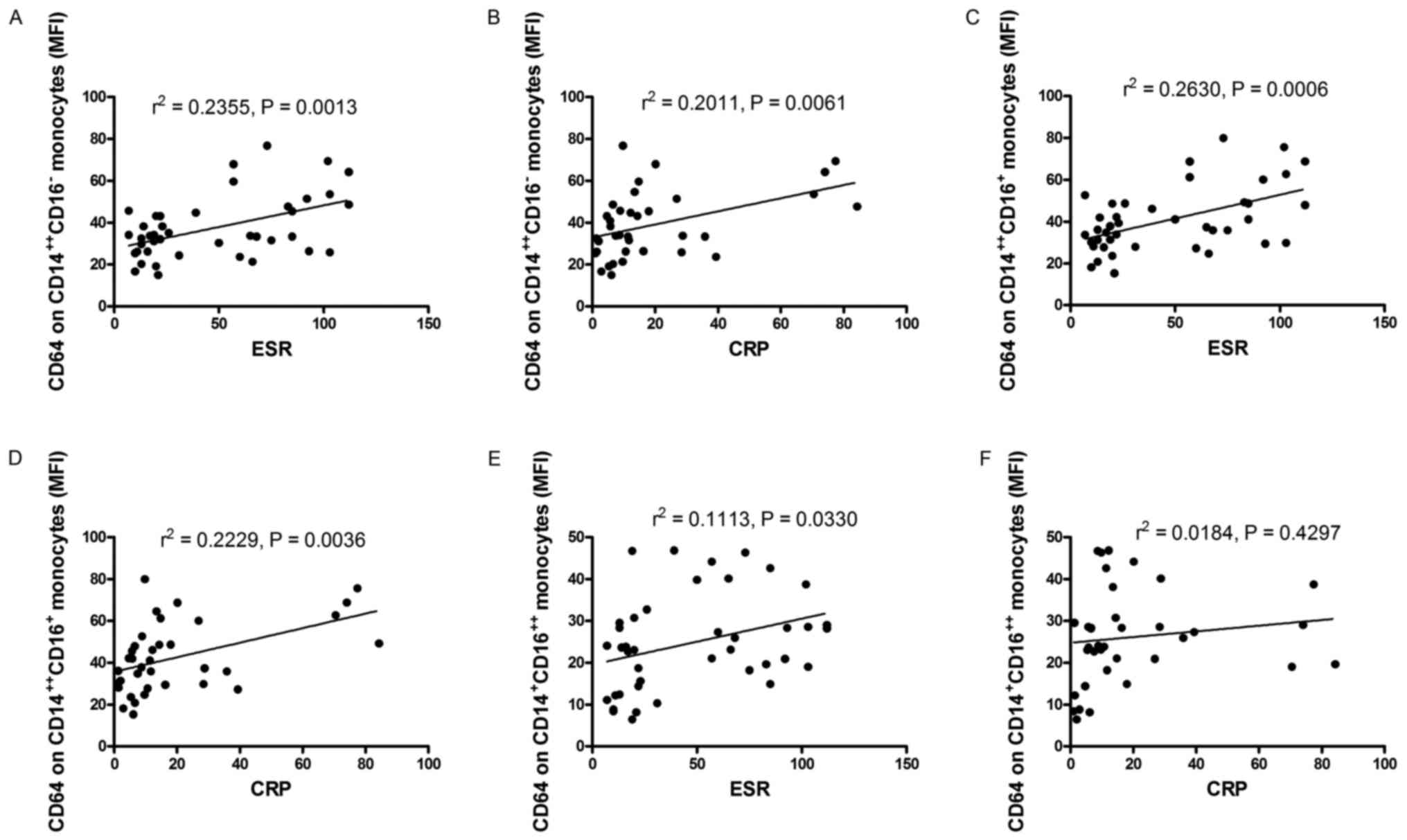
Expression of CD64 on monocyte subsets
correlates with inflammatory markers
Patients with RA frequently have elevated levels of
inflammatory markers. To determine the relationship between the
expression of CD64 on monocyte subsets and inflammatory markers,
inflammatory markers, such as ESR, CRP, white blood cell (WBC),
neutrophil count, the percent of neutrophil, IgG, C3 and C4, were
determined and analyzed for their relationship with the expression
of CD64 on CD14++CD16− monocytes,
CD14++CD16+ monocytes and
CD14+CD16++ monocytes in patients with RA.
