Introduction
Chronic hepatitis C virus (HCV) infection is a
common cause of liver cirrhosis and hepatocellular carcinoma (HCC)
(1,2). HCC is the third leading cause of
cancer-related death in Japan, and the annual risk of developing
HCC among HCV-infected patients with compensated cirrhosis is
reportedly 1.8–8.3% (3). The goal of
anti-HCV therapy is to successfully eradicate HCV to resolve liver
disease. Currently, interferon (IFN)-based therapies, usually in
combination with ribavirin (RBV), are commonly used to eradicate
HCV infection, but this combination therapy causes severe adverse
effects, such as hematological toxicity and depression (4–7);
therefore, direct-acting antiviral (DAA) agents are increasingly
used for the treatment of HCV infection (8). To date, the effects of IFN-free DAA
therapy on depressive symptoms has not been well-documented.
Therefore, the aims of the present study were to evaluate the
efficacy and tolerability of various IFN-free treatment regimens in
Japanese patients with HCV genotype-1 infection and to evaluate the
severity of depression symptoms using the Beck Depression
Inventory-II (BDI-II) questionnaire (9,10).
Materials and methods
Patients
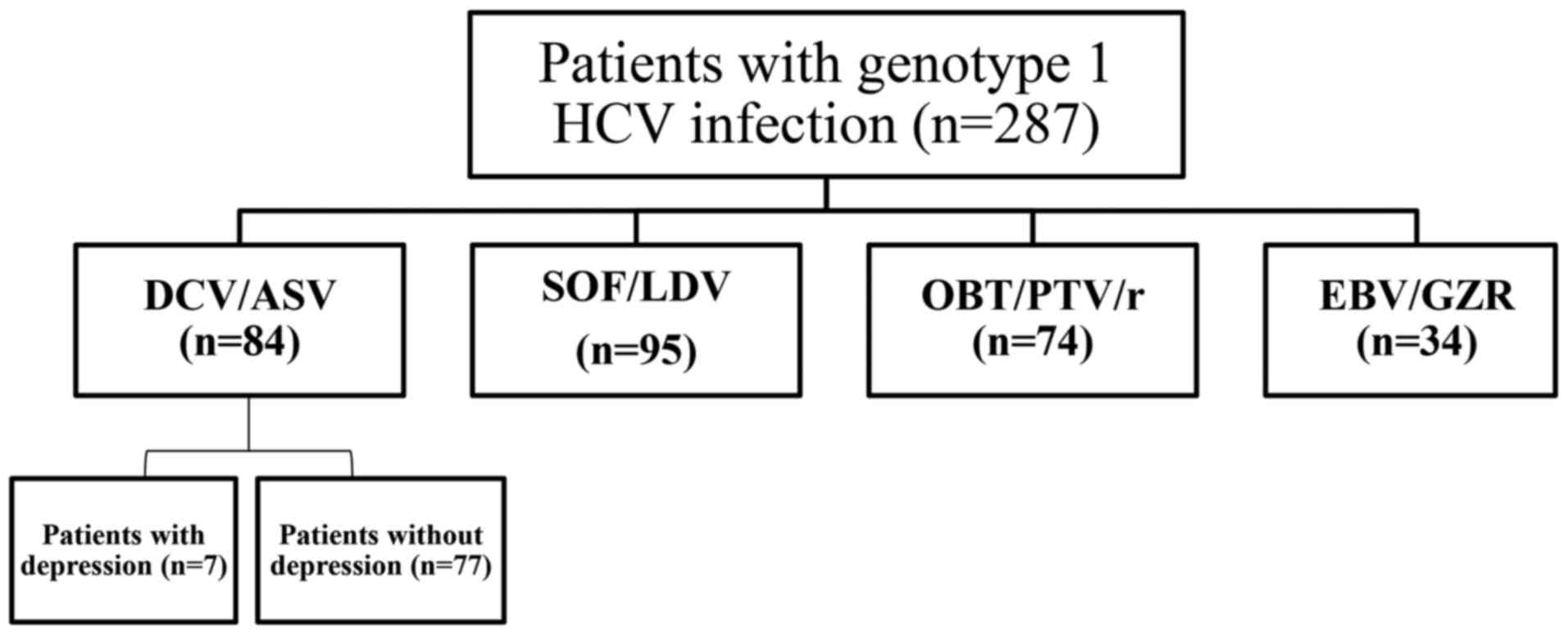
The study cohort consisted of 287 consecutive
patients with HCV genotype-1 infection who were treated at Nara
Medical University Hospital (Kashihara, Japan) from November 2013
to July 2015. The remaining 287 patients included 84 who were
treated for 24 weeks with daclatasvir/asunaprevir (DCV/ASV;
Bristol-Myers Squibb, Princeton, NJ, USA), 95 treated for 12 weeks
with sofosbuvir/ledipasvir (SOF/LDV; Gilead Sciences, Inc., Foster
City, CA, USA), 74 treated for 12 weeks with
ombitasvir/paritaprevir/ritonavir (OBV/PTV/r; AbbVie Inc., North
Chicago, IL, USA), and 34 treated for 12 weeks with
elbasvir/grazoprevir (EBV/GZR; MSD, Tokyo, Japan) (Fig. 1). The presence of NS5A
resistance-associated substitutions (RASs), which decrease the
sustained the sustained virological response (SVR) rate (11,12), was
assessed in all patients receiving DAAs prior to the start of
therapy by direct sequencing (13).
Exclusion criteria were coinfection with another virus, pregnancy,
history of clinical hepatic decompensation, or the use of
immunosuppressants. The study protocol was approved by the Ethics
Committee of Nara Medical University Hospital and conducted in
accordance with the tenets of the Tokyo revision of the Declaration
of Helsinki (1975).
BDI-II questionnaire
All patients enrolled in the study also completed
the BDI-II, which is a 21-question, self-reported, screening
instrument used to assess characteristic attitudes and symptoms of
depression. Each item is assigned a score of 0–3, with 3 indicating
the most severe symptoms. A cumulative score is determined by
adding the scores of the individual items. BDI-II scores were
determined using the guidelines set forth in the BDI-II manual
(14,15). In general, a score of <9 indicates
no or minimal depression, that of 10–18 indicates mild-to-moderate
depression, that of 19–29 indicates moderate-to-severe depression,
and that of >30 indicates severe depression. The clustering of
depressive symptoms was further examined using specific, somatic,
and cognitive-affective symptom dimensions described by Beck et
al (16).
