Introduction
Ischemia/reperfusion (I/R) causes severe damage to
vital organs, including the brain, heart, lungs and kidneys
(1–4). I/R injury encompasses stroke, which is
characterized by a sudden focal or global neurological impairment
and is one of the most prevalent causes of mortality and disability
worldwide (5–7).
In principle, cerebral ischemia is caused by
cerebral blood flow reduction leading to a series of pathological
changes, including ionic homeostasis loss, energy failure,
increased oxidative stress, apoptosis, irreversible tissue/organ
damage and neurological and behavioral deficits (8,9). I/R
injury-induced damage is associated with dysregulation of complex
interactions, inflammatory responses and extracellular matrix (ECM)
remodeling (10). The ECM forms a
key component of the basement membrane, and is essential for the
permeability and integrity of blood-brain barrier (BBB), while
matrix metalloproteinases (MMPs) can exert proteolytic activity to
degrade all ECM components (1,11).
Abnormal degradation of ECM components in rats with
cerebral I/R injury reduces the integrity and permeability of the
BBB, which may lead to leakage of fluids from the blood to the
brain and subsequent brain edema (1). Type IV collagen is a key component of
the ECM complex and a major proteolytic substrate of MMP2 and MMP9
(1). Overexpression of MMP2 and MMP9
has previously been reported in I/R injured tissues, including the
heart, liver and kidneys (1,3,12).
Furthermore, breakdown of the BBB in rats may be induced by
upregulated proteolytic activity of MMP2 and MMP9, which
subsequently results in over-degradation of type IV collagen to
reduce BBB integrity and promote BBB permeability (1).
At present, typical pharmacological agents used to
treat cerebral I/R injury therapy are neuroprotective,
antioxidative, antiapoptotic or anti-inflammatory agents (13,14).
Previous studies have reported the protective effect of
morroniside, an active extract of Cornus officinalis,
against neuronal apoptosis, oxidative stress and inflammation
(8,15–17).
Furthermore, the neuroprotective effect of morroniside has been
reported in rats with focal cerebral ischemia (8). However, to the best of our knowledge,
the effect of morroniside on MMP2/9 expression and neuron apoptosis
in cerebral I/R injury has yet to be reported.
The aim of the present study was to elucidate the
effect of morroniside on MMP2/9 expression and neuron apoptosis in
rats with cerebral I/R injury. Rats with focal cerebral I/R injury
were administrated with morroniside (30, 90 and 270 mg/kg) for 7
days, following which MMP2/9 expression and neuronal apoptosis were
detected and comparatively analyzed.
Materials and methods
Animal model and treatment
A total of 50 adult male Sprague Dawley rats (age,
7–8 weeks; weight, 260–280 g) were purchased from Beijing Vital
River Laboratory Animal Technology Co., Ltd. (Beijing, China). All
rats were housed at 25±2°C and 50% humidity, with a 12-h light/dark
cycle, and food and water ad libitum. Ethical approval was
granted by the Medical Ethics Committee of the Affiliated Ganzhou
Hospital of Nanchang University (Nanchang, China).
Rats were randomly assigned into five groups (n=10
in each). Rats in the control group underwent sham surgery. All
other rats underwent suture-occluded surgery as described by Longa
et al (18), with a 0.26 mm
nylon monofilament (Beijing Shandong Biotech Co., Ltd., Beijing,
China) inserted through the right common carotid artery and were
divided into groups as follows: The cerebral I/R injury model group
(model), no treatment; low dose group, 30 mg/kg/day morroniside
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) by gavage;
moderate dose group, 90 mg/kg/day morroniside by gavage; high dose
group, 270 mg/kg/day morroniside by gavage. Rats in the control and
model groups received an equal volume of normal saline. Longa's
five-grade scale methods were used to score neurological deficit
following surgery (18) and rats
with a score of 0 or 4 were excluded from the current study.
Immunofluorescence assay
Following 7 days of morroniside treatment, rats were
anesthetized intravenously (30 mg/kg 5% pentobarbital sodium; Sigma
Aldrich; Merck KGaA, Darmstadt, Germany) and brains were harvested.
