Introduction
Liver resection remains the treatment of choice for
primary liver cancer. However, patients with cirrhosis and portal
hypertension may develop portal hyperperfusion (PHP) following
major hepatectomy, which may lead to post-hepatectomy liver failure
(PHLF) (1–4). In literature, the reported mortality of
posthepatectomy liver failure is <5% and morbidity is 15-30%.
Around 3-8% of patients develop liver failure after major
hepatectomy (5,6). The elevated hepatic sinusoidal pressure
caused by PHP may damage hepatocytes and sinusoidal endothelial
(SE) cells and eventually impede liver regeneration (3,4). It was
hypothesized that artificially decreasing the portal flow following
major hepatectomy may alleviate the damage caused by PHP (2,7). The
liver receives portal as well as arterial blood, and is modulated
by the hepatic arterial buffer response, as the increase of portal
blood flow may cause a decrease in hepatic arterial blood flow and
vice versa (8,9). With PHP, the hepatic arterial blood
flow may decrease, leading to decreased oxygen supply, which may
cause further damage to the future liver remnant (FLR). Therefore,
the hepatic arterial blood flow may also be modulated to increase
the oxygen supply in order to protect and promote the regeneration
of the FLR. However, studies on the damage of PHP to the liver and
its effects on liver regeneration are based on living donor liver
transplantation and defined as ‘small-for-size’ syndrome, rather
than on major hepatectomy for patients with cirrhosis. A
considerable proportion of primary liver cancer patients have
concurrent cirrhosis and portal hypertension; furthermore, hepatic
hemodynamics is complex and major hepatectomy may further
complicate this condition. In addition, there are more cases of
major hepatectomy compared with living donor liver transplantation.
In order to mimic the clinical conditions, a rat model of cirrhosis
was established in this study, followed by major hepatectomy and
stenosis of the portal vein (PV) and/or hepatic artery (HA) to
establish different hepatic inflow volumes, and to evaluate the
effect of hepatic inflow on liver ultrastructure, function and
regeneration.
Materials and methods
Reagents and instruments
Experimental reagents
Anhydrous ethanol, xylene, hydrochloric acid,
embedding paraffin, neutral gum, 2.5% glutaraldehyde fixative,
Masson staining reagents, and other related chemical reagents were
purchased from Sinopharm Group (Shanghai, China); PAS staining kit
was purchased from Wuhan Servicebio Company (Wuhan, China).
CCL4 was purchased from Suzhou Second Chemical Research
Institute (Suzhou, China); hematoxylin was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Experimental instruments
The RM 2016 Microtome was obtained from Leica
Microsystems GmbH (Wetzlar, Germany); the automatic biochemical
analyzer was purchased from Beckman Coulter, Inc., (Brea, CA, USA);
the CX31 Upright Microscope was purchased from Olympus Corporation,
(Tokyo, Japan); the Laser Speckle Imager was from Perimed AB
(Järfälla, Sweden); and the transmission electron microscope was
obtained from JEOL, Ltd., (Tokyo, Japan).
Experimental protocol
Establishment of rat model of
cirrhosis
A total of 50 male Sprague-Dawley rats, aged ~8
weeks and weighing 300-320 g, were purchased from Zhaoyan New Drug
Research Center Co., Ltd., (Suzhou, China) (animal quality
certificate: SCXK 2013-0003). The animals were treated humanely
following the Institutional Guidelines for Animal Use and Care at
the Institute of Zoology, the Chinese Academy of Sciences. The
present study also complied with the management rules of the
National Health and Family Planning Commission of China and was
approved by the Ethics Committee of Zhejiang Cancer Hospital. All
animals were kept clean and in sanitary conditions, with the room
temperature maintained at 20±2°C, relative humidity at 65±10%, in a
diurnal cycle. The animals were randomly assigned to
CCL4-treated (model, n=43) and
non-CCL4-treated (n=7) groups. The process of
establishment of the cirrhosis model was divided into induction and
modeling stages. During the induction stage, the rats were fed a
normal diet and a solution of 0.35 g phenobarbital sodium per liter
of distilled water was administered as the only drinking water for
1 week. During the modeling stage, 40% CCL4 neutral
rapeseed oil solution was injected intraperitoneally (i.p.) at a
dose of 0.5 ml/100 g twice a week for 4 consecutive weeks; 10%
ethanol solution was given as the only drinking water. From the
fifth week, 50% CCL4 neutral rapeseed oil solution was
injected i.p. at a dose of 0.5 ml/100 g at the same frequency for 4
consecutive weeks; 30% ethanol solution was given as the only
drinking water until the end of the eighth week. In the
non-CCL4-treated group, saline at a dose of 0.3 ml/100 g
body weight was injected i.p. twice weekly, and the animals were
given ordinary drinking water and food.
Liver resection + hepatic inflow
control during the first laparotomy
Rats with liver cirrhosis were fasted for 12 h with
access to water ad libitum. After being anesthetized with i.p.
injection of 0.3% sodium pentobarbital (40 mg/kg), the rats
underwent laparotomy by a middle incision in the upper abdomen. The
liver's texture, PV system and possible presence of ascites were
evaluated; the left lateral lobe and left medial lobe of the liver
(accounting for ~45% of the total liver volume) were removed
(10). The liver specimens of the
non-CCL4-treated group were collected for pathological
analysis. After liver resection, different hepatic blood inflow was
established for the CCL4-treated group and these animals
were assigned to different groups (n=7 per group) as follows:
Group A: Control group, no blood inflow
intervention.
Group B: Low-flow PV + high-flow HA group (the PV
was narrowed and the splenic artery was ligated).
Group C: Low-flow PV + low-flow HA group (the PV and
the HA were narrowed).
Group D: High-flow PV + high-flow HA group (the
splenic artery was ligated).
Group E: High-flow PV + low-flow HA groups (the HA
was narrowed).
The procedure undertaken to narrow the PV was as
follows: The PV was exposed, a no. 8 needle was placed in parallel
with the main PV trunk, the PV was double-ligated and then the
needle was withdrawn, ensuring that the diameter of the narrowed PV
was approximately equal to that of the needle. Intrahepatic blood
flow was measured by laser speckle contrast analysis (LASCA) to
ensure a 30% decrease compared to the data after major hepatectomy.
To narrow the HA, an insulin needle was used, with the same method
applied as for narrowing the PV. To establish high flow to the HA,
the splenic artery was ligated.
Liver function tests
Serum was collected before and after establishment
of the cirrhosis model, and at 1, 3 and 5 days after hepatic inflow
regulation and liver resection, and was analyzed by an automatic
biochemical analyzer.
Measurement of the liver blood flow
with LASCA
During the first surgery, the data of liver blood
flow were collected at three different time points: Before
hepatectomy, after hepatectomy and after hepatectomy + blood flow
regulation. At 5 days after the first surgery, the second
laparotomy was performed and the data of liver blood flow were
again collected. Of note, due to the need to excise the left
lateral and left medial lobes of the liver, the LASCA probe must be
aimed at the right lateral and right medial lobes of the liver
(FLR) to measure the blood flow changes throughout the experiment
(11).
Weight of FLR
After the liver blood flow measurement in the second
laparotomy, the animals were sacrificed humanely and the whole
liver was harvested and weighed after removal of excessive fluid
and surrounding tissues.
Pathological examination
The liver specimens were collected at different time
points and treated as follows: Liver tissue was placed in 10%
formaldehyde for 48 h, rinsed with water, and dehydrated using
different concentrations of ethanol, followed by embedding in
paraffin, sectioning, heating and dewaxing. The sections were
stained with hematoxylin and eosin (H&E) for morphological
evaluation and Masson trichrome (MT) to assess the degree of
fibrosis. PAS staining was performed following the manufacturer's
protocol and the sample was collected and examined.
