Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune
disease characterized by symmetrical arthritis, with a morbidity
rate among the highest of all autoimmune connective tissue diseases
(1,2). The average global incidence of RA is
0.5–1%, whereas in China, the incidence rate reaches 0.32–0.36%
(3). Globally, the highest incidence
rates of RA are observed in individuals aged between 40 and 60
years, and the number of female patients with RA is 2- to 3-fold
higher than that of male RA patients (4). RA mainly affects synovial joints, and
also affects tunica serosa, the heart, lungs, blood vessels,
nerves and eyes (5). It is accepted
that RA is caused by pathological changes in humoral and cellular
immunity (6). The pathogenesis of RA
includes synovial lining cell hyperplasia, interstitial
inflammatory cell infiltration, angiogenesis, and destruction of
cartilage and bone tissues (7).
Clinically, RA is mainly treated with drugs such as nonsteroidal
anti-inflammatory drugs, disease-modifying anti-rheumatic drugs,
glucocorticoids, biological agents and botanical drugs (8–10).
Tetrandrine (Tet), typically administered as an oral
tablet, is a bisbenzylisoquinoline calcium antagonist that acts to
reduce total peripheral vascular resistance and blood pressure
(11). Tet also increases cardiac
output and exerts muscle relaxant, antipyretic, analgesic and
anti-inflammatory effects, and may inhibit cell proliferation and
induce apoptosis (12,13). In addition, Tet tablets may be used
for the treatment of simple silicosis, anthracosilicosis, mild
hypertension, rheumatalgia, arthralgia and neuralgia (14–16).
However, it is not clear whether Tet is effective in the treatment
of patients with RA and the mechanism of action of Tet on RA
remains unknown. In the present study, a rat model of RA was used
to investigate the index scores and expression of relative factors
such as interleukin (IL)-6, IL-1β, and tumor necrosis factor
(TNF)-α in the blood, as well as the effects of the Tet tablet on
RA.
Materials and methods
Animals
A total of 60 Wistar rats (100–200 g; sex ratio,
1:1; mean age, 5–7 weeks) were purchased from the Teng Xin
Experimental Animals Company (Chongqing, China). The rats were
raised in an environment at 24±2°C with 55±5% humidity and a 12 h
light/dark cycle. All rats had free access to food and water. They
were randomly divided into the following six groups (n=10 per
group): Blank (negative control, NC), RA model, methotrexate (MTX;
3 mg/kg body weight), high-dose Tet (31.25 mg/kg body weight),
medium-dose Tet (18.75 mg/kg body weight) and low-dose Tet (6.25
mg/kg body weight). According to previous methods (17), all rat groups excluding the blank
group were injected subcutaneously with 0.1 ml complete Freund's
adjuvant (F5881; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
into the right rear toe to induce inflammation. On day 2 following
adjuvant administration, intragastric drug treatments were
initiated. The MTX group received intragastric administration of
MTX (2.5 mg/tablet once every 3 days; 20150403; Shanghai Xinyi
Pharmaceutical Co., Ltd., Shanghai, China) while the Tet groups
received intragastric administrations of different concentrations
of Tet (20 mg/tablet; H20063332; Beihai Yangguang Pharmaceutical
Company Ltd., Beihai, China) at 11 a.m. every day. The blank group
received saline alone (daily). All animal experiments were
conducted according to the ethical guidelines of Zaozhuang
Municipal Hospital (Zaozhuang, China).
Right toe swelling test
Prior to establishment of the RA model, the initial
volume of the right rear toe was determined using a swelling
measuring instrument (PV-200; Chengdu Techman Software Co., Ltd.,
Chengdu, China) according to a previously published method
(18). Following injection of
complete Freund's adjuvant, the volume of the right rear toe was
measured once a week. The toe swelling rate was calculated
according to the following formula: (Current volume - volume before
experiments)/volume before experiment ×100%.
