Introduction
Chronic kidney disease (CKD) is defined as kidney
damage or a glomerular filtration rate <60 ml/min/1.73
m2 for ≥3 months, irrespective of the cause (1). A total of ~500 million adults are
diagnosed with CKD all over the world, whichhas become a global
public health problem due to its high prevalence and the
accompanying risk of end-stage renal disease (2). The Global Burden of Disease Study 2013
reported that, over the past 23 years, CKD is the most increased
non-communicable cause of mortality (3). Angiotensin converting enzyme inhibitors
and angiotensin receptor blockers have been used to treat CKD;
however, these agents do not completely prevent the progression of
CKD (4). In China, traditional
Chinese medicine (TCM) has been widely used to treat CKD (5,6).
Modified Huangqi Chifeng decoction (MHCD) has previously been
reported to be an effective treatment for CKD (7). However, the underlying mechanisms of
MHCD in CKD remain to be elucidated.
The role of autophagy in CKD has previously been
studied and it was reported that kidney cells differentiate by
acquiring specialized membranous components (8). However, the phenomenon of autophagy in
the kidney was not clearly defined at that time. Over the past few
decades, autophagy in CKD has attracted increased attention.
Several studies have demonstrated that autophagy is a major
protective mechanism against podocyte aging and glomerular injury
in rat kidney cells, suggesting that autophagy serves a role in
ameliorating human glomerular disease and aging-associated loss of
renal function (9–13). It is therefore important to elucidate
the relevant molecular mechanisms associated with autophagy in
CKD.
A previous study by our group demonstrated that MHCD
attenuated renal fibrosis by inhibiting excessive autophagy and
upregulating basal autophagy in rats with Doxorubicin-induced
nephropathy (14), which is an
experimental model of progressive kidney disease (15,16). In
the present study, Doxorubicin-induced nephrotic rats with
proteinuria were used as an experimental model. The aim of the
present study was to investigate whether MHCD was able to regulate
autophagy in rats to minimize podocyte and glomerular injuries via
the phosphoinositide-3 kinase/mammalian target of rapamycin
(PI3K/mTOR) signaling pathway.
Materials and methods
Drugs and antibodies
MHCD, comprised of 30 g Sheng Huang-qi
(Astragalus membranaceus Bge), 20 g Qian Shi (Euryale
ferox Salish), 20 g Jin Ying-zi (Rosae Laevigatae
Michx), 10 g Chi Shao (Paeonia lactiflora Pall), 10 g Fang
Feng (Saposhnikovia divaricata Schischk), 10 g Di Long
(Pheretima vulgaris Chen) and 10 g Bai Hua-she-she-cao
(Hedyotis diffusa Wild), was provided by the Pharmacy
Department of Xiyuan Hospital of China Academy of Chinese Medical
Sciences. The herbs were initially soaked in water for 1 h at room
temperature, followed by 1 h of decoction at 100°C. The extracts
harvested from the decoction were vacuum dried. Water-soluble
extracts of MHCD were dissolved in double-distilled water
(ddH2O). Telmisartan (Micardis; 80 mg/pill) was
purchased from Boehringer Ingelheim International GmbH (Ingelheim
am Rhein, Germany). Doxorubicin hydrochloride for injection
(instant; also known as Doxorubicin) was purchased from Pfizer
Italia Srl (Rome, Italy). Anti-beclin1 (1:1,000; cat. no.
ab210498), anti-LC3 (1:1,000; cat. no. ab48394), anti-PI3K
(1:1,000; cat. no. ab86714) and anti-mTOR (1:1,000; cat. no.
ab2732) primary antibodies were purchased from Abcam (Cambridge,
MA, USA). The secondary antibodies used were part of a
general-purpose two-step immunohistochemical kit (cat. no. PV-6000;
ZSGB Biological Technology; OriGene Technologies, Inc., Rockville,
MD, USA). The DAB kit was purchased from ZSGB Biological Technology
(OriGene Technologies, Inc.).
Animal grouping and treatment
A total of 40 male Sprague-Dawley rats (weight,
220±18 g; age, 2–3 months) were purchased from Beijing HFK
Bioscience Co., Ltd. (Beijing, China). The rats were housed in
humidity-controlled rooms (60±10%) at 24±1°C with a 12-h light/dark
cycle and free access to standard food and tap water. All rats were
housed in metabolic cages and acclimated to laboratory conditions
for 7 days, following which they were randomly divided into either
the blank (n=10) or Doxorubicin-induced nephrosis (n=30) group. The
Doxorubicin-induced nephrosis group was treated once with
Doxorubicin at a dose of 6.2 mg/kg, which was injected into a tail
vein, whereas the blank group was treated once with normal saline
by intravenous injection into a tail vein. The Doxorubicin-induced
nephrosis group was further divided into the model (n=10),
telmisartan (n=10) and MHCD (n=10) groups. After 2 weeks, rats in
the blank and model groups received normal saline, (0.1 ml/10 g)
the telmisartan group received telmisartan (8.33 mg/kg) and the
MHCD group received MHCD (11.46 g/kg) by intragastric
administration once a day for 6 weeks. All drugs were diluted with
distilled water and the dosages were evaluated by body surface
coefficient conversion between humans and rats. Urine was collected
from rats in the metabolic cages to determine the 24-h protein
level. Urine collection was performed once every 2 weeks. Blood was
obtained from the abdominal aorta following intragastric
administration at week 8 to determine albumin (ALB), total
cholesterol (TCH), triacylglyceride (TG) and serum creatinine (Scr)
levels using a Roche Cobas-800 automatic biochemical analyzer
(Roche Diagnositics GmbH, Mannheim, Germany). The rats were then
sacrificed and the kidneys were harvested. The Animal Care and Use
Committee of Xiyuan Hospital of China Academy of Chinese Medical
Sciences approved the experimental protocol.
Histopathological analysis
Sections of cortical tissues from the right kidney
were fixed in 10% buffered formalin at room temperature for 48 h,
embedded in paraffin and sliced to 3 µm thick sections. The
sections were stained at room temperature with hematoxylin-eosin
(HE) for 3 min, Masson's trichome for 30 sec-5 min and periodic
acid-Schiff (PAS) for 30 sec-5 min, following which they were
visualized using light microscopy (magnification, ×400). Other
sections of the right kidneys were fixed in 2.5% glutaraldehyde at
4°C for 24 h, washed with phosphate-buffered saline and sliced to
70–90 nm. Following staining with osmium tetroxide and lead citrate
at room temperature for 5–10 min, the ultrastructure of the kidneys
was observed under a Hitachi H-600 transmission electron microscope
(magnification, ×8,000 and ×20,500; Hitachi, Ltd., Tokyo,
Japan).
Immunohistochemistry
To evaluate the expression and distribution of
microtubule-associated proteins 1A/1B light chain 3B (LC3),
beclin-1, mTOR and PI3K, the paraffin-embedded renal cortical
sections were dewaxed with xylene and rehydrated with a descending
alcohol series. Sections were incubated with 3% hydrogen peroxide
(H2O2) and washed with PBS, following which
they were incubated with primary antibodies (1:200) at 4°C
overnight and washed with PBS. Next, the sections were incubated
with the secondary antibodies (1:2,500) and washed with PBS.
Sections were subsequently stained with DAB at room temperature for
10 sec-2 min, washed with PBS, dehydrated with a descending alcohol
series, permeabilized with xylene, mounted and viewed using light
microscopy (magnification, ×400). The expression of proteins was
demonstrated by the ratio of integral optical density (IOD).
IOD=average optical density × positive area.