The expression of CD64 on CD14++CD16−
monocytes positively correlated with ESR and CRP in RA patients
(r=0.4853, P=0.0013, Fig. 4A;
r=0.4484, P=0.0061, Fig. 4B), the
expression of CD64 on CD14++CD16+ monocytes
positively correlated with ESR and CRP in RA patients (r0.5128,
P=0.0006, Fig. 4C; r=0.4721,
P=0.0036, Fig. 4D), the expression
of CD64 on CD14+CD16++ monocytes positively
correlated with ESR (r=0.3336, P=0.0330, Fig. 4E), whereas the expression of CD64 on
CD14+CD16++ monocytes did not correlate with
CRP (r=0.1356, P=0.4297, Fig. 4F).
However, no obvious relationship was found between the expression
of CD64 on CD14++CD16− monocytes,
CD14+CD16++ monocytes,
CD14++CD16+ monocytes and WBC, neutrophil
count, the percent of neutrophil, IgG, C3, C4 (data no show).
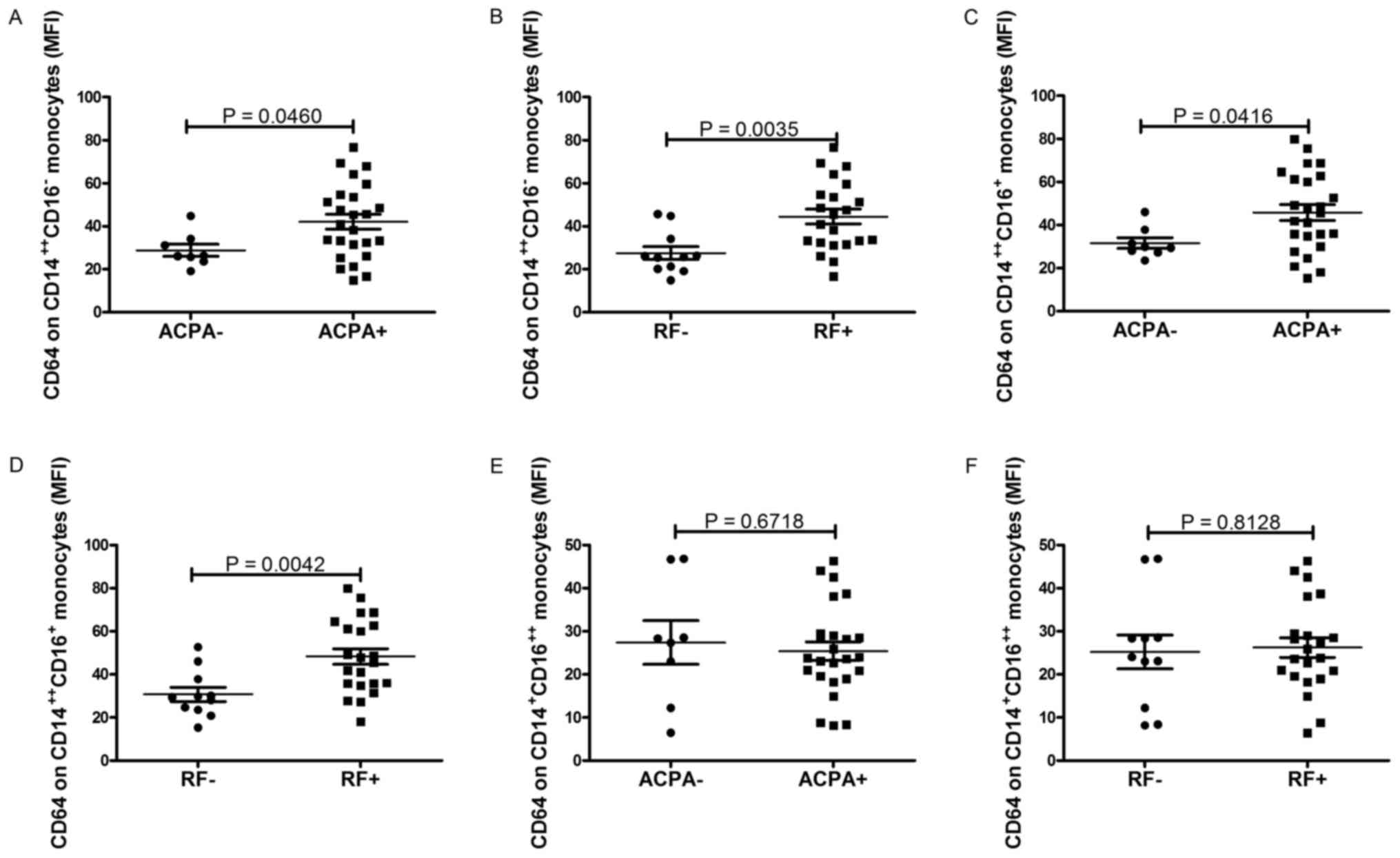
Expression of CD64 on monocyte subsets
correlates with markers of autoimmune response
The hallmark antibodies of RA, such as RF and ACPA,
were determined and analyzed for their correlation with the
expression of CD64 on monocyte subsets. As shown in Fig. 5, the expression of CD64 on
CD14++CD16− monocytes and
CD14++CD16+ monocytes were significantly
increased in patients with positive ACPA and RF respectively
(P=0.0460, Fig. 5A; P=0.0035,
Fig. 5B; P=0.0416, Fig. 5C; P=0.0042, Fig. 5D). However, no obvious relationship