Arthritis index
The extent of arthritis in rats was scored every
three days following injection with complete Freund's adjuvant.
Arthritis index was determined according to the degree and area of
erythema and the joint swelling and deformation status of the rear
toe after immunization (19).
Scoring was as follows: 0, rats with normal joint status; 1, rats
with mild erythema and swelling at the rear ankle; 2, rats with
erythema and swelling at the rear ankle joint and tarsal; 3, rats
with erythema and moderate swelling from the ankle to metatarsal or
metacarpal joint; and 4, rats with severe swelling and erythema
from the ankle to metatarsal.
Immune organ index
A primary function of the thymus is to produce T
lymphocytes and secrete thymic hormone, and thus the thymus is
principally involved in cellular immunity. High levels of
lymphocytes and macrophages are present in the spleen, though there
is a greater proportion of B lymphocytes present (20). Therefore, the spleen is more closely
associated with humoral immunity. Indices of the thymus or spleen
are expressed as the weight of the thymus or spleen (mg) per 10 g
body weight. Thymus and spleen indices are dependent on the
proliferation of lymphocytes and reflect immune function. On day 28
following adjuvant administration, all rats were sacrificed by
decapitation and the spleen and thymus were removed and wet-weighed
to calculate indices of the thymus spleen. Indices of the thymus or
spleen were calculated as follows: Weight of thymus or spleen
(mg)/body weight (g) ×103.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
On day 28, rats were sacrificed and blood was
collected from the abdominal aorta. Peripheral blood mononuclear
cells (PBMCs) were isolated from the blood according to the
manufacturer's protocol (TBD2011RAT; Haoyang Biological Manufacture
Co., Ltd., Tianjin, China) and lysed using TRIzol reagent
(10606ES60; Yeasen Corp., Shanghai, China). Total RNA (30 µl) was
extracted using the phenol chloroform method according to the
manufacturer's protocol (RP2401; BioTeke Corporation, Beijing,
China). The purity of RNA was determined using ultraviolet
spectrophotometry (Nanodrop ND1000; Thermo Scientific, Waltham, MA,
USA) according to the ratio of absorbance (A) at 260 and 280 nm
(A260/A280 ratio) following a previously published method (21). cDNA was subsequently obtained by
reverse transcription using a TIANScript II cDNA Kit (KR107;
Tiangen Biotech Co., Ltd., Beijing, China) from 1 µg RNA and stored
at −20°C. For qPCR, the following primers were used: Cyclooxygenase
(COX)-2, forward 5′-CAGCCATACAGCAAATCCTTG-3′ and reverse
5′-CAAATGTGATCTGGATGTCAAC-3′; and β-actin, forward
5′-CACCAGGGCGTGATGGT-3′ and reverse 5′-CTCAAACATGATCTGGGTCAT-3′.
The qPCR reaction system (20 µl) contained 10 µl SYB-RGreen
qPCR-Mix, 0.5 µl upstream primers, 0.5 µl downstream primers, 2 µl
cDNA and 7 µl ddH2O (all provided in the SuperReal
PreMix SYBR-Green kit; FP204, Tiangen Biotech Co., Ltd.). qPCR was
performed using an iCycler iQ5 real-time PCR detection system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and the PCR
protocol was as follows: Initial denaturation at 95°C for 30 sec,
followed by 40 cycles of denaturation at 95°C for 5 sec and
annealing at 60°C for 34 sec. The 2−ΔΔCq method
(22) was used to calculate the
relative expression of COX-2 against the expression of β-actin.
Each sample was tested in triplicate.