Brain tissues were mounted in optimal cutting temperature medium
(Sakura Finetek USA, Inc., Torrance, CA, USA), frozen at −20°C in a
Leica cryostat device (Leica Microsystems GmbH, Wetzlar, Germany)
and cut into 4-µm sections. Sections were treated with 3%
H2O2 solutions for 20 min at 37°C, blocked
with normal goat serum (Sigma Aldrich; Merck KGaA) for 30 min at
37°C, and incubated with anti-MMP2 (cat. no. sc-13594) and
anti-MMP9 (cat. no. sc-21733; 1:200; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) antibodies at 4°C for 20 h. Sections were
subsequently incubated with Cy3®-labelled goat
anti-mouse immunoglobulin (Ig)G (cat. no. ab97035; 1:100; Abcam,
Cambridge, MA, USA) in the dark at 37°C for 2 h. DAPI solution was
used for nuclear staining at 37°C for 10 min. Slides were observed
using a fluorescence microscope (magnification, ×200) and assessed
using Image-Pro Plus 6 software (Media Cybernetics, Inc.,
Rockville, MD USA).
Apoptotic cell analysis
Apoptotic cells in brain tissues were detected using
a TUNEL assay. Briefly, ischemic penumbra cortex area was resected
and the frozen sections (4-µm-thick) were fixed using 4%
polyoxymethylene at room temperature for 20 min. and and a
colorimetric TUNEL kit (EMD Millipore, Billerica, MA, USA) was used
according to the manufacturer's protocol for in situ
apoptosis detection. The sections were washed by PBS and then
incubated with prepared TUNEL solution for 1 h at 37°C in a dark
chamber. A 50 µl DAB solution (2.5 µl DAB, 0.5 µl 30%
H2O2 and 47 µl PBS) was added for nuclear
staining at 37°C for 20 min. The TUNEL-positive cells were observed
and counted under a light microscope at a magnification of ×200.
Apoptotic cells were stained brown and the mean was calculated from
10 independent fields.
Western blotting
Brain tissues were homogenized and lysed in
radioimmunoprecipitation assay buffer (Sigma Aldrich; Merck KGaA).
Proteins were quantified using BCA method and equal amounts (50 µg)
were separated by 8% SDS-PAGE. Proteins were transferred to a
polyvinylidene fluoride membrane, blocked with 5% non-fat milk at
37°C for 1 h, and then was subsequently incubated with specific
primary antibodies against MMP2 (cat. no. sc-13594), MMP9 (cat. no.
sc-21733), active Caspase-3 (cat. no. sc-56052), B-cell lymphoma 2
(Bcl-2; cat. no. sc-56015) and Bcl-2-associated X protein (Bax;
cat. no. sc-70407) and GAPDH (cat. no. sc-47724; all 1:1,000; Santa
Cruz Biotechnology, Inc.) at 4°C overnight. The membrane was
incubated with horseradish peroxidase-conjugated goat anti-rabbit
IgG (cat. no. sc-2004; 1:1,000, Santa Cruz Biotechnology, Inc.) for
2 h at 37°C followed by enhanced chemilluminescence reagents (GE
Healthcare Life Sciences, Little Chalfont, UK). Quantity One 4.4.1
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used
to quantify bands with GAPDH as an internal control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to measure the relative
expression of MMP2 and MMP9 mRNA. Total RNA was extracted from
brain tissues using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and RT was performed to synthesize cDNA.
