Introduction
A high-sugar diet (HSD) includes more and more types
of foods, such as baked goods, convenience foods and a variety of
sugary drinks. Dietary sugar is the main source of sugars in the
body; sugar is ingested and absorbed, converted into
monosaccharides, and then transported by the blood to cells and
tissues for metabolism (1). However,
a long-term, excessively high-sugar or high-calorie diet damages
the homeostasis of glucose metabolism in the body and causes
obesity, which further leads to metabolic disorders and other
problems such as hypertension, fatty liver, cancer, and especially
the onset of Type 2 diabetes (T2D) (2). T2D, a complex metabolic disease
characterized by insulin resistance, is related to metabolic
abnormalities, such as high blood glucose levels and weight loss,
and can cause serious blindness, amputation and disability, renal
failure, uremia, and so on (3).
Currently, the prevalence of this type of chronic disease is
increasing; while though T2D has traditionally only developed among
adults, the disease has begun to appear in children (4). The current treatment of T2D consists
mainly oral hypoglycemic agents and insulin injections, and the
side effects and safety of these agents need to be further studied
(5).
Traditional medicinal plants, which are low-cost,
easy to obtain, and have the advantages of small side effects, have
long been widely used around the world (6). Among them is Flos Chrysanthemi
Indici (CI), the capitulum of the perennial herb Chrysanthemum
indicum L. of Compositae. The chemical composition of CI
includes sesquiterpenes, flavonoids, and phenolic compounds. CI
extracts, which have anti-inflammatory, anti-oxidative and
anti-microbial activities, exhibit inhibitory activity against rat
lens aldose reductase, a mediator of pathogens involved in diabetic
complications (7,8). However, the regulatory effect of CI on
abnormal metabolic functions induced by a high sugar diet is poorly
understood.
Drosophila melanogaster (Drosophila)
has become an excellent model for investigating T2D because
approximately 74% of human disease-causing genes are conserved in
this species, and more importantly, the mechanisms of glucose
homeostasis are highly conserved between mammals and
Drosophila (4,9). In a number of previous studies,
Drosophila were fed with an HSD to establish models of T2D
that exhibit features of T2D patients, including hyperglycemia and
insulin resistance (10). Therefore,
we analyzed the effects of aqueous CI extracts on improving
T2D-like features in an HSD-induced Drosophila model. The
results indicated that CI improved HSD-induced metabolic
abnormalities as well as growth rate, body size, lifespan,
productive capacity and fat storage. In addition, CI improved fat
metabolism and cell size in S6k and Akt1 mutant
flies. These results provide a valuable reference for preclinical
drug discoveries that take the CI of this medicinal plant into
account.
Materials and methods
Fly stocks and culture conditions
Wild-type w1118 and Da-Gal4
flies were obtained from the Bloomington Drosophila Stock
Center (Bloomington, IN, USA), S6kl-1/TM6B flies
were obtained from Tian Xu, and Akt1 RNAi flies were
obtained from the Tsinghua Fly Center (Beijing, China). Fly stocks
were maintained on standard cornmeal-yeast medium at 25±1°C and
60±5% humidity under a 12-h light/12-h dark cycle.
Preparation of CI aqueous extract and
Drosophila growth medium
CI was purchased from the Renmin Tongtai Pharmacy
(Harbin, China). Aqueous CI extract was obtained as previously
described (11). Chopped capitula
(20 g) were soaked overnight in deionized water (200 ml; yield,
~5–14%) at room temperature and then heated until boiling for 3 h.
The extraction process was repeated twice and the filtrate was
collected and concentrated to 100 ml. The LSD (low-sugar diet) and
HSD contained 0.15 and 1 M of sucrose, respectively. Aside from
sucrose, no additional sugar was added to any of the growth media.
Flies fed the LSD or HSD media containing the CI extracts comprised
the experimental groups, and the final concentrations of the CI
extracts were 5 or 10% in weight/volume. The choice of extract
concentration was based in previous tests performed in flies which
showed that CI aqueous extract did not affect the size and growth
rate of Drosophila (data not shown).
Lifespan
To test the lifespan, after mating for 24 h, males
and females were separated into vials containing experimental
media. The flies were transferred to vials with fresh food once
every 2 days. The number of dead flies were recorded at the time of
transfer until all flies were dead. Each vial contained 30 flies,
and each lifespan assay was repeated 4 times independently.
Body weight, pupal and larvae
volume
Newly enclosed adult flies (less than 8 h old) of
each group were collected and maintained on the fresh respective
medium for 24 h. Then, males and females from each group were
separated under CO2 anesthesia and weighed on a balance.
Five experiments per group were performed and the mean body mass
was calculated. To determine the pupal or larvae volume, the pupae
and larvae were photographed and the volumes were calculated with
the formula 4/3π(L/2)(l/2)2 (L, length; l, width) using
ImageJ software (V1.47; National Institutes of Health, Bethesda,
MD, USA) (12).
Fecundity and hatching rate
Five-day-old adult flies were placed on apple juice
agar plates containing yeast as the only food source. The apple
juice agar plates were replaced every 2–3 h and the numbers of eggs
on each plate were counted. The egg production was calculated by
dividing the total egg production by the total number of h in each
cage. After 22 h, the number of 1st instar larvae (L1) on each
plate was counted again. The hatching rate was calculated by
dividing the total number of larvae by the total number of
fertilized eggs on each plate.
BODIPY and Phalloidin staining
assay
Phalloidin staining was performed as previously
described (13). The fat body was
dissected and fixed for 30 min with 4% paraformaldehyde in PBS at
room temperature. Then, the dissected tissue was stained with
Phalloidin and BODIPY (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) for 30 min each in a humidified chamber and washed three times
for 5 min in PBST. The tissues stained with DAPI for 10 min and
mounted using SlowFade Diamond Antifade Mountant (Thermo Fisher
Scientific, Inc.). Fluorescence was analyzed using a Zeiss Axioplan
2 microscope (Zeiss AG, Oberkochen, Germany). The cell and lipid
droplet areas were measured using ImageJ software.
Wing and cell area assay
To determine the wing and cell sizes, 19 wings from
males were analyzed. Cell size was estimated by counting the number
of trichomes in a defined area of the wing blade. The wing area was
measured using ImageJ software (V1.47; National Institutes of
Health).
