Introduction
Hand, foot and mouth disease (HFMD) is one of the
most common infectious diseases in children under 5 years. Typical
clinical manifestations of HFMD include mild fever, herpangina and
limb herpes. A few patients with HFMD may have severe neurological
symptoms, including aseptic meningitis, acute flaccid paralysis,
brainstem encephalitis, and neurogenic pulmonary edema, or even
death (1,2). Since the 1990s, HFMD has emerged in
countries in the Asia-Pacific region, and large-scale outbreaks
have occurred in Taiwan, Malaysia and Singapore (3–5). In
mainland China, the disease has spread throughout the country since
the large-scale EV71 related HFMD outbreak in 2007 in Linyi City of
Shandong Province and Fuyang City of Anhui Province in 2008
(6,7). Chinese government began to classify
HFMD as a category C notifiable infectious disease in 2008. From
2008 to 2017, a total of 18.18 million cases of hand-foot-mouth
disease were reported in mainland, including 3,632 deaths
(www.chinacdc.cn).
HFMD can be caused by a variety of human
enteroviruses, among which enterovirus 71 (EV71) and coxsackievirus
type A (CVA) types 16, 10 and 6 are the most common. EV71 is
neurotropic and causes severe nervous system infections and even
death. Studies have shown that ~80% of severe cases and >93% of
deaths are caused by EV71 infection (8). However, there are still no effective
antiviral drugs available for anti-EV71 infection, and the use of
vaccines has not been popularized. Therefore, blocking the
transmission of EV71 plays an important role in controlling its
infection. HFMD can be transmitted through close contact, air and
other means. Contact with contaminated hands and objects can also
cause transmission. Spread of HFMD through close contact has been
widely confirmed (9), but
transmission through environmental factors has not been well
studied. Goh et al (10)
found through case-control studies that exposure to contaminated
household items, such as toys, stationery, tableware, toiletries,
and tabletops, is a risk factor for the spread of HFMD. Li et
al (11) found that contact with
the hands of contaminated guardians and public playgrounds also led
to the spread of HFMD. However, the above studies were only risk
analysis or RT-qPCR-based assays, and isolation of EV71 from
environmental samples should be performed to elucidate the homology
between case isolates and environmental strains, so as to elucidate
the spread of the virus.
Because environmental specimens have the
characteristics of low virus concentration and complex components,
it is difficult to isolate viruses using traditional cell culture
methods. The use of polymerase chain reaction (PCR) is challenged
by impurities in environmental specimens, causing a false positive
or false negative. PCR can be used to detect the nucleic acid of
virus, but it is not able to detect virus infectivity.
Immunomagnetic enrichment (IME) is an antigen-antibody-based
immunological binding technique that can efficiently capture and
separate intact virus particles, which in turn enables the
enrichment of viruses and remove impurities. This technology
combined with RT-qPCR has been successfully applied to the
detection of norovirus and hepatitis A virus in complex
environmental samples such as feces, food and sewage, and high
sensitivity and specificity were achieved (12–14).
However, there have been no reports of isolation of viruses by
combination of IME and cell culture. In view of the above, in this
study, immunomagnetic beads of EV71 were prepared. Combined with
cell culture, RT-PCR and RT-qPCR techniques, a sensitive and
effective method for EV71 virus isolation from environmental
specimens was established.
Materials and methods
Reagents and instruments
EV71 virus strain (FJLY08) was donated by Dr Weng
Yuwei, director of the Department of Virology, Fujian Provincial
Center for Disease Control and Prevention. RD cells were provided
by the Polio Laboratory of the Chinese Center for Disease Control
and Prevention. Magnetic beads (11201D; Dynabeads® M-280
Sheep anti-mouse IgG; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and magnetic separator were purchased from Invitrogen (Thermo
Fisher Scientific, Inc.). EV71 monoclonal antibody was a gift from
Professor Xia Ningshao of Xiamen University School of Public
Health. AMV reverse transcriptase, random primers, Taq DNA
polymerase, and RNase inhibitor were purchased from Takara
Biotechnology Co., Ltd. (Dalian, China). Virus nucleic acid
extraction kit and gel extraction kit were purchased from Qiagen
GmbH (Hilden, Germany). EV71 nucleic acid detection kit was
purchased from Jiangsu Shuo Shi Technology Co., Ltd. The study was
approved by the Ethics Committee of The School of Public Health,
Fujian Medical University (Fuzhou, China).