RNA was converted into cDNA using a High Capacity cDNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. MMP2 and MMP9 mRNA
expression was detected on an ABI 7500 fast real time PCR platform
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using a SYBR
Premix Ex Taq™ kit (Takara Bio, Inc., Otsu, Japan) and the
following primers: MMP2, forward 5′-CTGGGCCACGCCATCGCTGC-3 and
reverse 5′-GCTTGCGGGGAAAGAAGTTG-3′; MMP9, forward
5′-GGCAGCCCCTGCTCCTGGTG-3′ and reverse 5′-CCTTTAGTGTCTCGCTGTCC-3′;
GAPDH, forward 5′-CTCCTCCTGGCCTCGCTGT-3′ and reverse
5′-GCTGTCACCTTCACCGTTCC-3′. Temperature protocols were as follows:
95°C for 4 min followed by 40 cycles of 95°C for 30 sec, 60°C for
35 sec and 72°C for 20 sec and a final extension step at 65°C for 6
min. The relative mRNA expression level was analyzed using the
2−∆∆Cq method with GAPDH as the internal reference gene
(19). Each reaction was run in
triplicate.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. SPSS 17.0 software was used for statistical analyses
(SPSS, Inc., Chicago, IL, USA). One-way analysis of variance
followed by Scheffe's test was used to assess differences between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Morroniside reduces the expression of
MMP2 and MMP9 in an I/R injury model
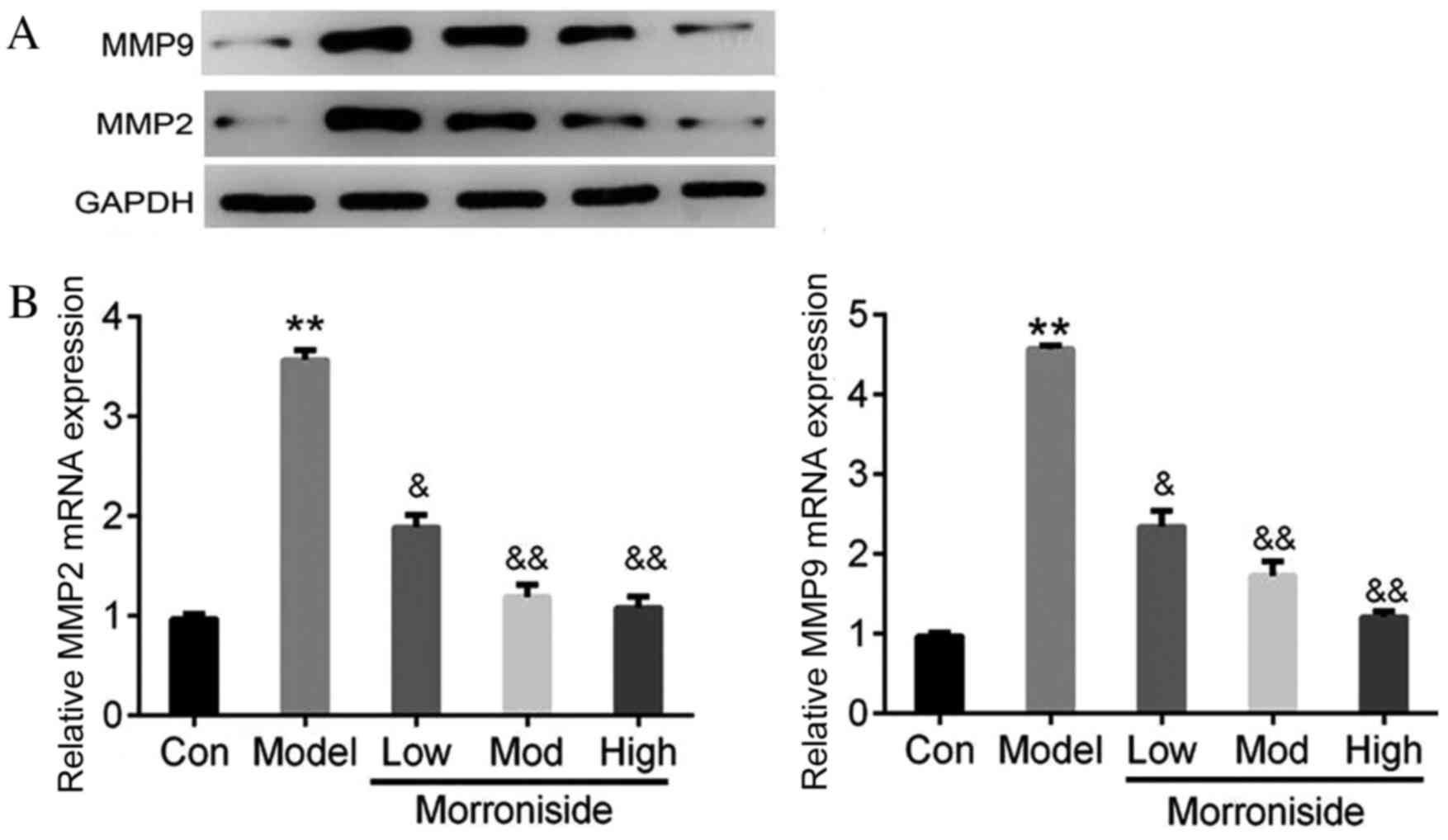
The results of western blotting and RT-qPCR revealed
that MMP2 and MMP9 protein (Fig. 1A)
and mRNA (P<0.01; Fig. 1B)
expression was increased in the model group compared with the
control group. However, treatment with morroniside reduced the
expression of MMP2 and MMP9 mRNA (Fig.