Statistical analysis
The data are representative of at least three
independent experiments, and images were analyzed using ImageJ
(v.1.47; National Institutes of Health). The Kaplan-Meier method
was used to analysis survival and performed using SPSS Statistics
v.19.0 software (IBM Corporation, Armonk, NY, USA), and survival
significances were used by log-rank test and performed using
GraphPad Prism v.6.0 software (GraphPad Software, Inc., La Jolla,
CA, USA) (P-values were calculated for HSD and LSD <0.0167 was
considered statistically significant, P-values were calculated for
HSD and HSD+CI <0.00833 was considered statistically
significant). The remaining statistical analyses were performed
through one-way ANOVA with post hoc Dunnett test using GraphPad
Prism 6.0 software (P-values >0.05 indicated no significance;
*P<0.05; **P<0.01; ***P<0.001). Error bars indicate the
means ± standard error of the means.
Results
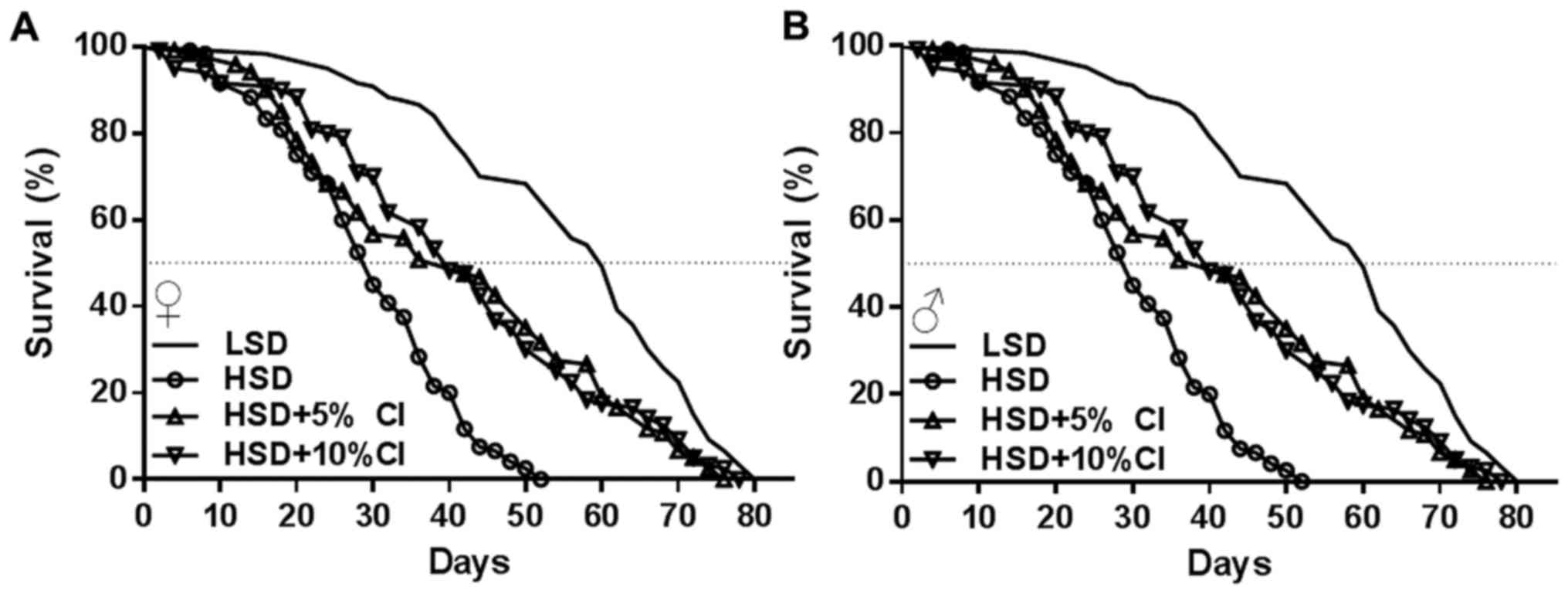
CI extracts increase the lifespan of
flies fed an HSD
Consumption of an HSD is often associated with a
decreased survival rate in flies (14). To assess whether CI could increase
the lifespan of flies fed an HSD, the flies were fed an HSD either
alone or supplemented with CI. We showed that the mean lifespan,
50% survival rate and maximum lifespan of the adult flies were
obviously decreased in the HSD group compared with those of the LSD
group (Table I, Fig. 1). However, supplementation with 5 or
10% CI significantly extended the HSD-induced lifespan in female
and male flies (Table I). The mean
lifespan was 33.1 and 29.7 days in HSD-fed female and male flies,
respectively; however, the lifespan was significantly increased by
more than 10 days in both females and males when supplemented with
5 or 10% CI, respectively. Furthermore, the 50% survival rates
increased by more than 8 or 10 days in the 5 and 10% CI groups
compared with those of the HSD group, and the maximum lifespan
increased by more than 20 and 24 days in females and males,
respectively. These results suggest that CI can limit the adverse
effects of the HSD and can increase the lifespan of flies. The
above results indicated that the effect of supplementation with 10%
CI was better than that of 5%. Thus, the subsequent experiments
were performed using 10% CI.
 | Table I.Lifespan of flies fed diets
supplemented with and without CI extract. |
Table I.
Lifespan of flies fed diets
supplemented with and without CI extract.
