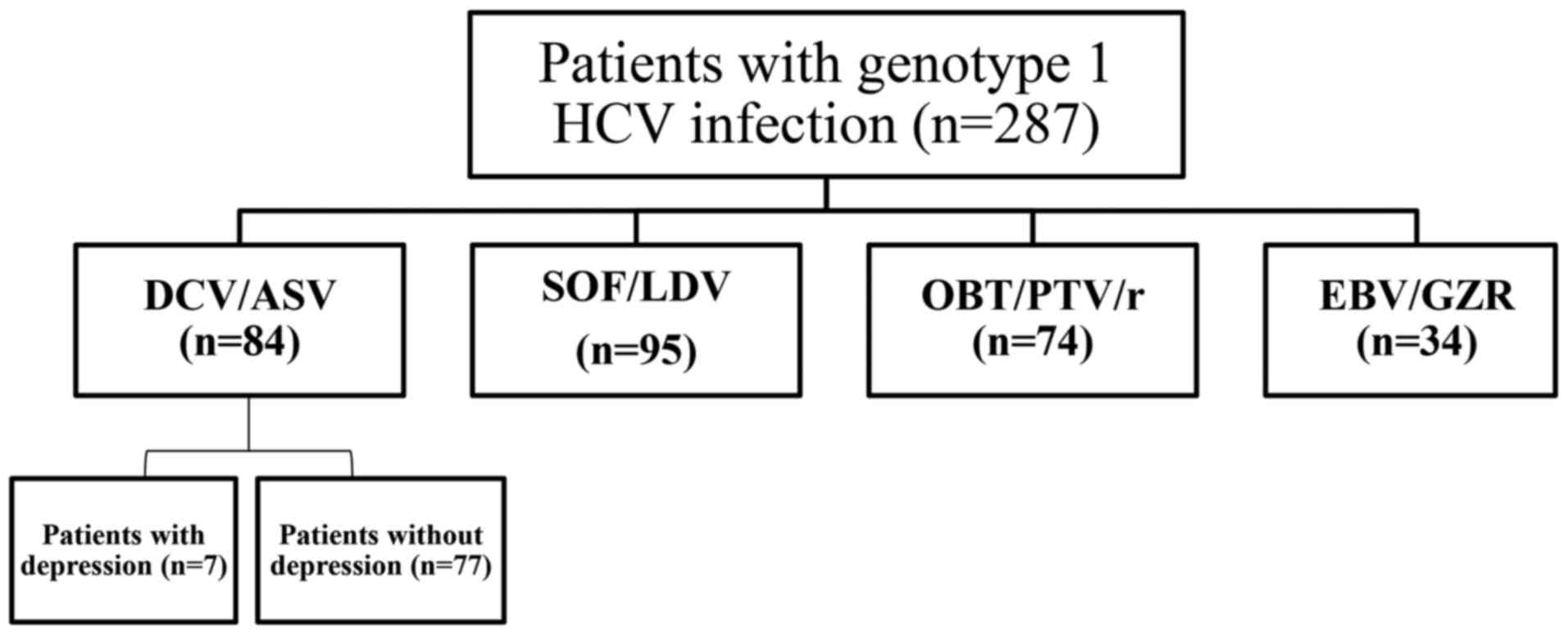
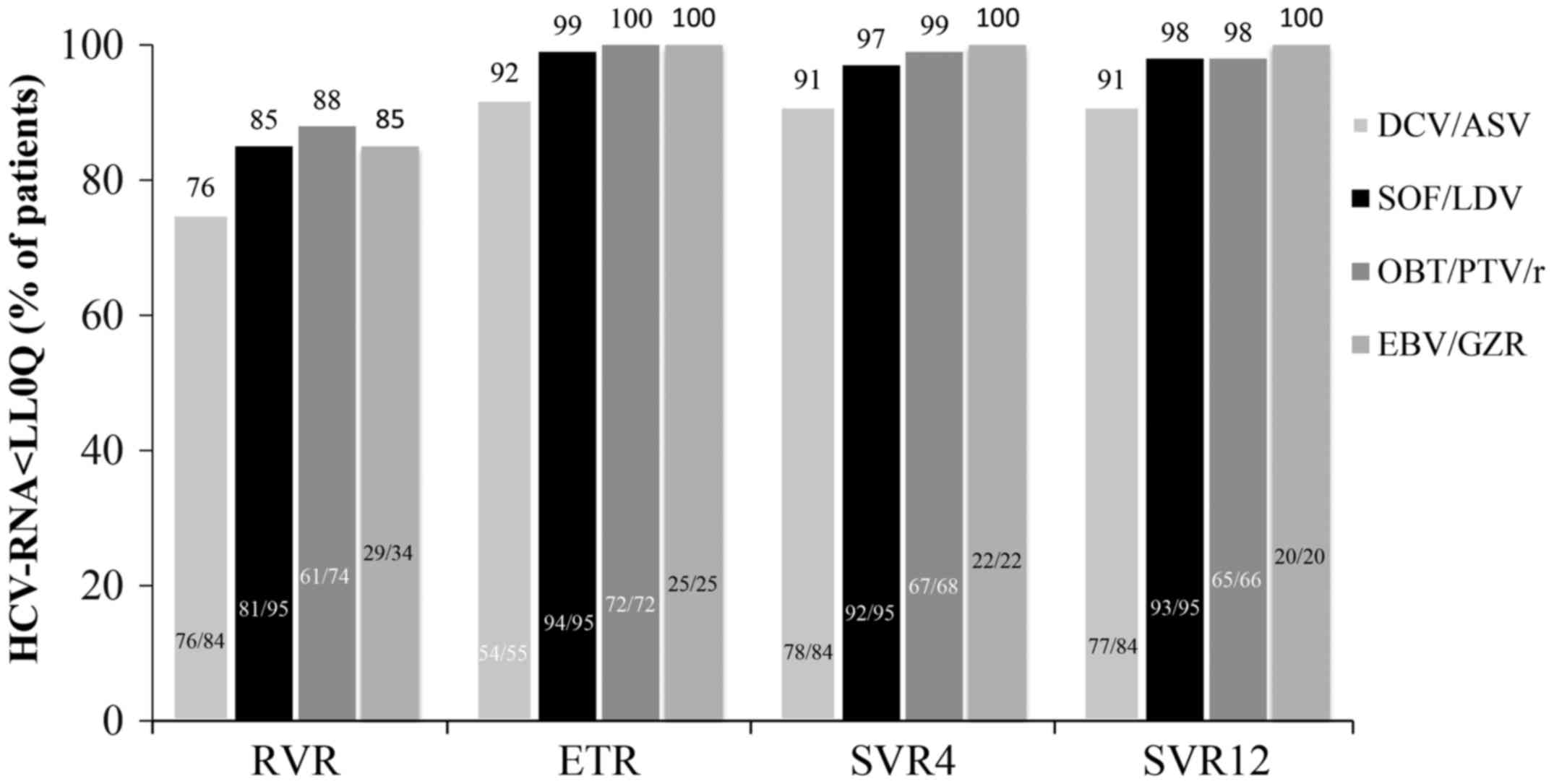
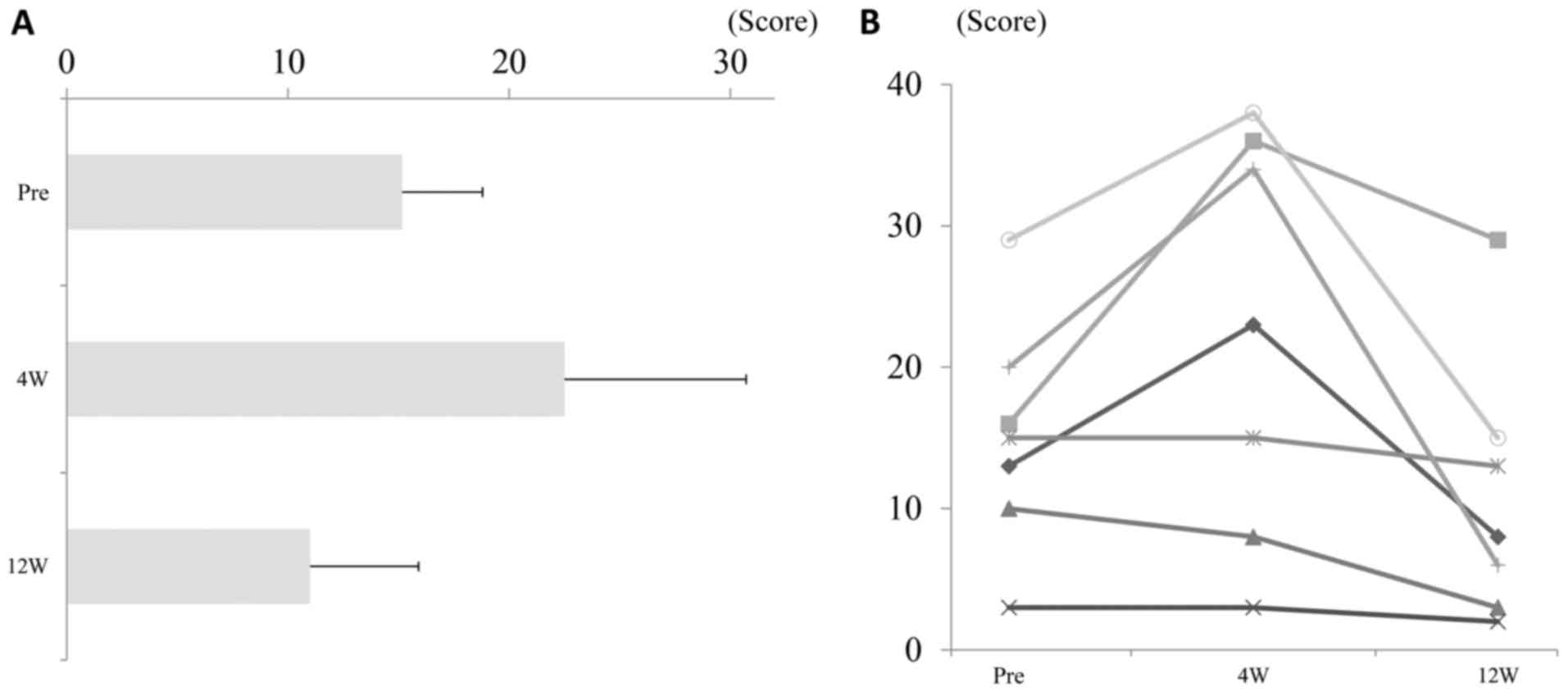
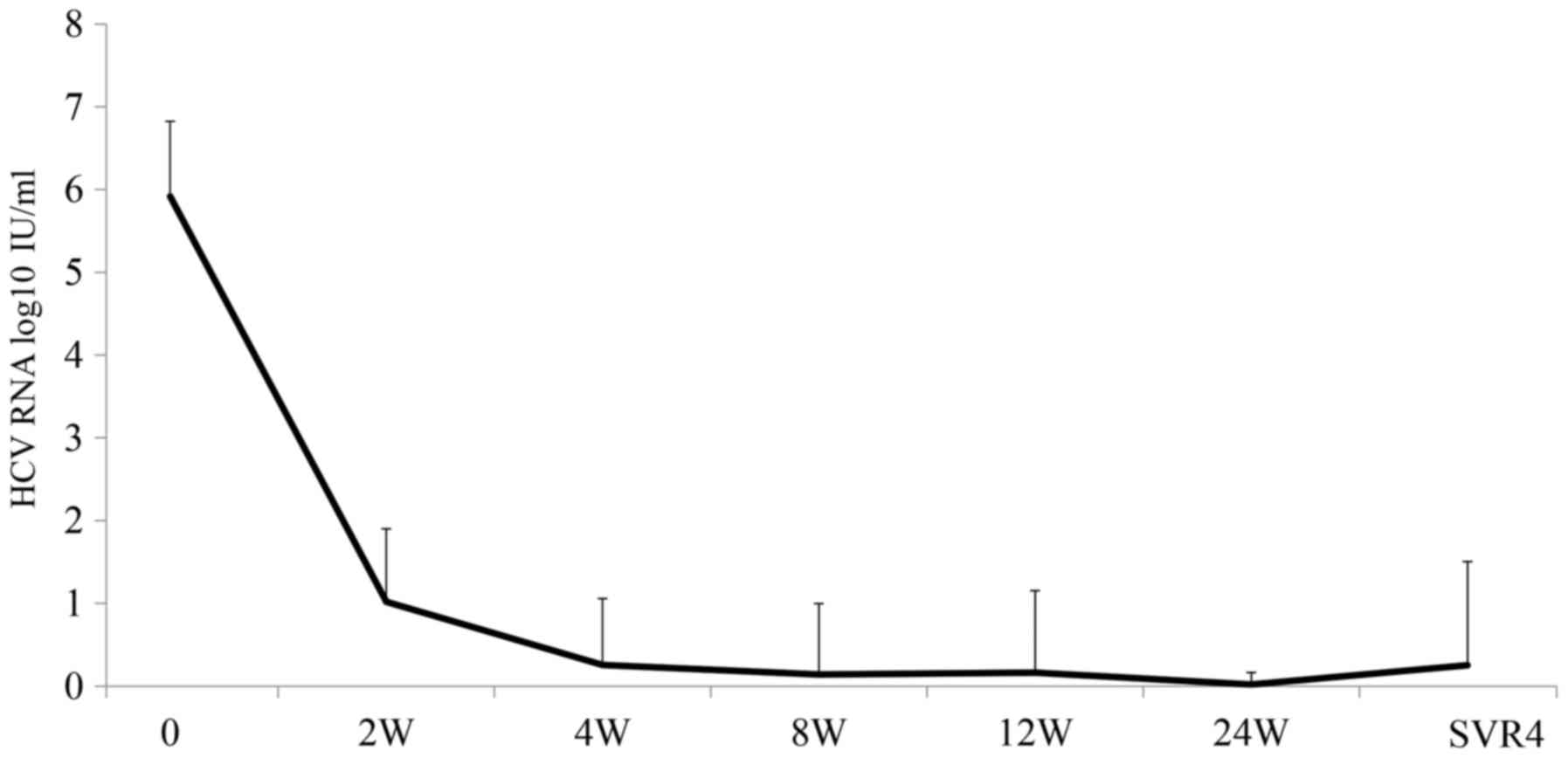
Spandidos Publications style
Takeda K, Noguchi R, Namisaki T, Moriya K, Akahane T, Kitade M, Kawaratani H, Shimozato N, Kaji K, Takaya H, Takaya H, et al: Efficacy and tolerability of interferon‑free regimen for patients with genotype‑1 HCV infection. Exp Ther Med 16: 2743-2750, 2018.