was found between the expression of CD64 on
CD14+CD16++ monocytes and ACPA, RF (P=0.6718,
Fig. 5E; P=0.8128, Fig. 5F).
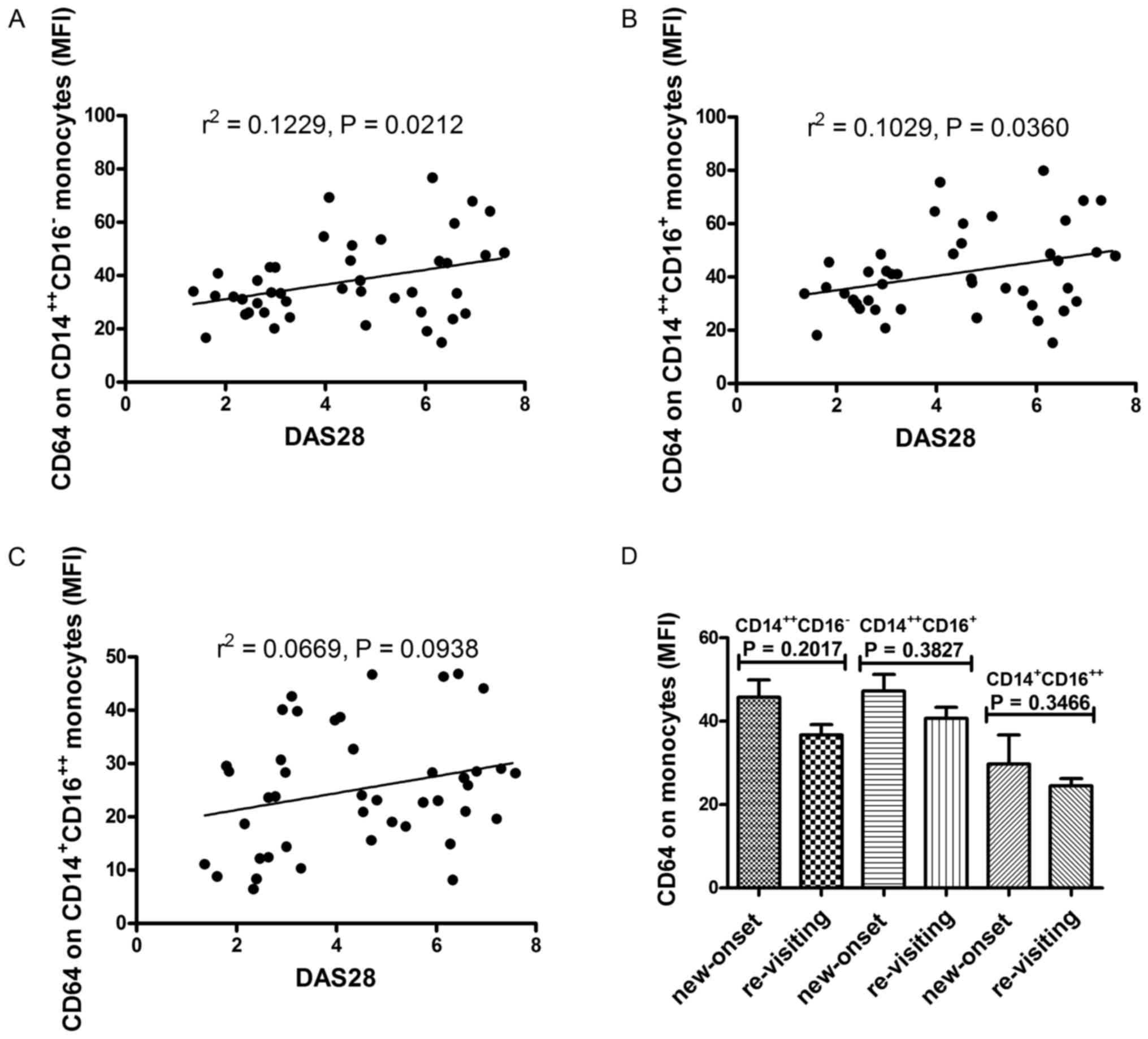
Expression of CD64 on monocyte subsets
correlates with disease activity of RA
Aforementioned data indicated that the expression of
CD64 on monocyte subsets was correlated with markers of
inflammation and autoimmune response. Thus, the correlation between
the expression of CD64 on monocyte subsets and disease activity
were investigated. Data showed that both the expression of CD64 on
CD14++CD16− monocytes and
CD14++CD16+ monocytes were positively
correlated with DAS28 score (r=0.3506, P=0.0212; r=0.3208,
P=0.0360) (Fig. 6A and B), while the
expression of CD64 on CD14+CD16++ monocytes
did not correlate with DAS28 score (r=0.2587, P=0.0938; Fig. 6C).
Subsequently, we compared the CD64 expression on
monocyte subsets between patients with new-onset and re-visiting
RA. Data showed that the expression of CD64 on monocytes subsets
tends to be elevated in patients with new-onset RA, but a
significant difference was not reached (P>0.0500; Fig. 6D).
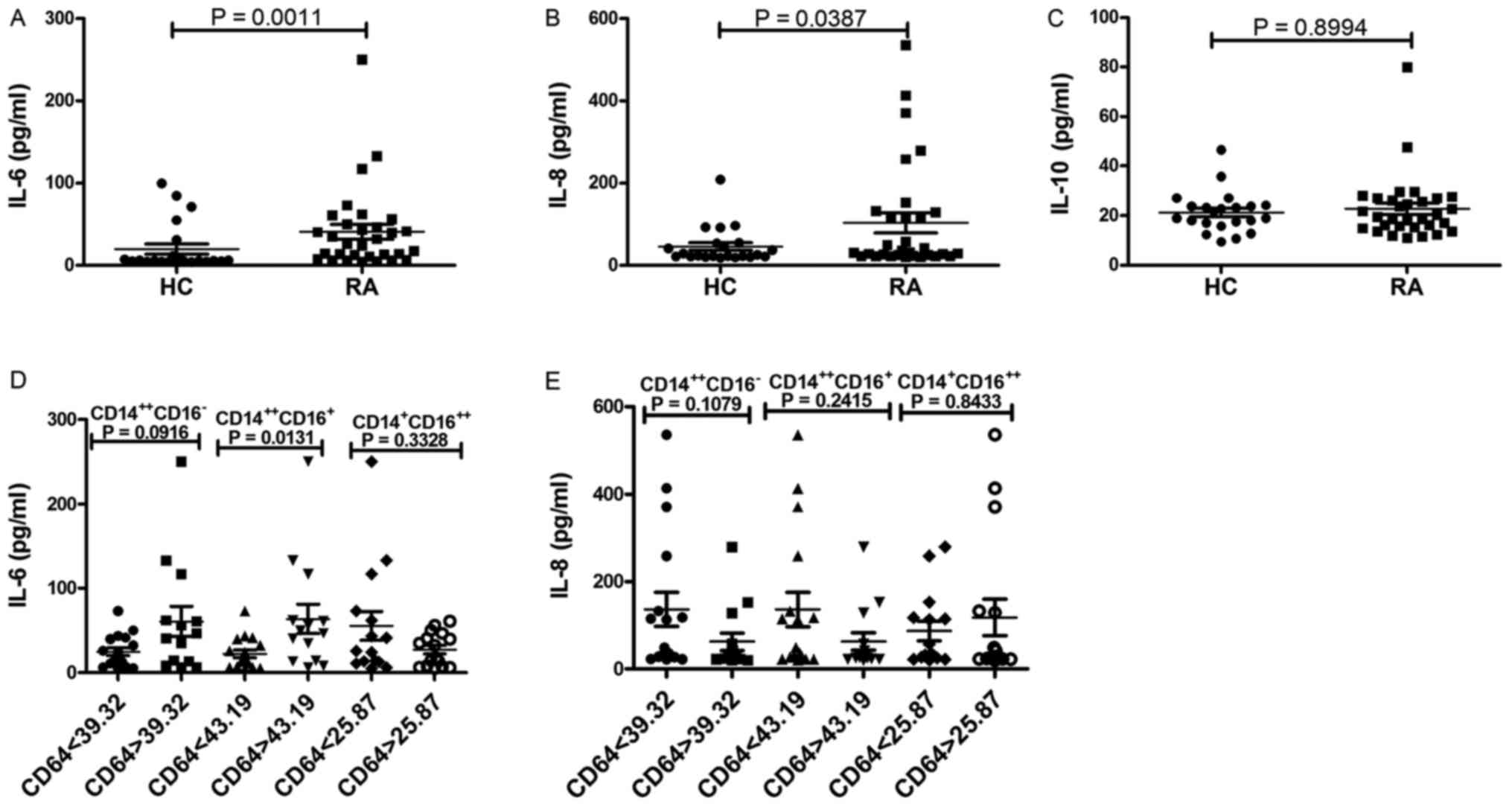
Association between the expression of
CD64 on monocyte subsets and serum cytokine concentration
Among the three serum inflammatory cytokines, levels
of IL-6 and IL-8 were significantly higher in patients with RA than