Evaluation of liver cell
ultrastructure
The samples were fixed in 2.5% glutaraldehyde,
washed and postfixed in 1% osmium tetroxide, followed by
dehydration in a series of ethanols and propylene oxide. The
tissues were then embedded in an epoxy resin. Ultrathin sections
were cut and mounted on a grid, and the grid was stained with
uranyl acetate and lead acetate and observed under a TEM.
Ki-67 expression analysis
Liver tissue was collected and fixed in
formaldehyde, washed and dehydrated using graded ethanols, embedded
in paraffin, sectioned, and subjected to antigen retrieval and
endogenous peroxidase activity inhibition. The primary antibody was
prepared with a dilution ratio of 1:100. Each section was incubated
with the primary antibody at 4°C, rinsed 3 times with
phosphate-buffered saline (PBS) for 5 min each time, followed by
the addition of secondary antibody working fluid and incubation at
room temperature in a wet box for 10-15 min. The secondary antibody
was then discarded, and the sections were rinsed 3 times with PBS
for 5 min each time; each section was placed in the wet box with
streptomycin-avidin peroxidase solution at room temperature for
10-15 min, and then the working fluid was discarded and the
sections were rinsed with PBS 3 times for 5 min each time, followed
by DAB dye staining, hematoxylin re-staining, alcohol dehydration,
xylene transparentization and other steps. The final sample was
observed under the microscope.
Statistical analysis
SPSS v.19.0 statistical software (SPSS Inc.,
Chicago, IL, USA) was used for data analysis. All data are
expressed as mean ± standard deviation (SD). One-way analysis of
variance followed by the Fisher's least significant difference test
was used to compare two different groups. P<0.05 was considered
to indicate statistically significant differences which were
plotted with GraphPad Prism v.6 software (GraphPad, Software, Inc.,
La Jolla, CA, USA).
Results
General observations
In the CCL4-treated group, 3 rats died
during the modeling process due to acute liver injury and liver
failure. Compared with the non-CCL4-treated group, all
animals in the CCL4-treated group were in low spirits
and exhibited a lower rate of body weight gain (data not shown). A
total of 5 rats died following major hepatectomy due to bleeding
(n=3) and PHLF (n=2). The livers of the CCL4-treated
group developed moderate cirrhosis, with formation of a few
portosystemic collateral vessels; no obvious ascites was
observed.
Pathological identification of
cirrhosis
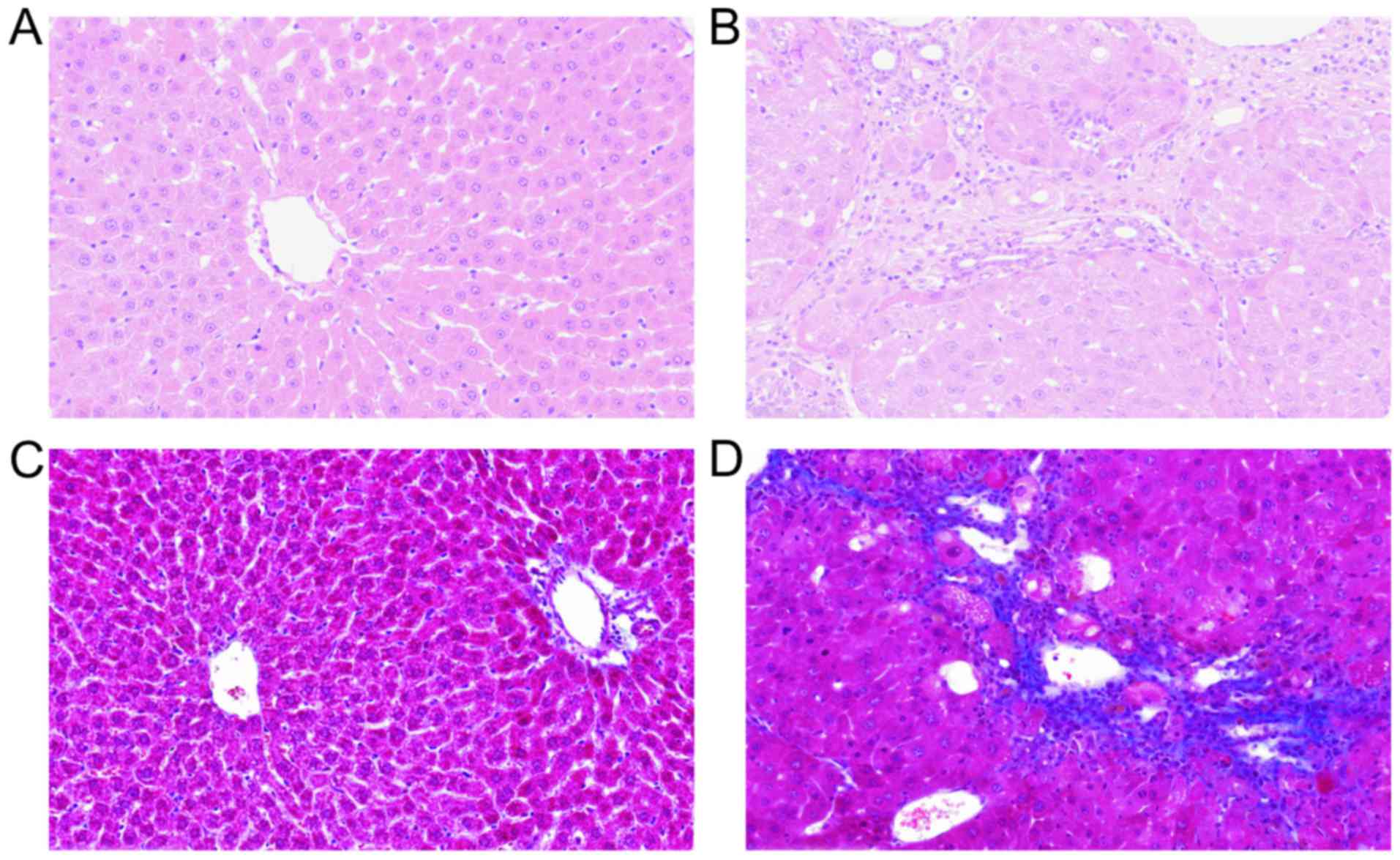
As shown in Fig. 1,
the pathological examination revealed disrupted liver architecture,
with collagen deposition, diffuse fibrosis and formation of false
lobules after the establishment of the model.