Western blotting
To evaluate the expression and distribution of
LC3-I, LC3-II, beclin-1, mTOR and PI3K protein, total protein was
extracted using radioimmunoprecipitation assay buffer, following
which the protein concentration was determined using a BCA Protein
assay kit (cat. no. 23225; Bio-Rad Laboratories Inc., Hercules, CA,
USA). Proteins were added to protein sample buffer, boiled in water
for 5 min and separated by SDS-PAGE. Proteins were transferred onto
polyvinylidene fluoride membranes and Blocked at 4°C overnight with
Tris-buffered saline/Tween 20 (TBST) containing 5% non-fat dried
milk. Membranes were incubated with anti-beclin1 (1:1,000),
anti-LC3 (1:1,000), anti-PI3K (1:1,000), anti-mTOR (1:1,000) and
anti-GAPDH antibodies (1:2,500; cat. no. sc-365062; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) primary antibodies, at 4°C
overnight. The membranes were rinsed three times with PBS Tween20
and then incubated with the secondary antibodies (1:2,500) for 2 h
at room temperature. The protein bands were visualized using an
enhanced chemiluminescent kit (EMD Millipore, Billerica, MA, USA).
ImageJ software (Version 4.0; National Institutes of Health,
Bethesda, MD, USA) was used to analyze the grayscale values of each
group and GAPDH was used as the internal reference.
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance followed by a post-hoc Bonferroni test with
SPSS software 20.0 (IBM Corp., Armonk, NY, USA). Data are expressed
as the mean ± standard deviation and P<0.05 was considered to
indicate a statistically significant difference.
Results
General conditions
The model group rats were in emotional distress and
exhibited diet reduction, messy hair, rough tail, diarrhea and a
difference in color and luster of their hair after 3 days (data not
shown). Two rats exhibited a slight ulcer in their tails (data not
shown). Compared with the blank group, Doxorubicin-induced
nephrotic rats exhibited mild edema in the testicles (data not
shown). The rats in the blank group exhibited free movement, a
normal diet, smooth body hair and no diarrhea or other abnormal
reactions (data not shown). No significant differences in the
weight were observed among the blank (509±8 g), model (496±30 g),
Telmisartan (500±32 g) and MHCD group (498±29 g) at week 8.
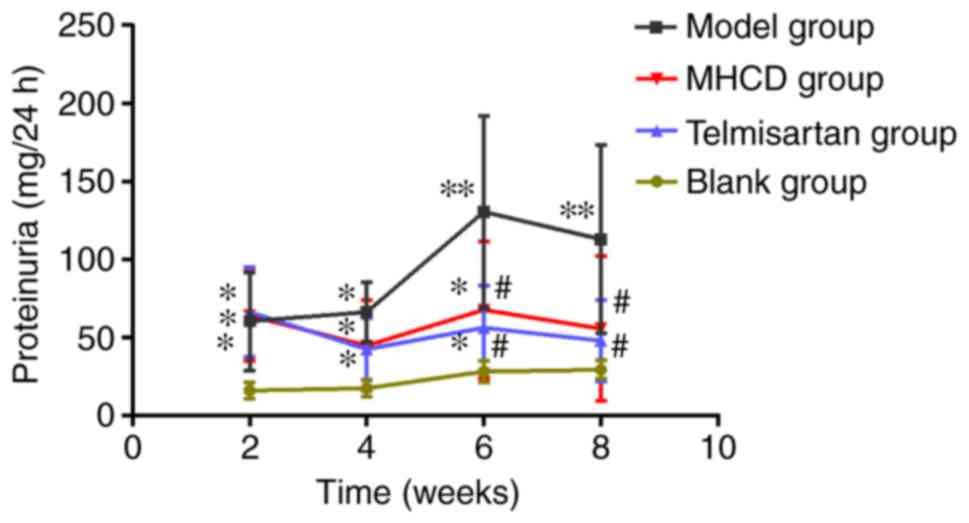
MHCD ameliorates proteinuria
As demonstrated in Fig.
1, proteinuria in Doxorubicin-induced nephrotic rats increased
to peak levels at week 6. Proteinuria was higher at weeks 2, 4
(both P<0.05), 6 and 8 (both P<0.01) in the model group
compared with the blank group, indicating the successful
establishment of the model. Proteinuria in the MHCD and telmisartan
groups increased at weeks 2, 4 and 6 compared with the blank group
(all P<0.05). However, at weeks 6 and 8, 24-h proteinuria
significantly decreased in the telmisartan and MHCD groups compared
with the model group (P<0.05). However, no significant
differences were observed between the telmisartan and MHCD groups.
At week 8, no significant differences were observed between the
MHCD and blank groups. These results indicate that MHCD ameliorates
proteinuria in Doxorubicin-induced nephrotic rats.
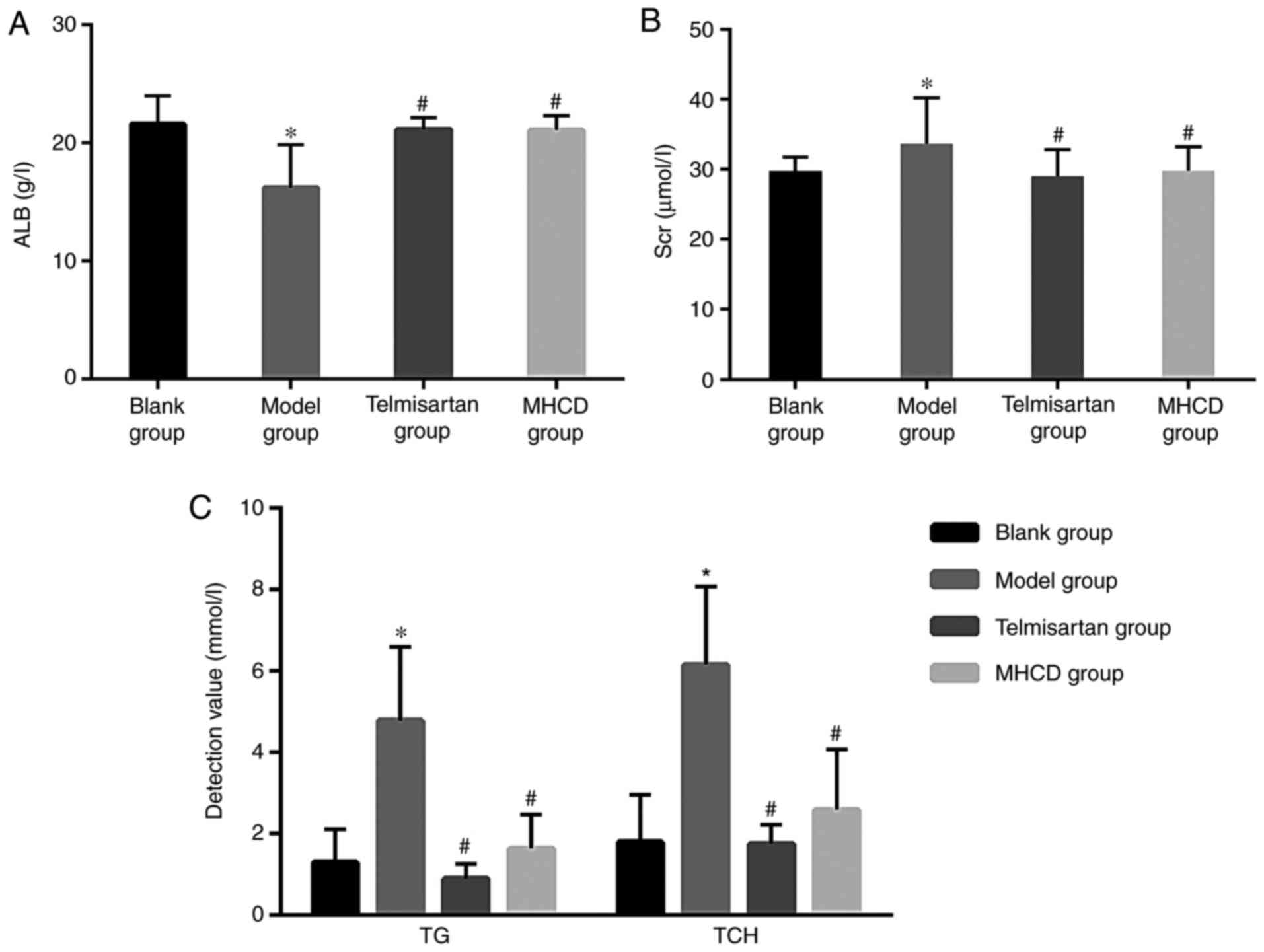
MHCD ameliorates the levels of ALB,
Scr, TG and TCH
As demonstrated in Fig.