in HV (P=0.0011, Fig. 7A; P=0.0387,
Fig. 7B), no significant difference
was observed in the levels of IL-10 between patients with RA and HV
(P=0.8994; Fig. 7C). To determine
whether increased levels of CD64 on monocyte subsets play a role in
the secretion of serum cytokines (IL-6 and IL-8), RA patients were
divided into two groups according to their CD64 levels (average of
MFI) on monocytes subsets: RA high(CD64 on
CD14++CD16− >39.32, CD64 on
CD14++CD16+ >43.19, CD64 on
CD14+CD16++ >25.87) and RA
low(CD64 on CD14++CD16− <39.32,
CD64 on CD14++CD16+ <43.19, CD64 on
CD14+CD16++ <25.87). RA patients with high
levels of CD64 on CD14++CD16+ monocytes
exhibited significantly higher levels of IL-6 compared with RA
patients with low levels of CD64 on
CD14++CD16+ monocytes (P=0.0131; Fig. 7D). No significant difference was
observed in the levels of IL-6 between RA patients with high levels
of CD64 on CD14++CD16− or
CD14+CD16++ monocytes and low levels of CD64
on CD14++CD16− or
CD14+CD16++ monocytes (P>0.05; Fig. 7D). And, no significant difference was
observed in the levels of IL-8 between RA patients with high levels
of CD64 on each monocyte subset and low levels of CD64 on each
monocyte subset (P>0.05; Fig.
7E). These results indicate that increased levels of CD64 on
CD14++CD16+ monocytes in RA patients are
associated with increased secretion of IL-6.
Discussion
Monocytes are a heterogeneous cell population
composed of classical monocytes
(CD14++CD16−), intermediate monocytes
(CD14++CD16+) and nonclassical monocytes
(CD14+CD16++). The three subsets of monocytes
perform different functions. The classical subset is rapidly
recruited to the sites of inflammation and appears to act as
phagocytic scavenger cells and regulators of inflammation (22,23). The
intermediate monocytes play a proinflammatory role, being increased
in blood from patients with acute inflammation (24,25). The
nonclassical monocytes are often referred to as patrolling
monocytes (26). Previous researchs
have reported monocytes play a important role in the progression of
RA. The onset and severity of RA might also be due to the
seasonality of monocyte subsets was aberrant.
Although an increase in
CD14++CD16+ monocytes and an decreased in
CD14++CD16− monocytes in patients with RA
have been reported, the increase in
CD14+CD16++ monocytes remained controversial
(27,28). In consistent with the report of
Patricia Lacerte (28), this study
demonstrate that circulating CD14++CD16+ and
CD14+CD16++ monocytes are increased, while
circulating CD14++CD16− monocytes are
decreased in patients with RA. The reasons for these outcomes are
probably due to differences in the disease duration and ongoing
treatments.