| Strain | Sex | Dieta | Mean lifespan (days
± SE)b | Change of mean
lifespan (%) | P-value for all
fliesc | 50% Survival
(days) | Maximum lifespan
(days ± SE) | Change of maximum
lifespan (%)d |
|---|
|
w1118 | Female |
|
|
|
|
|
|
|
|
|
| LSD | 57.5±1.4 |
|
| 58.0±2.2 | 76.7±0.7 |
|
|
|
| HSD | 33.1±1.1 | −42.4 |
<0.0001e | 34.0±1.7 | 48.3±0.6 | −37.0 |
|
|
| HSD+5% CI | 43.9±1.8 | −23.7 |
<0.0001f | 48.0±2.6 | 68.5±0.4 | −10.7 |
|
|
| HSD+10% CI | 44.9±1.9 | −21.9 |
<0.0001f | 42.0±2.1 | 73.5±0.6 | −4.2 |
|
| Male |
|
|
|
|
|
|
|
|
|
| LSD | 56.3±1.5 |
|
| 60.0±1.6 | 75.8±0.8 |
|
|
|
| HSD | 29.7±1.0 | −47.2 |
<0.0001e | 30.0±1.2 | 46.2±0.9 | −39.1 |
|
|
| HSD+5% CI | 40.9±1.8 | −27.4 |
<0.0001f | 42.0±4.1 | 70.8±0.7 | −7.0 |
|
|
| HSD+10% CI | 41.6±1.8 | −26.1 |
<0.0001f | 40.0±2.3 | 72.0±0.8 | −5.0 |
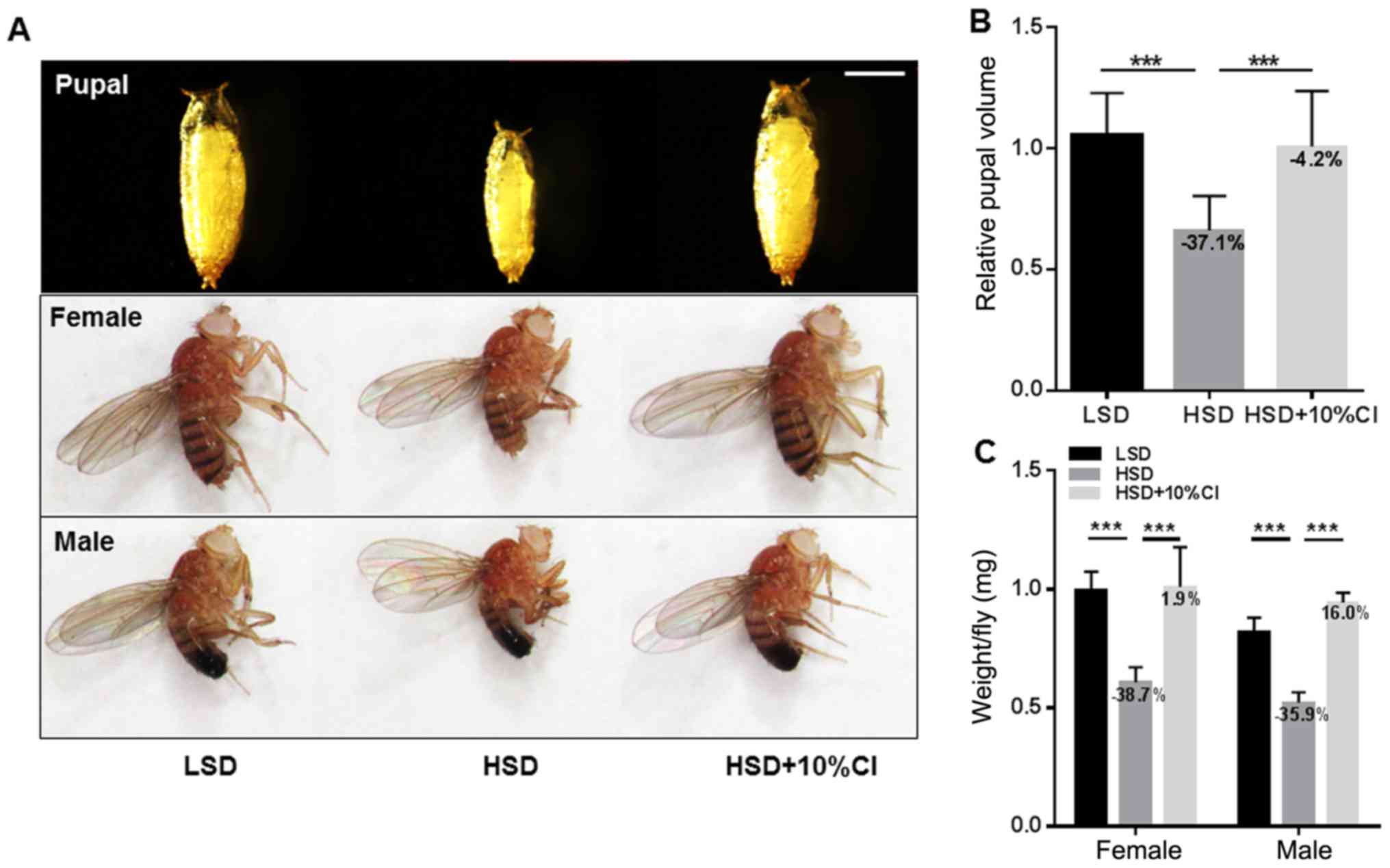
CI increases the body weight and pupal
volume of flies fed an HSD
An HSD decreases the size of both larvae and adults
due to insulin resistance (4). To
determine whether supplementation with CI in an HSD changes the
size of individual flies, we calculated the pupal volume and adult
body weight. The results showed that the pupal volume and adult
weight of flies fed an HSD were significantly decreased compared
with those fed LSD (Fig. 2A).
However, this small size was improved markedly when CI added to the
HSD. The pupal volume increased by 52.4% and the weights of both
male and female flies increased by 81.0 and 66.1%, respectively, in
flies fed diets supplemented with CI as compared with those fed an
HSD (Fig. 2B and C). These results
suggest that CI can reverse the phenomenon in which individuals
decrease due to feeding with an HSD.
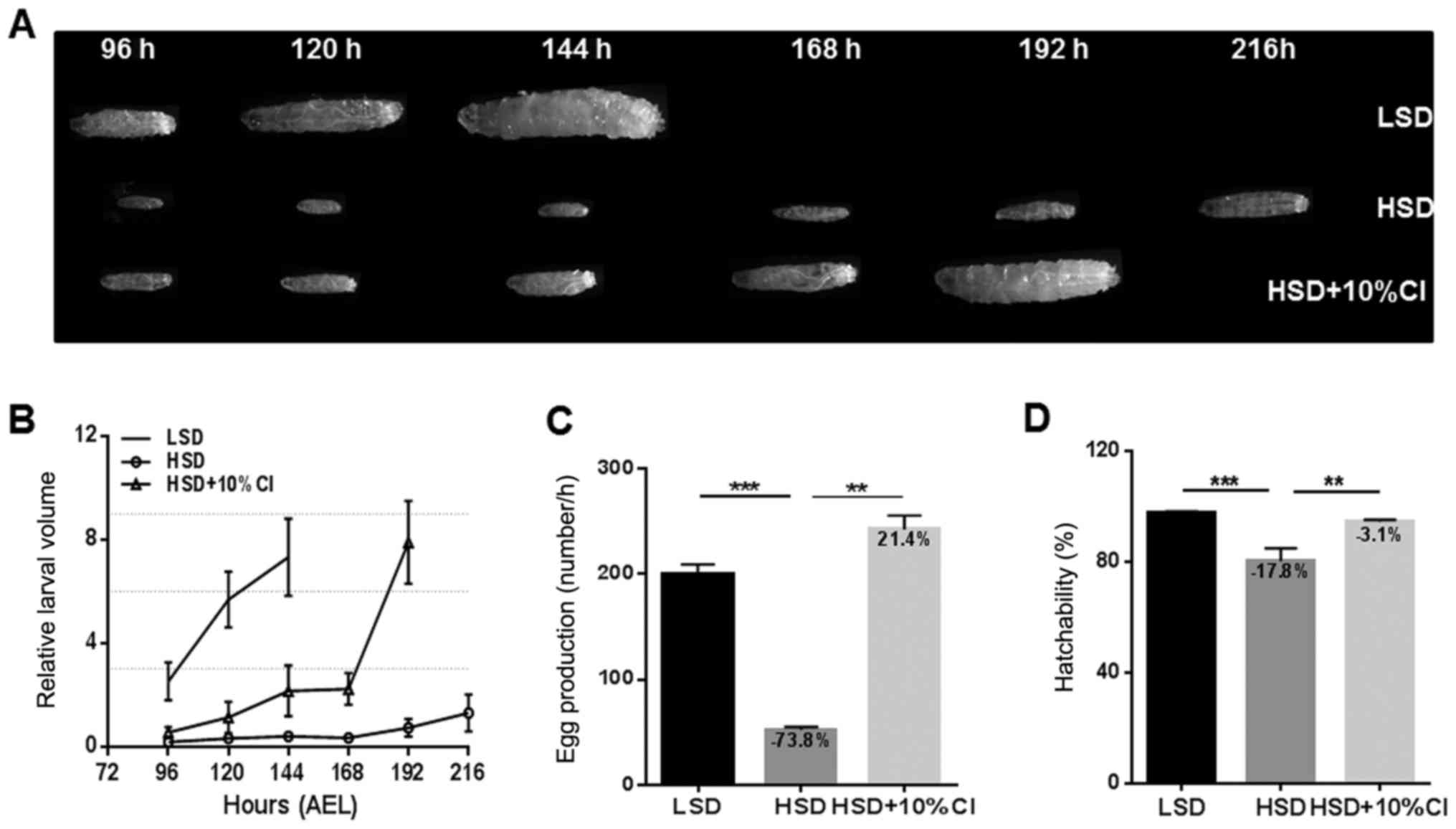
CI promotes larval development and
increases the fecundity of female flies fed an HSD
HSD can severely decrease the speed of larval
development (15). To analyze the
effects of CI on the speed of larval development, we recorded the