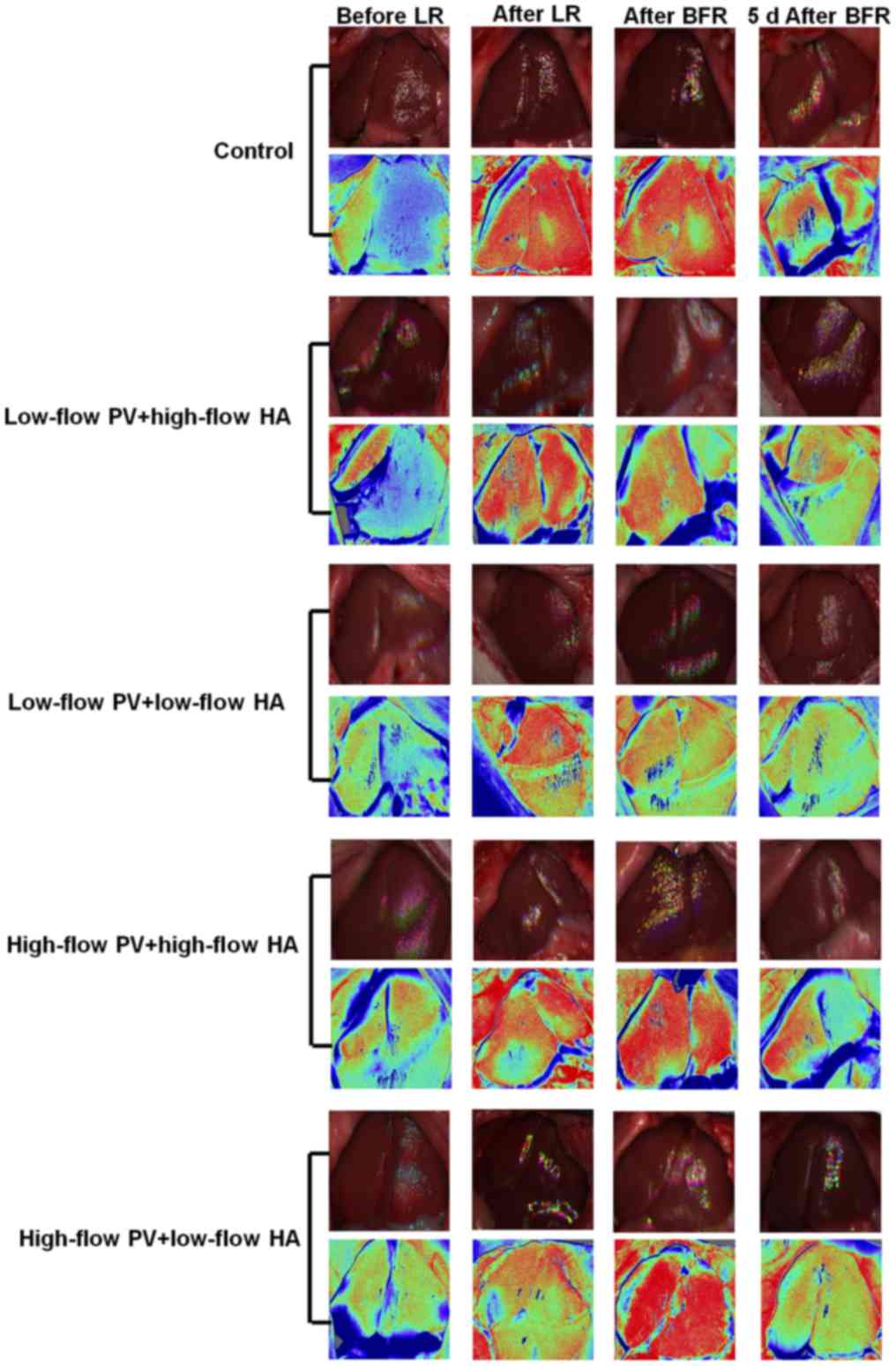
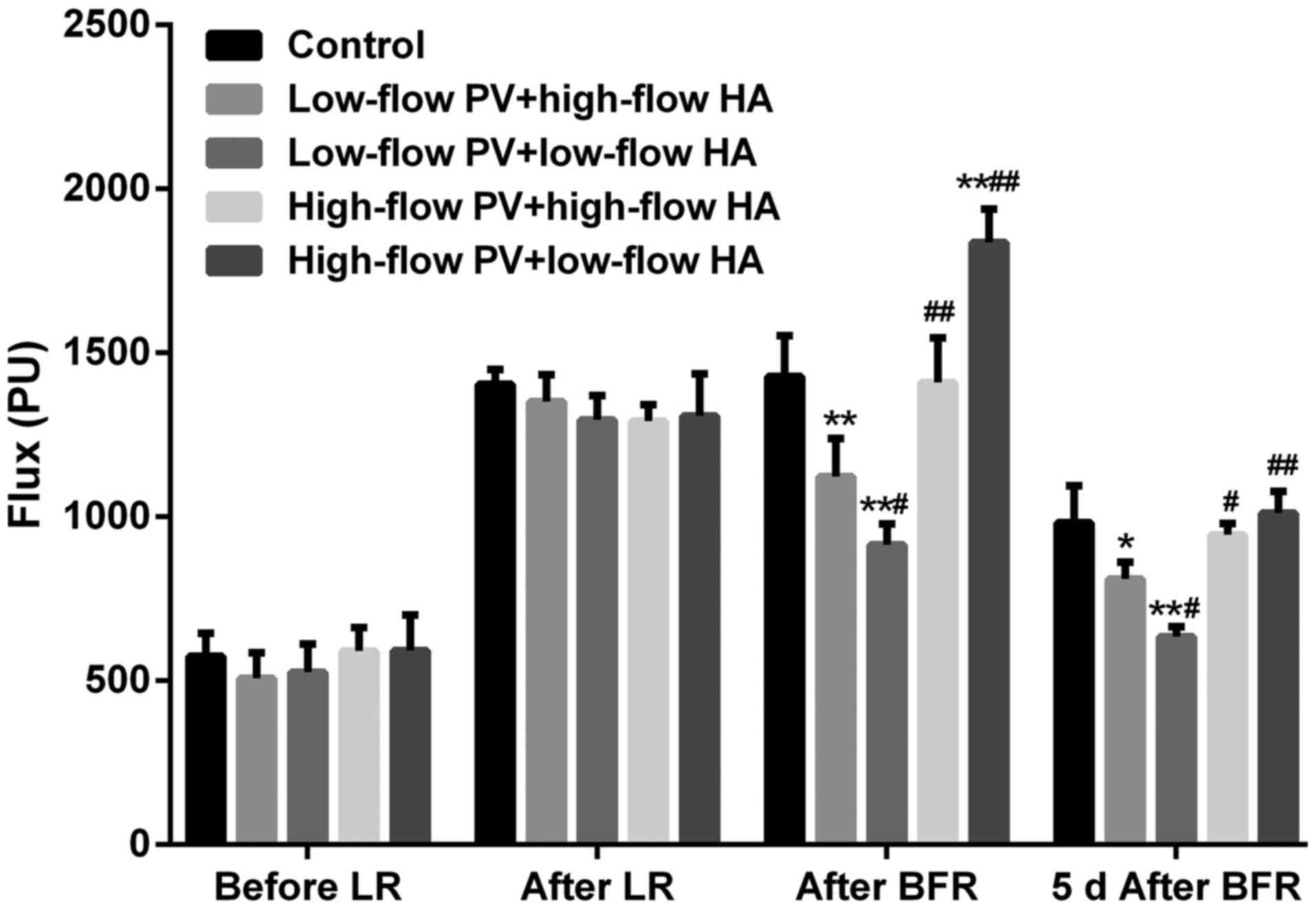
Hepatic hemodynamics
There were no significant differences in the
intrahepatic blood flow among groups prior to liver resection.
After major hepatectomy, the blood flow of the remnant liver in all
groups increased significantly (compared with that prior to
hepatectomy, P<0.05). After major liver resection + hepatic
inflow regulation, the intrahepatic blood flow in groups B and C
was significantly reduced (compared with groups A, D and E,
P<0.05). The intrahepatic blood flow in groups D and E was
increased to a different degree (compared with groups A, B and C,
P<0.05). At 5 days after liver resection + hepatic inflow
regulation, the aforementioned changes remained, but the
differences were less prominent (Figs.
2 and 3).