Experimental methods
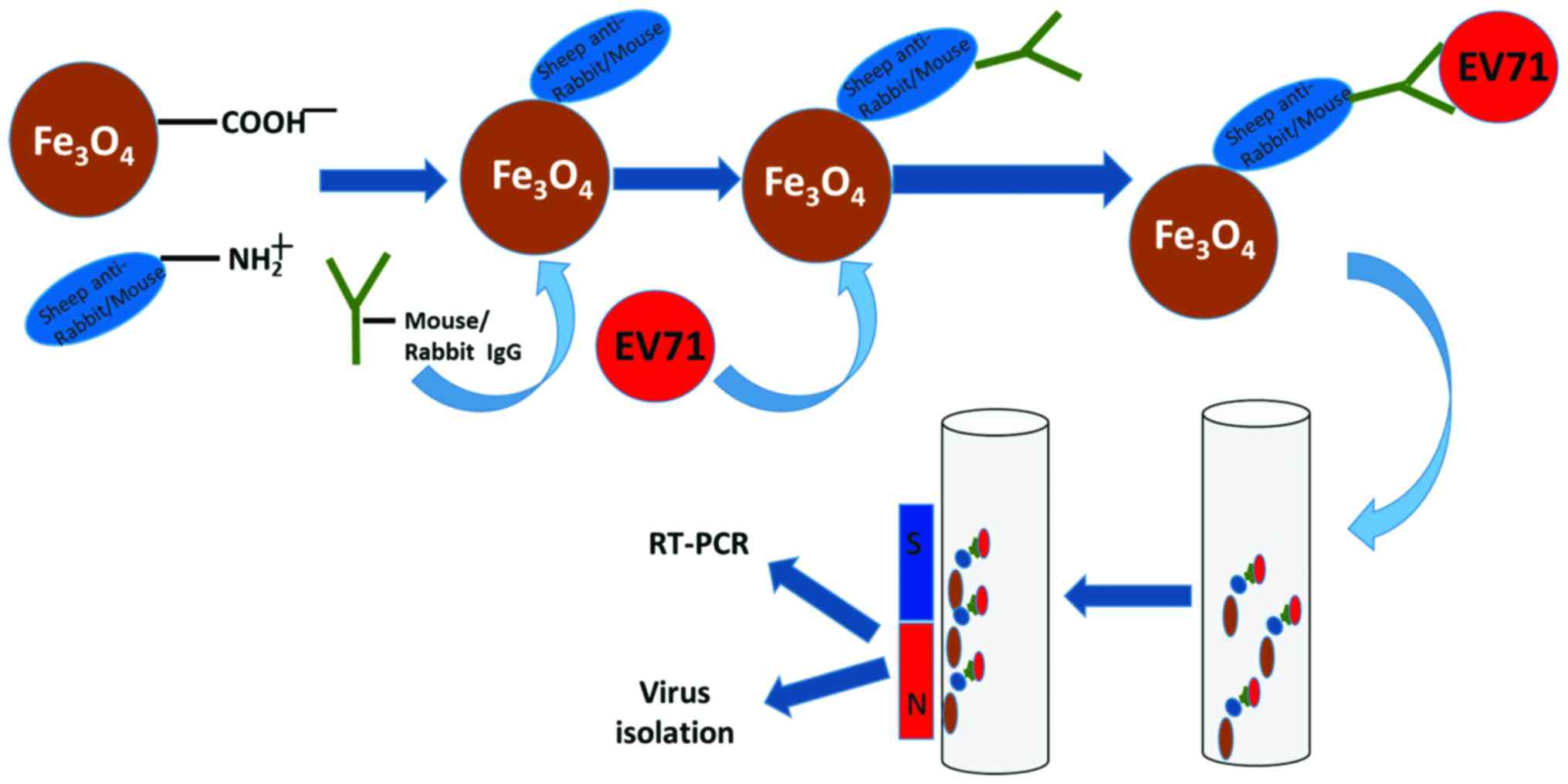
The process of EV71 enrichment of
using immunomagnetic beads
Magnetic beads in kit were coated with goat
anti-mouse antibody (11201D, Dynabeads® M-280 Sheep
anti-Mouse IgG; Thermo Fisher Scientific, Inc.). Monoclonal
antibodies against EV71 were incubated with magnetic beads to form
EV71 specific immunomagnetic beads (EV71-IMB). The EV71-IMB were
used to capture EV71 virus to form immunomagnetic bead-virus
complexes. Immunomagnetic bead-virus complex was adsorbed and
washed using a magnetic separator and then resuspended to form
immunomagnetic bead-virus solution for RT-qPCR, RT-PCR detection
and cell culture (Fig. 1).
Preparation of EV71-IMB. According to the
instructions of kit, magnetic beads were shaken well, and 50 µl of
the magnetic beads suspension was transferred to a 1.5 ml EP tube,
and 1 ml washing solution (PBS, 0.1% BSA, 2 mM EDTA, pH 7.4) was
added. The tube was placed on magnetic separator and supernatant
was discarded. This procedure was repeated 3 times. Then 200 µl of
antibody (65H12) was added and incubated at room temperature with
shaking. Supernatant was discarded and the beads were washed 3
times with washing solution. Finally, 50 µl of stock solution (PBS,
0.1% BSA, 0.05% NaN3) was added and stored at 4°C before
use.
Detection of sensitivity and
specificity of EV71 enrichment using immunomagnetic beads
FJLY08 strain with a known titer (107.5
TCID50/100 µl) was serially diluted in PBS and the virus
diluted at 102 TCID50/100 µl −10−4
TCID50/100 µl was enriched with EV71-IMB and detected by
cell culture, RT-PCR, and RT-qPCR. The maximum dilution that can be
detected by each method is the detection limit of the method.
Detection was performed 3 times, and positive signals in all 3
repeats were defined as positive. Quantification by RT-qPCR was
also performed. 102 TCID50 EV71, CVA6, CVA10,
CVA16, echovirus 6, and echovirus 7 were simultaneously mixed in 1
ml of PBS. After the mixture incubation with EV71-IMB for 2 h,
nucleic acids of immunomagnetic bead-virus complex suspension were
extracted. RT-PCR amplification was performed using universal
primers for enterovirus (292, 5′-MIGCIGYIGARACNGG-3′; 222,
5′-CICCIGGIGGIAYRWACAT-3′). PCR products were sequenced and
analyzed.