Statistical analysis
BDI-II subscale scores were evaluated using one-way
analysis of variance followed by the Bonferroni multiple-comparison
test. Bivariate analyses of nominal parameters were performed using
the chi-squared test. A paired t-test was used to evaluate changes
in body mass index (BMI). Statistical analyses were performed using
GraphPad Prism version 6.04 software (GraphPad Software, Inc., La
Jolla, CA, USA). All tests were two-tailed and a probability
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline characteristics of patients
treated with IFN-free DAAs
Overall, 287 patients were enrolled in this study
(Table I). The study cohort
comprised 61.0% female patients, 81.5% patients with chronic
hepatitis, 98.3% with HCV genotype 1b, and 38.3% who received
previous IFN therapy, and the mean age was 66.3±23.2 years. NS5A
RASs were identified in 18.4% patients.
 | Table I.Baseline characteristics of patients
treated with IFN-free DAA therapies (n=287). |
Table I.
Baseline characteristics of patients
treated with IFN-free DAA therapies (n=287).
| Parameters | Value |
|---|
| Mean age, years
(SD) | 66.3 (23.2) |
| Sex (%) |
|
|
Male | 112 (39.0) |
|
Female | 175 (61.0) |
| Liver disease
(%) |
|
| Chronic
hepatitis | 234 (81.5) |
| Liver
cirrhosis | 53 (18.5) |
| Genotype (%) |
|
| Ia | 5 (1.7) |
| Ib | 282 (98.3) |
| Previous IFN
therapy (%) | 110 (38.3) |
| Y93/L31
resistance-associated variants (%) | 53 (18.4) |
| Mean serum HCV-RNA
level, log10 IU/ml (SD) | 5.6 (2.1) |
| Mean aspartate
transaminase, IU/l (SD) | 40 (17) |
| Mean alanine
aminotransferase, IU/l (SD) | 47 (24) |
| Mean platelet
count, 103/µl (SD) | 16 (6.2) |
| Mean albumin, g/dl
(SD) | 4.1 (1.7) |
| Mean total
bilirubin, mg/dl (SD) | 0.5 (0.1) |
| SSRI use (before
DAA therapy) (%) | 4 (1.4) |
| Antipsychotic use
(before DAA therapy) (%) | 10 (3.5) |
| Benzodiazepine use
(before DAA therapy) (%) | 2 (0.7) |
| Tricyclic or
Tetracyclic antidepressants (before DAA therapy) (%) | 5 (1.7) |
| No medication
(before DAA therapy) (%) | 273 (95.1) |
| SSRI use (after DAA
therapy) (%) | 4 (1.4) |
| Antipsychotic use
(after DAA therapy) (%) | 10 (3.5) |
| Benzodiazepine use
(after DAA therapy) (%) | 2 (0.7) |
| Tricyclic or
Tetracyclic antidepressants (after DAA therapy) (%) | 5 (1.7) |
| No medication
(after DAA therapy) (%) | 273 (95.1) |
The baseline characteristics of seven patients with
depression who received the 24-week DCV/ASV treatment regimen are
summarized in Table II. Among the
seven patients, six (85.7%) had chronic hepatitis, six (85.7%) had
HCV genotype 1b, none had RASs, and six (85.7%) were female.
 | Table II.Baseline characteristics of
depressive patients with DCV/ASV therapy (n=7). |
Table II.
Baseline characteristics of
depressive patients with DCV/ASV therapy (n=7).
| Parameters | Value |
|---|
| Mean age, years
(SD) | 59.1 (23.2) |
| Sex (%) |
|
|
Male | 1 (14.3) |
|
Female | 6 (85.7) |
| Liver disease
(%) |
|
| Chronic
hepatitis | 6 (85.7) |
| Liver
cirrhosis | 1 (14.3) |
| Genotype (%) |
|
| Ib | 6 (85.7) |
| I | 1 (14.3) |
| Previous IFN
therapy (%) | 3 (42.9) |
| Y93/L31
resistance-associated variants (%) | 0 (0) |
| Mean serum HCV-RNA
level, log10 IU/ml (SD) | 5.7 (0.6) |
| Mean aspartate
transaminase, IU/l (SD) | 38.0 (21.0) |
| Mean alanine
aminotransferase, IU/l (SD) | 32.6 (29.2) |
| Mean platelet
count, 103/µl (SD) | 19.2 (5.1) |
| Mean albumin, g/dl
(SD) | 4.0 (1.8) |
| Mean total
bilirubin, mg/dl (SD) | 0.3 (0.2) |
| SSRI use (%) | 1 (14.3) |
| Antipsychotic use
(%) | 5 (71.4) |
| Benzodiazepine use
(%) | 1 (14.3) |
| Tricyclic or
Tetracyclic antidepressants (%) | 2 (28.6) |
| No medication
(%) | 2 (28.6) |
Baseline characteristics of patients
treated with IFN-free DAAs
Eighty four Japanese patients received 24 weeks of
DCV/ASV (SVR, 97%), 95 received 12 weeks of SOF/LDV, 74 received 12
weeks of OBT/PTV/r, and 34 received 12 weeks of EBV/GZR (Table III). Patients aged ≥65 years were
more often treated with EBV/GZR (85.2%, 29/34) than DCV/ASV (52.4%,
44/84), SOF/LDV (69.5%, 66/95), or OBT/PTV/r (62.2%, 46/74)
(P<0.01). Patients with cirrhosis were more commonly treated
with DCV/ASV [29.8% (25/84)] than SOF/LDV (18.9%. 18/95), OBT/PTV/r
(10.8%, 7/74), or EBV/GZR (8.8%, 3/34) (P<0.01). The NS5A
resistance-associated variant Y93H was not detected at baseline in
any patient treated with OBV/PTV/r. Patients with NS5A resistance
were more commonly treated with DCV/ASV (3.5%, 3/84) than SOF/LDV
(40.0%, 38/95) or EBV/GZR (35.3%, 12/34) (P<0.01). Patients who
received IFN-based therapy were more commonly treated with DCV/ASV
(61.9%, 52/84) than SOF/LDV (40.0%, 38/95), OBT/PTV/r (27.2%,
20/74), or EBV/GZR (35.3%, 12/34) (P<0.01). Among the four
treatment groups, the percentage of non-responders to IFN was
greatest among patients treated with ASV/DCV (P<0.01). Patients
with impaired renal function were more commonly treated with
EBV/GZR (23.5%, 8/34) than OBT/PTV/r (11%, 9/74), SOF/LDV (0%, 0),
or DCV/ASV (0%, 0) (P<0.01). There were no statistical
differences between the depression rates between the four
groups.
 | Table III.Baseline demographics and disease
characteristics of the patients with HCV infection. |
Table III.
