Introduction
Rheumatoid arthritis (RA) frequently involves other
organs, including the lung, kidney, eyes and skin, apart from the
joints. RA-induced kidney damage may have various clinical and
pathological manifestations, but to date, RA-induced complement 3
glomerulonephritis (C3GN) has not been reported, to the best of our
knowledge. C3GN is caused by dysregulation of the alternative
pathway of complement diseases (1).
C3GN may be caused by acquired or congenital defects. Acquired C3GN
refers to the antibodies in the body against activated complement
factors, including the C3 nephritis factor or anti-H factor
antibodies, which continually activate the alternative pathway of
complements. C3GN caused by congenital defects refers to a genetic
abnormality or gene mutation in the alternative pathway of
complements that causes excessive activation (2). Studies have indicated that monoclonal
immunoglobulinopathies may also activate the alternative pathway of
complements and thus induce C3GN (3,4). In
addition, a study by Alexander et al (5) on C3GN and autoimmune disease suggests
that an autoimmune milieu may serve as a trigger for the
development of C3GN in patients who carry C3
glomerulopathy-associated risk alleles by leading to dysregulation
of the alternative pathway of complements. The study also suggested
that the short-term prognosis of C3GN associated with autoimmune
disorders is excellent. The present case study reports on renal
involvement in a patient with RA after 18 years, who was diagnosed
with C3GN through renal biopsy. The patient's treatment, follow-up
and outcome are presented.
Case report
A 63-year-old Chinese woman presented at the Second
Hospital of Jilin University (Changchun, China) in January 2015 due
to polyarthritis with pain, stiffness and swelling for 18 years, as
well as acute bilateral leg swelling and urinary abnormalities for
1 week. She had been diagnosed as having RA 18 years ago when she
presented with spontaneous symmetrical polyarthritis involving the
small joints of her hands and knee joint with significant morning
stiffness, and she received symptomatic treatment (the specific
treatment is unknown). Subsequently, she achieved remission of the
symptoms, but the symptoms of joint pain and swelling were
recurrent, so she took medication, including analgesics, Chinese
herbal supplements and various home remedies; however, her medical
condition did not stabilize. The patient had stopped using these
herbal supplements for >1 year prior to her presentation at
hospital. One week prior to her presentation at hospital, she had
bilateral pitting leg edema with no obvious predisposing causes.
The urine analysis revealed hematuria 3+ and proteinuria 3+ and
thus, the patient was scheduled for further treatment. The
patient's medical history included cholecystitis for 6 years, which
was stable, and bilateral inguinal hernia 3 years previously, which