APA
Takeda, K., Noguchi, R., Namisaki, T., Moriya, K., Akahane, T., Kitade, M. ... Yoshiji, H. (2018). Efficacy and tolerability of interferon‑free regimen for patients with genotype‑1 HCV infection. Experimental and Therapeutic Medicine, 16, 2743-2750. https://doi.org/10.3892/etm.2018.6481
MLA
Takeda, K., Noguchi, R., Namisaki, T., Moriya, K., Akahane, T., Kitade, M., Kawaratani, H., Shimozato, N., Kaji, K., Takaya, H., Sawada, Y., Seki, K., Fujinaga, Y., Tsuji, Y., Kubo, T., Sato, S., Saikawa, S., Nakanishi, K., Furukawa, M., Kitagawa, K., Ozutsumi, T., Kaya, D., Mitoro, A., Mashitani, T., Okura, Y., Yamao, J., Yoshiji, H."Efficacy and tolerability of interferon‑free regimen for patients with genotype‑1 HCV infection". Experimental and Therapeutic Medicine 16.3 (2018): 2743-2750.
Chicago
Takeda, K., Noguchi, R., Namisaki, T., Moriya, K., Akahane, T., Kitade, M., Kawaratani, H., Shimozato, N., Kaji, K., Takaya, H., Sawada, Y., Seki, K., Fujinaga, Y., Tsuji, Y., Kubo, T., Sato, S., Saikawa, S., Nakanishi, K., Furukawa, M., Kitagawa, K., Ozutsumi, T., Kaya, D., Mitoro, A., Mashitani, T., Okura, Y., Yamao, J., Yoshiji, H."Efficacy and tolerability of interferon‑free regimen for patients with genotype‑1 HCV infection". Experimental and Therapeutic Medicine 16, no. 3 (2018): 2743-2750. https://doi.org/10.3892/etm.2018.6481