Baseline demographics and disease
characteristics of the patients with HCV infection.
| Characteristic | DCV/ASV (n=84) | SOF/LDV (n=95) | OBT/PTV/r
(n=74) | EBV/GZR (n=34) | P-value |
|---|
| Age (years) | 64±15.3 | 68±10.5 | 67±24.9 | 71±8.6 | n.s |
| Age ≥65 years | 44 (52.4) | 66 (69.5) | 46 (62.2) | 29 (85.2) | P<0.01 |
| Male | 42 (50.0) | 35 (36.8) | 21 (32.3) | 14 (41.2) | P<0.05 |
| Cirrhosis | 25 (29.8) | 18 (18.9) | 7 (10.8) | 3 (8.8) | P<0.01 |
| Genotype 1b | 79 (94.0) | 85 (89.5) | 63 (96.9) | 30 (88.2) | n.s |
| HCV-RNA
(log10 IU/ml) | 5.6±0.9 | 5.9±0.7 | 5.9±0.6 | 5.2±0.5 | n.s |
|
Resistance-associated variants | 3 (3.5) | 38 (40.0) | 0 (0.0) | 12 (35.3) | P<0.01 |
| Prior IFN-based
therapy | 52 (61.9) | 38 (40.0) | 20 (27.2) | 12 (35.3) | P<0.01 |
| Intolerable | 5 (9.6) | 8 (21.1) | 4 (20.0) | 4 (33.3) | n.s |
|
Breakthrough/relapse | 12 (23.1) | 18 (47.3) | 8 (40.0) | 4 (33.3) | n.s |
| Non-response | 35 (67.3) | 12 (31.6) | 8 (40.0) | 6 (50.0) | P<0.01 |
| eGFR >30
ml/min/1.73 m2 or hemodialysis | 0 | 0 | 9 (11.0) | 8 (23.5) | P<0.01 |
| Depression | 7 (8.3) | 5 (5.2) | 5 (6.8) | 2 (5.9) | n.s |
Viral suppression after initiation of
IFN-free therapy, end of treatment response, and SVR
Fig. 2 shows the
percentages of patients in the four groups who achieved viral
suppression based on the duration after the start of therapy, while
treatment was still ongoing, at week 4 (rapid virological response,
RVR), at week 12 (end of treatment, ETR), and at 4 and 12 weeks
after the end of treatment (SVR4 and SVR12). The RVR, ETR, SVR4,
and SVR12 rates were 76% (64/84), 92% (54/55), 93% (78/84), and 92%
(77/84), respectively, in patients treated with DCV/ASV; 85%
(81/95), 99% (94/95), 97% (92/95), and 98% (93/95) for those
treated with SOF/LDV; 88% (65/74), 100% (72/72), 99% (67/68), and
98% (65/66) for those treated with OBV/PTV/r; and 85% (29/34), 100%
(25/25), 100% (22/22), and 100% (20/20) for those treated with
EBV/GZR. No significant differences were observed in the RVR, ETR,
SVR4, and, SVR12 rates among the four groups.
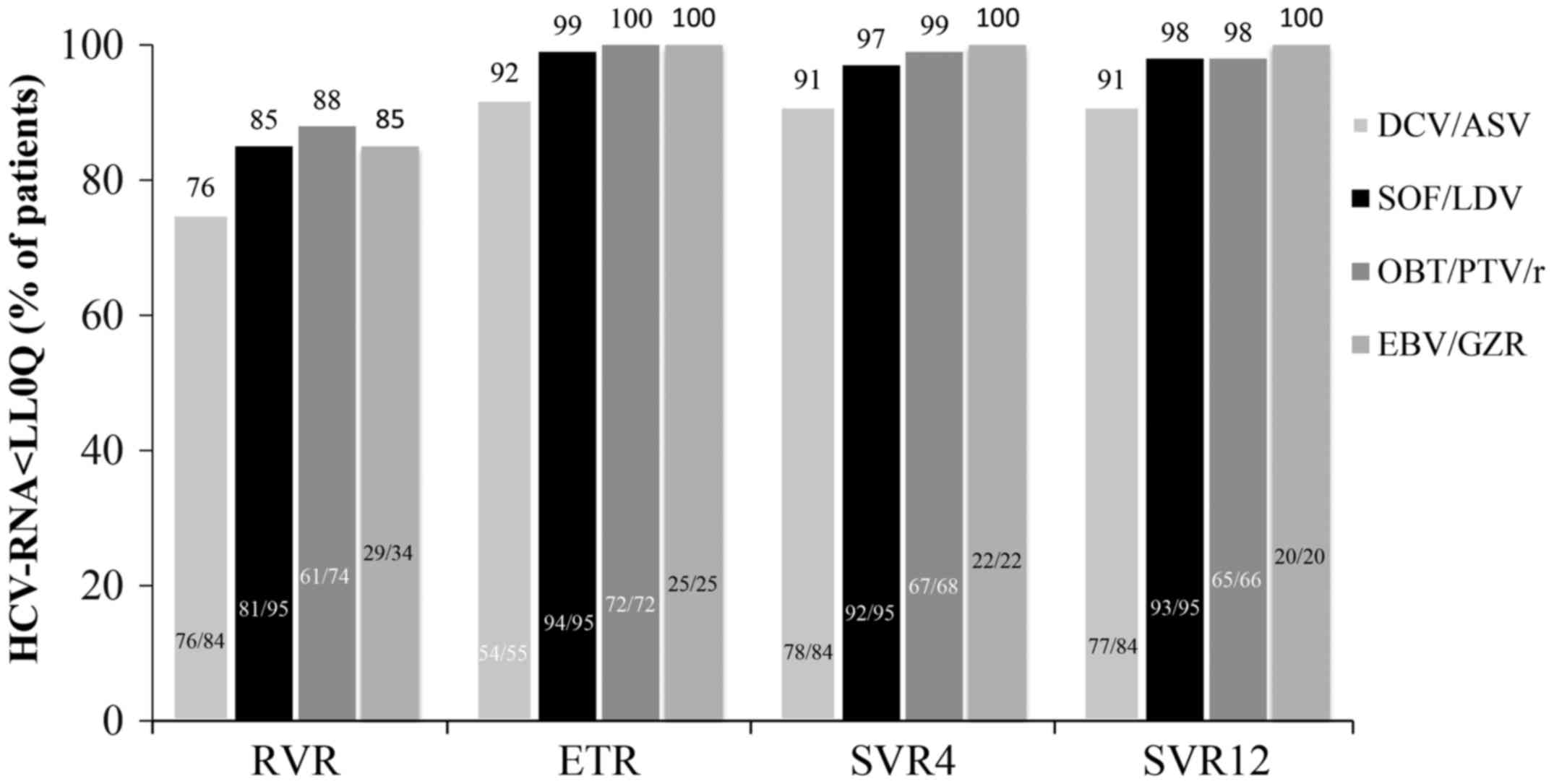 | Figure 2.The proportion of HCV-infected
patients exhibiting viral suppression in response to DAA treatment.