was surgically treated. The patient had no history of hypertension,
diabetes or coronary disease, no known drug or food allergy, no
known family history of hypertension, diabetes, autoimmune disease
or kidney disease, and she was a non-smoker and did not consume any
alcohol. The physical examination revealed the following: Underarm
temperature, 36.5°C; pulse, 80 beats/min; respiratory rate, 18
breaths/min; blood pressure, 145/85 mmHg; height, 153 cm; and
weight, 48 kg. She had mucosal edema of the face with anemic
palpebral conjunctiva, and mild dropsy of the bilaretal eyelids and
legs. Her hands had an ulnar deviation and swan-neck deformity on
the first to fourth fingers. The proximal interphalangeal joint,
metacarpophalangeal joint and wrist joint had tenderness. Her
wrist, elbow and knee joints had a slightly limited range of
motion. No subcutaneous nodules were observed on the joints of her
four extremities. The laboratory test results are presented in
Table I. Apart from serum creatinine
and urea nitrogen, which are normal values, the remainder of the
results in Table I are abnormal. The
chest computed tomography scan revealed bilateral pleural effusion,
predominantly on the left side. The bone marrow morphology test
results indicated hyperplastic anemia with an iron deficiency
(cellular iron and exocellular iron were negative). Histological
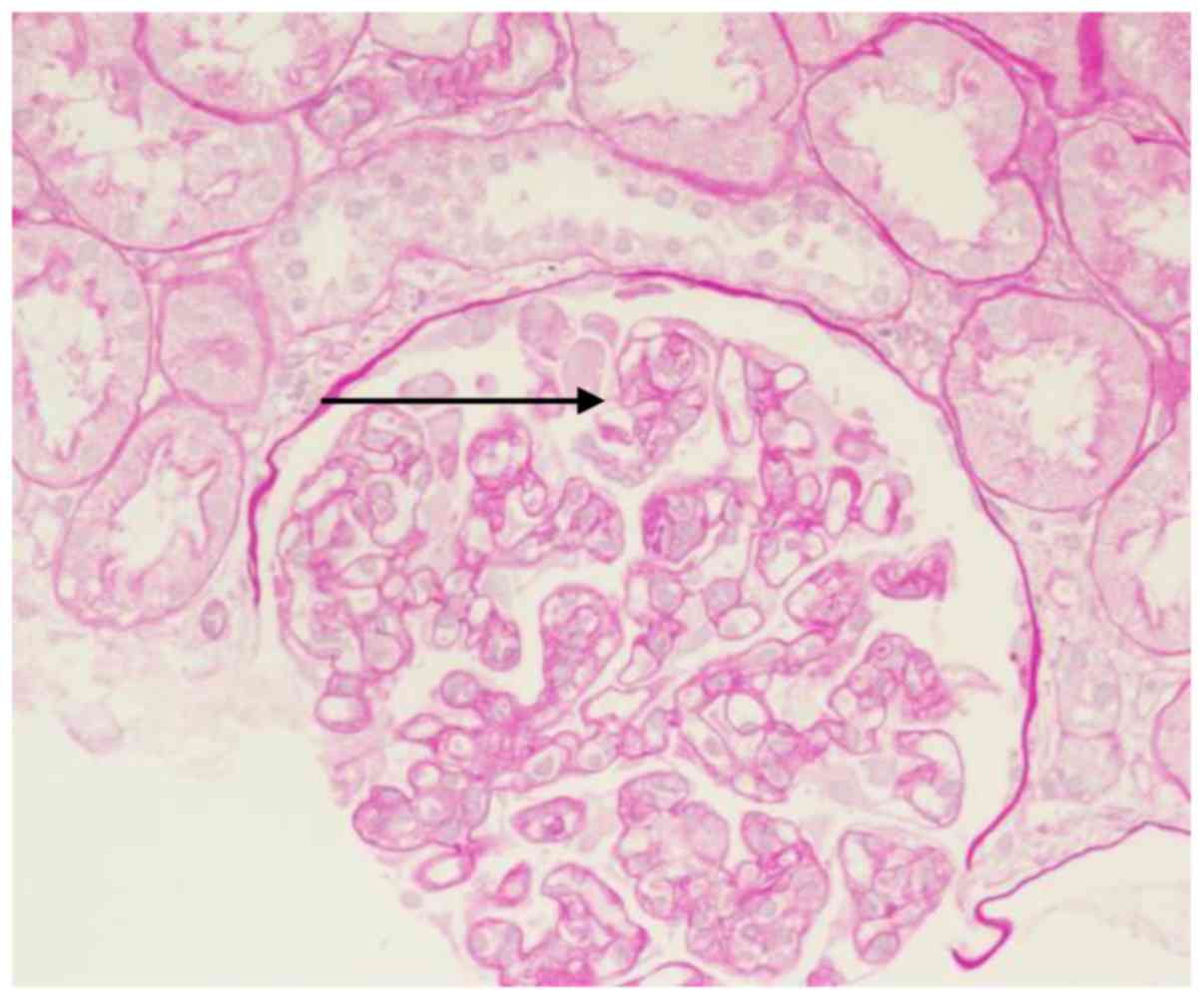
analysis of the renal biopsy indicated the following: 15 whole