Liver function
The serum levels of alanine aminotransferase (ALT),
aspartate aminotransferase (AST) and total bilirubin (TBIL) of
CCL4-treated group increased gradually during the
modeling process (Table I). These
liver function index of each group increased significantly after
hepatectomy + hepatic inflow regulation. The postoperative serum
levels of ALT, AST and TBIL in groups B and C were significantly
lower compared with those in the control group and groups D and E
(P<0.05; Table II). The albumin
levels remained stable throughout the experiment.
 | Table I.Liver function changes during the
establishment of rat cirrhosis model (n=7, each group). |
Table I.
Liver function changes during the
establishment of rat cirrhosis model (n=7, each group).
|
| ALT (U/l) | AST (U/l) | ALB (g/l) | TBiL (µmol/l) |
|---|
|
|
|
|
|
|
|---|
|
| 0 week | 8 week | 10 week | 0 week | 8 week | 10 week | 0 week | 8 week | 10 week | 0 week | 8 week | 10 week |
|---|
|
Non-CCL4-treated | 38.9±2.1 | 37.7±2.1 | 35.7±7.3 | 116.4±6.0 | 113.3±8.7 | 115.6±7.3 | 30.2±1.3 | 29.4±1.6 | 30.1±2.4 | 0.59±0.2 | 0.7±0.2 | 0.7±0.3 |
| CCL4-treated | 38.3±2.6 | 132.1±7.5 | 245.3±22.8 | 116.9±8.2 | 327.0±11.8 | 1056.3±109.6 | 29.9±0.8 | 30.4±1.9 | 29.4±1.8 | 0.53±0.2 | 6.1±1.2 | 22.0±4.8 |
| P-value | =0.66 | <0.01 | <0.01 | =0.91 | <0.01 | <0.01 | =0.61 | =0.29 | =0.53 | =0.55 | <0.01 | <0.01 |
 | Table II.Liver function changes following
major hepatectomy and hepatic blood inflow regulation (n=7, each
group). |
Table II.
Liver function changes following
major hepatectomy and hepatic blood inflow regulation (n=7, each
group).
|
| ALT (U/l) | AST (U/l) | ALB (g/l) | TBiL (µmol/l) |
|---|
|
|
|
|
|
|
|---|
| Group | 1 day | 3 day | 5 day | 1 day | 3 day | 5 day | 1 day | 3 day | 5 day | 1 day | 3 day | 5 day |
|---|
| A | 676.9±41.7 | 574.9±28.0 | 436.1±32.7 | 1141±83 | 941±28 | 789±30 | 28.6±2.4 | 27.6±2.2 | 27.1±1.9 | 30.3±1.4 | 26.5±0.8 | 22.1±1.2 |
| B | 460.9±31.7 | 331.0±22.0 | 285.6±15.8 | 791±27 | 662±33 | 552±24 | 27.7±1.1 | 29.0±1.6 | 28.1±2.6 | 20.4±1.5 | 16.1±1.0 | 13.5±0.6 |