Therapies consisted of DCV/ASV, SOF/LDV, OBV/PTV/r, and EBV/GZR.
The cumulative proportions of patients in the four different groups
who achieved viral suppression at 4 weeks after the start of
treatment, during treatment ongoing, at the ETR, and at 4 and 12
weeks after the end of treatment (SVR4 and SVR12) were shown.
Patient numbers are also shown in the bar graph. No significant
differences were observed in the RVR, ETR, SVR4, and SVR12 rates
among the four groups. ETR, end of treatment; HCV, hepatitis C
virus; DCV/ASV, daclatasvir/asunaprevir; SOF/LDV,
sofosbuvir/ledipasvir; OBT/PTV/r,
ombitasvir/paritaprevir/ritonavir; EBV/GZR, elbasvir/grazoprevir;
RVR, rapid virological response; SVR, sustained virological
response. |
Change in depressive symptoms in
patients treated with IFN-free DAA therapies
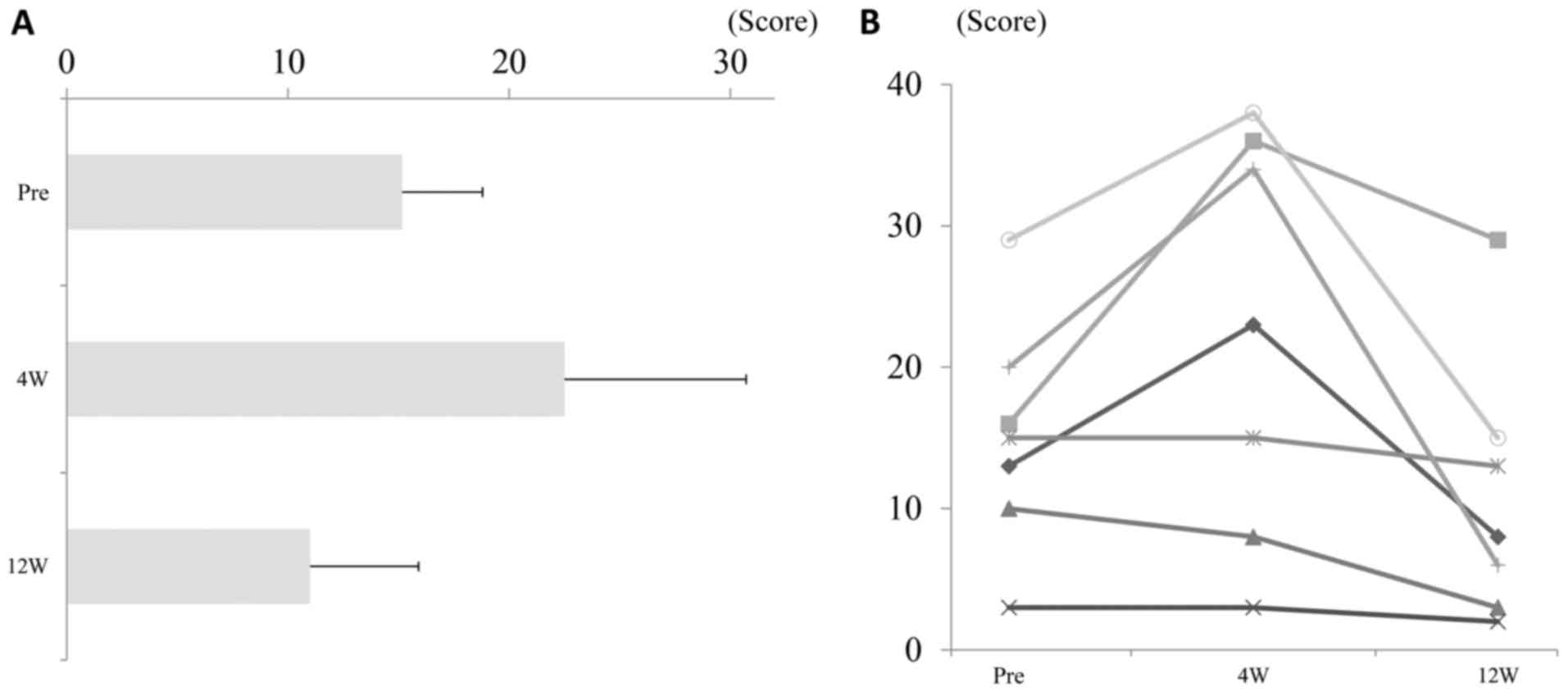
In seven patients with depression who received a
24-week DCV/ASV treatment regimen, the BDI-II scores had increased
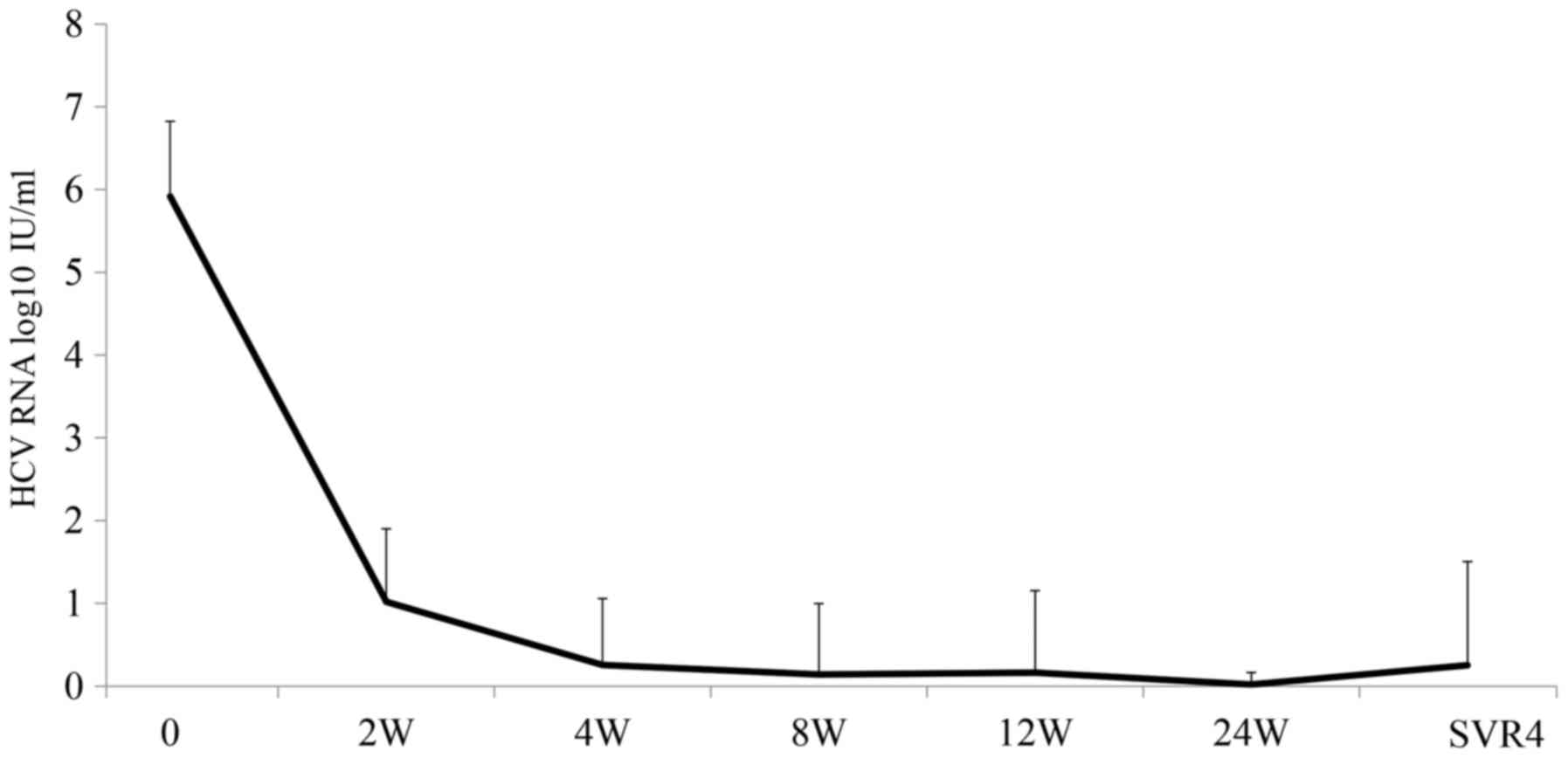
at week 4, as compared to baseline and at week 12 (Fig. 3) in spite of the rapid decline of
serum HCV levels after intiation of DCV/ASV therapy (Fig. 4). The patients treated with DCV/ASV,
OBT/PTV/r, SOF/LDV, and EBR/GZR were divided according to their
median BDI-II scores at baseline into BDI-II relatively high group