glomeruli were observed, part of the glomerular volume was
increased, 3 glomeruli were globally sclerotic, 2 cells were
fibrotic, 1 small cellular crescent formation was present and 2
capillary loops exhibited segmental fibroid necrosis. Glomerular
endothelial cells exhibited diffuse hyperplasia, certain capillary
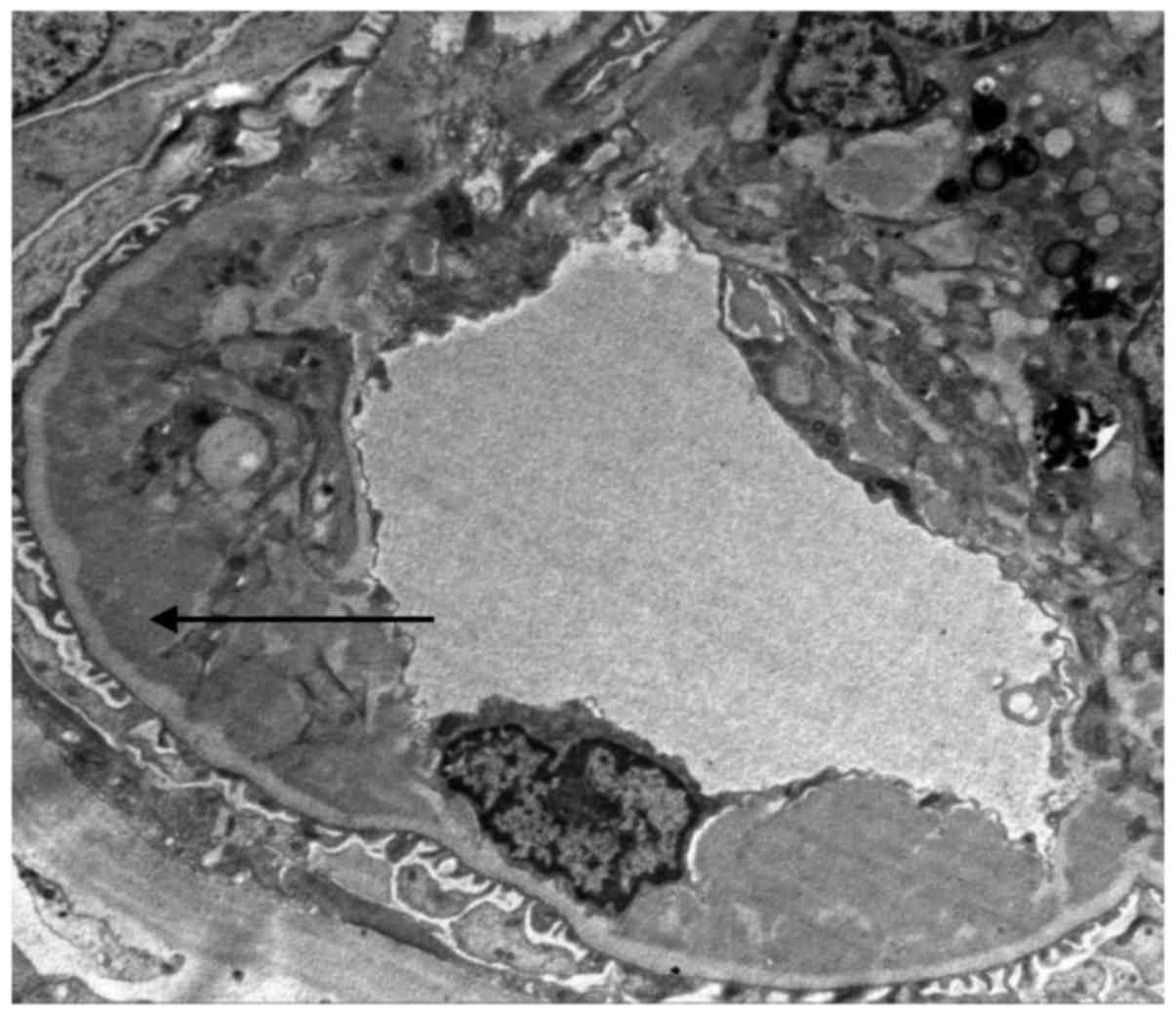
loops displayed limited opening (Fig.
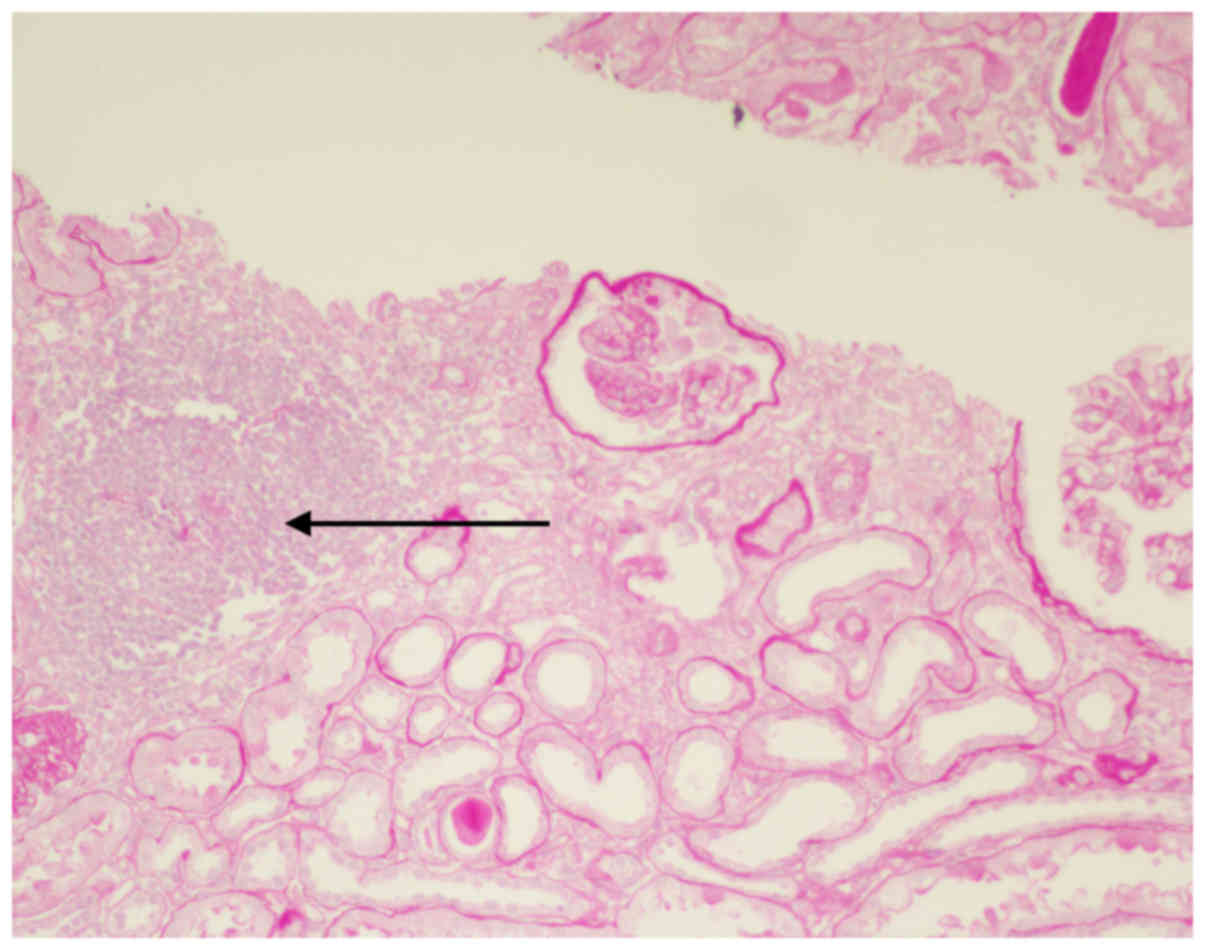
1), renal tubular epithelial cells were diffuse with granular
degeneration, tubular focal atrophy was present, protein and red
blood cell cast was visible, mild edema was observed in the renal
interstitium, small focal fibrosis and focal inflammatory-cell
infiltration were present, an inflammatory cell cluster was
observed and the prevailing cell type was mononuclear cells
(Fig. 2). The small arteries were
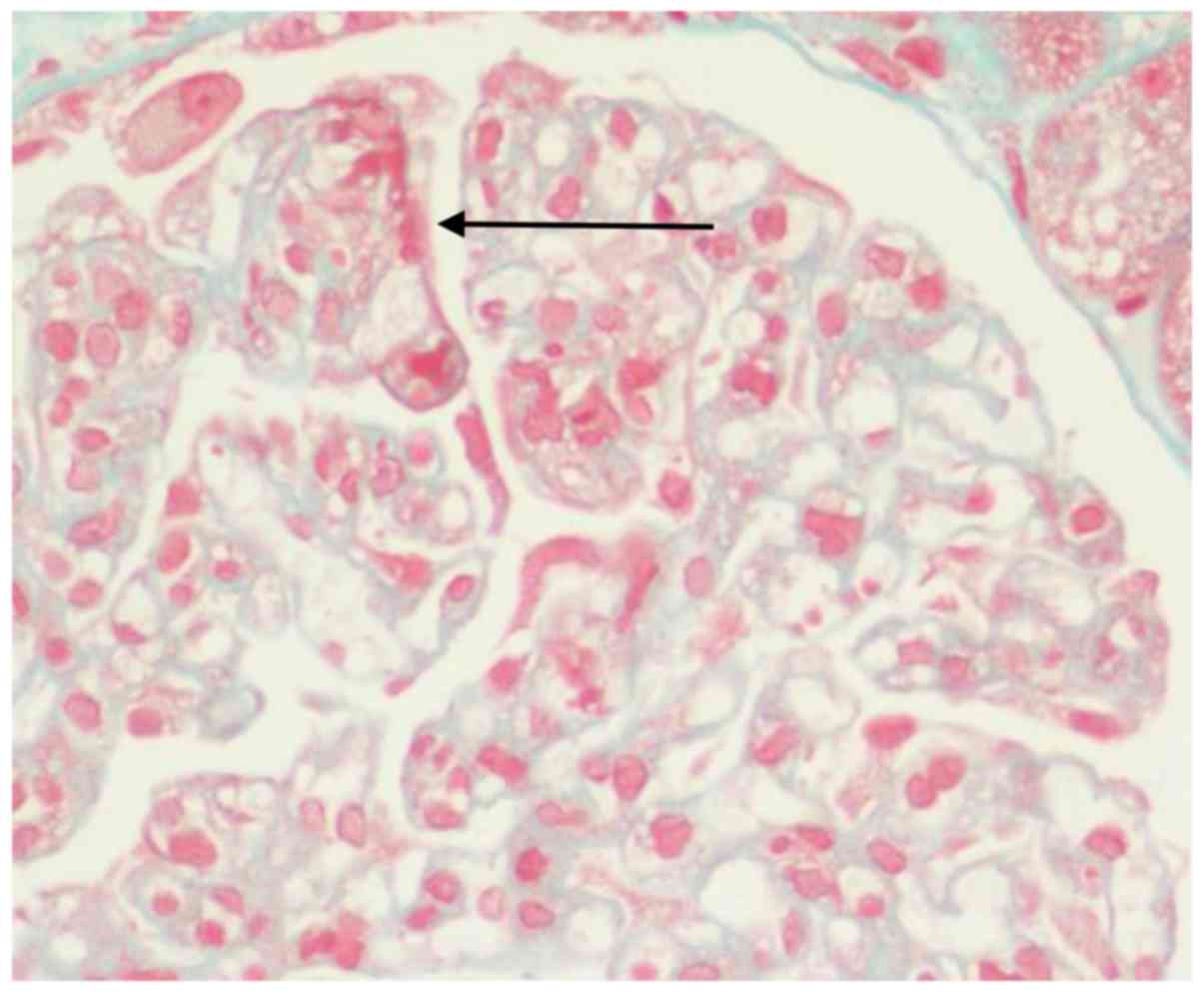
unremarkable. Periodic-acid methenamine silver and Masson staining
indicated no spikes or double tracking, and there was a deposition
of a segmental eosinophilic substance in the subendothelial area
(Fig. 3). Congo red staining was
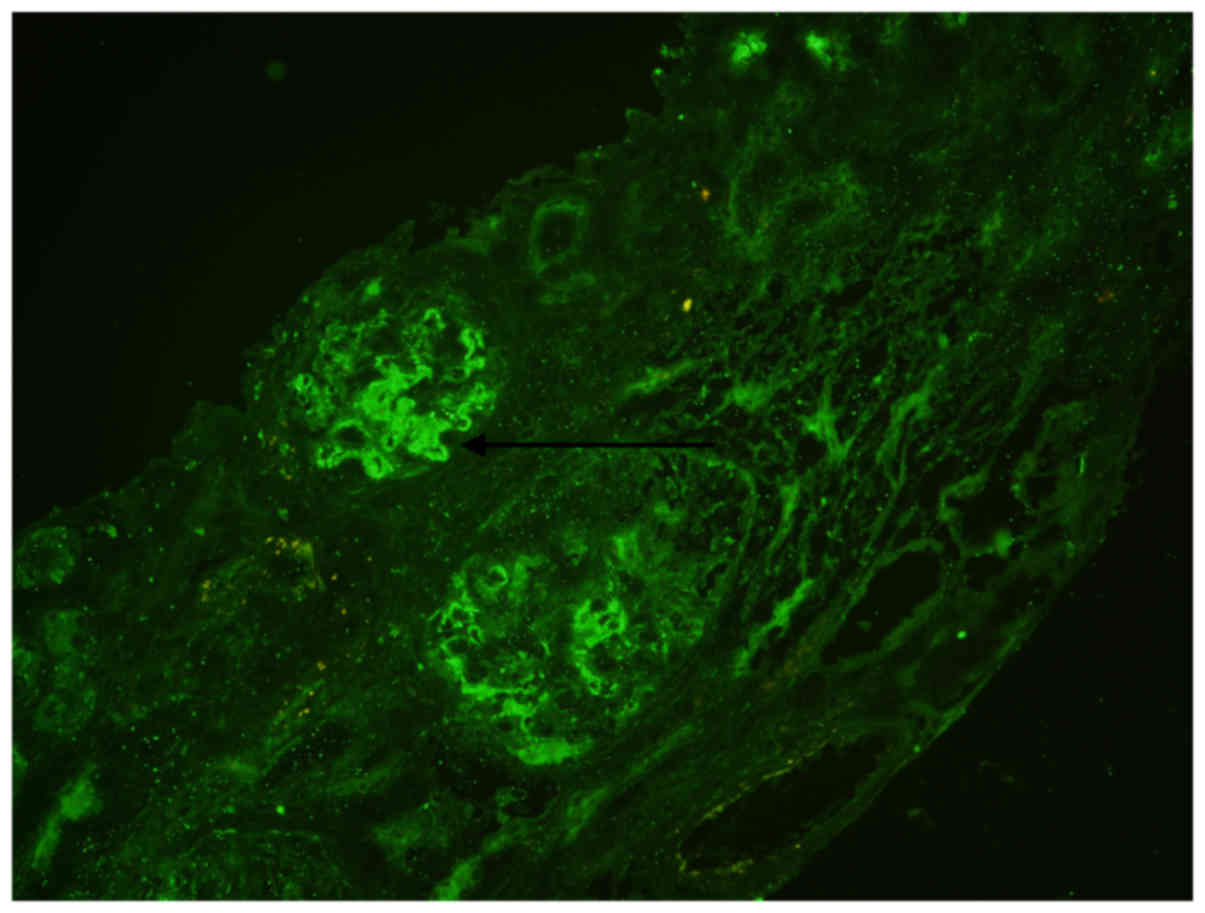
negative (data not shown). Immunofluorescence microscopy revealed
bright C3 staining (2+) in the mesangium and granular deposition
along the capillary walls (Fig. 4),
while immunofluorescent reactivity was negative for immunoglobulin
(Ig)G, IgM, IgA, C4, C1q and fibrillarin (results not shown).