1A) and protein (low dose, P<0.05; moderate and high doses,
P<0.01; Fig. 1B) in a dose
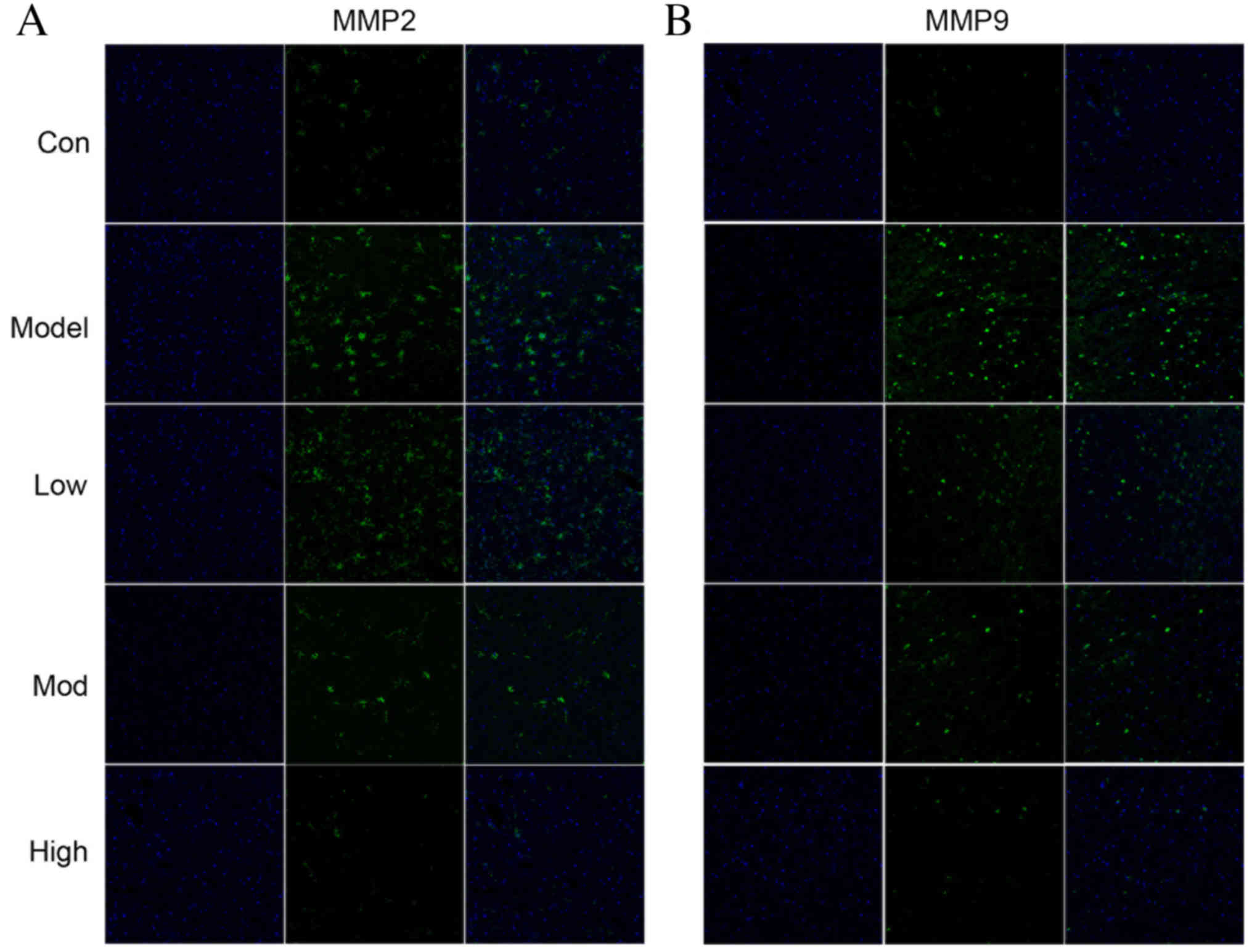
dependent manner compared with the model group. Immunofluorescence
results revealed that the pattern of MMP2 and MMP9 positive cells
in cortical infarctions followed a similar trend to protein
expression (Fig. 2). The MMP2 and
MMP9 fluorescence intensity in the brains of rats was increased in
the model group compared with the control (Fig. 2). However, treatment with morroniside
markedly reduced MMP2 and MMP9 fluorescence compared with the model
group (Fig. 2).