| C | 551.0±33.0 | 429.9±28.5 | 359.1±22.9 | 937±56 | 809±36 | 648±38 | 28.3±1.6 | 28.41.9± | 29.0±1.6 | 22.9±1.5 | 22.6±0.8 | 17.9±1.3 |
| D | 674.7±27.6 | 588.9±22.6 | 454.0±18.5 | 1156±61 | 937±27 | 803±28 | 29.1±2.4 | 27.8±1.8 | 28.9±2.3 | 29.6±1.6 | 27.5±0.9 | 23.3±0.9 |
| E | 857.0±26.8 | 727.6±29.8 | 579.1±38.2 | 1438±73 | 1175±59 | 931±44 | 29.1±1.1 | 29.2±2.6 | 28.8±1.2 | 38.0±1.7 | 32.8±1.7 | 28.8±1.7 |
|
P-valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | >0.05 | >0.05 | >0.05 | <0.05 | <0.001 | <0.001 |
| P-valueb | 0.925 | 0.379 | 0.193 | 0.740 | 0.603 | 0.398 | 0.720 | 0.831 | 0.141 | 0.358 | 0.101 | 0.050 |
Weight of liver remnant
On postoperative day 5, after the hepatic blood flow
examination, the rats were sacrificed and the residual liver was
collected for weighing. The weight of the FLR was: Group A:
11.8±0.7 g, group B: 15.4±1.0 g, group C: 13.1±1.2 g, group D:
11.8±0.6 g, group E: 10.9±1.0 g, respectively. The results
demonstrated that the residual liver weight in the low-flow PV
groups was significantly higher compared with that in the control
group and the high-flow PV groups (P<0.05).
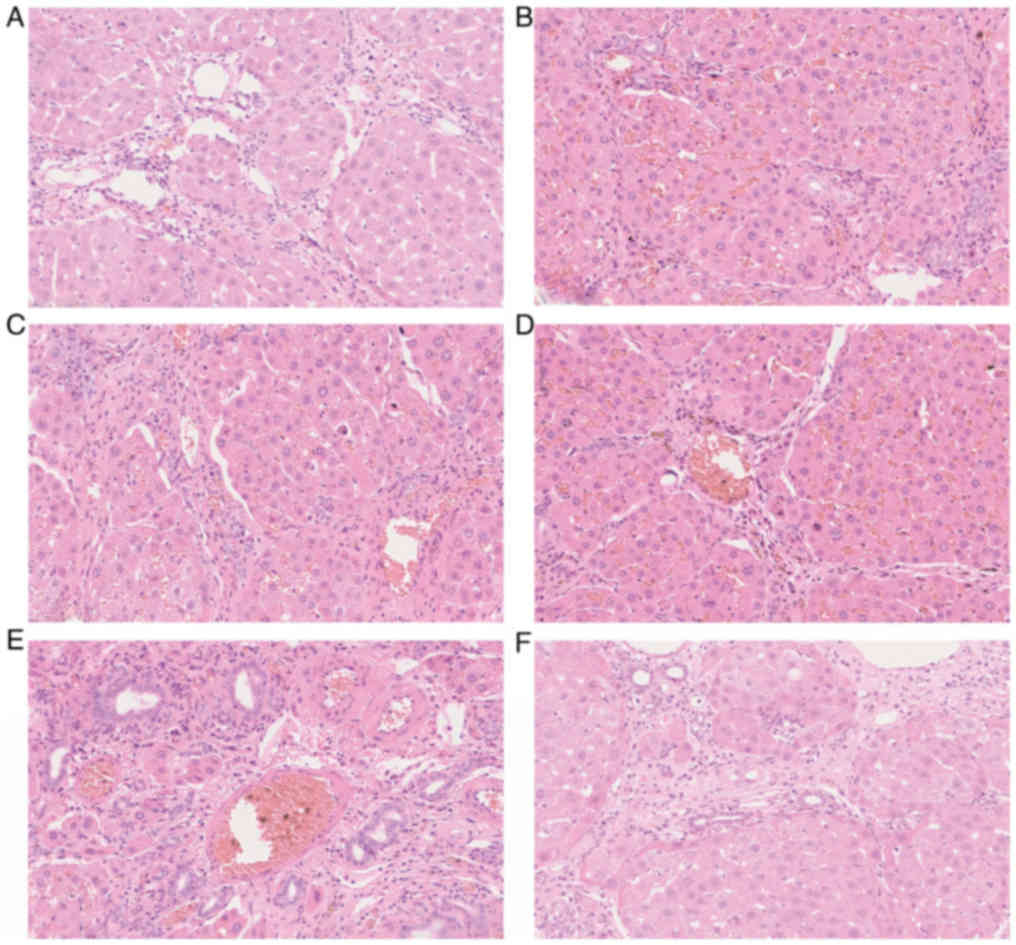
Liver pathological examination and
ultrastructure examination
Routine HE staining of the remnant liver tissues
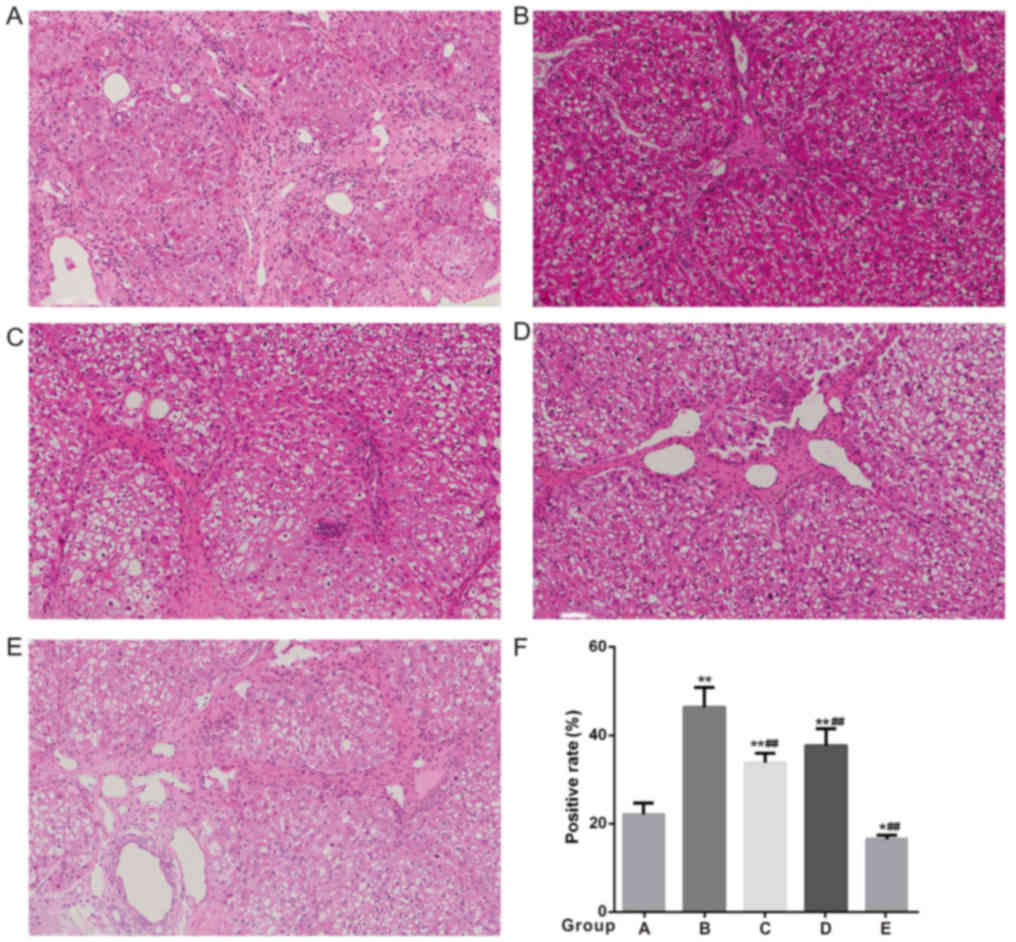
revealed the congestion was improved in the low-flow PV groups (B
and C), although it was still obvious in group B, and the hepatic
sinus structure and liver cell damage were significantly improved
compared with the high-flow PV groups (Fig. 4). The PAS staining of the remnant
liver tissues revealed that the accumulation of glycogen was
increased significantly in group B and group C (Fig. 5) In the high-flow PV groups, the
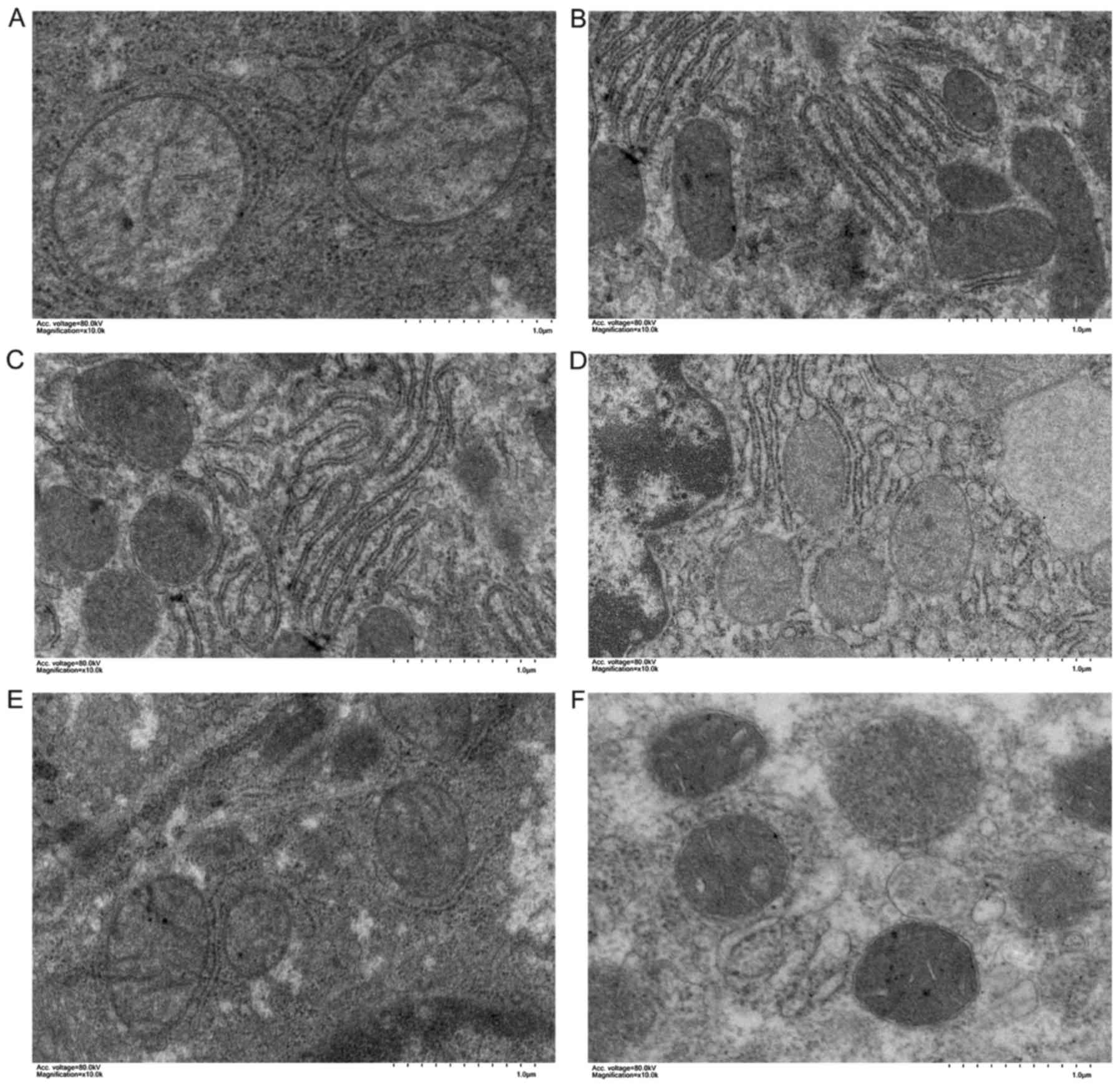
mitochondrial swelling of the hepatocytes was more prominent, the
mitochondrial ridge structure was loose and dissolved, the membrane
structure was blurred, and the endoplasmic reticulum was
significantly decreased (Fig. 6).