2A, ALB levels in the model group decreased significantly
compared with the blank group (P<0.05). Compared with the model
group, the MHCD and telmisartan groups exhibited significantly
increased levels of ALB (both P<0.05). Scr levels in the model
group increased significantly compared with the blank group
(P<0.05; Fig. 2B). Compared with
the model group, the MHCD and telmisartan groups exhibited
significantly decreased levels of Scr (both P<0.05).
Furthermore, levels of TG and TCH in the model group increased
significantly compared with the blank group (P<0.05; Fig. 2C). Compared with the model group, TG
and TCH were significantly decreased in the MHCD and telmisartan
groups (both P<0.05). These results indicate that MHCD
ameliorates Doxorubicin-induced changes in ALB, Scr, TG and TCH
levels in rats. Long-term proteinuria causes hyperlipidemia due to
dysfunctional hepatic lipid protein synthesis (17). MHCD may improve lipid levels by
reducing albuminuria in Doxorubicin-induced nephrotic rats.
However, it is unclear whether MHCD can directly improve lipid
levels.
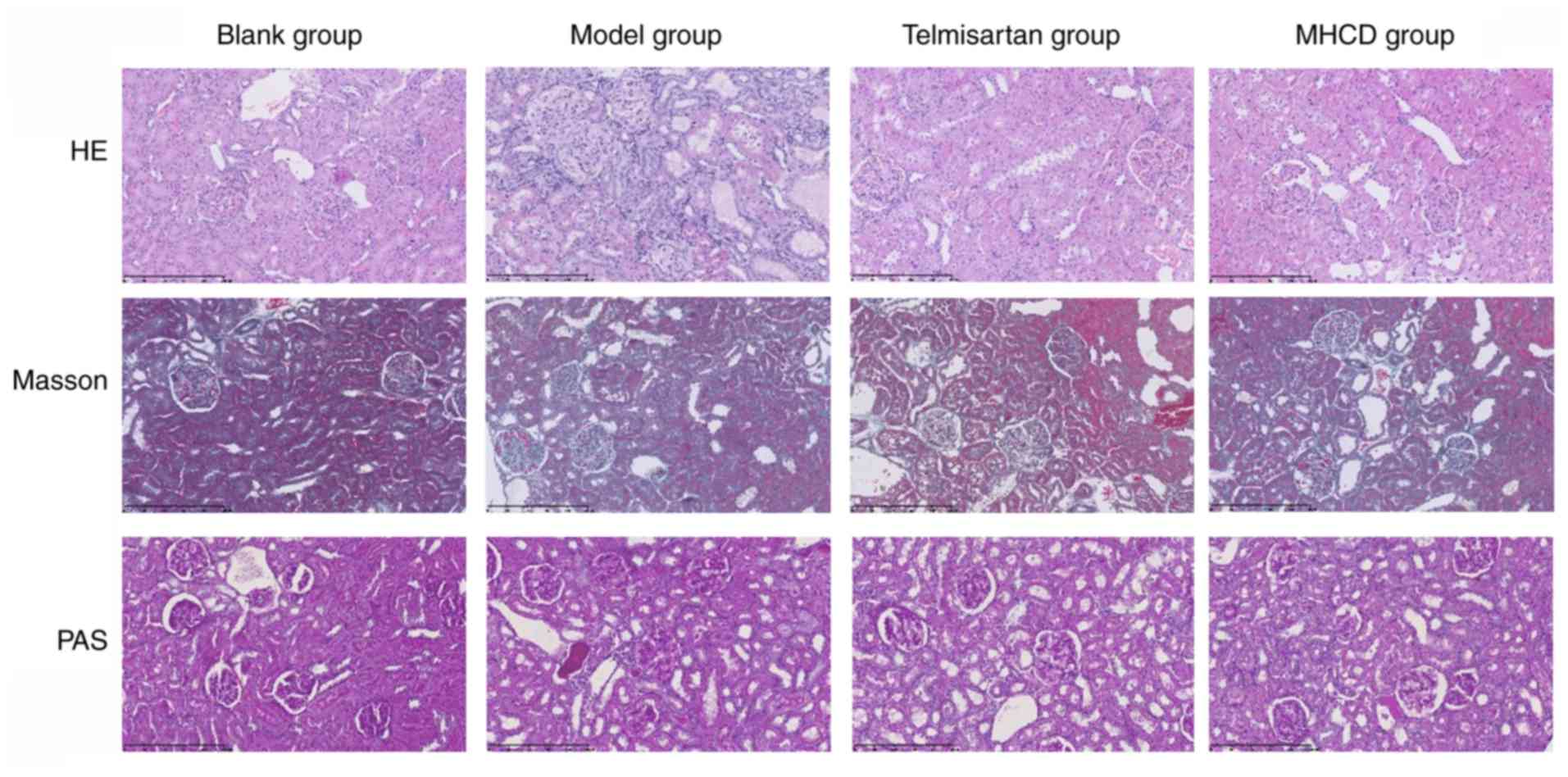
MHCD prevents glomerular and podocyte
injury
As demonstrated in Fig.
3, renal pathological changes were examined using HE, Masson's
trichome and PAS staining. Compared with the blank group, the model
group had a greater number of proliferative mesangial cells,
increased extracellular matrix deposition, a thickened glomerular
basement membrane and disorderly arranged tubular cells (Fig. 3). Compared with the model group,
telmisartan and MHCD treatments ameliorated the Doxorubicin-induced
renal pathological changes in rats. These results demonstrate the
protective effect of MHCD on the kidneys of Doxorubicin-induced
nephrotic rats.