An assessment of the expression of the
characteristic phenotypic markers CD40, CD64, CD163, CD206, HLA-DR,
CD80 and CD86 helped to characterize further the monocyte response
in patients with RA. In consistent with the results of other
researches (16,29), we found that the expression of CD64
on monocytes was significantly elevated in RA patients compared to
HV, no changes of other markers between patients with RA and HV.
The increased expression of CD64 on monocytes in patients with
active RA may suggest the progression of the disease (16), and may also reflect the activation of
the monocytes. Although an increase in CD64 on monocytes subsets in
patients with RA has been reported (30), the possibility of correlation between
the expression of CD64 on each monocytes subset and disease
activity in patients with RA has not yet been investigated. Our
results support previous observations (30) and show that the expression of CD64 on
CD14++CD16− monocytes and
CD14++CD16+ monocytes were significantly
elevated compared to CD14+CD16++ monocytes in
RA patients, and the expression of CD64 on
CD14++CD16− monocytes and
CD14++CD16+ monocytes were positively
correlated with DAS28 score.
Little is known about the possibility of
correlation between the expression of CD64 on each monocyte subset
and the proportion of each monocyte subset in patients with RA. We
found that the proportion of CD14++CD16+
monocytes positively correlated with the expression of CD64 on
CD14++CD16+ monocytes in RA patients, whereas
the proportion of CD14++CD16− monocytes
negatively correlated with the expression of CD64 on
CD14++CD16− monocytes. The reasons for the
results are probably due to the facts that the proportion of
intermediate monocytes positively correlated with the disease
activity of RA, whereas the proportion of classical monocytes
negatively correlated (27) and our
results showed that the expression of CD64 on
CD14++CD16− monocytes and
CD14++CD16+ monocytes were positively
correlated with DAS28 score.
It is well-known that RA is a autoimmune disease
characterized by the production of autoantibodies including RF,
ACPA and autoimmune response is a kind of chronic inflammation
against self antigens. In this study, the inflammatory markers,
DAS28, the hallmark antibodies of RA including RF and ACPA were
first determined and analyzed for their relation with the
expression of CD64 on monocyte subsets. Our results showed that the
expression of CD64 on CD14++CD16− and
CD14++CD16+ monocytes were positively related
with ESR, CRP and DAS28, whereas the expression of CD64 on
CD14+CD16++ monocytes did not correlate with
CRP and DAS28. In addition, we found the expression of CD64 on
CD14++CD16− monocytes and
CD14++CD16+ monocytes were significantly
increased in patients with positive RF and ACPA respectively,
whereas no obvious relationship was found between the expression of
CD64 on CD14++CD16+ monocytes and RF, ACPA.
This may be that (1)
CD14++CD16− monocytes and
CD14++CD16+ monocytes appears to act as
regulators of inflammation, whereas
CD14+CD16++ monocytes often referred to as
patrolling monocytes (22–26); (2)
CD64 is a high-affinity activating receptor that can bind IgG and
CRP and stimulate inflammatory processes (16,31,32).
In consistent with previous study (27), we showed here that levels of IL-6 and
IL-8 at baseline were significantly higher in patients with RA than
in HV. In addition, we observed that RA patients with high levels
of CD64 on CD14++CD16+ monocytes exhibited
significantly higher levels of IL-6 compared with the RA patients
with low levels of CD64 on CD14++CD16+
monocytes. These results suggested that the levels of CD64 on
CD14++CD16+ monocytes is indeed linked to the
secretion high concentrations of proinflammatory cytokines.
However, there are some limitations in the present
study. First is the relatively small sample size, especially the
sample of new-onset RA; these data may be confirmed in large-scale
studies. Second, we did not show that each monocyte subset are
directly associated with inflammatory cytokines in RA in
vitro. Third, No function study and experiments about the
mechanism of CD64 have been done in this study. The molecular
mechanisms underlying CD64 functions in RA still require further
investigation.
In conclusions, results presented in this study
demonstrate that blood monocyte subsets isolated from patients with
RA have high levels of CD64 and the levels of CD64 on
CD14++CD16− and
CD14++CD16+ monocytes correlates with the
disease activity of RA. In addition, the levels of CD64 on
CD14++CD16+ monocytes is linked to the high
secretion level of proinflammatory cytokines.