and BDI-II relatively low group to examine the effects of DAA
therapies on BDI-II scores (Fig. 5).
The BDI-II scores had decreased, but not significantly, at weeks 4
and 12, as compared to pretreatment values among patients treated
with DCV/ASV in both BDI-II relatively high and low groups
(Fig. 5A and B). BDI-II scores
declined significantly from baseline to week 12 among patients
treated with SOF/LDV and EBR/GZR in both BDI-II relatively high and
low groups and OBT/PTV/r in BDI-II relatively high group (Fig. 5C-G). However, BDI-II scores had
decreased significantly from baseline to week 4, but not week 12,
in BDI-II relatively low group (Fig.
5H).
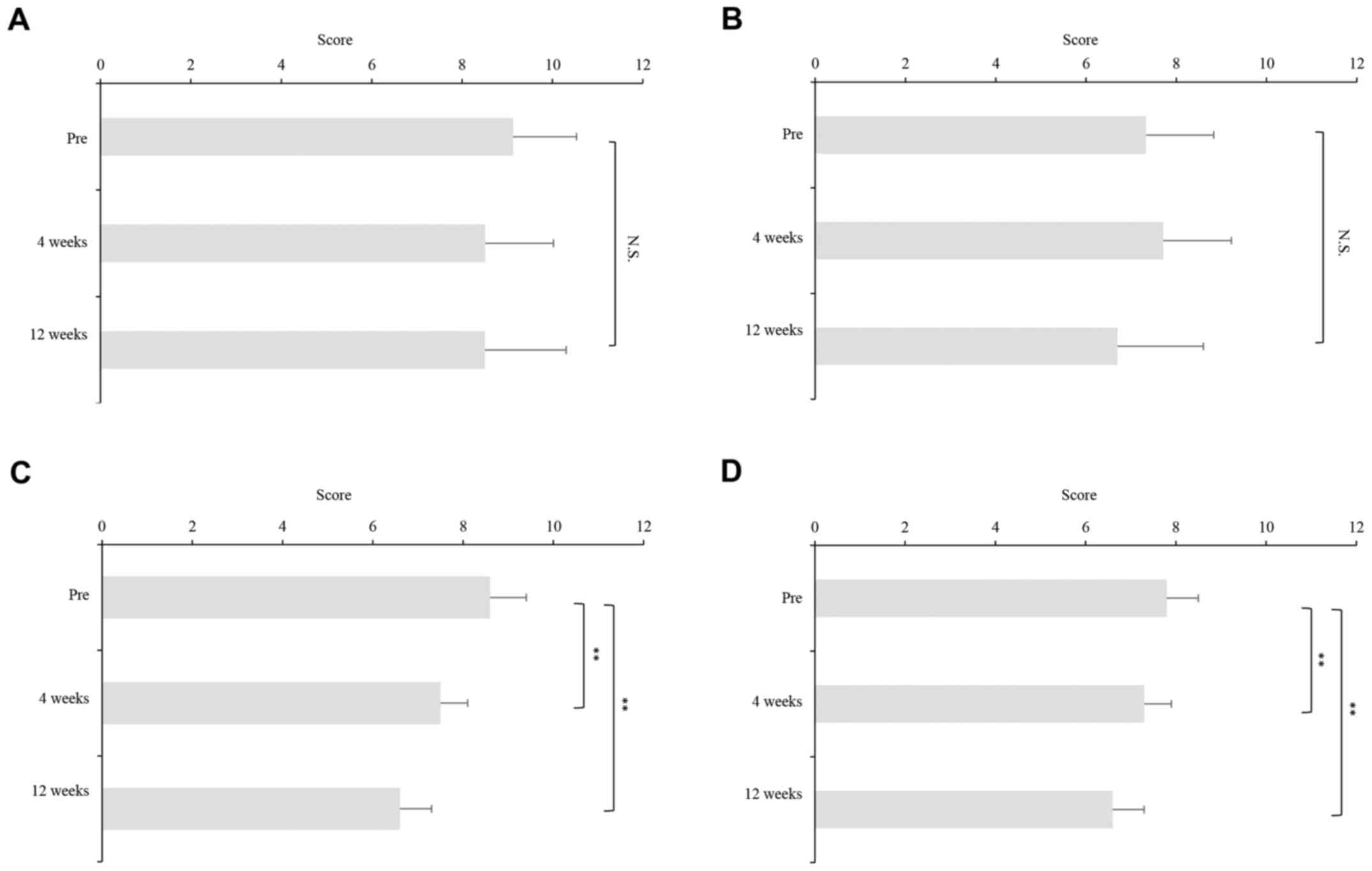 | Figure 5.Change in BDI-II scores during DAA
therapies. The patients treated with DCV/ASV, OBT/PTV/r, SOF/LDV,
and EBR/GZR were divided according to their median BDI-II scores at
baseline into BDI-II relatively high group and BDI-II relatively
low group to examine the effects of DAA therapies on BDI-II scores.
The BDI-II scores had decreased, but not significantly, at weeks 4
and 12 as compared to pretreatment values among patients treated
with DCV/ASV in both BDI-II relatively (A) high and (B) low groups.
BDI-II scores declined significantly from baseline to week 12 among