developmental time and the size of larvae that hatched from
fertilized eggs for 96 h starting after egg laying (AEL) until the
larvae reached the 3rd instar stage at different time points. There
was no significant difference in size or developmental speed
between the HSD and HSD+CI groups before 96 h (data not shown). The
HSD significantly retarded larval development by delaying the time
to become pupa, and the individual sizes of HSD-fed larvae were
smaller than those of the LSD-fed larvae. However, larvae volume
was significantly increased when CI was added to the HSD, and CI
accelerated larval growth and increased individual size (Fig. 3A and B). Fecundity and development
are inseparable; therefore, we determined the fecundity of
5-day-old female flies in different experimental groups by
measuring the egg production over 1 h. Compared with the LSD group,
egg production was noticeably decreased by 73.8% in flies fed an
HSD. However, the egg production was significantly increased when
the HSD was supplemented with CI, and the numbers of eggs were
similar to those of the LSD group (Fig.
3C). Though the hatching rate of fertilized eggs in the HSD
group decreased by 17.8% compared with LSD, feeding with CI
significantly increased the hatching rate by 17.9% (Fig. 3D). These results suggest that CI can
improve the HSD-induced larvae developmental time, female fecundity
and egg hatching rate.
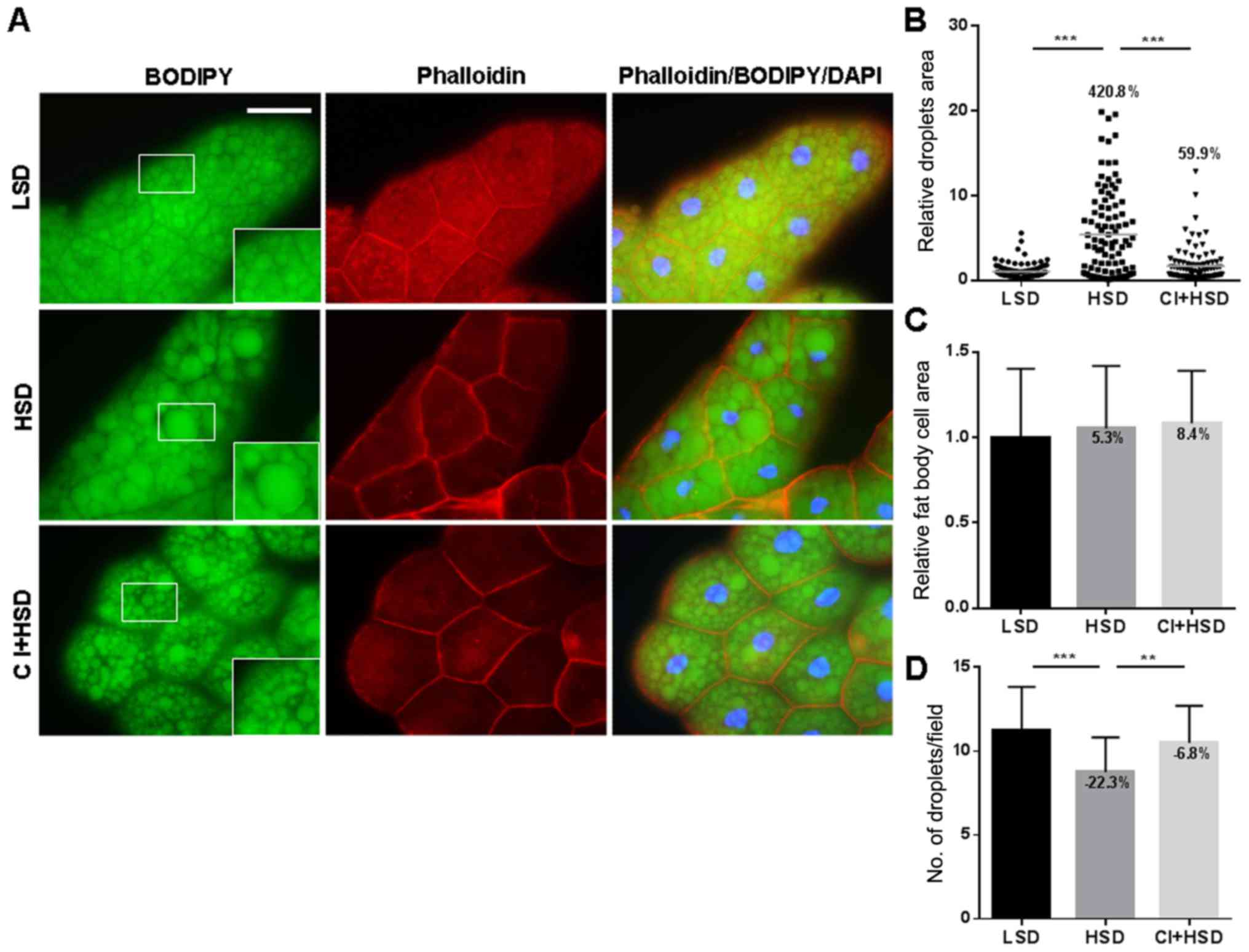
CI reduces lipid accumulation in the
fat bodies of HSD-fed larvae
During the initial stage, T2D is often accompanied
by obesity (16). The fat body of
Drosophila is a functional homolog of the liver and white
fat in vertebrates and is used to store fat (17). To analyze whether CI controls the
accumulation of lipids, we stained the lipid droplets of the larval
fat body with BODIPY. The results showed that after feeding with an
HSD, the lipid droplets of fat bodies were significantly larger
than those in the LSD group (Fig.