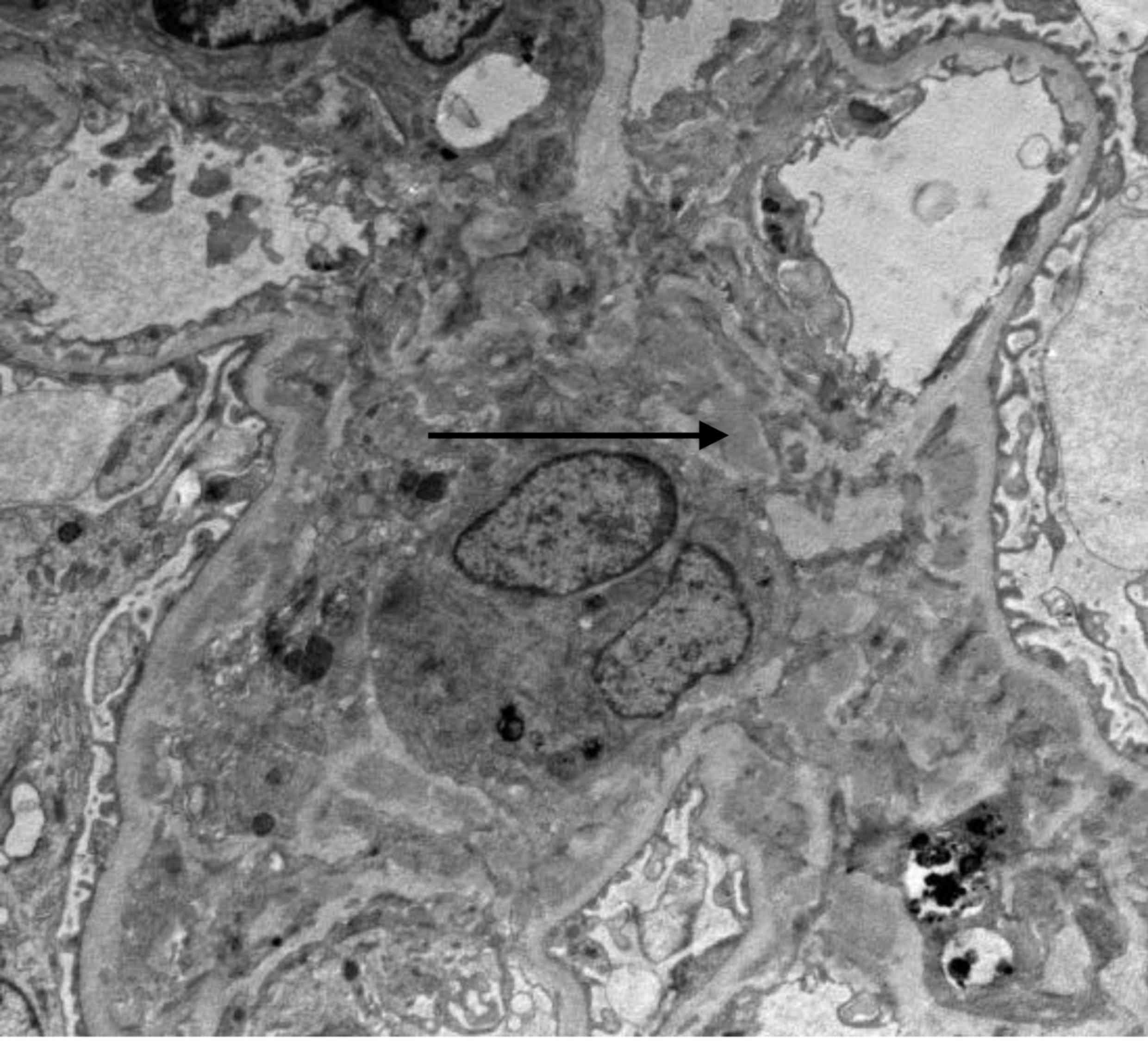
Electron microscopy indicated glomerular mesangial cell
proliferation and mild hyperplasia of the mesangial matrix, diffuse
endothelial cell proliferation, a widened inner tectorium of the
basal membrane segment, electron-dense deposits in the
subendothelial, mesangial and paramesangial area, fusion of most
foot processes and increased lysosome in renal tubular epithelial
cells; part of the microvilli exhibited shedding, atrophy, mild
edema of the tubular interstitium, a small amount of lymphocytes
and mononuclear-cell infiltration with collagen fiber hyperplasia
(Figs. 5 and 6). The clinical diagnoses were RA, C3GN,
hypothyroidism and iron-deficiency anemia. The patient was treated
with a daily dose of prednisone acetate (40 mg) combined with a
lipid-lowering drug, anticoagulant and supplements of thyroxine and
iron. The patient's follow-up results are presented in Table II. These included changes in each
index during the follow-up (including urinary protein, 24 h urinary
protein quantification, erythrocyte, hemoglobin, albumin and
complement). It was observed that the 24 h urinary protein
quantification rapidly decreased, the urine red blood cells
gradually decreased, the serum albumin gradually increased and
hemoglobin gradually increased to normal. The patient now receives
small doses of prednisone acetate maintenance therapy (10 mg per
day).
 | Table I.Patient's laboratory examination