Specimen collection and
processing
When EV71 clustered cases were found, the following
three types of specimens were collected: i) throat swab specimens
of the cases and their guardians; ii) swab specimens of the toys,
stationery, tableware, toiletries, table tops at patients home, and
hands of guardian; iii) environment swab specimens such as table
tops, railings, toys, and playgrounds of kindergartens with
clustered cases. Throat swabs were stored in 5 ml of MEM with 5%
FBS. Environmental swabs and hand swabs were soaked in PBS and
swabbed three times in a 100–200 cm2 range and then
stored in 5 ml of PBS. All samples were stored at 4°C and sent to
the laboratory within 12 h. Swab specimens were mixed on a vortex
shaker for 5 min and centrifuged at 4,000 × g for 10 min.
Supernatant was filtered through a 0.22 µm aperture needle filter
and stored at −80°C before use.
Enrichment of EV71 virus in specimen
using EV71-IMB
A total of 50 µl of EV71-IMB was mixed with 1.5 ml
treated sample solution, followed by incubation for 2 h at room
temperature with shaking. The tube was placed on a magnetic
separator, and beads were washed 3 times with washing solution.
Magnetic bead-virus complex was resuspended in 150 µl MEM and
stored at 4°C for follow-up detection. The above steps were
repeated in case sample volume >1.5 ml.
Detection of EV71 after IME
Cell culture: immunomagnetic bead-virus complex
suspension was inoculated into monolayer RD cells (200 µl/well).
CPE was observed daily. If no CPE was observed after 7 days,
subculture was performed, followed by observation for another 7
days. If CPE still did not appear, the sample as judged as
negative.
RT-qPCR and RT-PCR: RNA was extracted from 140 µl of
immunomagnetic beads-virus complexes using the QIAamp Viral RNA
Mini kit (52906; Qiagen GmbH), followed by detection of EV71
through RT-qPCR using EV71 nucleic acid detection kit (JC20102;
Perfectus, Jiangsu Bioperfectus Technologies Co., Ltd., Taizhou,
China). EV71-specific primer (2372, 5′-GCAGCCCAAAAGAACTTCAC-3′;
3454, 5′-AAGTCGCGAGAGCTGTCTTC-3′) were used in ordinary RT-PCR to
amplify full length EV71 VP1. PCR products were sequenced by
Shanghai Biosune Biotechnology Co., Ltd. (Shanghai, China).
EV71 direct detection without
enrichment (non-IME)
Virus culture, RT-qPCR and RT-PCR were used to
detect EV71 directly from sample solution without enrichment as
mentioned above.
Statistical analysis
Positive rate of the two detection methods was
compared using McNermar test (α=0.05). The comparison of rates was
performed using the Pearson Chi-square test (α=0.05) or Fisher's
exact test. Statistical analyses were performed using SPSS (version
22.0; IBM Corp., Armonk, NY, USA). Nucleotide and amino acid
sequence alignment and homology analysis were performed using
Bio-Edit software (V7.0.1). Phylogenetic tree was constructed by
MEGA software (V6.0) using neighbor-joining method.
Results
Optimization of preparation conditions
for immunomagnetic beads
Optimization of coated antibody
quantity
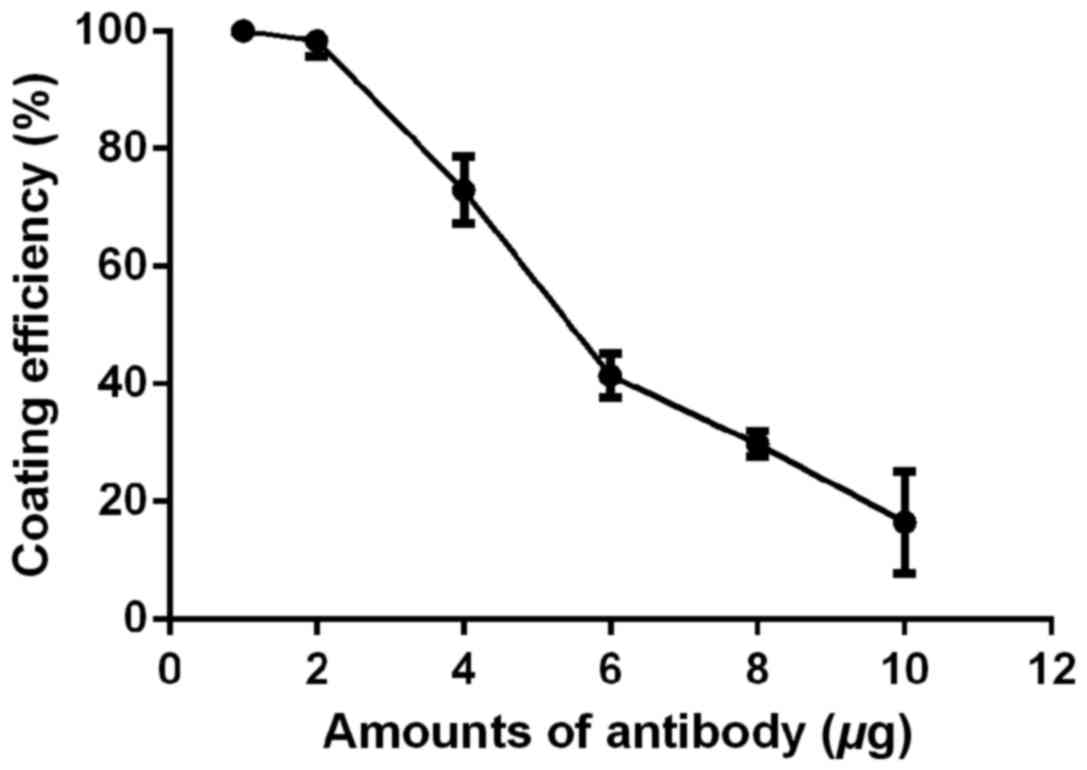
Different amounts (1, 2, 4, 6, 8, and 10 µg) of
antibody were added into an EP tube containing 50 µl of magnetic
beads. After incubation at room temperature for 16 h, the amount of
antibodies before and after coating was measured. Coating
efficiency = (amount of antibody before coating - amount of
antibody after coating)/amount of antibody before coating ×100%. As
shown in Fig. 2, when the amount of
antibody is <2 µg/50 µl of magnetic beads, the coating
efficiency is 100%, and as the amount of antibody increases, the
coating efficiency of magnetic beads gradually decreases. Although
the coating efficiency is the highest when the amount of antibody
added is <2 µg/50 µl of magnetic beads, amount of antibodies may
be insufficient. Therefore, in order to obtain the maximum coating
efficiency and ensure that the amount of antibody is sufficient, 4
µg/50 µl of magnetic beads was used in the following
experiments.