Western blotting
PBMCs were trypsinized and the resulting lysates
collected. Pre-cooled radioimmunoprecipitation assay lysis buffer
(600 µl; 50 mM Tris-base, 1 mM EDTA, 150 mM NaCl, 0.1% SDS, 1%
TritonX-100 and 1% sodium deoxycholate; Beyotime Institute of
Biotechnology, Haimen, China) was then added to the samples (final
volume, 620 µl). Following lysis for 50 min on ice, the mixture was
centrifuged at 12,000 × g at 4°C for 5 min. The protein
concentration of the resulting supernatant was determined using a
bicinchoninic acid protein concentration determination kit
(RTP7102; Real-Times Biotechnology Co., Ltd., Beijing, China).
Protein samples (20 µg) were then mixed with SDS loading buffer
prior to denaturation in a boiling water bath for 5 min. Protein
samples (50 µg) were then subjected to 10% SDS-polyacrylamide gel
electrophoresis. Resolved proteins were transferred to
polyvinylidene difluoride membranes on ice (100 V; 2 h) and blocked
with 5% skimmed milk at room temperature for 1 h. Membranes were
then incubated with rabbit anti-mouse COX-2 polyclonal primary
antibody (1:1,000; ab52237; Abcam, Cambridge, UK) and rabbit
anti-mouse β-actin primary antibody (1:5,000; ab129348; Abcam) at
4°C overnight. After 3×15 min washes with PBST, membranes were
incubated with goat anti-rabbit horseradish peroxidase-conjugated
secondary antibody (1:3,000; ab6721; Abcam) for 1 h at room
temperature, before 3×15 min washes with PBST. Membranes were
developed for imaging using an enhanced chemiluminescence detection
kit (Sigma-Aldrich; Merck KGaA). Image Lab 3.0 software (Bio-Rad
Laboratories, Inc.) was used to detect and analyze imaging signals.
The relative content of COX-2 protein was expressed as a
COX-2/β-actin ratio.
Enzyme-linked immunosorbent assay
(ELISA)
Blood samples were centrifuged at 1,200 × g for 10
min under room temperature to isolate serum. Levels of interleukin
IL-6, IL-1β and TNF-α were measured using ELISA kits (ERC003.96,
ERC007.96 and ERC102a.96, respectively; Neobioscience, Shenzhen,
China), according to the manufacturer's protocol. Absorbance at 450
nm was measured using a microplate reader (DG5033A; Nanjing Huadong
Electronics Group Co., Ltd., Nanjing, China) within 15 min of
terminating the reactions.
Statistical analysis
Results were analyzed using SPSS 18.0 software
(SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean ±
standard deviation. Multiple sets of measurement data were
initially compared using one-way analysis of variance to test for
normality. For data exhibiting homogeneity of variance, data were
analyzed using Least Significant Difference and
Student-Newman-Keuls methods. For data exhibiting heterogeneity of
variance, Tamhane's T2 or Dunnett's T3 methods were used. Each
assay was repeated a minimum of 3 times. P<0.05 was considered
to indicate a statistically significant difference.