Morroniside inhibits neuron
apoptosis
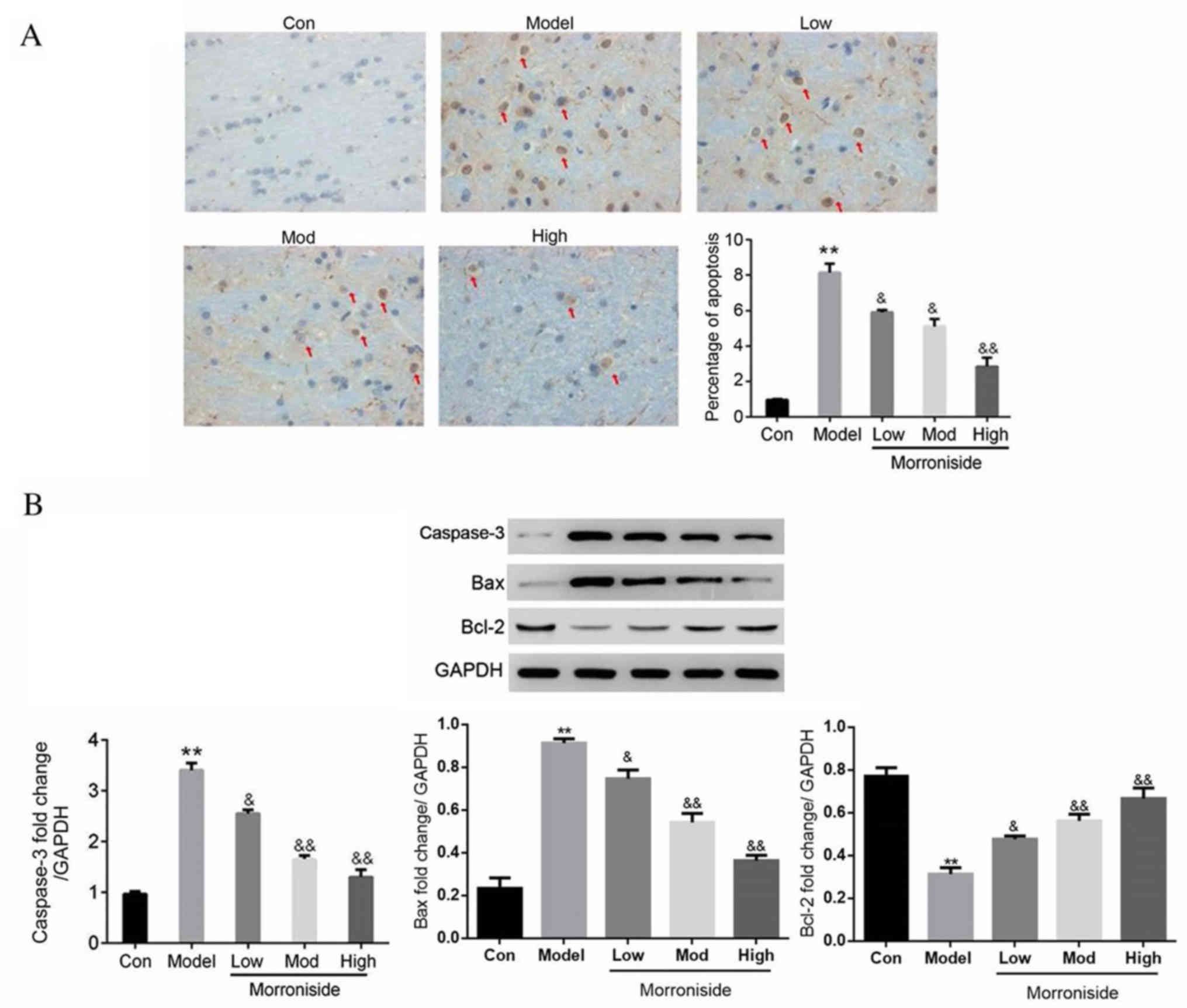
Apoptotic neurons on the sections from ischemic
penumbra cortex were detected using a TUNEL assay. The percentage
of apoptotic neurons was significantly increased in the model group
compared with the control (P<0.01; Fig. 3A). However, treatment with
morroniside significantly reduced I/R-associated neuron apoptosis
in a dose dependent manner (low and moderate doses, P<0.05; high
dose, P<0.01; Fig. 3A). The
expression of apoptosis-associated proteins was assessed using
western blotting. The results demonstrated that active caspase-3
and Bax were significantly upregulated in the model group compared
with the control group (P<0.01; Fig.