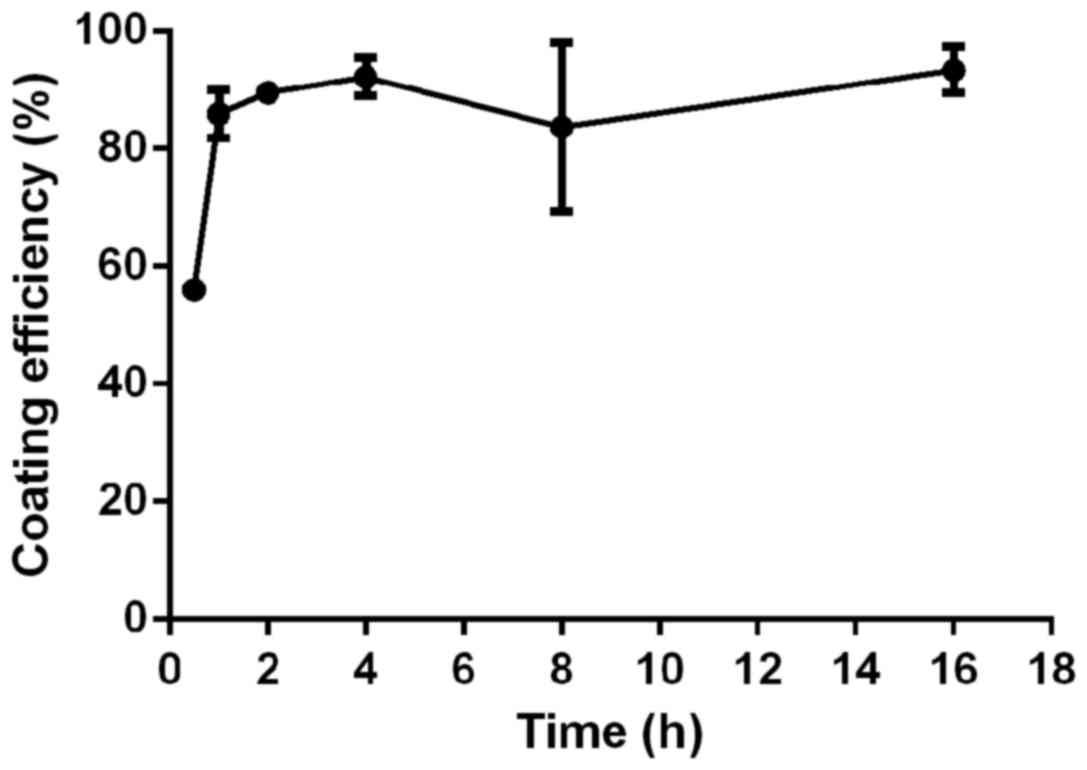
Optimization of coating time
A total of 4 µg of antibody was added into an EP
tube containing 50 µl of magnetic beads, followed by incubation for
0.5, 1, 2, 4, 8, and 16 h, respectively. The amount of antibody
before and after magnetic bead coating was measured. Coating
efficiency = (amount of antibody before coating - amount of
antibody after coating)/amount of antibody before coating ×100%. As
shown in Fig. 3, coating efficiency
increased 0.5 to 2 h. However, after 2 h, coating efficiency did
not change significantly with the increase of the coating time.
Therefore, the optimal coating time of the antibody was set at 2
h.
Sensitivity and specificity of EV71 enrichment using
immunomagnetic beads. After the enrichment of immunomagnetic beads,
detection limit of cell culture was 10−1
TCID50/100 µl, with an average of 5.47×104
copies/ml (95% CI: 3.53–7.42×104 copies/ml). Detection
limits of RT-qPCR and RT-PCR were 10−4
TCID50/100 µl and 1TCID50/100 µl,
respectively, and the average was 3.57×102 copies/ml
(95% CI: 1.36–5.79×102 copies/ml) and
5.30×105 copies/ml (95% CI: 2.81–7.78×105
copies/ml), respectively. EV71 and 3 other common HFMD-related type
(CVA6, 10, 16 group A) of virus and 2 types (echovirus 6 and 7
belong to group B) virus were mixed in equal amounts and tested by
EV71 IME method. The results showed that only EV71 was detected
specifically, other types of viruses were not detected. The data
suggest that EV71 immunomagnetic beads can specifically enrich the
EV71 virus without enriching other viruses.
Practical application of imunomagnetic
bead enrichment technology
Sample collection
A total of 346 specimens were collected from four
outbreaks of EV71 infection in Xiamen, Longyan, Ningde, and Sanming
City. Of these, 102 were throat swab samples from HFMD patients and
patients' guardians, 219 were environmental swab specimens from
home and kindergarten environments and 25 were swab specimens from
guardians' hand (Table I).
 | Table I.Specimens collected from four
epidemics of EV71 infection. |
Table I.