4A). Moreover, analysis using ImageJ showed a large
distribution of lipid droplets in the HSD group, which demonstrated
the uneven size of the average relative area of lipid droplets in
this group (Fig. 4B). However,
Phalloidin staining showed that though the size of the fat cells
did not increase, the numbers of lipid droplets decreased (Fig. 4C and D). We also showed that after
the addition of CI to the HSD group, the size of the lipid droplets
was significantly reduced, the distribution of lipid droplets was
smooth, and the number of lipid droplets was increased (Fig. 4A). These results suggest that the CI
extract can reduce HSD-induced lipid accumulation without changing
the size of the fat cells.
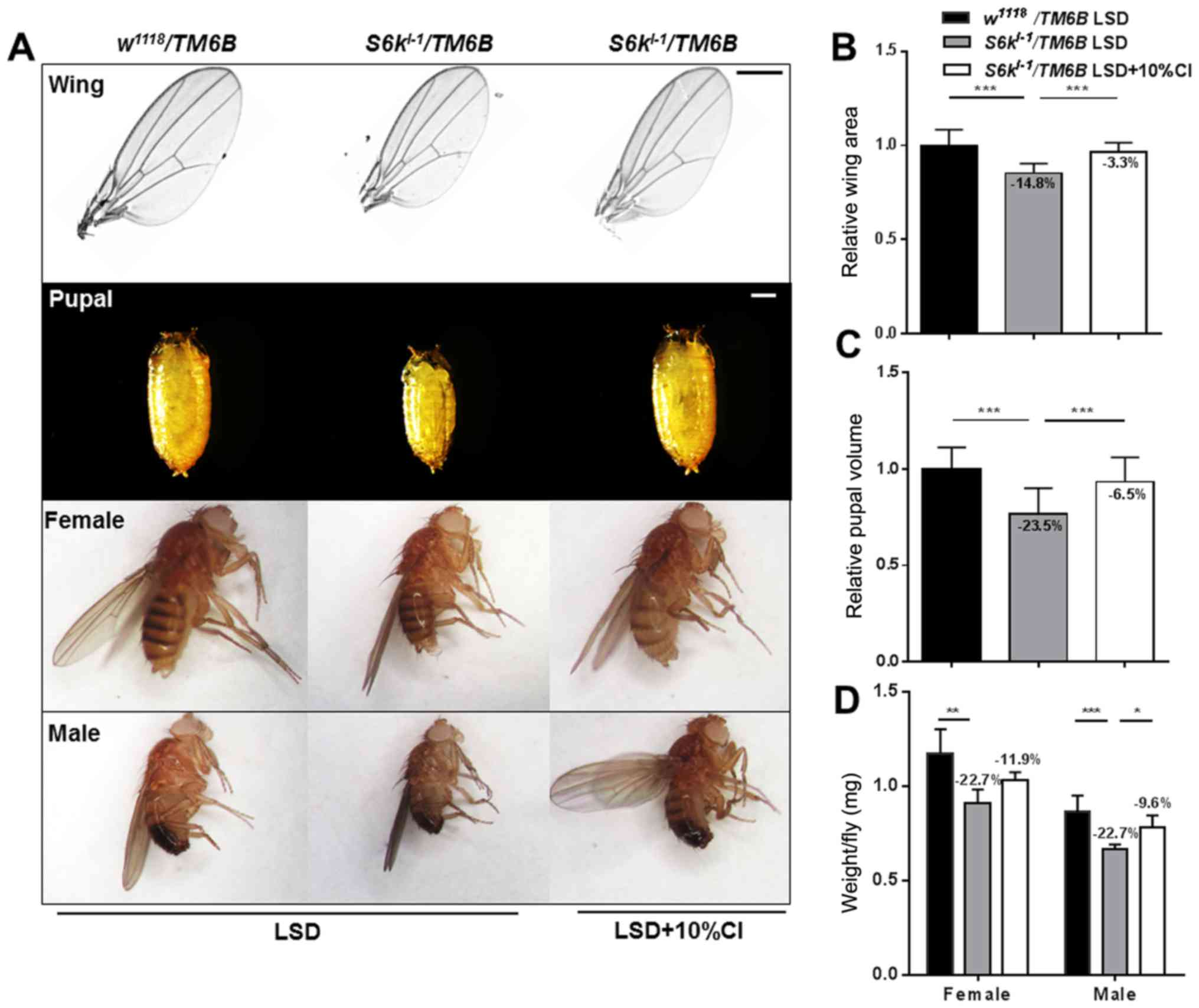
CI increases the body weight, pupal
volume and wing area in S6k mutants
Insulin and insulin-growth-factor-like signaling
(IIS) play vital roles during development by increasing the levels
of phosphatidylinositol 3,4,5-triphosphate through the activation
of 40S ribosomal protein S6 kinase (S6k, or dS6k in the case of
Drosophila S6k) and protein kinase B (PKB, or dAkt in case
of Drosophila protein kinase B) (18). S6k is involved in metabolic
processes, cell growth and reproduction (19), and though dS6Kl-1
mutants are viable, they have smaller body sizes due to a decrease
in cell size (20). We found that CI
significantly improved the HSD-induced disorders especially by
increasing the individual size. These results indicate that CI may
affect the insulin metabolic pathway to improve the state of
insulin resistance in Drosophila. To further investigate
whether CI regulates insulin metabolism in Drosophila, we
fed the S6k mutant diet containing CI. Compared with the control
flies, the S6k mutants showed 14.8 and 23.5% decreases in
wing size and pupal volume, respectively, and the body weight of
males decreased by 22.7% (Fig. 5).