patients treated with SOF/LDV in both BDI-II relatively (C) high
and (D) low groups, EBR/GZR in BDI-II (E) high and (F) groups, and
(G) OBT/PTV/r in BDI-II relatively high group. (H) However, BDI-II
scores had decreased significantly from baseline to week 4, but not
week 12, in BDI-II relatively low group. Asterisks indicate
statistically significant differences between indicated
experimental groups. (*P<0.05, **P<0.01) N.S, not
significant; DAA, direct acting antiviral; DCV/ASV,
daclatasvir/asunaprevir; SOF/LDV, sofosbuvir/ledipasvir; OBT/PTV/r,
ombitasvir/paritaprevir/ritonavir; EBV/GZR, elbasvir/grazoprevir;
BDI, Beck Depression Inventory. |
Change in psychotropic medication use
in patients treated with IFN-free DAAs
Among the seven patients with depression treated
with DCV/ASV, one (14.3%) received selective serotonin reuptake
inhibitors (SSRI), five (71.4%) received antipsychotic medication,
one (14.3%) received benzodiazepine, two (28.6%) received tricyclic
or tetracyclic antidepressants, and two (28.6%) received no
treatment (Table II). There was no
difference in psychotropic medication use before and after DAA
therapy (Table I).
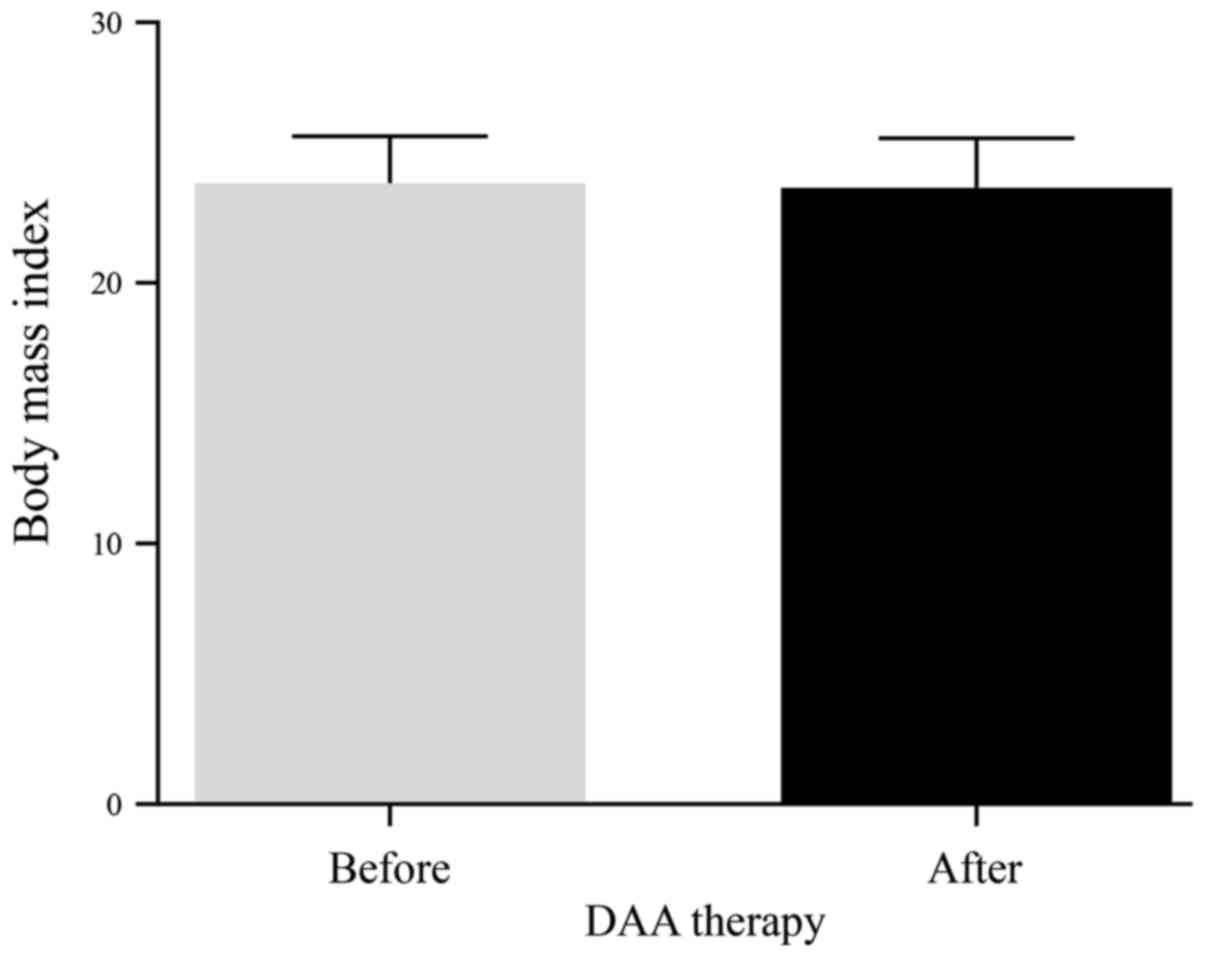
Change in BMI in patients treated with
IFN-free DAAs
There was no significant change in the BMI in
patients after DAA therapy (Fig.