results. |
Table I.
Patient's laboratory examination
results.
| Parameters | Value | Reference range |
|---|
| Proteinuria | 3+ | Negative |
| 24-h urine protein
(g) | 4.46 | 0–0.15 |
| Urine RBC count
(/HPF) | 52.0 | 0.0–5.0 |
| Hb (g/l) | 56 | 115–150 |
| RBC count
(×1012/l) | 2.63 | 3.80–5.10 |
| Mean corpuscular
volume (fl) | 71.9 | 82.0–100.0 |
| Mean corpuscular Hb
(pg) | 21.3 | 27.0–34.0 |
| Mean corpuscular Hb
concentration (g/l) | 296.0 | 316.0–354.0 |
| TP (g/l) | 64.7 | 65–85 |
| Albumin (g/l) | 31.1 | 40–55 |
| Total cholesterol
(mmol/l) | 8.75 | 2.9–5.17 |
| LDL cholesterol
(mmol/l) | 4.13 | 2.70–3.40 |
| Serum creatinine
(µmol/l) | 95 | 44–106 |
| Urea nitrogen
(mmol/l) | 7.08 | 1.79–7.14 |
| Anti-CCP level
(RU/ml) | 142.0 | 0–5 |
| AKA | Positive | Negative |
| APF | Weakly positive | Negative |
| P-ANCA | Positive | Negative |
| ANCA-MPO (RU/ml) | <20 | 0–20 |
| ANA ratio | 1:320;
homogeneous | <1:100 |
| RF (IU/ml) | <20 | 0.0–20.0 |
| ESR (mm) | 102.0 | <20 |
| CRP (mg/l) | 2.37 | 0.00–0.80 |
| C3 (mg/dl) | 68.90 | 79–152 |
| C4 (mg/dl) | 12.60 | 16–38 |
| IgA (g/l) | 5.91 | 0.82–4.53 |
| TSH (mIU/l) | >150.000 | 0.55–4.78 |
| FT3 (pmol/l) | 1.36 | 3.5–6.5 |
| FT4 (pmol/l) | 4.25 | 11.5–22.7 |
| TPOAb (U/ml) | 247.40 | 0–60 |
| TgAb (U/ml) | 127 | 0–60 |
| TT3 (nmol/l) | 0.36 | 0.92–2.79 |
| TT4 (nmol/l) | 24.80 | 58.1–140.6 |
| Ferritin (ng/ml) | 5.10 | 11.0–306.8 |
 | Table II.Follow-up results. |
Table II.