Specimens collected from four
epidemics of EV71 infection.
|
| Throat swab | Hand swab | Patients home
environment swab | Kindergarten
environment swab |
|---|
|
|
|
|
|
|
|---|
| Regions | Patients | Guardians | Guardians | Toys | Stationery | Toiletries | Table surface | Tableware | Total | Table surface | Railing | Toys | Playground | Others | Total |
|---|
| Longyan | 18 | 18 | 0 | 9 | 9 | 9 | 9 | 9 | 45 | 3 | 4 | 4 | 3 | 2 | 16 |
| Xiamen | 8 | 8 | 7 | 7 | 7 | 7 | 7 | 7 | 35 | 3 | 4 | 5 | 4 | 0 | 16 |
| Ningde | 8 | 8 | 7 | 7 | 7 | 7 | 7 | 7 | 35 | 4 | 4 | 4 | 4 | 1 | 17 |
| Sanming | 17 | 17 | 11 | 11 | 11 | 11 | 11 | 11 | 55 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 51 | 51 | 25 | 34 | 34 | 34 | 34 | 34 | 170 | 10 | 12 | 13 | 11 | 3 | 49 |
Test results of IME and non-IME
The above-mentioned 346 specimens were detected
directly and subjected to IME followed by detection and direct
detection. Results showed (Tables
II and III) that, after
enrichment by immunomagnetic beads, the positive rates of RT-qPCR,
RT-PCR, and cell culture were 20.52, 5.78 and 9.25%, respectively,
which were significantly higher than those of direct detection
(15.89, 3.47 and 4.05%, respectively) (P<0.05). In the case
specimens, the positive rate of cell culture after enrichment using
magnetic beads was 43.14%, which was significantly higher than that
of direct cell culture (27.45%) (P<0.05). In household product
samples, EV71 was not isolated directly from the cell culture, but
the isolation rate of EV71 after enrichment with immunomagnetic
beads was 5.29% (9/170). The positive rates of RT-qPCR was
significantly increased from 8.24 to 14.71% (P<0.05).
 | Table II.Results of EV71 detection from
different types of samples. |
Table II.
Results of EV71 detection from
different types of samples.
|
|
|
| IME | Non-IME |
|---|
|
|
|
|
|
|
|---|
| Sample from | Sample types | Number | RT-qPCR | RT-PCR | Cell culture | RT-qPCR | RT-PCR | Cell culture |
|---|
| Patient | Throat swab | 51 | 37 | 16 | 22 | 36 | 12 | 14 |
| Guardian | Throat swab | 51 | 6 | 1 | 1 | 4 | 0 | 0 |
| Guardian | Hand swab | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| Household item | Wipe swab | 170 | 25 | 3 | 9 | 14 | 0 | 0 |
| Kindergarten | Wipe swab | 49 | 3 | 0 | 0 | 1 | 0 | 0 |
| Total |
| 346 | 71 | 20 | 32 | 55 | 12 | 14 |
 | Table III.Comparison of EV71 detection results
between IME and non-IME. |
Table III.
Comparison of EV71 detection results
between IME and non-IME.
|
|
|
| Non-IME |
|
|---|
|
|
|
|
|
|
|---|
| Sample | Method |
| Positive | Negative | McNemar test |
|---|
| Patient | RT-qPCR | Positive | 35 | 2 |
|
|
|
| Negative | 1 | 13 |
|
|
| RT-PCR | Positive | 11 | 5 |
χ2=1.500, P>0.05 |
|
|
| Negative | 1 | 34 |
|
|
| Cell culture | Positive | 14 | 8 |
χ2=6.125, P<0.05 |
|
|
| Negative | 0 | 29 |
|
| Household item | RT-qPCR | Positive | 11 | 14 |
χ2=5.882, P<0.05 |
|
|
| Negative | 3 | 142 |
|
|
| RT-PCR | Positive | 0 | 3 |
|
|
|
| Negative | 0 | 167 |
|
|
| Cell culture | Positive | 0 | 9 |
|
|
|
| Negative | 0 | 161 |
|
| Total | RT-qPCR | Positive | 54 | 17 | χ2=12.5,
P<0.0001 |
|
|
| Negative | 1 | 274 |
|
|
| RT-PCR | Positive | 11 | 9 | χ2=4.9,
P=0.021 |
|
|
| Negative | 1 | 325 |
|
|
| Cell culture | Positive | 14 | 18 |
χ2=16.05, P<0.0001 |
|
|
| Negative | 0 | 314 |
|
Detection of EV71 in different
environment specimens
In environmental samples, positive results of one of
the three detection methods after enrichment were considered as
positive. Positive rates of environmental swabs from home and
kindergarten were 14.71% (25/170) and 6.12% (3/49), respectively,
and there was no significant difference between them (P>0.05).
In home environment specimens, positive rates for toys, stationery,
toiletries, tableware, and tabletops were 38.23% (13/34), 17.65%
(6/34), 5.88% (2/34), and 5.88% (2/34) and 5.88% (2/34),
respectively, and there were significant differences among them
(P<0.05). Most of the 25 positive specimens were detected in
toys and stationery, of which 13 were toys (52.00%) and 6 were
stationery (24.00%). In kindergarten environmental samples, there
was no difference in the positive rate among different
environmental samples (P>0.05) (Table IV).
 | Table IV.The detection results of EV71 in
environmental samples via IME. |
Table IV.