6).
Discussion
The results of the present study clearly show that
various 12-week IFN-free treatment regimens were highly effective
and tolerable in patients with HCV genotype-1 infection. The
percentages of patients aged ≥65 years with cirrhosis who received
IFN-based therapy and non-responders to IFN-based therapy were
highest in those who received 24 weeks of DCV/ASV, probably because
DCV/ASV is the first IFN-free regimen for the treatment of HCV
infection. Chronic HCV infection has a profound negative impact on
mental health disorders, including depression (17–20). To
the best of our knowledge, this study is the first to evaluate the
effects of different IFN-free DAA regimens on the psychometric
properties of the BDI-II in Japanese patients with HCV genotype-1
infection. In the present study, the BDI-II scores decreased from
baseline to the end of the 12-week DAA therapies. Younossi et
al (5,7,21–23) have
reported that 12 weeks of SOF/LDV therapy improve the
health-related quality of life during treatment. Consistent with
the findings of the present study, the use of SOF/LDV regimen has
been reportedly associated with improved BDI-II scores both during
and after treatment (24). In
contrast, in the present study, patients with depression treated
with DCV/ASV for 24 weeks had higher BDI-II scores at week 4, but
lower scores at week 12 than at pretreatment in spite of the rapid
decline of serum HCV levels. Ichikawa et al (25) demonstrated that 24-week DAA treatment
eliminated HCV-RNA and improved psychologic distress. Furthermore,
the results of a recent study demonstrated that 24-week DAA
treatment with DCV plus ASV did not affect mental component scores
at either 12 or 24 weeks after treatment initiation (26). The discrepancy in tolerability of
24-week DAA treatment may be explained in part by the fact that
clinical profiles of patients differ between studies. The 24-week
DAA treatment would have temporary negative effects by leading to
the development of anxiety over a long term therapy but would have
also provided positive effects in the long term by yielding
clinical benefits following HCV eradication. These findings
reinforce the notion that the 12-week regimen was effective and
safe for patients with HCV genotype-1 infection, including those
with depression. Nevertheless, there were several limitations to
this study, including the small number of depressed patients with
HCV infection and the relatively short follow-up period, which
prevented evaluation of the effect on post treatment BDI-II
scores.
Collectively, all four DAA regimens achieved similar
high efficacy in Japanese patients with HCV genotype-1 infection.
The 24-week DAA treatment had temporary negative impact on the
mental health in patients with HCV infection. The BDI-II scores had
significantly decreased following a 12-week regimen of SOF/LDV or
EBR/GZR. Meanwhile, the tolerability was superior with the 12-week
DAA regimes and allowed more patients to reach SVR sooner and with
fewer side effects.
Acknowledegements
The authors acknowledge the support of AbbVie GK and
Bristol-Myers Squibb Company (New York City, NY, USA) for the
support of sequencing analysis of NS5A from patients with HCV
genotype 1b.
Funding
No funding was received.
Availability of data and materials
Raw data were generated at Nara Medical University
Hospital. Derived data supporting the findings of the present study
are available from the corresponding author on request.
Authors' contributions
KT, RN, KM, TA, MK, HK, NS, KKa, HT, YS, KS, YF, YT,
SSat, SSai, KN, MF, KKi, TK, TO, DK, AM, TM, YO and JY performed
data analysis. All statistical analyses in this current study were
supervised by TM. HY and TN made substantial contributions to
conception and design and analysis and interpretation of data.
Ethics approval and consent to
participate
Written informed consent for the use of resected
tissue was obtained from all patients and the study protocol was
approved by the Ethics Committee of Nara Medical University.
Patient consent for publication
All study participants or their legal guardians
provided written informed consent prior to study enrollment.
Competing interests
The authors declare that they have no conflicts of
interest.
Glossary
Abbreviations
Abbreviations:
|
HCV
|
hepatitis C virus
|
|
HCC
|
hepatocellular carcinoma
|
|
IFN
|
interferon
|
|
DAAs
|
direct-acting antivirals
|
|
DCV/ASV
|
daclatasvir/asunaprevir
|
|
SOF/LDV
|
sofosbuvir/ledipasvir
|
|
OBV/PTV/r
|
ombitasvir/paritaprevir/ritonavir
|
|
EBR/GZR
|
elbasvir/grazoprevir
|
|
BDI-II
|
Beck Depression Inventory-II
|
|
RBV
|
ribavirin
|
|
RVR
|
rapid virological response
|
|
ETR
|
end of treatment
|
|
SVR
|
sustained virologic response
|
References
|
1
|
Toshikuni N, Arisawa T and Tsutsumi M:
Hepatitis C-related liver cirrhosis-strategies for the prevention
of hepatic decompensation, hepatocarcinogenesis and mortality.
World J Gastroenterol. 20:2876–2887. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sievert W, Altraif I, Razavi HA, Abdo A,
Ahmed EA, Alomair A, Amarapurkar D, Chen CH, Dou X, El Khayat H, et
al: A systematic review of hepatitis C virus epidemiology in Asia,
Australia and Egypt. Liver Int. 31 Suppl 2:S61–S80. 2011.