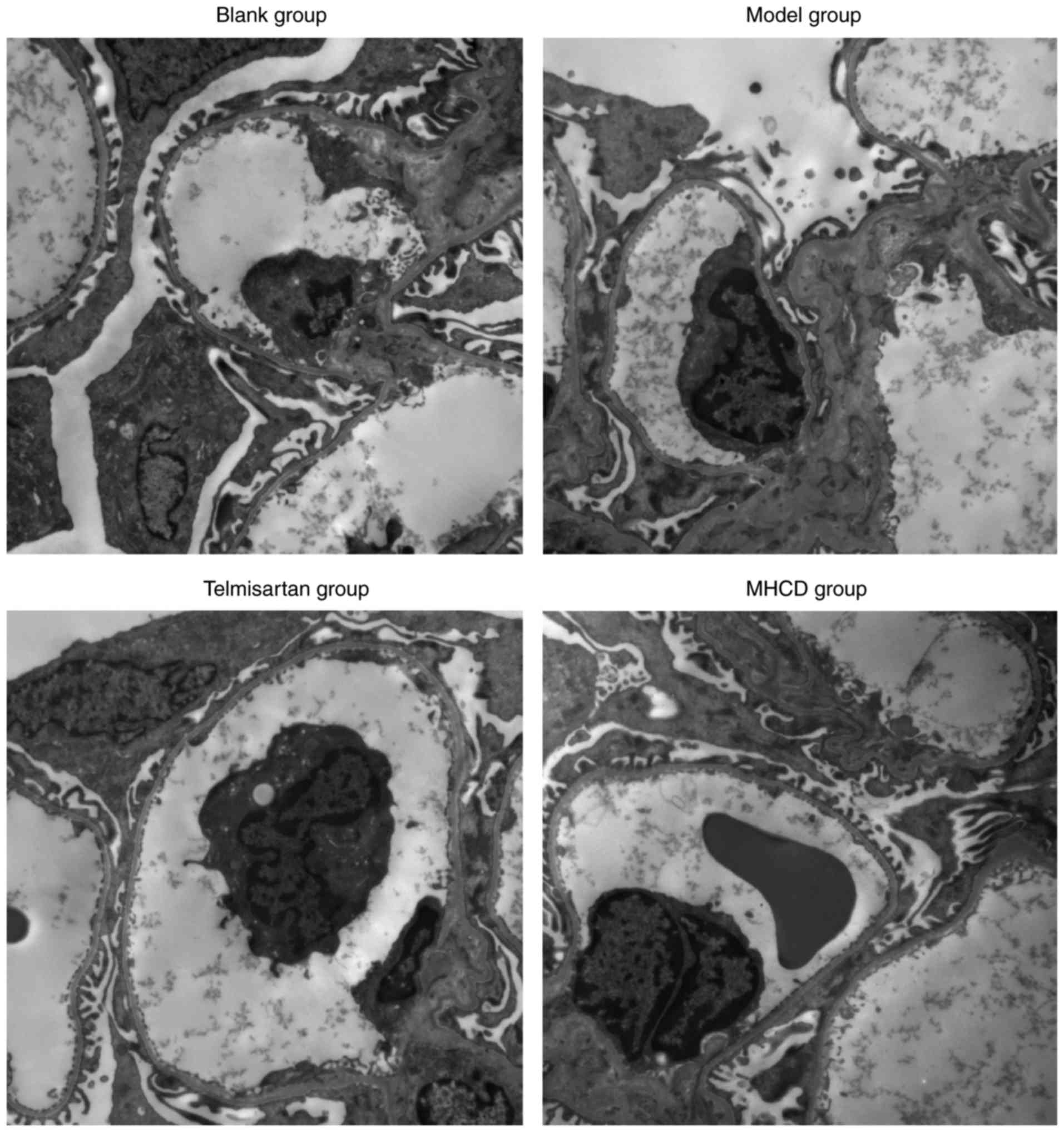
As demonstrated in Fig.
4, the blank group exhibited normal morphology. The podocytes
in the model group were flattened or fused and an exposed basement
membrane and increased number of proliferating mesangial cells were
observed. In addition, lipid vacuoles were observed in a number of
endothelial cells. Compared with the model group, telmisartan and
MHCD treatments alleviated the degree of podocyte foot process
fusion. Additionally, only a small number of swollen endothelial
cells were observed following MHCD treatment, further supporting
the findings.
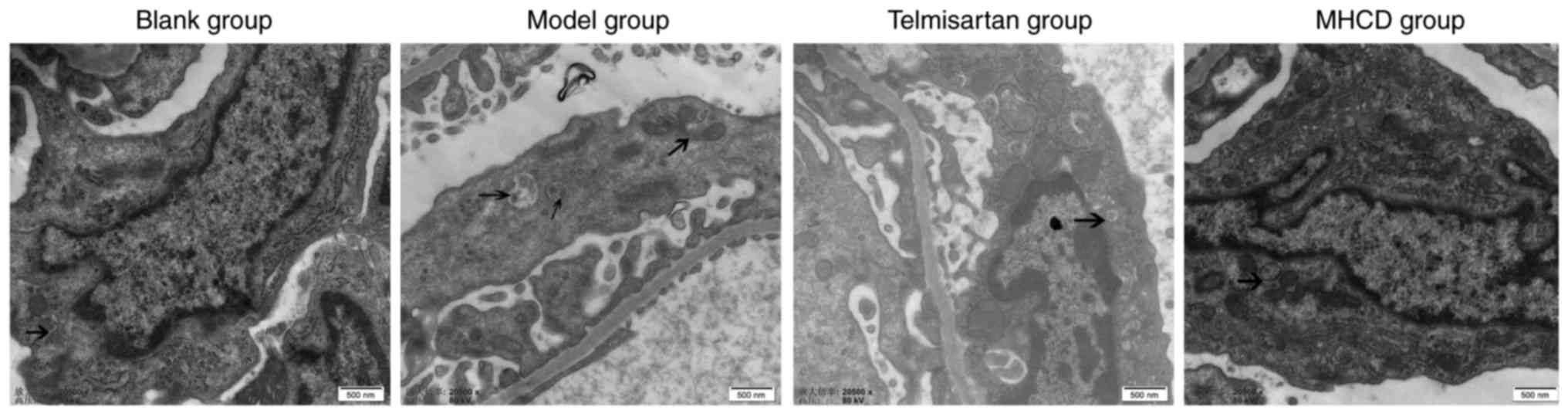
MHCD inhibits excessive autophagy
Autophagy is a highly conserved cellular process
that is important in anaphase cells, including neurocytes and
podocytes (18,19). Autophagy in podocytes was observed
using transmission electron microscopy. Podocytes in the blank
group exhibited a normal nucleus, mitochondria, endoplasmic
reticulum and Golgi apparatus (Fig.
5), whereas podocytes in the model group had a marked increase
in the number of autophagosomes, dilated endoplasmic reticula and
swollen mitochondria. A lower number of autophagosomes were
observed in the MHCD and telmisartan groups compared with the model
group. These results demonstrate that MHCD inhibits excessive
autophagy in rats with Doxorubicin-induced nephrosis.