3B), while Bcl-2 was significantly downregulated (P<0.01;
Fig. 3B). The expression of active
caspase-3 and Bax was significantly downregulated by morroniside
treatment in a dose-dependent manner (low dose, P<0.05; moderate
and high doses, P<0.01; Fig. 3B),
while the expression of Bcl-2 was significantly upregulated (low
dose, P<0.05; moderate and high doses, P<0.01; Fig. 3B).
Discussion
The upregulation of MMPs, in particular MMP2 and
MMP9, results in the degradation of type IV collagen, which in turn
impairs BBB integrity and enhances I/R injuries in the brain
(1). The present study is the first
to demonstrate the effect of morroniside treatment on MMP2/9
expression and neuron apoptosis and in I/R. The results revealed
that MMP2 and MMP9 are upregulated in the brains of rats with
cerebral I/R injury, whereas the administration of morroniside
significantly ameliorated this effect. These results suggest that
morroniside may convey a protective effect in the brains of rats
with I/R injury.
Increased MMP2 and MMP9 expression has been reported
in I/R injuries of the lung, heart, skeletal muscle, kidneys and
other organs (1–4). Previous studies have revealed that
MMP2/9 overexpression disrupts the BBB and results in edema
(20,21). It has also been reported that the
inhibition of MMP2/9 may protect against cerebral ischemia and
improve organ function (12,22–25).
However, MMP2 knockout does not protect against acute focal
ischemia injury in brain (26). Kato
et al (10) demonstrated that
MMP2 deletion was able to impair poly ADP-ribose polymerase 1
degradation, enhance MMP9 activity and exacerbate hepatic I/R
injury in mice (10). It has also
been reported that whereas MMP9 deficiency may protect against
hepatic I/R injury (12). Together,
these reports suggest that effect of MMP2/9 in I/R injury is
complex and, while MMP2/9 inhibition may be beneficial, it is not
necessarily required to improve I/R injury. However, the results of
the present study demonstrated that morroniside administration
inhibits MMP2 and MMP9 in I/R injured brains, revealing morroniside
was beneficial to protect against cerebral I/R injury.
The results of the present study revealed that
morroniside significantly reduces the percentage of apoptotic
neurons, inhibits caspase-3 activation and increases the Bcl-2/Bax
protein ratio in rats with cerebral I/R injury. Cerebral I/R injury
results in neurological and behavioral deficits, which are
accompanied by neuron apoptosis (9,27,28).
Wang et al (16) reported
that morroniside was able to reduce
H2O2-induced SH-SY5Y cell apoptosis,
intracellular Ca2+ accumulation and mitochondrial
membrane potential, while also inhibiting the
H2O2-induced decrease in superoxide dismutase
activity (16). The protective
effect of morroniside on cell apoptosis and other diseases has been
widely reported (8,29,30). The
results of the present study suggest that morroniside treatment has
a protective effect against I/R-induced neuron apoptosis; future
studies should investigate the effect of MMP2 or MMP9 in rats with
I/R injury to confirm the mechanisms of morroniside.
In conclusion, the present study revealed that
protective effect of morroniside in rats with cerebral I/R injury.
Morroniside inhibits the I/R-induced upregulation of MMP2/9 and
neuron apoptosis, suggesting that morroniside may promote
behavioral deficit repair following cerebral I/R injury. However,
the underlying mechanism responsible for these effects remains to
be elucidated.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GZ wrote the manuscript and conducted the majority
of the experiments. WD, YL and MS collected and analyzed the data.