Follow-up results.
| Parameters | 1 month | 2 months | 4 months | 8 months | 14 months | 18 months |
|---|
| Proteinuria | 2+ | 3+ | 2+ | 1+ | 1+ | 1+ |
| 24-h urine protein
(g) |
| 3.53 | 0.5 |
| 0.35 | 0.34 |
| Urine RBC count
(/HPF) | 27.5 | 13.3 | 4 | 4.5 | 2.7 | 2.8 |
| Hb (g/l) | 86 | 103 | 109 | 120 | 148 | 155 |
| RBC count,
(×1012/l) | 3.51 | 3.87 | 4.34 | 4.57 | 4.85 | 5.08 |
| TP (g/l) | 55.6 | 52.1 | 63.1 | 55.6 | 70.8 | 67.2 |
| Albumin (g/l) | 29 | 30 | 36.7 | 34.3 | 39.7 | 37.2 |
| FT3 (pmol/l) | 1.89 | 2.32 | 3.31 |
| 3.98 | 4.78 |
| FT4 (pmol/l) | 12.21 | 15.78 | 22.47 |
| 23.73 | 22.74 |
| TSH (mIU/l) | 53.408 | 26.229 | 10.985 |
| 6.534 | 5.576 |
| Anti-CCP (RU/ml) |
|
|
|
| 88 | 18 |
| C3 (mg/dl) |
|
|
|
|
| 76.00 |
| C4 (mg/dl) |
|
|
|
|
| 14.80 |
| RF (IU/ml) |
|
| <20 | <20 | <20 | <20 |
| AKA |
|
|
|
| Negative | Negative |
Discussion
C3 glomerulopathy has been gradually recognized in
recent years. Fakhouri et al (1) proposed the concept of C3 nephropathy
for the first time in 2010. Subsequently, the definition of C3
nephropathy was further substantiated and revised by
multidisciplinary experts in 2012 (2). To date, numerous studies on C3
glomerulopathy from China and abroad have been published (6,7). C3
nephropathy results from dysregulation of the alternative pathway
of C3, and the major pathological characteristics are the
deposition of complement C3 on glomeruli, with or without minimal
Ig deposition and without complement C4 and C1q deposition. C3
nephropathy is divided into dense deposit disease and C3GN
according to the characteristics on electron microscopy. The
electron-dense deposits of C3GN may be identified in the basement
membrane, as well as the mesangial, subendothelial and
subepithelial areas (2,8). The histopathological changes in the
present case were endocapillary proliferative changes with certain
crescentic formatins, C3 granules deposited in the subendothelial
and mesangial areas, and electron-dense deposits in mesangial and
subendothelial areas. The following diseases were excluded: i)
Acute post-streptococcal glomerulonephritis, as the complement
remained low at 1 year and there were no subepithelial humplike
dense deposits observed under electron microscopy; ii)
membranoproliferative glomerulonephritis type I, as there was no
mesangial cell proliferation, and mesangial cells were not
identified in the endothelial cells under light microscopy. In
addition, there was only complement C3 deposition and no
immunoglobulin deposition observed under immunofluorescence
microscope; iii) systemic lupus erythematosus, although the patient
had joint swelling and pain, nephritis and low complementemia, the
ANA were negative and there was no deposition of various immune
complex deposits observed under immunofluorescence microscopy; iv)
cryoglobulinemia, the test results were negative for the hepatitis
C antibody, normal for the rheumatoid factor and negative for
cryoglobulin in the sera. No thrombus-like substance in the loop
cavity was identified; and v) monoclonal Ig disease associated with
C3 nephritis, as the results of chains λ in serum, urine M protein
and cryoglobulin in the sera were all negative. The bone marrow
biopsy did not reveal abnormal plasma cell proliferation.
Therefore, the diagnosis of C3GN was confirmed.
RA-induced GN is rare, and to date, RA with C3GN has
not been reported in the relevant literature. In 2016, Alexander
et al (5) reported on a
cohort of 85 patients diagnosed with C3GN from 2007 to 2014, and of
these, 10 patients (3 men and 7 women; 12%) also had autoimmune
diseases. These 10 patients had antinuclear antibody titer
abnormalities, and in 6 patients, anti-double-stranded DNA
antibodies were detected. The incidence of autoimmune diseases in
this previous study was higher than that in the general population.