The detection results of EV71 in
environmental samples via IME.
|
|
|
| Detection |
|---|
|
|
|
|
|
|---|
| Sample from | Sample types | Number | Positive | Negative | Fishers exact
test |
|---|
| Household item | Toy | 34 | 13 | 21 | P<0.05 |
|
| Stationery | 34 | 6 | 28 |
|
|
| Toiletry | 34 | 2 | 32 |
|
|
| Tableware | 34 | 2 | 32 |
|
|
| Tabletop | 34 | 2 | 32 |
|
| Kindergarten | Toy | 13 | 1 | 12 | P>0.05 |
|
| Tabletop | 10 | 0 | 10 |
|
|
| Handrail | 12 | 0 | 12 |
|
|
| Playground | 11 | 1 | 10 |
|
|
| Others | 3 | 1 | 2 |
|
|
| Total | 219 | 28 | 191 |
|
Homology of EV71 isolated from
environment and patients
Full-length VP1 was amplified from EV71 obtained
from patients, guardians, and environment samples and was
sequenced. Results showed (Table V)
that the nucleotide and amino acid homology of the virus strains in
4 outbreaks of 4 regions were 92.8–100% and 97.9–100%,
respectively. Environmental strains and case strains obtained in
the same epidemic in the same area were closely related. Nucleotide
and amino acid homology was 98.0–100% and 99.6–100%, respectively,
indicating that the environmental strains and the case strains were
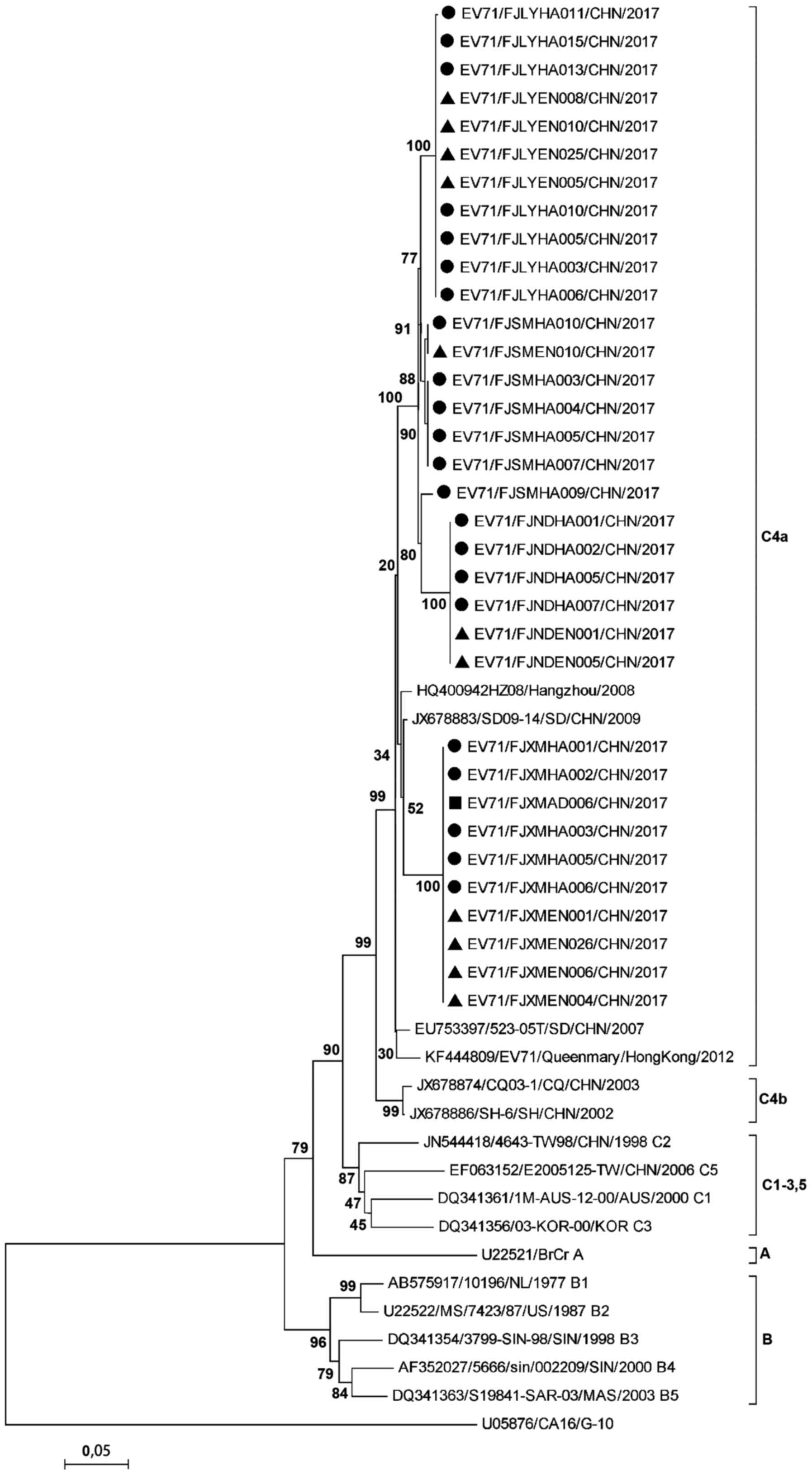
in the same transmission chain. Phylogenetic tree also showed
similar results. The strains in the four regions belonged to the
same branch and all belonged to the C4a subtype. Strains isolated
from the same area were all grouped in the same cluster, except
that EV71/FJSMHA009/2017 belonged to the Ningde isolate cluster.
But the homology of the EV71/FJSMHA009/2017 strain with other
Sanming strains was 98.0%, and with the Ningde strains was 96.9%.