Results
Tet treatment alleviates the severity
of rear toe swelling in a rat model of RA
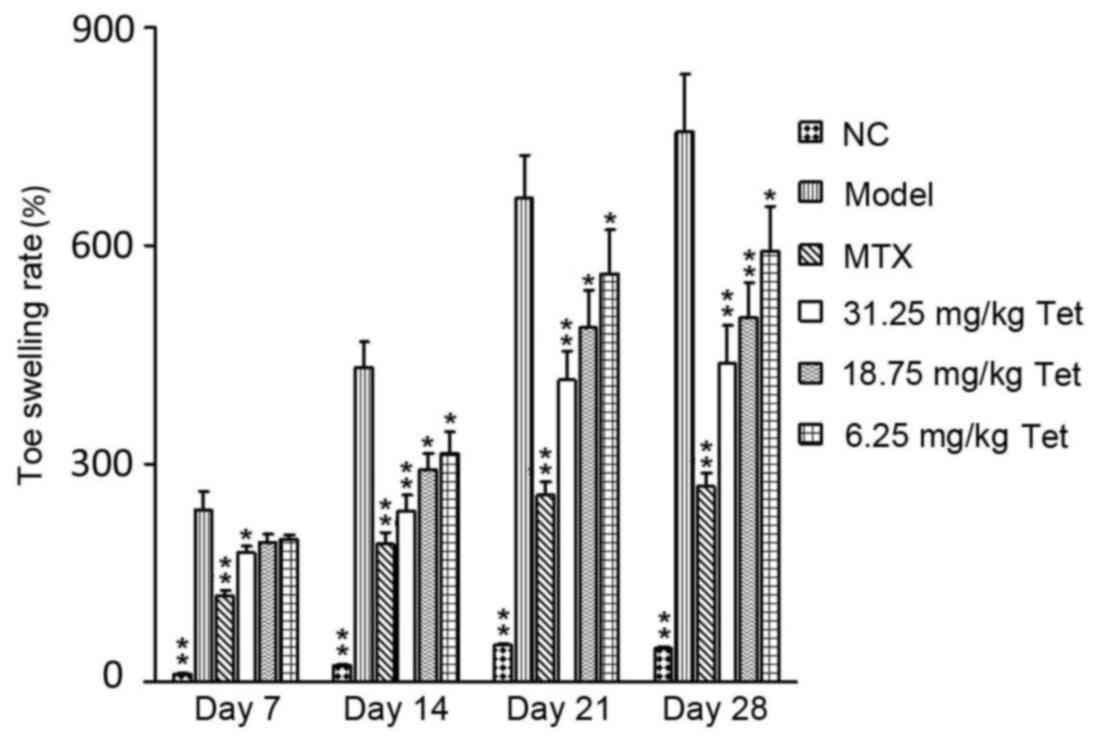
To determine the effect of Tet on toe swelling in RA
rats, a swelling measuring instrument was used. Following injection
of complete Freund's adjuvant, visual observation showed that right
toe swelling was apparent by 24 h in rats of the model group, and
became aggravated on day 7. On day 21, the swelling became more
severe, and the right rear toe exhibited partial loss of movement.
On day 28, the majority of movement in the right rear toe had been
lost. By contrast, swelling was alleviated in rats in the high dose
group treated with Tet on day 7. Quantification showed that toe
swelling rates in the MTX and high dose groups were significantly
lower than that in model group on day 7, and those in the MTX and
all dose groups were significantly lower than the model group on
days 14, 21 and 28 (P<0.05 compared with model group; Fig. 1). These data suggest that treatment
with Tet may alleviate the severity of RA-induced rear toe swelling
in rats.
Tet exerts anti-inflammatory effects
in a rat model of RA
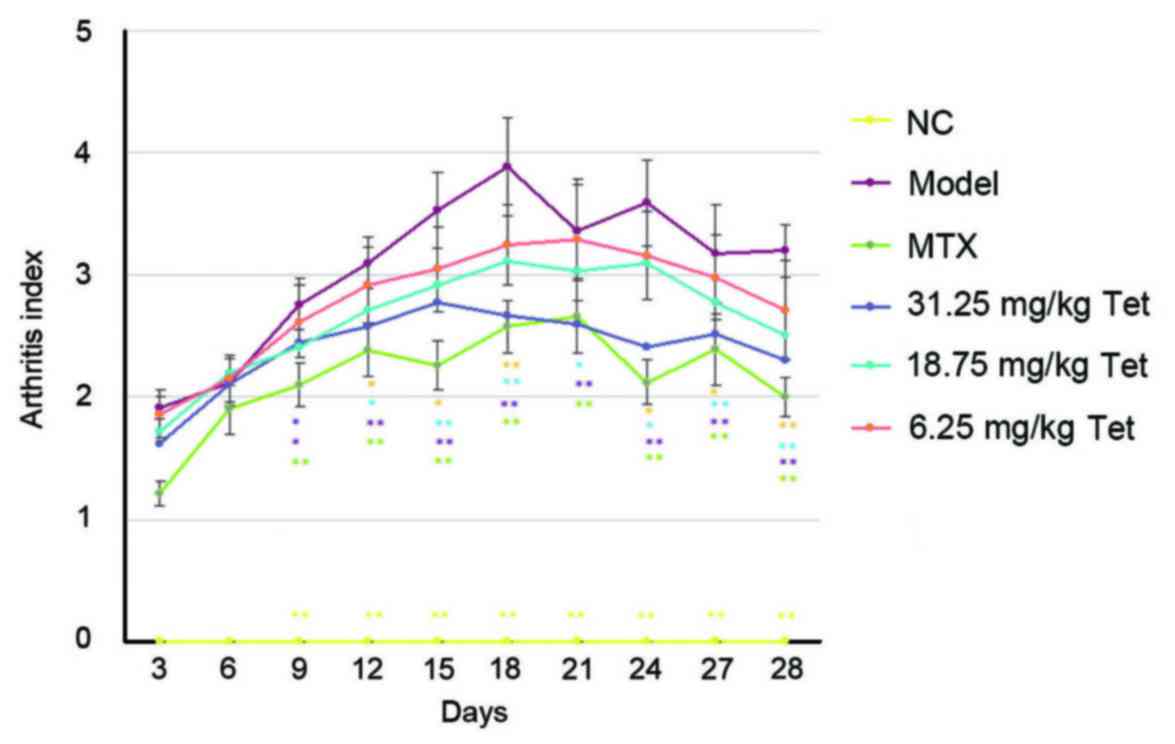
To determine the effect of Tet on the extent of
arthritis in rats, an arthritis index was calculated. It was
observed that the arthritis index in rats of the model group
gradually increased within the first 18 days, reaching a peak on
day 18, before decreasing between days 18 and 28. Following
treatment, the arthritis indices of rats in the MTX, high dose and
medium dose groups were significantly lower than that of rats in
model group from day 12 to day 28 (all P<0.05 compared with
model group; Fig. 2). These results
indicate that Tet may exert anti-inflammatory effects in rats with
RA.
Tet exerts immunosuppressive effects
in a rat model of RA
To evaluate the effect of Tet on immune organ