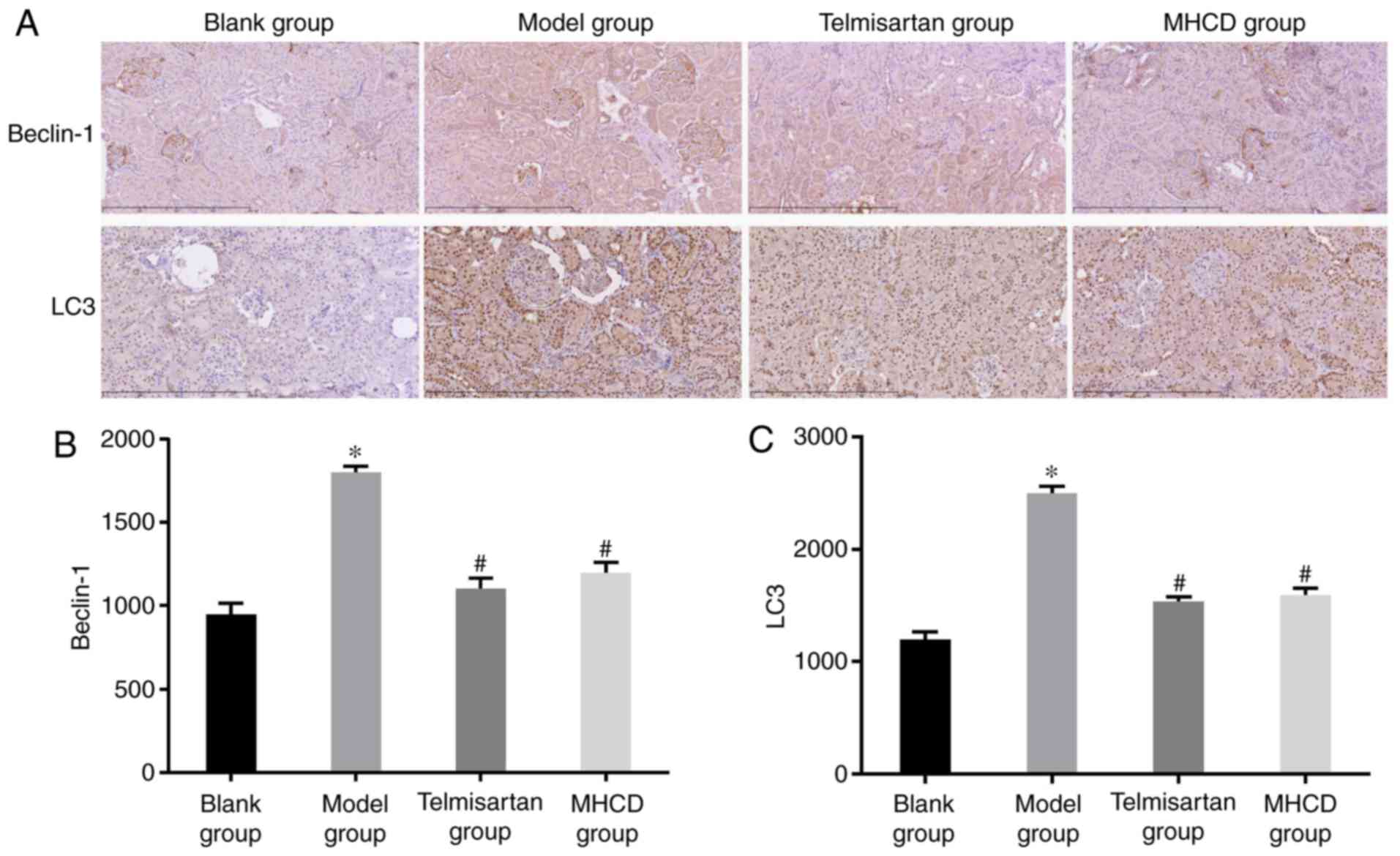
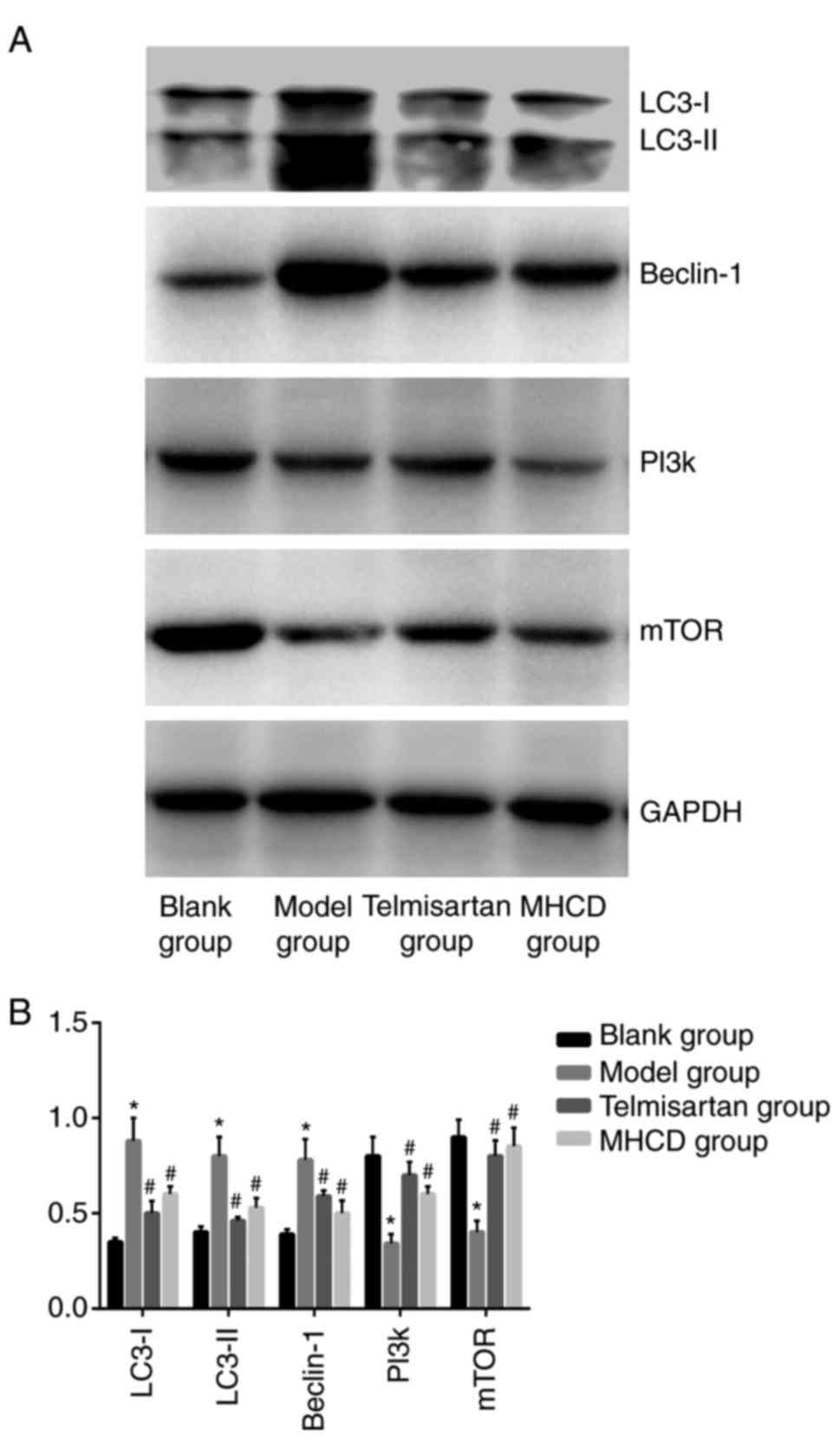
To assess whether autophagy is affected by MHCD, the
expression of beclin-1 and LC3 were measured using
immunohistochemistry (Fig. 6A) and
western blotting (Fig. 7A). Beclin-1
(Figs. 6B and 7B), LC3 (Fig.
6C), LC3-I and LC3-II (Fig. 7B;
all P<0.05) expression was significantly higher in the model
group compared with the blank group. These increases were
significantly inhibited following treatment with MHCD or
telmisartan (all P<0.05).