There was more endoplasmic reticulum in the low-flow PV groups, the
mitochondrial swelling was alleviated (it was more obvious in group
C compared with that in group B), and the mitochondrial ridge was
clear.
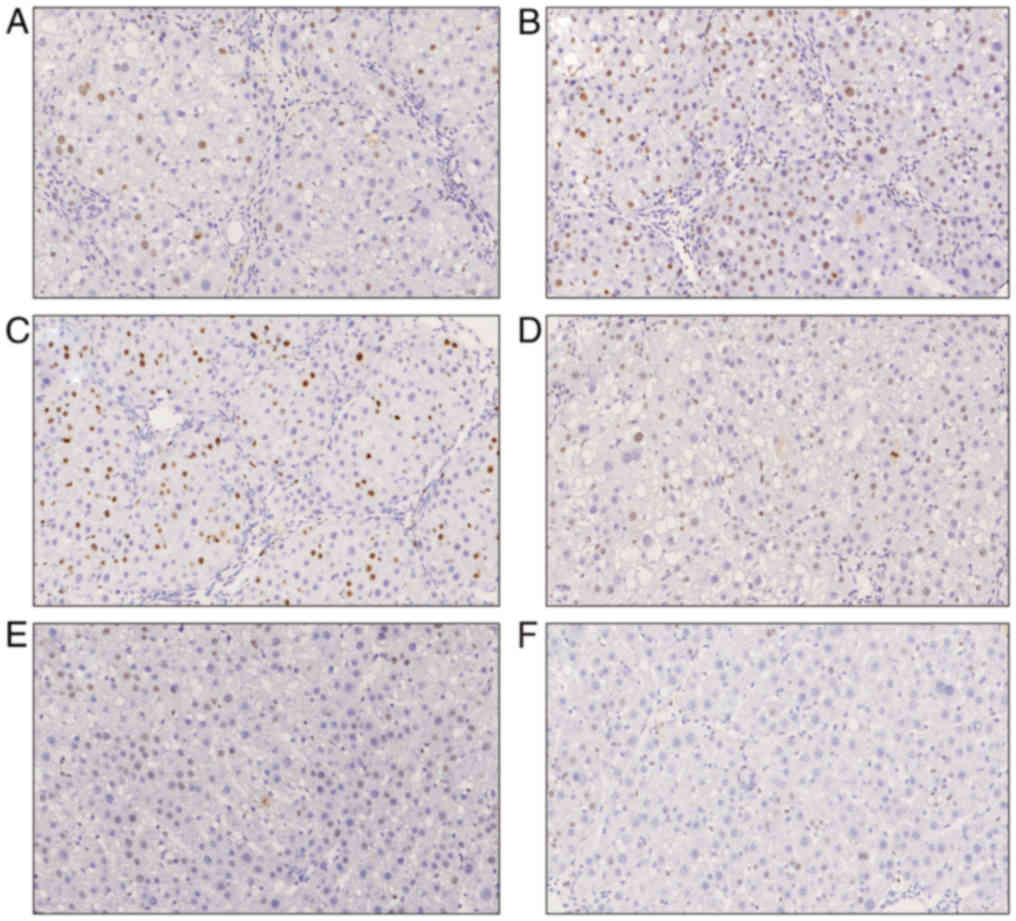
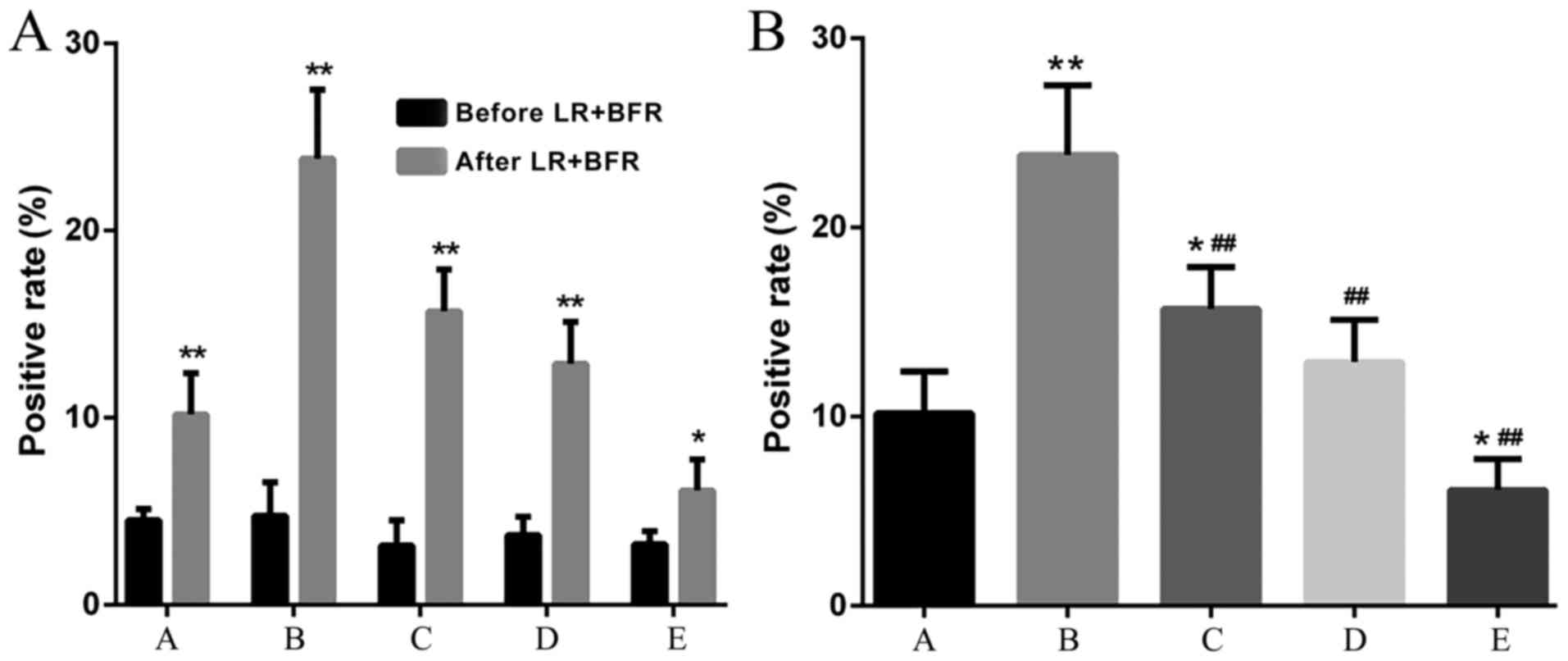
Ki-67 expression
There was no significant difference in Ki-67
expression among groups prior to liver resection + blood inflow
regulation. The Ki-67 expression in groups B and C was
significantly increased, particularly in group B (compared with
groups A, D and E, P<0.05). The expression of Ki-67 in group D
was also increased compared with group A, but was lower compared
with that in groups B and C. The Ki-67 expression in group E was
significantly lower compared with that in the control group
(P<0.05) (Figs. 7 and 8).
Discussion
In human, resection of three or more segments, or ≥4
liver segments was defined as major hepatectomy. In the present
study, the removal of the left lateral lobe and left medial lobe of
the liver (accounting for ~45% of the total liver volume) was
defined as major hepatectomy (10,12).