indices, weights of the spleen and thymus following the
experimental period (28 days) were used to determine thymus and
spleen indices. The data indicated that treatment with MTX or Tet
(low, medium and high dose groups) significantly decreased the
thymus index (for MTX group and all Tet dose groups, P<0.05
compared with model group; Fig. 3A)
and spleen index (for MTX group and all Tet dose groups, P<0.05
compared with model group; Fig. 3B).
Thus, Tet may exert immunosuppressive effects in a rat model of
RA.
Tet reduces COX-2 expression in the
PBMCs of RA rats
To determine the effect of Tet on COX-2 expression
at the mRNA and protein level in the PBMCs of rats, RT-qPCR and
western blotting were performed, respectively. It was observed that
COX-2 was significantly upregulated at the mRNA and protein level
in the PBMCs of the model group, relative to the blank (NC) group
(both P<0.01). Following treatment, levels of COX-2 mRNA and
protein in MTX group and all Tet dose groups were significantly
reduced compared with the model group (for MTX and Tet dose groups,
P<0.05 compared with model group; Fig. 4). These results indicate that Tet may
downregulate the expression of COX-2 in the PBMCs of RA rats.
Tet reduces the concentration of
inflammatory factors in the serum of RA rats
Using ELISA, levels of inflammatory factors in the
serum of RA rats were measured. The data indicated that levels of
of IL-1β (P<0.01), IL-6 (P<0.01) and TNF-α (P<0.05) in the
model group were significantly higher than those in the NC group.
In turn, treatment with MTX or Tet (low, medium and high doses)
significantly reduced levels of inflammatory factors in the serum
compared with the model group (MTX group and all Tet dose groups,
P<0.05 compared with model group; Fig. 5). Therefore, Tet may lower the
concentration of inflammatory factors in the serum of RA rats.