The S6k mutants after feeding with CI, the wing size and
pupal volume increased by 13.5 and 22.1%, respectively, and the
body weight of males increased by 17.0% (Fig. 5).
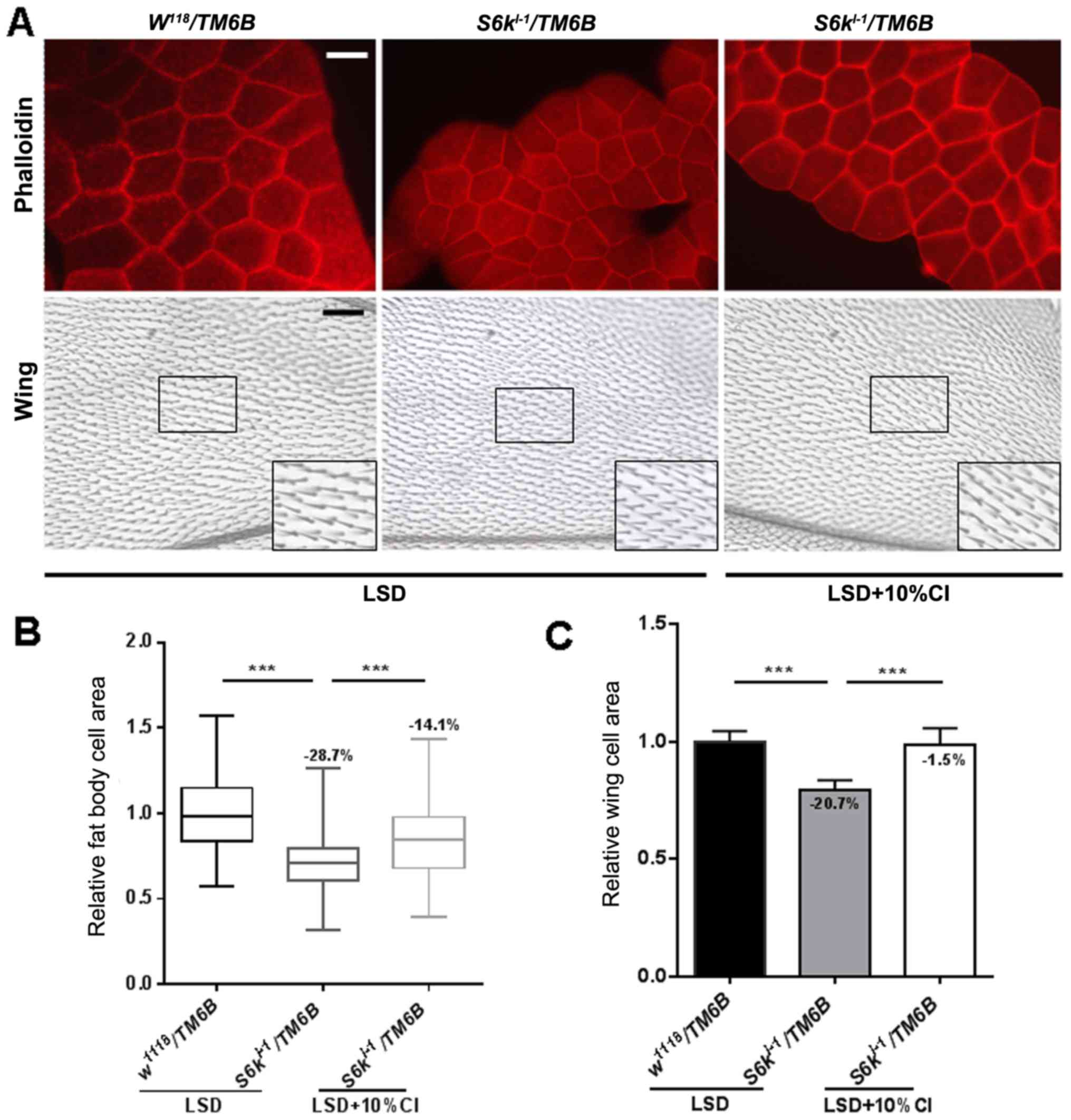
Next, we analyzed the sizes of both fat and wing
cells. Phalloidin was used to stain the membrane of fat cells, and
the relative average cell area was calculated using ImageJ. In
addition, we measured the number of trichomes, a type of single
bristle that accessorizes each cell of the wing blade, in a defined
area of the wing blade. Though the loss of S6k can
significantly decrease both fat cell and wing cell size by 28.7 and
20.7%, respectively, the cell sizes of S6k mutant were
significantly increased by 20.6 and 24.2%, respectively, after the
addition of 10% CI to the LSD (Fig.
6). These results suggest that the CI extract can restore the
developmental defects observed in S6k mutants and can
increase body weight and the cell size of peripheral tissue
(thereby increasing the wing and pupa sizes).