LD designed the study and approved the manuscript for
submission.
Ethics approval and consent to
participate
Ethical approval was granted by the Medical Ethics
Committee of the Affiliated Ganzhou Hospital of Nanchang University
(Nanchang, China).
Consent for publication
Not applicable.
Competing interests
All of the authors have no conflict of interest in
this research.
References
|
1
|
Roach DM, Fitridge RA, Laws PE, Millard
SH, Varelias A and Cowled PA: Up-regulation of MMP-2 and MMP-9
leads to degradation of type IV collagen during skeletal muscle
reperfusion injury; protection by the MMP inhibitor, doxycycline.
Eur J Vasc Endovasc Surg. 23:260–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yano M, Omoto Y, Yamakawa Y, Nakashima Y,
Kiriyama M, Saito Y and Fujii Y: Increased matrix metalloproteinase
9 activity and mRNA expression in lung ischemia-reperfusion injury.
J Heart Lung Transplant. 20:679–686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheung PY, Sawicki G, Wozniak M, Wang W,
Radomski MW and Schulz R: Matrix metalloproteinase-2 contributes to
ischemia-reperfusion injury in the heart. Circulation.
101:1833–1839. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang X, Sakamoto T, Hata Y, Kubota T,
Hisatomi T, Murata T, Ishibashi T and Inomata H: Expression of
matrix metalloproteinases and their inhibitors in experimental
retinal ischemia-reperfusion injury in rats. Exp Eye Res.
74:577–584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Navarro-Sobrino M, Rosell A,
Hernández-Guillamon M, Penalba A, Boada C, Domingues-Montanari S,
Ribó M, Alvarez-Sabín J and Montaner J: A large screening of
angiogenesis biomarkers and their association with neurological
outcome after ischemic stroke. Atherosclerosis. 216:205–211. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feigin VL, Forouzanfar MH, Krishnamurthi
R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L,
Truelsen T, et al: Global and regional burden of stroke during
1990–2010: Findings from the Global burden of disease study 2010.
Lancet. 383:245–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen L, Xiang Y, Kong L, Zhang X, Sun B,
Wei X and Liu H: Hydroxysafflor yellow A protects against cerebral
ischemia-reperfusion injury by anti-apoptotic effect through
PI3K/Akt/GSK3β pathway in rat. Neurochem Res. 38:2268–2275. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang W, Xu J and Li L, Wang P, Ji X, Ai H,
Zhang L and Li L: Neuroprotective effect of morroniside on focal
cerebral ischemia in rats. Brain Res Bull. 83:196–201. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Q, Sun AY, Simonyi A, Jensen MD,
Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE,
Weisman GA and Sun GY: Neuroprotective mechanisms of curcumin
against cerebral ischemia-induced neuronal apoptosis and behavioral
deficits. J Neurosci Res. 82:138–148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato H, Duarte S, Liu D, Busuttil RW and
Coito AJ: Matrix metalloproteinase-2 (MMP-2) gene deletion enhances
MMP-9 activity, impairs PARP-1 degradation, and exacerbates hepatic
ischemia and reperfusion injury in mice. PLoS One. 10:e01376422015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosenberg GA, Estrada E, Kelley RO and
Kornfeld M: Bacterial collagenase disrupts extracellular matrix and
opens blood-brain barrier in rat. Neurosci Lett. 160:117–119. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hamada T, Fondevila C, Busuttil RW and
Coito AJ: Metalloproteinase-9 deficiency protects against hepatic
ischemia/reperfusion injury. Hepatology. 47:186–198. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ai J, Wan H, Shu M, Zhou H, Zhao T, Fu W
and He Y: Guhong injection protects against focal cerebral
ischemia-reperfusion injury via anti-inflammatory effects in rats.
Arch Pharm Res. 40:610–622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao P, Zhou R, Zhu XY, Hao YJ, Li N, Wang
J, Niu Y, Sun T, Li YX and Yu JQ: Matrine attenuates focal cerebral
ischemic injury by improving antioxidant activity and inhibiting
apoptosis in mice. Int J Mol Med. 36:633–644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu HQ, Hao HP, Zhang X and Pan Y:
Morroniside protects cultured human umbilical vein endothelial
cells from damage by high ambient glucose. Acta Pharmacol Sin.