MHCD inhibits excessive autophagy by
inducing the PI3K/mTOR signaling pathway
Excessive autophagy leads to cell death and it has
been demonstrated that the PI3K/mTOR signaling pathway serves a
critical role in regulating this process (20,21). To
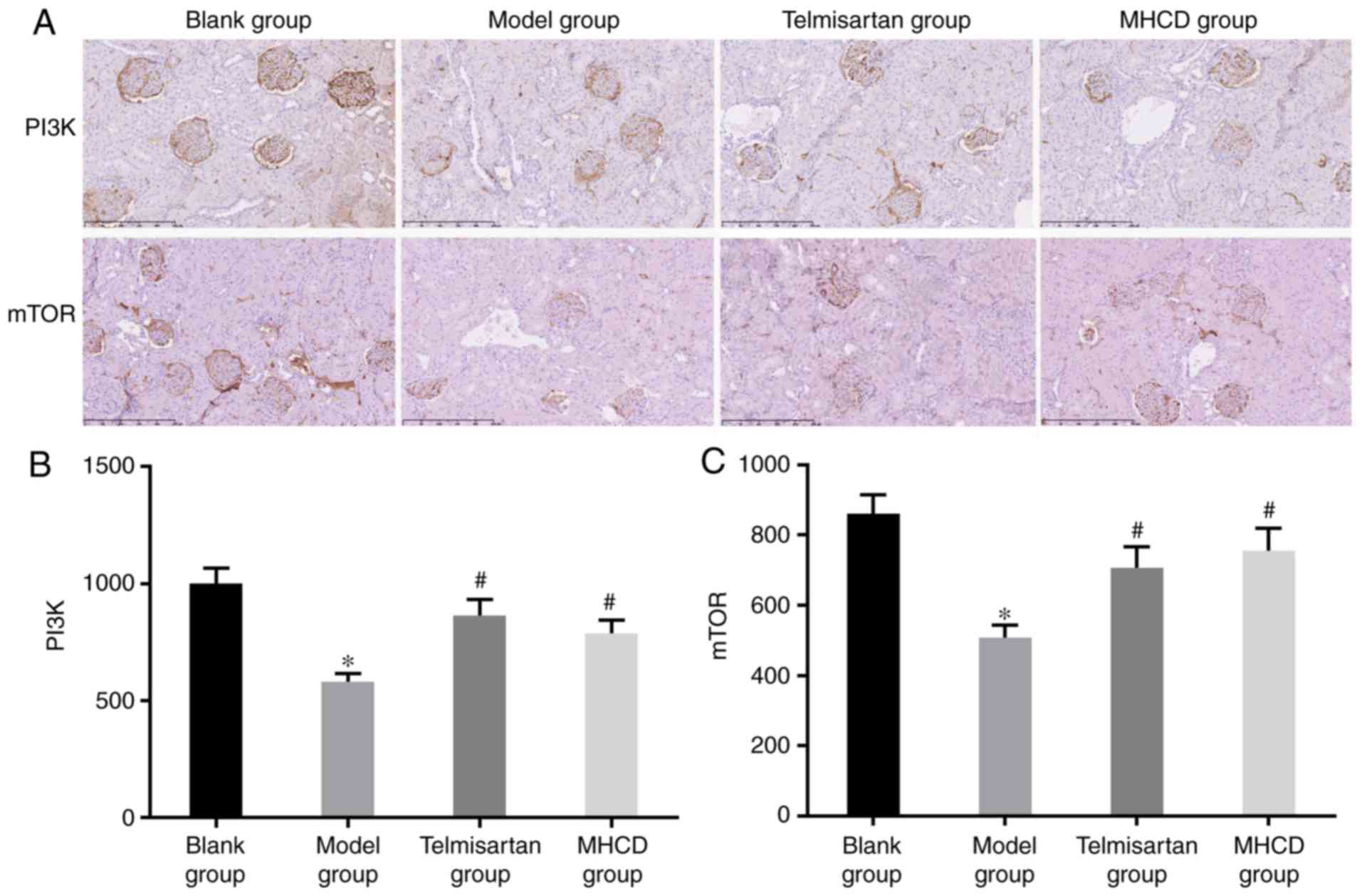
assess whether MHCD inhibits excessive autophagy via the PI3K/mTOR
signaling pathway, PI3K and mTOR expression were measured by
western blotting (Fig. 7A) and
immunohistochemistry (Fig. 8A). As
presented in Figs. 7B, 8B and C, the expression of PI3K and mTOR
was significantly lower in the model group compared with the blank
group (all P<0.05). When Doxorubicin-induced nephrotic rats were
treated with MHCD or telmisartan, PI3K and mTOR expression
increased significantly compared with the model group (all
P<0.05). However, as presented in Fig. 6, autophagic activity in the model
group was significantly higher compared with the blank group,
indicating that the inhibition of the PI3K/mTOR signaling pathway
resulted in excessive autophagy. These results indicate that MHCD
inhibits excessive autophagy by inducing the PI3K/mTOR signaling
pathway.
Discussion
The aim of the present study was to investigate
whether the regulatory effects of MHCD in autophagy are mediated
via the PI3K/mTOR signaling pathway. It was revealed that the
protective effects of MHCD in Doxorubicin-induced nephrotic rats
are achieved by suppressing excessive autophagy via activation of
the PI3K/mTOR pathway.
Proteinuria, hypoalbuminemia and hyperlipidemia are
typical manifestations of Doxorubicin-induced nephrosis in rats
(22) and these characteristics were
observed in rats in the present study. Histological changes,
including focal segmental glomerularsclerosis, tubulointerstitial
inflammation, fibrosis and podocyte fusion, typical of
Doxorubicin-induced nephropathy, were also observed in the present
study (23). In TCM, MHCD has been
demonstrated to reduce urinary protein and cholesterol levels,
increase serum albumin levels and prevent glomerular and podocyte
injuries in Doxorubicin-induced nephrotic rats (24). These results may be used to develop
novel methods for delaying glomerulosclerosis using TCM.
Autophagy is a process by which cellular components
are recycled (25) and injured
organelles and proteins are removed (26). Autophagy comprises mechanistically
distinct steps, including the induction, identification and
selection of cargo, the formation of vesicles, autophagosome
vacuole fusion, the breakdown of cargo and the release of
degradation products into the cytoplasm (27). The formation of autophagosomes serves
an important role in autophagy and requires the recruitment of
ubiquitin-like-conjugating enzyme ATG (Atg) (28). LC3 has a molecular mass of ~17 kDa
and is a mammalian ortholog of yeast Atg8 (29). Previous studies have demonstrated
that LC3 is recruited into autophagosomal membranes, indicating
that LC3 is a marker for autophagy (30,31).
Beclin-1, another key regulator of autophagy, is the mammalian
ortholog of yeast Atg6 (32,33). Beclin-1 has been demonstrated to
induce autophagy via regulating PI3K VPS34 (Vps-34) to promote the
formation of beclin 1-Vps34-Vps15 core complexes (34). As such, LC3 and beclin-1 are
effective biomarkers for monitoring autophagy. Cells typically
trigger autophagy to reduce cellular damage following infection,
ischemia, starvation or growth factor deficiency (35). Autophagy, which occurs under basal
conditions, serves an important role in cell growth, development
and homeostasis by maintaining a balance between the synthesis and
subsequent recycling of cellular products (36). However, excessive or sustained
autophagy triggers non-apoptotic programmed cell death due to
excessive self-digestion and degradation of essential cellular
constituents (20,37,38). It
has been reported that excessive autophagy serves a role in kidney
disease (39); furthermore,
excessive autophagy has been demonstrated to result in cell death
(40). The results of the present
study are in agreement with previous reports, indicating that MHCD
attenuates excessive autophagy in the kidney by attenuating LC3 and
beclin-1 overexpression and upregulating basal autophagy to protect
Doxorubicin-induced nephrotic rats.