Among clustered cases in Xiamen, the EV71/FJXMEN001/CHN/2017 strain
was isolated from children's toy specimens, and
EV71/FJXMHA001/CHN/2017 and EV71/FJXMHA002/CHN/2017 strains were
isolated from sibling throat swabs, respectively. These three
viruses were derived from the same family, and their nucleotide
homology was 100%, indicating the presence of family
cross-infection. EV71/FJXMAD008/CHN/2017 was the isolate of throat
swab specimens from other cases of guardian, and its homology with
the isolates in Xiamen was 100% (Fig.
4).
 | Table V.Comparison of nucleotide and amino
acid homology of EV71 isolates in different regions. |
Table V.
Comparison of nucleotide and amino
acid homology of EV71 isolates in different regions.
|
| Nucleotide homology
(%) |
|---|
|
|
|
|---|
|
| Xiamen | Ningde | Longyan | Sanming |
|---|
| Xiamen | 100 |
|
|
|
| Ningde | 92.8 | 100 |
|
|
| Longyan | 93.7–93.8 | 96.1–96.2 | 99.8–100 |
|
| Sanming | 94.0–94.6 | 96.6–96.9 | 97.4–98.3 | 98.0–100 |
|
|
| Amino acid
homology (%) |
|
|
|
|
| Xiamen | Ningde | Longyan | Sanming |
|
| Xiamen | 100 |
|
|
|
| Ningde | 98.9 | 100 |
|
|
| Longyan | 97.9–98.3 | 98.9–99.3 | 99.6–100 |
|
| Sanming | 98.6 | 99.6 | 99.3–99.6 | 100 |
Discussion
EV71 virus can be excreted with the excreta of
infected persons, respiratory tracts and skin rashes. Results of
epidemiological studies have clearly shown that articles
contaminated with excreta or secretions from children can cause
indirect contact infection, and experimental studies have found
that these contaminants indeed contain virus-associated antigens or
nucleic acids, but no live virus has been isolated from these
contaminants (10,11). It is difficult to isolate live virus
from contaminated environmental samples, mainly due to the low
concentration of virus in environmental specimens, which is lower
than the detection limit of conventional detection methods. Complex
components in environmental specimens contain impurities that
interfere with subsequent cell culture or RT-PCR assays. For
example, organic substances can interfere with PCR and prevent the
template from being amplified, thus causing false negative or false
positive results. Therefore, finding effective ways to concentrate,
enrich viruses, and remove impurities is the key to virus detection
in environmental samples.
IME technology is based on the antigen-antibody
immune binding reaction. This technique only isolates antigenically
intact virus particles, and rarely adsorb naked nucleic acid
(13). Therefore, IME technology can
simultaneously detect the antigenicity and nucleic acid of the
virus for the assessment of the risk of virus infection. Compared
with common PCR methods, IME technology has a higher degree of
confidence, and it also provides a more effective tool for
assessing the infection risk of viruses that cannot be cultivated
yet. The EV71 monoclonal antibody immunomagnetic beads prepared in
this study have high specificity. After enriching the immune
magnetic beads, the antigen-antibody complexes need not be
separated from the magnetic beads, and the cells can be directly
inoculated without affecting the normal cell growth. Cytopathic
effect caused by virus infection was observed, indicating that this
enrichment method does not affect the virus infectivity.
EV71 can remain in the intestine and upper
respiratory tract for 2–4 weeks. Therefore, specimens such as
feces, rectal swabs, and throat swabs are often used for virus
detection and isolation. Among them, the throat swab specimens are
considered to be the most appropriate specimens for HFMD detection
because of their high detection rate and convenient sampling, and
are suitable for outpatient and inpatient cases (15). In this study, the positive rate of
virus isolation was significantly higher in cases of throat swabs
enriched with immunomagnetic beads compared to direct cell culture.
This shows that the combination of immunomagnetic beads enrichment
and cell culture can increase the detection rate of case pathogens.
Therefore, in the outbreak of HFMD, immune magnetic bead enrichment
will be an effective tool for virus isolation. National Center for
Immunization and Respiratory Diseases of the United States found
that adults with latent infection do not show any clinical
symptoms, but they can still spread the virus to others (http://www.cdc.gov/hand-fo-mouth/About/Transmission.html),
and they are very important sources of infection. In this study,
the EV71 virus was detected in child guardians from throat swab.
The guardian isolate has 100% homology with the case isolate. This
suggests that child guardians can serve as vehicles for the
transmission of EV71 and play an important role in the transmission
of EV71. Considering that adult infections are mostly asymptomatic
and the range of activities of adults is much greater than that of
children, it is very important to strengthen monitoring of
guardians.
In outbreak of EV71 infection, kindergartens are the
main control site. Closing kindergartens and performing
comprehensive disinfection are considered to be the most effective
control measures. This study failed to separate EV71 from
kindergarten specimens, which may be related to the large-scale
disinfection of kindergartens before the collection of
environmental specimens. Disinfection is well planned and strictly
executed in Chinese kindergartens. EV71 virus was detected in part
of kindergarten environmental swabs through IME-RT-qPCR, suggesting
that toys and other articles are indeed contaminated with the
virus. If they are not disinfected in time, the spread of the
disease may occur.