Therefore, C3GN may be secondary to autoantibodies that directly
activate the alternative pathway of C3 or indirectly lead to its
dysregulation. Autoimmune diseases occur early in the course of
C3GN. In addition, Alexander et al (5) reported that the treatment was based on
the use of prednisone alone or in combination with
cyclophosphamide. The serum creatinine and urine protein level
decreased significantly, suggesting the presence of C3GN with
autoimmune diseases and a good prognosis. The patient of the
present study was treated with hormone therapy alone and given
maintenance treatment following their diagnosis; the patient's
nephritis reached complete clinical remission at 4 months and the
condition remained stable after a follow-up of 18 months with
low-dose hormone maintenance therapy.
The patient of the present study suffered from
kidney damage after years of RA, and at the same time, autoimmune
thyroiditis occurred, so the presentation of C3GN may be secondary
to autoimmune diseases. However, no direct evidence is available to
prove that C3RN is caused by RA and/or autoimmune thyroiditis. The
patient responded well to hormone therapy and the condition
remained stable for a long time, which suggests that the C3GN may
be associated with autoimmune diseases. It should also be noted
that the patient's renal histopathological examination indicated
the formation of crescents and segmental fibroid necrosis in
certain capillary loops, while perinuclear anti-neutrophil
cytoplasmic antibody (ANCA) was present; therefore, the combined
diagnosis of ANCA-associated vasculitis (AAV) should be considered.
A review of the literature indicated that conditions including SLE
(9), scleroderma (10) and RA (11), may all occur in combination with AAV,
and that the syndromes tend to overlap (9,11).
However, the patient had normal renal function and massive
proteinuria, immunofluorescence and electron microscopic analysis
revealed the deposition of immune complexes, and myeloperoxidase
and protease 3 were negative. These results do not support the
diagnosis of AAV, and it is therefore unlikely that this patient
had AAV.
Acknowledgements
The authors would like to thank Dr Fan Yang (The
Second Hospital of Jilin University, Changchun, China) for
capturing the images.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
DZ conceived the present study. XW analyzed the data
and prepared the manuscript. XW and LH performed the data analyses
and wrote the manuscript. HL assisted with the data analysis.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of their data and associated images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
C3
|
complement 3
|
|
GN
|
glomerulonephritis
|
|
RA
|
rheumatoid arthritis
|
|
Ig
|
immunoglobulin
|
|
AAV
|
ANCA-associated vasculitis
|
References
|
1
|
Fakhouri F, Frémeaux-Bacchi V, Noël LH,
Cook HT and Pickering MC: C3 glomerulopathy: A new classification.
Nat Rev Nephrol. 6:494–499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pickering MC, D'Agati VD, Nester CM, Smith
RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M,
et al: C3 glomerulopathy: Consensus report. Kidney Int.
84:1079–1089. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bridoux F, Desport E, Frémeaux-Bacchi V,
Chong CF, Gombert JM, Lacombe C, Quellard N and Touchard G:
Glomerulonephritis with isolated C3 deposits and monoclonal
gammopathy: A fortuitous association? Clin J Am Soc Nephrol.
6:2165–2174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang LH, Chen Z, Xu F, Zhang T, Zhang HT,
Ge YC, Zhou Y, Zeng CH, Hu WX, Liu ZH and Tang Z: C3
glomerulonephritis associated with monoclonal gammopathy. Chin J
Nephrol Dial Transplant. 24:507–511. 2015.
|
|
5
|
Alexander MP, Fervenza FC, De Vriese AS,
Smith RJH, Nasr SH, Cornell LD, Herrera Hernandez LP, Zhang Y and
Sethi S: C3 glomerulonephritis and autoimmune disease: More than a
fortuitous association? J Nephrol. 29:203–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang T, Zhang HT, Xu F, Huang Q, Wang JQ,
Zeng CH, Chen HM and Liu ZH: Clinicopathologic features of patients
with C3 glomerulopathy. Chin J Nephrol Dial Transplant. 23:426–431.
2014.(In Chinese).
|
|
7
|
Hou J, Markowitz GS, Bomback AS, Appel GB,
Herlitz LC, BarryStokes M and D'Agati VD: Toward a working
definition of C3 glomerulopathy by immunofluorescence. Kidney Int.
85:450–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang HT, Chen HP, Zeng CH, Deng KP, Li
SJ, Zheng CX and Liu ZH: C3 glomerulonephritis: A clinicopathologic
analysis of 17 cases. Chin J Nephrol Dial Transplant. 20:307–311.
2011.(In Chinese).
|
|
9
|
Hervier B, Hamidou M, Haroche J, Durant C,
Mathian A and Amoura Z: Systemic lupus erythematosus associated
with ANCA-associated vasculitis: An overlapping syndrome?
Rheumatology International. 32:3285–3290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Derrett-Smith EC, Nihtyanova SI, Harvey J,
Salama AD and Denton CP: Revisiting ANCA-associated vasculitis in
systemic sclerosis: Clinical, serological and immunogenetic
factors. Rheumatology (Oxford). 52:1824–1831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Draibe J and Salama AD: Association of
ANCA associated vasculitis and rheumatoid arthritis: A lesser
recognized overlap syndrome. Springerplus. 4:502015. View Article : Google Scholar : PubMed/NCBI
|