The specific mechanism by which MHCD regulates
autophagy has not previously been elucidated, however it has been
reported that PI3K/mTOR pathway activation is involved in autophagy
(41,42). Based on this, the effect of MHCD on
PI3K/mTOR pathway activation was investigated. PI3K is a family of
lipid kinases (43) and mTOR is a
highly conserved serine/threonine kinase (44). In the present study, the expression
of PI3K and mTOR in the renal cortical tissues of
Doxorubicin-induced nephrotic rats was examined. The results
revealed that MHCD activates the PI3K/mTOR signaling pathway. Luo
et al (45) reported that
excessive autophagy resulted in delayed cell death due to
inhibition of the PI3K/mTOR signaling pathway. Furthermore,
excessive autophagy is often observed when the PI3K/mTOR signaling
pathway is chemically blocked (46).
A previous study revealed that autophagy was one of the main
mechanisms of cell death when the PI3K/mTOR signaling pathway is
inhibited (47). Wang et al
(48) revealed that PI3K/Akt
signaling was associated with the protection of neurons via
autophagy inhibition. Xing et al (49) reported that mTOR upregulation
inhibited autophagy. The results of the present study revealed that
MHCD attenuates excessive autophagy and activates the PI3K/mTOR
signaling pathway in Doxorubicin-induced renal injury. This
suggests that MHCD attenuates excessive autophagy by inhibiting the
PI3K/mTOR signaling pathway.
Podocyte activity has previously been assessed in
vitro using CCK-8 and it was revealed that Doxorubicin promoted
autophagy compared with the autophagy inhibitor 3-methyladenine
(50). The aim of the present study
was to assess whether MHCD inhibited excessive autophagy caused by
Doxorubicin only; no other agents were assessed. In future studies
additional autophagy inhibitors and PI3K/mTOR signaling pathway
inhibitors should be assessed in vivo to confirm the results
of the present study. Furthermore, autophagy is a complicated
process; elucidating the specific effects of individual MHCD
components in autophagy requires the synthesis of specific small
molecule compounds for validation.
In the present study, it rats with untreated
Doxorubicin-induced nephrosis exhibited a marked and sustained
increase in proteinuria until week 6. In contrast, proteinuria in
MHCD- and telmisartan-treated rats increased only slightly between
weeks 2 and 4. Future studies should include a group of individuals
treated with MHCD for 2 weeks prior to Doxorubicin treatment to
further demonstrate that MHCD has protective roles in
Doxorubicin-induced nephrotic rats. In conclusion, the results of
the present study suggest that MHCD ameliorates proteinuria,
increases ALB levels and decreases Scr, TG and TCH levels.
Additionally, it protects against glomerular and podocyte injuries
by inhibiting excessive autophagy via the PI3K/mTOR signaling
pathway and upregulating basal autophagy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Subjective
Selected Subjects Program of China Academy of Chinese Medical
Sciences (grant no. ZZ0708105).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ conceived and designed the animal experiments and
helped to draft the paper. ZKY, BY, LSL, JNZ and WH performed the
experiments. ZKY and BY analyzed the data and wrote the paper.
Ethics approval and consent to
participate
The Animal Care and Use Committee of Xiyuan Hospital
of China Academy of Chinese Medical Sciences (Beijing, China)
approved the experimental protocol.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MHCD
|
modified Huangqi Chifeng decoction
|
|
ALB
|
albumin
|
|
TCH
|
total cholesterol
|
|
TG
|
triacylglyceride
|
|
Scr
|
serum creatinine
|
|
LC3
|
microtubule-associated proteins 1A/1B
light chain 3B
|
|
PI3K
|
phosphoinositide-3 kinase
|
|
mTOR
|
mammalian target of rapamycin
|
|
CKD
|
chronic kidney disease
|
|
TCM
|
traditional Chinese medicine
|
|
PAS
|
periodic acid Schiff
|
References
|
1
|
Kirsztajn GM, Filho NS, Draibe SA, Netto
MV, Thomé FS, Souza E and Bastos MG: Fast reading of the KDIGO
2012: Guidelines for evaluation and management of chronic
kidneydisease in clinical practice. J Bras Nefrol. 36:63–73.
2014.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mills KT, Xu Y, Zhang W, Bundy JD, Chen
CS, Kelly TN, Chen J and He J: A systematic analysis of worldwide
population-based data on the global burden of chronic kidney
disease in 2010. Kidney Int. 88:950–957. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carney EF: Epidemiology: Global burden of
disease study 2013 reports that disability caused by CKD is
increasing worldwide. Nat Rev Nephrol. 11:4462015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barnett AH, Bain SC, Bouter P, Karlberg B,
Madsbad S, Jervell J and Mustonen J; Diabetics Exposed to
Telmisartan and Enalapril Study Group, : Angiotensin-receptor
blockade versus converting-enzyme inhibition in type 2 diabetes and
nephropathy. N Engl J Med. 351:1952–1961. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gong XZ, Zhou LF, Wang Q, Tang XC, Qian
YR, Wang YR, Lu L and Zhou JJ: Effect of Chuanhuang No.1 recipe on
renal function and micro-inflammation in phase 3 chronic kidney
disease patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 35:137–141.
2015.(In Chinese). PubMed/NCBI
|
|
6
|
Dong F, Cheng J, Lin S, Hu Z, Chen G and
He L: The clinical research on serum cystatin-C alteration on stage
II chronic kidney disease with gubenquduyishen decoction treatment.
J Ethnopharmacol. 131:581–584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Yu: Clinical observation on 50 cases
of chronic nephritis proteinuria treated with modified Huangqi
Chifeng Decoction. Chin Med Guides. 36:137–138. 2007.(In
Chinese).
|
|
8
|
Clark SL Jr: Cellular differentiation in
the kidneys of newborn mice studied with the electron microscope. J
Biophys Biochem Cytol. 3:349–362. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berkenstam A, Ahlberg J and Glaumann H:
Isolation and characterization of autophagic vacuoles from rat
kidney cortex. Virchows Arch B Cell Pathol Incl Mol Pathol.
44:275–286. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asanuma K, Tanida I, Shirato I, Ueno T,
Takahara H, Nishitani T, Kominami E and Tomino Y: MAP-LC3, a
promising autophagosomal marker, is processed during the
differentiation and recovery of podocytes from PAN nephrosis. FASEB
J. 17:1165–1167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizushima N, Yamamoto A, Matsui M,
Yoshimori T and Ohsumi Y: In vivo analysis of autophagy in response
to nutrient starvation using transgenic mice expressing a
fluorescent autophagosome marker. Mol Biol Cell. 15:1101–1104.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hartleben B, Gödel M, Meyer-Schwesinger C,
Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ,
Lindenmeyer MT, et al: Autophagy influences glomerular disease
susceptibility and maintains podocyte homeostasis in aging mice. J
Clin Invest. 120:1084–1096. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng C, Fan Y, Wu J, Shi S, Chen Z, Zhong
Y, Zhang C, Zen K and Liu Z: Podocyte autophagic activity plays a
protective role in renal injury and delays the progression of
podocytopathies. J Pathol. 234:203–213. 2014.PubMed/NCBI
|
|
14
|
Yu Z, Yang B and Zhang Y: Protective
effect of modified Huangqi Chifeng decoction on Doxorubicin
Nephrosis (AN) rats kidney and its regulated effect on its
autophagy level. Zhongguo Zhong Yi Ji Chu Yi Xue Za Zhi.
23:477–479+563. 2017.(In Chinese).
|
|
15
|
Faleiros CM, Francescato HD, Papoti M,
Chaves L, Silva CG, Costa RS and Coimbra TM: Effects of previous
physical training on adriamycin nephropathy and its relationship
with endothelial lesions and angiogenesis in the renal cortex. Life
Sci. 169:43–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee VW and Harris DC: Adriamycin
nephropathy: A model of focal segmental glomerulosclerosis.