In outbreaks of HFMD, the family sanitation and
disinfection work is more easily overlooked. In this study, viral
nucleic acids were detected in environmental specimens by RT-qPCR
after IME. Positive rate was significantly increased, and the virus
was isolated, revealing that immunomagnetic beads enrichment
technology can improve detection sensitivity. Nucleotide sequences
of the isolates from family environment specimens and case isolates
were highly consistent, and the same strains were isolated in the
siblings and toys of the same family, indicating that the EV71 can
be spread through family cross-infection. EV71 has a high detection
rate in home environment specimens, especially in toys and
stationery. Therefore, in the prevention of HFMD epidemic,
disinfection of the family environment should also be
performed.
In conclusion, we established EV71 immunomagnetic
bead enrichment technique, and combination of this technique and
routine detection methods improved the detection sensitivity of
EV71. This method is applied to the detection of peripheral
environmental swabs in children and viruses can be isolated from
environmental samples. Viruses isolated from environmental
specimens and case specimens were highly homologous. It was
confirmed that children's toys and stationery played an important
role in the transmission of EV71.
Acknowledgements
We would like to extend our deep gratitude to the
laboratory support from Department of Immunization Planning, Fujian
Center for Disease Control and Prevention. We are grateful for the
support during the sampling work from Qianjin Chen, Yuanhui Xiao,
Heshen Chen and Daobin Zhu, the directors of Fujian Municipal
Center for Disease Control and Prevention.
Funding
This study was supported by Fujian Health System
Youth Backbone Talents training project ‘environmental sewage
applied to enterovirus epidemic rule’ (2013-ZQN-ZD-10) and leading
(key) project of social development in Fujian Province: changes in
the epidemic of enterovirus and prevention and control strategy of
PVII vaccine strain in the population and the external environment
after the polio vaccine conversion (2017Y0011).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ drafted the manuscript. WZ, XY and YZ were mainly
devoted to specimen collection and processing. WZ and XY helped
with enrichment of EV71 virus in specimen using immunomagnetic
beads. WZ, XY and YY were responsible for the conception and design
of the study. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The School of Public Health, Fujian Medical University (Fuzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ
and Solomon T: Clinical features, diagnosis, and management of
enterovirus 71. Lancet Neurol. 9:1097–1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yi EJ, Shin YJ, Kim JH, Kim TG and Chang
SY: Enterovirus 71 infection and vaccines. Clin Exp Vaccine Res.
6:4–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT,
Tsai SF, Wang JR and Shih SR: Taiwan Enterovirus Epidemic Working
Group: An epidemic of enterovirus 71 infection in Taiwan. N Engl J
Med. 341:929–935. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herrero LJ, Lee CS, Hurrelbrink RJ, Chua
BH, Chua KB and McMinn PC: Molecular epidemiology of enterovirus 71
in peninsular Malaysia, 1997–2000. Arch Virol. 148:1369–1385. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan KP, Goh KT, Chong CY, Teo ES, Lau G
and Ling AE: Epidemic hand, foot and mouth disease caused by human
enterovirus 71, Singapore. Emerg Infect Dis. 9:78–85. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Tan XJ, Wang HY, Yan DM, Zhu SL,
Wang DY, Ji F, Wang XJ, Gao YJ, Chen L, et al: An outbreak of hand,
foot, and mouth disease associated with subgenotype C4 of human
enterovirus 71 in Shandong, China. J Clin Virol. 44:262–267. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang F, Ren L, Xiong Z, Li J, Xiao Y, Zhao
R, He Y, Bu G, Zhou S, Wang J, et al: Enterovirus 71 outbreak in
the People's Republic of China in 2008. J Clin Microbiol.
47:2351–2352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xing W, Liao Q, Viboud C, Zhang J, Sun J,
Wu JT, Chang Z, Liu F, Fang VJ, Zheng Y, et al: Hand, foot, and
mouth disease in China, 2008–12: An epidemiological study. Lancet
Infect Dis. 14:308–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park SK, Park B, Ki M, Kim H, Lee K, Jung
C, Sohn YM, Choi SM, Kim DK, Lee DS, et al: Transmission of
seasonal outbreak of childhood enteroviral aseptic meningitis and
hand-foot-mouth disease. J Korean Med Sci. 25:677–683. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goh KT, Doraisingham S, Tan JL, Lim GN and
Chew SE: An outbreak of hand, foot, and mouth disease in Singapore.
Bull World Health Organ. 60:965–969. 1982.PubMed/NCBI
|
|
11
|
Li P, Li T, Gu Q, Chen X, Li J, Chen X,
Chen Y, Zhang D, Gao R, He Z, et al: Children's caregivers and
public playgrounds: Potential reservoirs of infection of
hand-foot-and-mouth disease. Sci Rep. 6:363752016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Casas N and Suñén E: Detection of
enteroviruses, hepatitis A virus and rotaviruses in sewage by means
of an immunomagnetic capture reverse transcription-PCR assay.
Microbiol Res. 157:169–175. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu P, Kim M, Schlesinger D, Kranz C, Ha
S, Ha J, Slauch J, Baek S and Moe C: Immunomagnetic separation
combined with RT-qPCR for determining the efficacy of disinfectants
against human noroviruses. J Infect Public Health. 8:145–154. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kobayashi S, Natori K, Takeda N and Sakae
K: Immunomagnetic capture RT-PCR for detection of norovirus from
foods implicated in a foodborne outbreak. Microbiol Immunol.
48:201–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ooi MH, Solomon T, Podin Y, Mohan A, Akin
W, Yusuf MA, del Sel S, Kontol KM, Lai BF, Clear D, et al:
Evaluation of different clinical sample types in diagnosis of human
enterovirus 71-associated hand-foot-and-mouth disease. J Clin
Microbiol. 45:1858–1866. 2007. View Article : Google Scholar : PubMed/NCBI
|