Discussion
Tet is a bisbenzylisoquinoline alkaloid extracted
from the root of Stephaniae tetrandrae Radix, a plant
belonging to the menispermaceae family. Tet comprises ~1% of the
root of Stephaniae tetrandrae Radix (23), and has been documented to have
numerous pharmacological effects (24), including anti-myocardial ischemia
activities, inhibitory effects on platelet aggregation (25) and spasmolytic (26), anti-tumor (27), analgesic, anti-inflammatory (28), anti-ulcer and hepatoprotective
effects (29,30). Tet has also been implicated in the
regulation of immunity (31),
hypoxia tolerance (14) and
suppression of rheumatalgia, arthralgia and neuralgia (32,33).
RA is pathological change specific to the joints
that is characterized by hyperplasia of synovial tissues, vascular
exclusion and the formation of granulation tissues (34,35). In
the pathogenesis of RA, inflammation that originates in the
synovial membrane spreads to cartilage and bone tissues, leading to
destruction of the joint structure. Inflammatory mechanisms also
induce angiogenesis, which further accelerates joint damage and
leads to joint deformity, rigidity and loss of function (36). RA is a chronic process and patients
typically experience episodes of alternating occurrence and
alleviation. Although individual cases differ, the morbidity rate
of RA may reach 60–70% (37).
Therefore, studies providing insight into the underlying mechanism
of RA are warranted to identify novel therapeutic drugs. To date,
it has been documented that a number of mRNA and microRNA molecules
are involved in the pathogenesis of RA (38), and numerous inflammatory cytokines,
including TNF-α and IL-1, may participate in the damaging
inflammatory responses observed in RA (39,40).
In the present study, rate of toe swelling and an
arthritis index were measured, as indicators of the potential
therapeutic effects of Tet on RA. It was observed that treatment
with Tet alleviated swelling of the right rear toe of the rats. In
particular, the effects of high-dose Tet (31.25 mg/kg body weight)
were similar to that of MTX. As an approved drug for RA, MTX is
more effective in the treatment of adjuvant-induced arthritis
models compared with Tet (41).
However, MTX may suppress the formation of bone marrow and affect
liver and kidney functions. By contrast, Tet does not exert these
side effects. The present study investigated the effects of Tet to
compare the effects of Tet and MTX and to determine whether future
studies into the combined use of both drugs are warranted.
Arthritis index measurements were comparable to
those of the toe swelling assay. However, there was a discrepancy
between the immunosuppressive effects of Tet and changes in the toe
swelling rate and arthritis index, possibly due to the effects of
Tet on lymphocyte activity. Nonetheless, thymus and spleen indices
may serve as rough estimates of immune function.
The thymus and spleen are major immune organs in the
body, with the thymus functioning as a central immune organ and the
spleen as a peripheral immune organ. Changes in the thymus and
spleen indices are considered to reflect the overall immune
function of the body (42,43). The present study demonstrated that
Tet decreased indices of the thymus and spleen, suggesting that Tet
may inhibit the functions of the thymus and spleen in RA rats.
COX-2 is a major inflammatory enzyme that has a
rate-limiting role in the synthesis of prostaglandin (44). COX-2 also converts arachidonic acid
into prostaglandin endoperoxides, which are subsequently converted
to thromboxane A2 and prostaglandin to exert inflammatory effects
(45). High expression of COX-2 is
considered to be a key sign of inflammation (46). Results of the present study indicated
that Tet reduced the expression of COX-2 at the mRNA and protein
level in the PBMCs of RA rats, suggesting that Tet may inhibit
inflammatory responses in the body. It has also been documented
that pro-inflammatory cytokines, including TNF-α, IL-1β and IL-6,
are upregulated in RA foci (47).