25:412–415. 2004.PubMed/NCBI
|
|
16
|
Wang W, Sun F, An Y, Ai H, Zhang L, Huang
W and Li L: Morroniside protects human neuroblastoma SH-SY5Y cells
against hydrogen peroxide-induced cytotoxicity. Eur J Pharmacol.
613:19–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park CH, Noh JS, Kim JH, Tanaka T, Zhao Q,
Matsumoto K, Shibahara N and Yokozawa T: Evaluation of morroniside,
iridoid glycoside from Corni Fructus, on diabetes-induced
alterations such as oxidative stress, inflammation, and apoptosis
in the liver of type 2 diabetic db/db mice. Biol Pharm Bull.
34:1559–1565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pérez-Hernández M, Fernández-Valle ME,
Rubio-Araiz A, Vidal R, Gutiérrez-López MD, O'shea E and Colado MI:
3,4-Methylenedioxymethamphetamine (MDMA, ecstasy) produces edema
due to BBB disruption induced by MMP-9 activation in rat
hippocampus. Neuropharmacology. 118:157–166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tasaki A, Shimizu F, Sano Y, Fujisawa M,
Takahashi T, Haruki H, Abe M, Koga M and Kanda T: Autocrine MMP-2/9
secretion increases the BBB permeability in neuromyelitis optica. J
Neurol Neurosurg Psychiatry. 85:419–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin HB, Cadete VJ, Sra B, Sawicka J, Chen
Z, Bekar LK, Cayabyab F and Sawicki G: Inhibition of MMP-2
expression with siRNA increases baseline cardiomyocyte
contractility and protects against simulated ischemic reperfusion
injury. Biomed Res Int. 2014:8103712014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan P, Chen KJ, Wu J, Sun L, Sung HW,
Weisel RD, Xie J and Li RK: The use of MMP2 antibody-conjugated
cationic microbubble to target the ischemic myocardium, enhance
Timp3 gene transfection and improve cardiac function. Biomaterials.
35:1063–1073. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin H, Sra B, Cadete VJ, Sawicka J,
Cayabyab FS and Sawicki G: 774 MMP-2 siRNA Protects Against
Contractile Dysfunction in Cardiomyocytes Subjected to
Ischemia/Reperfusion. Canadian J Cardiology. 28:S396–S397. 2012.
View Article : Google Scholar
|
|
25
|
Zhang HT, Zhang P, Gao Y, Li CL, Wang HJ,
Chen LC, Feng Y, Li RY, Li YL and Jiang CL: Early VEGF inhibition
attenuates blood-brain barrier disruption in ischemic rat brains by
regulating the expression of MMPs. Mol Med Rep. 15:57–64. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Asahi M, Sumii T, Fini ME, Itohara S and
Lo EH: Matrix metalloproteinase 2 gene knockout has no effect on
acute brain injury after focal ischemia. Neuroreport. 12:3003–3007.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hua F, Ma J, Ha T, Xia Y, Kelley J,
Williams DL, Kao RL, Browder IW, Schweitzer JB, Kalbfleisch JH and
Li C: Activation of toll-like receptor 4 signaling contributes to
hippocampal neuronal death following global cerebral
ischemia/reperfusion. J Neuroimmunol. 190:101–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang K, Ye Y, Wang Y, Zhang J and Li C:
Formononetin mediates neuroprotection against cerebral
ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2
ratio and upregulation PI3K/Akt signaling pathway. J Neurol Sci.
344:100–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Huang W, Li L, Ai H, Sun F, Liu C
and An Y: Morroniside prevents peroxide-induced apoptosis by
induction of endogenous glutathione in human neuroblastoma cells.
Cell Mol Neurobiol. 28:293–305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yokozawa T, Yamabe N, Kim HY, Kang KS, Hur
JM, Park CH and Tanaka T: Protective effects of morroniside
isolated from Corni Fructus against renal damage in
streptozotocin-induced diabetic rats. Biol Pharm Bull.
31:1422–1428. 2008. View Article : Google Scholar : PubMed/NCBI
|