Following major hepatectomy, the cross-section of the intrahepatic
vascular bed is significantly reduced and the residual liver
receives the blood that formerly flowed through the whole liver,
resulting in PHP (13,14). Although a slightly increased portal
pressure on liver SE cells may promote liver regeneration (2), the damage caused by excessive pressure
and excessive flow may prevail. PHP may cause SE cells swelling and
detachment by increased shear stress, impede oxygen diffusion and
oxygen uptake by the liver tissue (2,3,15). Liver regeneration requires a rich
supply of oxygen and energy (16,17),
while PHP reduces the oxygen saturation in the liver tissue,
limiting the oxygenation of liver cells and reducing the
mitochondrial redox function, Dold et al revealed that the
injury to mitochondria by PHP following major hepatectomy may be
related to oxygen supply and oxygen uptake (13). In that study, hepatic tissue oxygen
pressure (pO2) was significantly decreased and was
associated with an increased expression of hypoxia-inducible
factor-1α (HIF-1α) under conditions of PHP after a 70 and 90%
hepatectomy; in line with these findings, 90% hepatectomy also
resulted in an increase of NADH fluorescence compared with the
baseline, indicating an impaired mitochondrial redox status of the
liver tissue. Mitochondria are the only energy-supplying organelles
in the cells; thus, mitochondrial damage may cause disordered
intracellular metabolism and lead to cell necrosis and apoptosis.
Following hepatectomy, mitochondrial DNA replication and
translation increase, and mitochondrial respiratory function is
significantly increased. These activities are closely associated
with liver regeneration (18–21).
Moreover, it is hypothesized that mitochondria may be a potential
regulatory site to initiate hepatocytes entering the division cycle
(18). In the present study,
pathological examination revealed that the congestion was
alleviated in the low portal flow groups; in addition, the
disruption in sinusoidal structure and hepatocyte injury were
significantly attenuated. The ultrastructural study by TEM revealed
that the damage to the mitochondria was more severe in the high
portal flow groups compared with that in the low portal flow
groups. These results show that correcting the state of PHP and
alleviating the damage of SE cells by PHP may improve the oxygen
supply of liver tissue and the consumption of oxygen by liver
tissue, thereby reducing the damage to mitochondria. It is the
limitation of the present study not to evaluate the function of
mitochondria. In the future study, we will examine at least two of
the respiratory complex including Complex I, Complex II, Complex
III or Complex IV in order to analyze the active status of the
mitochondria. It's also a limitation of the study that not perform
the Masson staining through the whole experiment process. In the
future study, we will perform Masson staining to monitor the
changes of the degree of fibrosis to evaluate the possible impact
of hepatic blood inflow on the liver fibrosis.
The regeneration of the FLR is the basis for the
recovery of liver function following major hepatectomy. Liver
regeneration is triggered 0-4 h after liver resection or acute
injury. There are three main stages of hepatocyte regeneration: The
initiation phase (G0-G1 phase), the proliferation phase (G1-S
phase) and the termination phase (G1-G0 phase) (22,23).
Ki-67 is located in the nucleus and is a marker of cell
proliferation; it can mark most cells, except those in the G0
phase. A high positive rate of Ki-67 in hepatocytes indicates a
higher proportion of cells in the proliferative cycle and faster
regeneration (24). As PHP has a
negative effect on the regeneration of the FLR, correcting PHP may
promote liver regeneration. A series of measures to decrease portal
pressure have been adopted in clinical settings to alleviate damage
and provide a more favorable physiological environment for the
regeneration of FLR (13,25,26). For
example, Bucur et al (14)
promoted the regeneration of the FLR and reduced the incidence of
PHLF by restricting portal flow with a ring-shaped device after
major hepatectomy in pigs. As the liver receives a dual blood
supply (PV and HA) and is regulated by the HA buffer response, the
PHP after major hepatectomy may affect HA blood flow and have an
additional significant impact. By improving the HA blood flow,
liver regeneration and liver function may improve. Eipel et
al (27) reported that removal
of ~85% of the liver in rats resulted in a 40% increase in residual
hepatic portal flow, a 50% decrease in liver tissue oxygen uptake
and a significant increase in the mortality rate in rats; when the
HA blood supply was increased by spleen resection, the prognosis of
rats also improved. In the present study, the expression of Ki-67
in the low portal flow groups, particularly in the low portal flow
+ high arterial flow group, was significantly increased and the
weight of FLR was significantly increased. The regeneration of the
FLR was consistent with the pathological changes following major
hepatectomy, and the blood inflow regulation may play an important
role through the protection of the liver structure: Limiting the
injury of liver tissue is associated with higher oxygen supply,
normal structure and function of the mitochondria, and more
efficient liver regeneration. Following major hepatectomy, the
epitelial bili, endotelial cells, Küpffer, as well as hepatocytes
begin to regenerate rapidly. We will detect the hepatocyte markers
including alfa-fetoprotein, hexokinase or piruvate kinase in the
future study to determine whether regeneration is primarily from
hepatocytes or other cells. The recovery of function of the FLR is
based on the intact structure and rapid regeneration of the FLR.
The present study demonstrated that the levels of serum ALT and
TBIL of the low portal flow groups were closest to normal, PAS
staining of the liver tissue showed that the glycogen synthesis by
hepatocytes of the low portal flow groups was better than other
groups, indicating that the decrease of the portal flow may
significantly improve the function of the FLR following major
hepatectomy. With the improvement of PHP, the increase of HA blood
flow may have a beneficial effect on the recovery of liver
function, likely due to the increased oxygen supply. However, on
the basis of PHP, increasing the HA flow cannot effectively
alleviate the tissue damage and improve liver function, suggesting
that the injury caused by PHP exceeds the improvement associated
with the increase of HA flow.
In the present study, the research was based on a
cirrhosis rat model, which was different from the majority of
previous studies. A considerable proportion of hepatectomies have
to be performed on patients with chronic hepatitis and cirrhosis,
which are often concomitant with portal hypertension. However,
further research is required to elucidate the mechanism underlying
the damage resulting from PHP after major hepatectomy. First, as
physical damage caused by PHP is a possible explanation, measures
should be taken to adequately reduce the pressure on SE cells and
hepatocytes; second, oxygen supply to the liver tissue and oxygen
consumption by liver tissue should be a focus of further research;
finally, as the key organelles of cellular oxygen metabolism, the
structure and function of mitochondria should also be investigated.
Clinically, preoperative liver blood flow should be evaluated,
especially for patients with cirrhosis. Intraoperatively, portal
flow as well as hepatic arterial flow should be assessed again
after major hepatectomy. If PHP exists, it is necessary to narrow
the portal vein properly to reduce the portal pressure and portal
flow to avoid damage to the liver. The spleen artery could be
appropriately narrowed to increase the hepatic arterial blood flow
in order to bring more oxygen to the liver. Further research may
help find strategies for protecting and promoting the regeneration
of the FLR, in order to ensure safe recovery following major
hepatectomy.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science
Foundation of Zhejiang Province (grant no. LY15H030002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WJ, JG, XW and YZ were responsible for the design
and implementation of experiments; SF and BY were responsible for
the management of animals, the collection of specimens and
pathological examinations; RD and BW were responsible for the data
collection and analysis, and the production of figures. WJ, YZ, JG
and XW were responsible for writing of the manuscript.