Acknowledgements
The authors would like to acknowledge the help from
Dr Rui Wu from the Department of Rheumatology, The First Affiliated
Hospital of Nanchang University, Nanchang, Jiangxi, China.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81360459), Jiangxi
Provincial Natural Science Foundation of China (grant nos.
20151BAB215031 and 20171BAB205113), the Science and Technology
Project of Health and Family Planning Commission of Jiangxi
Province of China (grant no. 20165094), the Science and Technology
Plan Project of the Education Department of Jiangxi Province (grant
no. GJ170008) and the Foundation for Distinguished Young Scientists
of the Jiangxi Province of China (grant no. 20171BCB23087).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
QL participated in designing the study, performed
statistical analyses and drafted the manuscript. PCX participated
in the design of the study and helped to revise the manuscript. XL
performed flow cytometry analysis and drafted the manuscript. ZD
performed statistical analyses and drafted the manuscript. CQ
performed data acquisition of markers of autoimmune response,
performed statistical analyses and drafted the manuscript. RGS
carried out data acquisition of markers of inflammation, performed
statistical analyses and drafted the manuscript. JQX performed data
acquisition of disease activity and severity, performed statistical
analyses, and drafted the manuscript. YG carried out the
experiments on the expression of cytokines and drafted the
manuscript. ZKH and JML conceived of the study, participated in its
design and coordination, and helped to draft the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Nanchang University (019) and was
carried out in compliance with the Helsinki Declaration. Written
informed consent was obtained from all participants before they
entered the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li X, Yuan FL, Lu WG, Zhao YQ, Li CW, Li
JP and Xu RS: The role of interleukin-17 in mediating joint
destruction in rheumatoid arthritis. Biochem Biophys Res Commun.
397:131–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan FL, Li X, Lu WG, Li CW, Xu RS and
Dong J: IL-33: A promising therapeutic target for rheumatoid
arthritis? Expert Opin Ther Targets. 15:529–534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McInnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. N Engl J Med. 365:2205–2219. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cascão R, Rosário HS, Souto-Carneiro MM
and Fonseca JE: Neutrophils in rheumatoid arthritis: More than
simple final effectors. Autoimmun Rev. 9:531–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi C and Pamer EG: Monocyte recruitment
during infection and inflammation. Nat Rev Immunol. 11:762–774.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM
and Wong SC: The three human monocyte subsets: Implications for
health and disease. Immunol Res. 53:41–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ziegler-Heitbrock L: The CD14+ CD16+ blood
monocytes: Their role in infection and inflammation. J Leukoc Biol.
81:584–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Skrzeczyńska-Moncznik J, Bzowska M, Loseke
S, Grage-Griebenow E, Zembala M and Pryjma J: Peripheral blood
CD14high CD16+ monocytes are main producers of IL-10. Scand J
Immunol. 67:152–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rossol M, Kraus S, Pierer M, Baerwald C
and Wagner U: The CD14 (bright) CD16+ monocyte subset is expanded
in rheumatoid arthritis and promotes expansion of the Th17 cell
population. Arthritis Rheum. 64:671–677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsukamoto M, Seta N, Yoshimoto K, Suzuki
K, Yamaoka K and Takeuchi T: CD14brightCD16+ intermediate monocytes
are induced by interleukin-10 and positively correlate with disease
activity in rheumatoid arthritis. Arthritis Res Ther. 19:282017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nimmerjahn F and Ravetch JV: Fcγ receptors
as regulators of immune responses. Nat Rev Immunol. 8:34–47. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amigorena S and Bonnerot C: Fc receptor
signalling and trafficking: A connection for antigen processing.
Immunol Rev. 172:279–284. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
García-García E and Rosales C: Signal
transduction during Fc receptor-mediated phagocytosis. J Leukoc
Biol. 72:1092–1108. 2002.PubMed/NCBI
|
|
14
|
Magnusson SE, Engström M, Jacob U, Ulfgren
AK and Kleinau S: High synovial expression of the inhibitory
FcgammaRIIb in rheumatoid arthritis. Arthritis Res Ther. 9:R512007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Vuuren AJ, van Roon JA, Walraven V,
Stuij I, Harmsen MC, McLaughlin PM, van de Winkel JG and Thepen T:
CD64-directed immunotoxin inhibits arthritis in a novel CD64
transgenic rat model. J Immunol. 176:5833–5838. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matt P, Lindqvist U and Kleinau S:
Elevated membrane and soluble CD64: A novel marker reflecting
altered fcγr function and disease in early rheumatoid arthritis
that can be regulated by anti-rheumatic treatment. PLoS One.