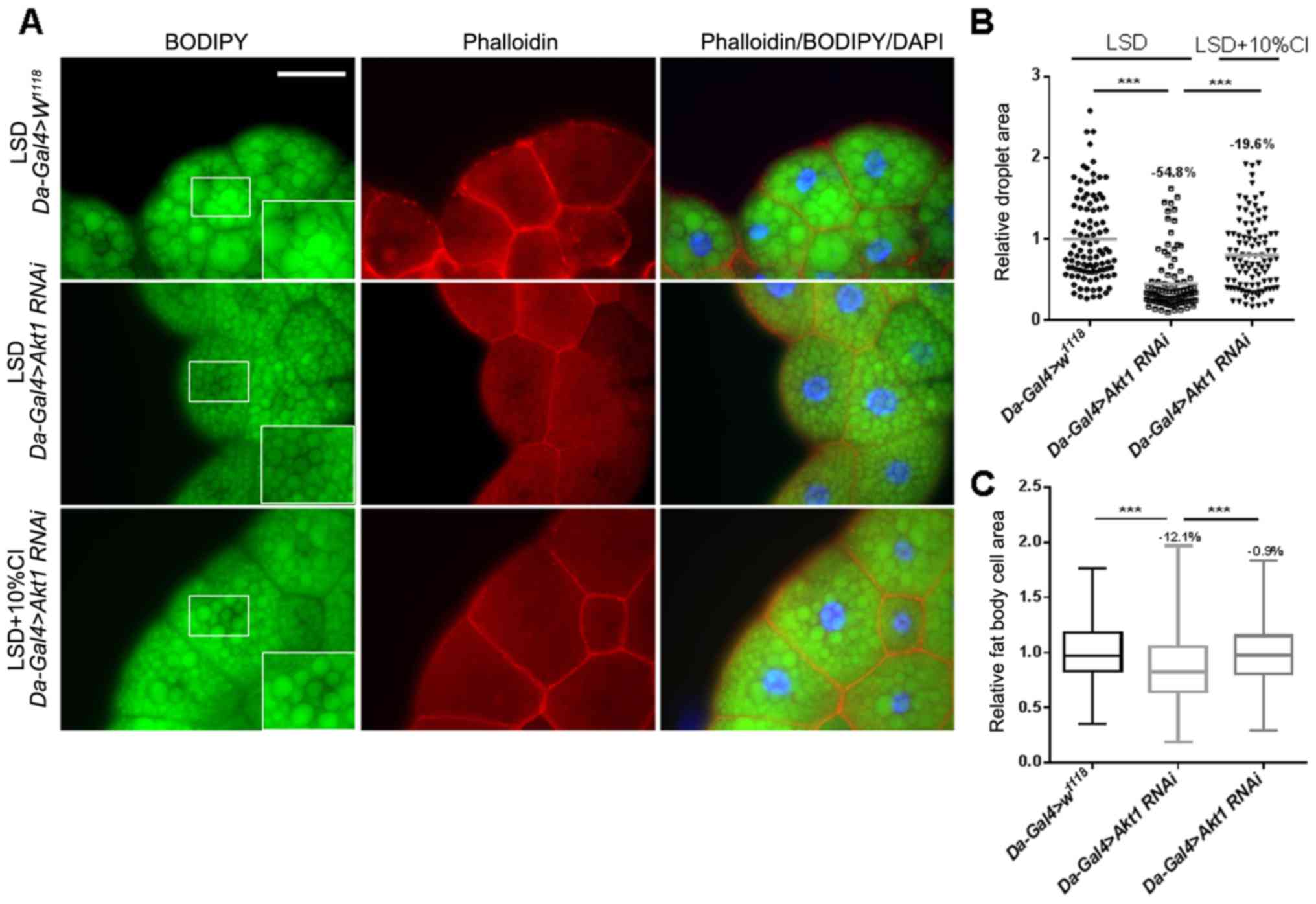
CI increases the fat cell area and
lipid accumulation in Akt1 larvae
Akt1, a downstream effector of PI3K that regulates
cell growth and organ size, can phosphorylate and antagonize the
transcription factor FOXO (19). As
previous experiments showed that CI can improve the knockdown of
S6k-induced defects in development, we next knocked down
Akt1 levels under the control of a UAS driver to further
confirm the role of CI in the insulin signaling pathway. Phalloidin
and BODIPY were used to stain the fat cell membranes and lipid
droplets, respectively. We showed that the cell area and the size
of lipid droplets were significantly decreased in fat bodies of
Akt1 knockdown larvae. However, when CI was added, the cell
area and lipid droplets were obviously increased by 12.6 and 78.1%,
respectively, and they were similar to normal size (Fig. 7). Altogether, these results suggest
that CI can increase the cell size and fat storage resulting from
Akt1 knockdown.
Discussion
CI has been widely used in the treatment of various
diseases due to its anti-inflammatory and antioxidative properties
(21). CI is also usually used in
Chrysanthemum tea, Chrysanthemum pillow, as a food additive, and in
medicated baths in folk medicine (22). Previous studies have demonstrated
that Chrysanthemi Flos, the same genus as CI, might have
therapeutic potential in diabetic complications (8). However, the effects of aqueous CI
extracts on T2D have not been previously characterized. Previous
studies have demonstrated that an HSD induces insulin-resistant
phenotypes in Drosophila; these phenotypes involve decreases
in the individual size, growth rate, fecundity and lifespan while
increasing fat deposition (10). Due
to the high level of conservation between Drosophila and
mammalian insulin metabolism (23),
our results provide a theoretical basis for exploring the potential
use of CI for the clinical treatment of diabetes.
In the present study, we observed that CI
significantly improved the HSD-induced disorders by increasing the
lifespan, individual size, growth rate, fecundity and hatching
rate. In addition, CI decreased the fat content, and the
developmental state of CI-supplemented flies was similar to those
of LSD group. These results indicate that CI may affect the insulin
metabolic pathway to improve the state of insulin resistance in
Drosophila. However, it is not clear whether this
improvement simply strengthens the absorption of excess
carbohydrates by the peripheral tissue in Drosophila. To
further confirm the pharmacological effects of CI, we used
transgenic flies in which important components of insulin signaling
pathway were knocked down. Our findings indicated that CI can
increase the individual size of HSD-fed flies. In addition,
previous studies have indicated that S6k and Akt
knockdown can decrease cell size (24). Therefore, we fed S6k and
Akt mutants with 10% CI, and the results indicated that CI
could significantly increase the sizes of fat cells in S6k
and Akt mutants and lipid droplets of Akt
mutants.
The two signaling pathways that control energy
metabolism in Drosophila are the insulin signaling and the
AKH signaling, respectively (25,26). The
way of lipid metabolism of Drosophila is similar to that of
mammals. Excess lipids are stored as fat droplets in the fat body
cells (4). Previous studies indicate
that the activation of insulin signaling pathways in non-fat
tissues leads to an increase in fat storage, and that fat bodies
regulate secretion of DILPs in the brain by sensing changes in
carbohydrate content in the diet (17,27).
Therefore, the storage of fat and insulin metabolism are
inextricably linked. Most T2D patients have long-term obesity
accompanied by the gradual onset of abnormal fat metabolism
(28). We observed similar symptoms
in HSD-fed flies, in which a large amount of fat accumulated in the
fat body; however, after the addition of CI, the excessive storage
of fat was significantly improved. Perhaps CI improves the
metabolic homeostasis by improving the fat storage of
Drosophila to further influence growth and development, but
the specific mechanism requires further exploration. Therefore, we
predict that aqueous CI extracts may have potential as antidiabetic
agents, and further experimentation is required to fully understand
the pharmacological functions of CI.
Acknowledgements
The authors would like to thank Professor Tian Xu
(Department of Genetics, Yale University School of Medicine, New
Haven, CT, USA) for the flies and The Bloomington and TsingHua Fly
Center for the fly stocks.
Funding
This work was supported by grants from the
Fundamental Research Funds for the Central Universities (grant no.