Nephrology (Carlton). 16:30–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rahman M, Yang W, Akkina S, Alper A,
Anderson AH, Appel LJ, He J, Raj DS, Schelling J, Strauss L, et al:
Relation of serum lipids and lipoproteins with progression of CKD:
The CRIC study. Clin J Am Soc Nephrol. 9:1190–1198. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu N, Xu L, Shi Y and Zhuang S: Podocyte
autophagy: A potential therapeutic target to prevent the
progression of diabetic nephropathy. J Diabetes Res.
2017:35602382017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mijaljica D, Prescott M and Devenish RJ:
The intriguing life of autophagosomes. Int J Mol Sci. 13:3618–3635.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stern ST, Adiseshaiah PP and Crist RM:
Autophagy and lysosomal dysfunction as emerging mechanisms of
nanomaterial toxicity. Part Fibre Toxicol. 9:202012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guan H, Piao H, Qian Z, Zhou X, Sun Y, Gao
C, Li S and Piao F: 2,5-Hexanedione induces autophagic death of
VSC4.1 cells via a PI3K/Akt/mTOR pathway. Mol Biosyst.
13:1993–2005. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Wang ZG and Liu Z: Changes of
glomerular fixed anionic charge sites in adriamycin nephrosis in
rats. Chin Med J (Engl). 104:128–131. 1991.PubMed/NCBI
|
|
23
|
Bertani T, Poggi A, Pozzoni R, Delaini F,
Sacchi G, Thoua Y, Mecca G, Remuzzi G and Donati MB:
Adriamycin-induced nephrotic syndrome in rats: Sequence of
pathologic events. Lab Invest. 46:16–23. 1982.PubMed/NCBI
|
|
24
|
Wang YL, Zhang Y, Wang HX and Hao W:
Impact of jiaweihuangqichifeng decoction on proteinuria, renal
cortex SOD and renal cortex MDA in nephrotic rats induced by
adriamycin. Chin J Basic Med Trad Chin Med. 17:505–507. 2011.(In
Chinese).
|
|
25
|
Costas MA and Rubio MF: Autophagy: A
strategy for cell survival. Medicina (B Aires). 77:314–320.
2017.(In Spanish). PubMed/NCBI
|
|
26
|
Yao ST and Qin SC: The relationship of
autophagy with endoplasmic reticulum stress and its role in
pathogenesis, prevention and therapy of atherosclerosis. Sheng Li
Xue Bao. 69:515–521. 2017.(In Chinese). PubMed/NCBI
|
|
27
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davis S, Wang J and Ferro-Novick S:
Crosstalk between the secretory and autophagy pathways regulates
autophagosome formation. Dev Cell. 41:23–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanida I, Ueno T and Kominami E: LC3 and
autophagy. Methods Mol Biol. 445:77–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shibata M, Yoshimura K, Tamura H, Ueno T,
Nishimura T, Inoue T, Sasaki M, Koike M, Arai H, Kominami E and
Uchiyama Y: LC3, a microtubule-associated protein1A/B light chain3,
is involved in cytoplasmic lipid droplet formation. Biochem Biophys
Res Commun. 393:274–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Glover K, Li Y, Mukhopadhyay S, Leuthner
Z, Chakravarthy S, Colbert CL and Sinha SC: Structural transitions
in conserved, ordered Beclin 1 domains essential to regulating
autophagy. J Biol Chem. 292:16235–16248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mei Y, Glover K, Su M and Sinha SC:
Conformational flexibility of BECN1: Essential to its key role in
autophagy and beyond. Protein Sci. 25:1767–1785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin Z, Pascual C and Klionsky DJ:
Autophagy: Machinery and regulation. Microb Cell. 3:588–596. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martinet W, Agostinis P, Vanhoecke B,
Dewaele M and De Meyer GR: Autophagy in disease: A double-edged
sword with therapeutic potential. Clin Sci (Lond). 116:697–712.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kroemer G and Jäättelä M: Lysosomes and
autophagy in cell death control. Nat Rev Cancer. 5:886–897. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Johnson-Lyles DN, Peifley K, Lockett S,
Neun BW, Hansen M, Clogston J, Stern ST and McNeil SE: Fullerenol
cytotoxicity in kidney cells is associated with cytoskeleton
disruption, autophagic vacuole accumulation, and mitochondrial
dysfunction. Toxicol Appl Pharmacol. 248:249–258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Klionsky DJ: Autophagy: From phenomenology
to molecular understanding in less than a decade. Nat Rev Mol Cell
Biol. 11:931–937. 2007. View Article : Google Scholar
|
|
41
|
Peng Y, Qiu L, Xu D, Zhang L, Yu H, Ding
Y, Deng L and Lin J: M4IDP, a zoledronic acid derivative, induces
G1 arrest, apoptosis and autophagy in HCT116 colon carcinoma cells
via blocking PI3K/Akt/mTOR pathway. Life Sci. 185:63–72. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang S, Li J, Du Y, Xu Y, Wang Y, Zhang Z,
Xu Z, Zeng Y, Mao X and Cao B: The class I PI3K inhibitor S14161
induces autophagy in malignant blood cells by modulating the Beclin
1/Vps34 complex. J Pharmacol Sci. 134:197–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
De Santis MC, Sala V, Martini M, Ferrero
GB and Hirsch E: PI3K signaling in tissue hyper-proliferation: From
overgrowth syndromes to kidney cysts. Cancers (Basel). 9(pii):
E302017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Reilly R, Mroz MS, Dempsey E, Wynne K,
Keely SJ, McKone EF, Hiebel C, Behl C and Coppinger JA: Targeting
the PI3K/Akt/mTOR signalling pathway in cystic fibrosis. Sci Rep.
7:76422017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Luo T, Liu G, Ma H, Lu B, Xu H, Wang Y, Wu
J, Ge P and Liang J: Inhibition of autophagy via activation of
PI3K/Akt pathway contributes to the protection of ginsenoside Rb1
against neuronal death caused by ischemic insults. Int J Mol Sci.
15:15426–15442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oh I, Cho H and Lee Y, Cheon M, Park D and
Lee Y: Blockage of autophagy rescues the dual PI3K/mTOR Inhibitor
BEZ235-induced growth inhibition of colorectal cancer cells. Dev
Reprod. 20:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Echeverry N, Ziltener G, Barbone D, Weder
W, Stahel RA, Broaddus VC and Felley-Bosco E: Inhibition of
autophagy sensitizes malignant pleural mesothelioma cells to dual
PI3K/mTOR inhibitors. Cell Death Dis. 6:e17572015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Y, Wang W, Li D, Li M, Wang P, Wen J,
Liang M, Su B and Yin Y: IGF-1 alleviates NMDA-induced
excitotoxicity in cultured hippocampal neurons against autophagy
via the NR2B/PI3K-AKT-mTOR pathway. J Cell Physiol. 229:1618–1629.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xing Y, Liu YX, Liu X, Wang SL, Li P, Lin
XH, Sui CL, Xu C, Qi B and Tong Q: Effects of Gui Zhu Yi Kun
formula on the P53/AMPK pathway of autophagy in granulosa cells of
rats with polycystic ovary syndrome. Exp Ther Med. 13:3567–3573.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li S: The effect of MHCD on proteinuria
and its cellular molecular mechanism [D]. Beijing Zhong Yi Yao Da
Xue. 2016.(In Chinese).
|