10:e01374742015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laurent L, Clavel C, Lemaire O, Anquetil
F, Cornillet M, Zabraniecki L, Nogueira L, Fournié B, Serre G and
Sebbag M: Fcγ receptor profile of monocytes and macrophages from
rheumatoid arthritis patients and their response to immune
complexes formed with autoantibodies to citrullinated proteins. Ann
Rheum Dis. 70:1052–1059. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hepburn AL, Mason JC and Davies KA:
Expression of Fcγ and complement receptors on peripheral blood
monocytes in systemic lupus erythematosus and rheumatoid arthritis.
Rheumatology (Oxford). 43:547–554. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arnett FC, Edworthy SM, Bloch DA, McShane
DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS,
et al: The american rheumatism assocaition 1987 revised criteria
for the classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Shan Y, Jiang Z, Feng J, Li C, Ma
L and Jiang Y: High frequencies of activated B cells and T
follicular helper cells are correlated with disease activity in
patients with new-onset rheumatoid arthritis. Clin Exp Immunol.
174:212–220. 2013.PubMed/NCBI
|
|
21
|
Prevoo ML, van't Hof MA, Kuper HH, van
Leeuwen MA, van de Putte LB and van Riel PL: Modified disease
activity scores that include twenty-eight-joint counts. Development
and validation in a prospective longitudinal study of patients with
rheumatoid arthritis. Arthritis Rheum. 38:44–48. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mehta NN and Reilly MP: Monocyte mayhem:
Do subtypes modulate distinct atherosclerosis phenotypes? Circ
Cardiovasc Genet. 5:7–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mobley JL, Leininger M, Madore S, Baginski
TJ and Renkiewicz R: Genetic evidence of a functional monocyte
dichotomy. Inflammation. 30:189–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Auffray C, Sieweke MH and Geissmann F:
Blood monocytes: Development, heterogeneity and relationship with
dendritic cells. Annu Rev Immunol. 27:669–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Belge KU, Dayyani F, Horelt A, Siedlar M,
Frankenberger M, Frankenberger B, Espevik T and Ziegler-Heitbrock
L: The proinflammatory CD14 + CD16 + DR++ monocytes are a major
source of TNF. J Immunol. 168:3536–3542. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cros J, Cagnard N, Woollard K, Patey N,
Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, et
al: Human CD14dim monocytes patrol and sense nucleic acids and
viruses via TLR7 and TLR8 receptors. Immunity. 33:375–386. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsukamoto M, Seta N, Yoshimoto K, Suzuki
K, Yamaoka K and Takeuchi T: CD14brightCD16+ intermediate monocytes
are induced by interleukin-10 and positively correlate with disease
activity in rheumatoid arthritis. Arthritis Res Ther. 19:282017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lacerte P, Brunet A, Egarnes B, Duchêne B,
Brown JP and Gosselin J: Overexpression of TLR2 and TLR9 on
monocyte subsets of active rheumatoid arthritis patients
contributes to enhance responsiveness to TLR agonists. Arthritis
Res Ther. 18:102016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wijngaarden S, van Roon JA, Bijlsma JW,
van de Winkel JG and Lafeber FP: Fcgamma receptor expression levels
on monocytes are elevated in rheumatoid arthritis patients with
high erythrocyte sedimentation rate who do not use anti-rheumatic
drugs. Rheumatology (Oxford). 42:681–688. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rossol M, Kraus S, Pierer M, Baerwald C
and Wagner U: The CD14brightCD16 monocyte subset is expanded in
rheumatoid arthritis and promotes expansion of the th17 cell
population. Arthritis Rheum. 64:671–677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bruhns P, Iannascoli B, England P,
Mancardi DA, Fernandez N, Jorieux S and Daëron M: Specificity and
affinity of human Fcgamma receptors and their polymorhic variants
for IgG subclasse. Blood. 113:3716–3725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu J, Marjon KD, Marnell LL, Wang R, Mold
C, Du Clos TW and Sun P: Recognition and functional activation of
the human IgA receptor (FcαRI) by C-reactive protein. Proc Natl
Acad Sci USA. 108:4974–4979. 2011. View Article : Google Scholar : PubMed/NCBI
|