2572016EAJ4) and Natural Science Foundation of Heilongjiang
Province of China (grant no. C2016010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LHJ made substantial contributions to conception and
design, and revised the manuscript critically for important
intellectual content. YB performed the experiments, and analyzed
the growth rate, lifespan, fat storage, cell size and wing size,
and was a major contributor in writing the manuscript. KL performed
and analyzed the data regarding the body weight. JYS performed the
experiments and analyzed the data regarding the reproductive
capacity. QXL performed statistical analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CI
|
Flos Chrysanthemi Indici
|
|
Drosophila
|
Drosophila melanogaster
|
|
HSD
|
high-sugar diet
|
|
T2D
|
Type 2 diabetes
|
|
LSD
|
low-sugar diet
|
|
AEL
|
after egg laying
|
|
S6k
|
S6 kinase
|
|
Akt
|
protein kinase B
|
References
|
1
|
Trommelen J, Fuchs CJ, Beelen M, Lenaerts
K, Jeukendrup AE, Cermak NM and van Loon LJ: Fructose and sucrose
intake increase exogenous carbohydrate oxidation during exercise.
Nutrients. 9(pii): E1672017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khan TA and Sievenpiper JL: Controversies
about sugars: Results from systematic reviews and meta-analyses on
obesity, cardiometabolic disease and diabetes. Eur J Nutr. 55 Suppl
2:S25–S43. 2016. View Article : Google Scholar
|
|
3
|
Vlassara H and Striker GE: Advanced
glycation endproducts in diabetes and diabetic complications.
Endocrinol Metab Clin North Am. 42:697–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Graham P and Pick L: Drosophila as a model
for diabetes and diseases of insulin resistance. Curr Top Dev Biol.
121:397–419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taylor C and Hobbs FD: Type 2 diabetes,
thiazolidinediones, and cardiovascular risk. Br J Gen Pract.
59:520–524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Y, Liu Z, Chen Y and Jin LH:
Identification of the protective effects of traditional medicinal
plants against SDS-induced Drosophila gut damage. Exp Ther Med.
12:2671–2680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luyen BT, Tai BH, Thao NP, Cha JY, Lee HY,
Lee YM and Kim YH: Anti-inflammatory components of Chrysanthemum
indicum flowers. Bioorg Med Chem Lett. 25:266–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Onoda T, Ishikawa C, Fukazawa T, Li W,
Obayashi M and Koike K: Inhibitory activities of selected Kampo
formulations on human aldose reductase. BMC Complement Altern Med.
14:4352014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bier E and Bodmer R: Drosophila, an
emerging model for cardiac disease. Gene. 342:1–11. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Owusu-Ansah E and Perrimon N: Modeling
metabolic homeostasis and nutrient sensing in Drosophila:
Implications for aging and metabolic diseases. Dis Model Mech.
7:343–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li W, Luo Q and Jin LH: Acanthopanax
senticosus extracts have a protective effect on Drosophila gut
immunity. J Ethnopharmacol. 146:257–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parisi F, Riccardo S, Zola S, Lora C,
Grifoni D, Brown LM and Bellosta P: dMyc expression in the fat body
affects DILP2 release and increases the expression of the fat
desaturase Desat1 resulting in organismal growth. Dev Biol.
379:64–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang G, Hao Y and Jin LH: Overexpression
of jumu induces melanotic nodules by activating Toll signaling in
Drosophila. Insect Biochem Mol Biol. 77:31–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ecker A, Gonzaga TKSDN, Seeger RL, Santos
MMD, Loreto JS, Boligon AA, Meinerz DF, Lugokenski TH, Rocha JBTD
and Barbosa NV: High-sucrose diet induces diabetic-like phenotypes
and oxidative stress in Drosophila melanogaster: Protective role of
Syzygium cumini and Bauhinia forficata. Biomed Pharmacother.
89:605–616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Musselman LP, Fink JL, Narzinski K,
Ramachandran PV, Hathiramani SS, Cagan RL and Baranski TJ: A
high-sugar diet produces obesity and insulin resistance in
wild-type Drosophila. Dis Model Mech. 4:842–849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tapp RJ, Shaw JE, Zimmet PZ, Balkau B,
Chadban SJ, Tonkin AM, Welborn TA and Atkins RC: Albuminuria is
evident in the early stages of diabetes onset: Results from the
Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Am J
Kidney Dis. 44:792–798. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Géminard C, Rulifson EJ and Léopold P:
Remote control of insulin secretion by fat cells in drosophila.
Cell Metab. 10:199–207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kozma SC and Thomas G: Regulation of cell
size in growth, development and human disease: PI3K, PKB and S6K.
Bioessays. 24:65–71. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Potter CJ, Pedraza LG, Huang H and Xu T:
The tuberous sclerosis complex (TSC) pathway and mechanism of size
control. Biochem Soc Trans. 31:584–586. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murillo-Maldonado JM, Sánchez-Chávez G,
Salgado LM, Salceda R and Riesgo-Escovar JR: Drosophila insulin
pathway mutants affect visual physiology and brain function besides
growth, lipid, and carbohydrate metabolism. Diabetes. 60:1632–1636.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Q, Liu H, Yuan Z, Wei D and Ye Y:
Evaluation of antioxidant activity of chrysanthemum extracts and
tea beverages by gold nanoparticles-based assay. Colloids Surf B
Biointerfaces. 92:348–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Hu Q, Jiang S, Li F, Lin J, Han L,
Hong Y, Lu W, Gao Y and Chen D: Flos Chrysanthemi Indici protects
against hydroxyl-induced damages to DNA and MSCs via antioxidant
mechanism. J Saudi Chem Soc. 19:454–460. 2014. View Article : Google Scholar
|
|
23
|
Rulifson EJ, Kim SK and Nusse R: Ablation
of insulin-producing neurons in flies: Growth and diabetic
phenotypes. Science. 296:1118–1120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garofalo RS: Genetic analysis of insulin
signaling in Drosophila. Trends Endocrinol Metab. 13:156–162. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Colombani J, Raisin S, Pantalacci S,
Radimerski T, Montagne J and Léopold P: A nutrient sensor mechanism
controls Drosophila growth. Cell. 114:739–749. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haselton A, Sharmin E, Schrader J, Sah M,
Poon P and Fridell YW: Partial ablation of adult Drosophila
insulin-producing neurons modulates glucose homeostasis and extends
life span without insulin resistance. Cell Cycle. 9:3063–3071.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vereshchagina N and Wilson C: Cytoplasmic
activated protein kinase Akt regulates lipid-droplet accumulation
in Drosophila nurse cells Development. 133:1–4735. 2006.
|
|
28
|
Snel M, Jonker JT, Hammer S, Kerpershoek
G, Lamb HJ, Meinders AE, Pijl H, de Roos A, Romijn JA, Smit JW and
Jazet IM: Long-term beneficial effect of a 16-week very low calorie
diet on pericardial fat in obese type 2 diabetes mellitus patients.
Obesity (Silver Spring). 20:1572–1576. 2012. View Article : Google Scholar : PubMed/NCBI
|