View Article : Google Scholar
|
|
3
|
Alazawi W, Cunningham M, Dearden J and
Foster GR: Systematic review: Outcome of compensated cirrhosis due
to chronic hepatitis C infection. Aliment Pharmacol Ther.
32:344–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Younossi Z and Henry L: Systematic review:
Patient-reported outcomes in chronic hepatitis C-the impact of
liver disease and new treatment regimens. Aliment Pharmacol Ther.
41:497–520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Younossi ZM, Stepanova M, Henry L, Nader F
and Hunt S: An in-depth analysis of patient-reported outcomes in
patients with chronic hepatitis c treated with different anti-viral
regimens. Am J Gastroenterol. 111:808–816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Younossi ZM, Stepanova M, Nader F, Lam B
and Hunt S: The patient's journey with chronic hepatitis C from
interferon plus ribavirin to interferon- and ribavirin-free
regimens: A study of health-related quality of life. Aliment
Pharmacol Ther. 42:286–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Younossi Z, Stepanova M, Omata M, Mizokami
M, Walters M and Hunt S: Health utilities using SF-6D scores in
Japanese patients with chronic hepatitis C treated with
sofosbuvir-based regimens in clinical trials. Health Qual Life
Outcomes. 15:252017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spengler U: Direct antiviral agents
(DAAs)-A new age in the treatment of hepatitis C virus infection.
Pharmacol Ther. 183:118–126. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patterson AL, Morasco BJ, Fuller BE,
Indest DW, Loftis JM and Hauser P: Screening for depression in
patients with hepatitis C using the Beck Depression Inventory-II:
Do somatic symptoms compromise validity? Gen Hosp Psychiatry.
33:354–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang SL, Hsieh CL, Wu RM and Lu WS:
Test-retest reliability and minimal detectable change of the Beck
Depression Inventory and the Taiwan geriatric depression scale in
patients with parkinson's disease. PLoS One. 12:e01848232017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pawlotsky JM: Hepatitis C virus resistance
to direct-acting antiviral drugs in interferon-free regimens.
Gastroenterology. 151:70–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeuzem S, Mizokami M, Pianko S, Mangia A,
Han KH, Martin R, Svarovskaia E, Dvory-Sobol H, Doehle B, Hedskog
C, et al: NS5A resistance-associated substitutions in patients with
genotype 1 hepatitis C virus: Prevalence and effect on treatment
outcome. J Hepatol. 66:910–918. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uchida Y, Kouyama J, Naiki K and Mochida
S: A novel simple assay system to quantify the percent HCV-RNA
levels of NS5A Y93H mutant strains and Y93 wild-type strains
relative to the total HCV-RNA levels to determine the indication
for antiviral therapy with NS5A inhibitors. PLoS One.
9:e1126472014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steer RA, Ball R, Ranieri WF and Beck AT:
Dimensions of the Beck Depression Inventory-II in clinically
depressed outpatients. J Clin Psychol. 55:117–128. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayden MJ, Dixon JB, Dixon ME and O'Brien
PE: Confirmatory factor analysis of the Beck Depression Inventory
in obese individuals seeking surgery. Obes Surg. 20:432–439. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beck AT, Ward CH, Mendelson M, Mock J and
Erbaugh J: An inventory for measuring depression. Arch Gen
Psychiatry. 4:561–571. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dbouk N, Arguedas MR and Sheikh A:
Assessment of the PHQ-9 as a screening tool for depression in
patients with chronic hepatitis C. Dig Dis Sci. 53:1100–1106. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Armstrong AR, Herrmann SE, Chassany O,
Lalanne C, Da Silva MH, Galano E, Carrieri PM, Estellon V, Sogni P
and Duracinsky M: The International development of PROQOL-HCV: An
instrument to assess the health-related quality of life of patients
treated for Hepatitis C virus. BMC Infect Dis. 16:4432016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Younossi Z, Park H, Henry L, Adeyemi A and
Stepanova M: Extrahepatic manifestations of hepatitis C: A
meta-analysis of prevalence, quality of life and economic burden.
Gastroenterology. 150:1599–1608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sockalingam S, Blank D, Banga CA, Mason K,
Dodd Z and Powis J: A novel program for treating patients with
trimorbidity: Hepatitis C, serious mental illness and active
substance use. Eur J Gastroenterol Hepatol. 25:1377–1384. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Younossi ZM, Stepanova M, Afdhal N,
Kowdley KV, Zeuzem S, Henry L, Hunt SL and Marcellin P: Improvement
of health-related quality of life and work productivity in chronic
hepatitis C patients with early and advanced fibrosis treated with
ledipasvir and sofosbuvir. J Hepatol. 63:337–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Younossi ZM, Stepanova M, Omata M,
Mizokami M, Walters M and Hunt S: Quality of life of Japanese
patients with chronic hepatitis C treated with ledipasvir and
sofosbuvir. Medicine (Baltimore). 95:e42432016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Younossi ZM, Stepanova M, Chan HL, Lee MH,
Yu Ml, Dan YY, Choi MS and Henry L: Patient-reported outcomes in
asian patients with chronic hepatitis C treated with ledipasvir and
sofosbuvir. Medicine (Baltimore). 95:e27022016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang LS, Masur J, Sims Z, Nelson A,
Osinusi A, Kohli A, Kattakuzhy S, Polis M and Kottilil S: Safe and
effective sofosbuvir-based therapy in patients with mental health
disease on hepatitis C virus treatment. World J Hepatol.
8:1318–1326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ichikawa T, Miyaaki H, Miuma S, Taura N,
Motoyoshi Y, Akahoshi H, Nakamura S, Nakamura J, Takahashi Y, Honda
T, et al: Hepatitis C virus-related symptoms, but not quality of
life, were improved by treatment with direct-acting antivirals.
Hepatol Res. 48:E232–E239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawakubo M, Eguchi Y, Okada M, Iwane S,
Oeda S, Otsuka T, Nakashita S, Araki N and Koga A: Chronic
hepatitis c treatment with daclatasvir plus asunaprevir does not
lead to a decreased quality of life. Intern Med. 2018.doi:
10.2169/internalmedicine.0091-17. View Article : Google Scholar
|