Ethical approval and consent to
participate
The animals were treated humanely following the
Institutional Guidelines for Animal Use and Care at the Institute
of Zoology, the Chinese Academy of Sciences. The study also
complied with the management rules of the National Health and
Family Planning Commission of China and was approved by the Ethics
Committee of Zhejiang Cancer Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Poon RT, Fan ST, Lo CM, Liu CL, Lam CM,
Yuen WK, Yeung C and Wong J: Improving perioperative outcome
expands the role of hepatectomy in management of benign and
malignant hepatobiliary diseases: Analysis of 1222 consecutive
patients from a prospective database. Ann Surg. 240:698–710.
2004.PubMed/NCBI
|
|
2
|
Fondevila C, Hessheimer AJ, Taurá P,
Sánchez O, Calatayud D, de Riva N, Muñoz J, Fuster J, Rimola A and
García-Valdecasas JC: Portal hyperperfusion: Mechanism of injury
and stimulus for regeneration in porcine small-for-size
transplantation. Liver Transpl. 16:364–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kinaci E and Kayaalp C: Portosystemic
shunts for ‘too small-for-size syndrome’ after liver
transplantation: A systematic review. World J Surg. 40:1932–1940.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiang L, Huang L, Wang X, Zhao Y, Liu Y
and Tan J: How much portal vein flow is too much for liver remnant
in a stable porcine model? Transplant Proc. 48:234–241. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rahbari NN, Garden OJ, Padbury R,
Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo
RP, Christophi C, et al: Posthepatectomy liver failure: A
definition and grading by the international study group of liver
surgery (ISGLS). Surgery. 149:713–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yadav K, Shrikhande S and Goel M: Post
hepatectomy liver failure: Concept of management. J Gastrointest
Cancer. 45:405–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li CH, Chen YW, Chen YL, Yao LB, Ge XL,
Pan K, Zhang AQ and Dong JH: Preserving low perfusion during
surgical liver blood inflow control prevents hepatic
microcirculatory dysfunction and irreversible hepatocyte injury in
rats. Sci Rep. 5:144062015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu C, Song JL, Lu WS, Yang JY, Jiang L,
Yan LN, Zhang JY, Lu Q, Wen TF, Xu MQ and Wang WT: Hepatic arterial
buffer response maintains the homeostasis of graft hemodynamics in
patient receiving living donor liver transplantation. Dig Dis Sci.
61:464–473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Audebert C, Bekheit M, Bucur P, Vibert E
and Vignon-Clementel IE: Partial hepatectomy hemodynamics changes:
Experimental data explained by closed-loop lumped modeling. J
Biomech. 50:202–208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andreou A, Vauthey JN, Cherqui D, Zimmitti
G, Ribero D, Truty MJ, Wei SH, Curley SA, Laurent A, Poon RT, et
al: Improved long-term survival after major resection for
hepatocellular carcinoma: A multicenter analysis based on a new
definition of major hepatectomy. J Gastrointest Surg. 17:66–77;
discussion p.77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li CH, Ge XL, Pan K, Wang PF, Su YN and
Zhang AQ: Laser speckle contrast imaging and oxygen to see for
assessing microcirculatory liver blood flow changes following
different volumes of hepatectomy. Microvasc Res. 110:14–23. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Etra JW, Squires MH III, Fisher SB, Rutz
DR, Martin BM, Kooby DA, Cardona K, Sarmiento JM, Staley CA III,
Maithel SK and Russell MC: Early identification of patients at
increased risk for hepatic insufficiency, complications and
mortality after major hepatectomy. HPB (Oxford). 16:875–883. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dold S, Richter S, Kollmar O, von Heesen
M, Scheuer C, Laschke MW, Vollmar B, Schilling MK and Menger MD:
Portal hyperperfusion after extended hepatectomy does not induce a
hepatic arterial buffer response (HABR) but impairs mitochondrial
redox state and hepatocellular oxygenation. PLoS One.
10:e01418772015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bucur PO, Bekheit M, Audebert C, Othman A,
Hammad S, Sebagh M, Allard MA, Decante B, Friebel A,
Miquelestorena-Standley E, et al: Modulating portal hemodynamics
with vascular ring allows efficient regeneration after partial
hepatectomy in a porcine model. Ann Surg. 268:134–142.
2018.PubMed/NCBI
|
|
15
|
Schleimer K, Stippel DL, Kasper HU,
Tawadros S, Allwissner R, Gaudig C, Greiner T, Hölscher AH and
Beckurts KT: Portal hyperperfusion causes disturbance of
microcirculation and increased rate of hepatocellular apoptosis:
Investigations in heterotopic rat liver transplantation with portal
vein arterialization. Transplant Proc. 38:725–729. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alexandrino H, Rolo A, Teodoro JS, Donato
H, Martins R, Serôdio M, Martins M, Tralhão JG, Caseiro Alves F,
Palmeira C and Castro E Sousa F: Bioenergetic adaptations of the
human liver in the ALPPS procedure - how liver regeneration
correlates with mitochondrial energy status. HPB (Oxford).
19:1091–1103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Satoh S, Tanaka A, Hatano E, Inomoto T,
Iwata S, Kitai T, Shinohara H, Tsunekawa S, Chance B and Yamaoka Y:
Energy metabolism and regeneration in transgenic mouse liver
expressing creatine kinase after major hepatectomy.
Gastroenterology. 110:1166–1174. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koyama H, Kurokawa T, Nonami T, Nakao A,
Sugiyama S, Murakami T, Shimomura Y and Takagi H: Increases in the
mitochondrial DNA replication and transcription in the remnant
liver of rats. Biochem Biophys Res Commun. 243:858–861. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alexandrino H, Varela AT, Teodoro JS,
Martins MA, Rolo AP, Tralhão JG, Palmeira CM, Castro E and Sousa F:
Mitochondrial bioenergetics and posthepatectomy liver dysfunction.
Eur J Clin Invest. 46:627–635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weng J, Li W, Jia X and An W: Alleviation
of ischemia-reperfusion injury in liver steatosis by augmenter of
liver regeneration is attributed to antioxidation and preservation
of mitochondria. Transplantation. 101:2340–2348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xing XK, Li MH, Guo ZW and Xu CS:
Expression profiles of genes associated with mitochondria-mediated
apoptosis and their roles in liver regeneration. Genet Mol Res.
15:2016. View Article : Google Scholar
|
|
22
|
Fujiyoshi M and Ozaki M: Molecular
mechanisms of liver regeneration and protection for treatment of
liver dysfunction and diseases. J Hepatobiliary Pancreat Sci.
18:13–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tao Y, Wang M, Chen E and Tang H: Liver
regeneration: Analysis of the main relevant signaling molecules.
Mediators Inflamm. 2017:42563522017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xia J, Zhou Y, Ji H, Wang Y, Wu Q, Bao J,
Ye F, Shi Y and Bu H: Loss of histone deacetylases 1 and 2 in
hepatocytes impairs murine liver regeneration through Ki67
depletion. Hepatology. 58:2089–2098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Troisi RI, Berardi G, Tomassini F and
Sainz-Barriga M: Graft inflow modulation in adult-to-adult living
donor liver transplantation: A systematic review. Transplant Rev
(Orlando). 31:127–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baccarani U, Pravisani R, Luigi Adani G,
Lorenzin D, Cherchi V, Toniutto P and Risaliti A: Safety and
efficacy of splenic artery embolization for portal hyperperfusion
in liver transplant recipients: A 5-year experience. Liver Transpl.
21:1457–1458. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eipel C, Abshagen K, Ritter J, Cantré D,
Menger MD and Vollmar B: Splenectomy improves survival by
increasing arterial blood supply in a rat model of reduced-size
liver. Transpl Int. 23:998–1007. 2010. View Article : Google Scholar : PubMed/NCBI
|