Cytokines may be key etiological factors in RA that affect the
destruction of joint and articular cartilage in patients with RA
(48). The current study
demonstrated that Tet treatment significantly reduced the
concentrations of TNF-α, IL-1β and IL-6 in the serum of RA rats,
which was consistent with results of the immune organ index and
COX-2 expression assays.
A limitation of the present study was the absence of
T- and/or B-cell activity assays, and thus these are warranted in
future studies. In conclusion, the present study demonstrated that
Tet alleviated the pathological manifestations of RA in rats. The
underlying mechanisms regarding the effects of Tet may involve
suppression of immune organs, downregulation of COX-2 and a
reduction in the release of blood inflammatory factors. Compared
with MTX, the pharmacological effects of Tet were not advantageous.
However, as Tet has fewer side effects, studies into the use of Tet
within combined or auxiliary drug therapies for the treatment of RA
are warranted.
References
|
1
|
Sandoo A, Veldhuijzen van Zanten JJ,
Metsios GS, Carroll D and Kitas GD: Vascular function and
morphology in rheumatoid arthritis: A systematic review.
Rheumatology (Oxford). 50:2125–2139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomas E, Symmons DP, Brewster DH, Black
RJ and Macfarlane GJ: National study of cause-specific mortality in
rheumatoid arthritis, juvenile chronic arthritis, and other
rheumatic conditions: A 20 year followup study. J Rheumatol.
30:958–965. 2003.PubMed/NCBI
|
|
3
|
Lu Z and Zhong N: Internal medicine. 7th
edition. Beijing: People's Medical Publishing House; pp.
8482008
|
|
4
|
Koushik S, Joshi N, Nagaraju S, Mahmood S,
Mudeenahally K, Padmavathy R, Jegatheesan SK, Mullangi R and
Rajagopal S: PAD4: Pathophysiology, current therapeutics and future
perspective in rheumatoid arthritis. Expert Opin Ther Targets.
21:433–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Worthington J, Barton A and John SL: The
epidemiology of rheumatoid arthritis and the use of linkage and
association studies to identify disease genes. The hereditary basis
of rheumatic disease. Rikard Holmdahl: Birkhäuser Basel;
Switzerland: pp. 1–28. 2006
|
|
6
|
Smolen JS, Aletaha D and McInnes IB:
Rheumatoid arthritis. Lancet. 388:2023–2038. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Venuturupalli S: Immune mechanisms and
novel targets in rheumatoid arthritis. Immunol Allergy Clin North
Am. 37:301–313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wolfe RM and Ang DC: Biologic therapies
for autoimmune and connective tissue diseases. Immunol Allergy Clin
North Am. 37:283–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diaper R, Wong E and Metcalfe SA: The
implications of biologic therapy for elective foot and ankle
surgery in patients with rheumatoid arthritis. Foot (Edinb).
30:53–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lampropoulos CE, Orfanos P, Manoussakis
MN, Tzioufas AG, Moutsopoulos HM and Vlachoyiannopoulos PG:
Treat-to-target biologic therapy in patients with rheumatoid
arthritis is more efficacious and safe compared to delayed
initiation of biologics: A real-world study. Clin Exp Rheumatol.
35:192–200. 2017.PubMed/NCBI
|
|
11
|
Xu XH, Gan YC, Xu GB, Chen T, Zhou H, Tang
JF, Gu Y, Xu F, Xie YY, Zhao XY and Xu RZ: Tetrandrine citrate
eliminates imatinib-resistant chronic myeloid leukemia cells in
vitro and in vivo by inhibiting Bcr-Abl/β-catenin axis. J Zhejiang
Univ Sci B. 13:867–874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim
SH, Huang E, Gao Y, Yang K, Wagner ER, et al: Tetrandrine inhibits
Wnt/β-catenin signaling and suppresses tumor growth of human
colorectal cancer. Mol Pharmacol. 79:211–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou Y, Guo T, Wu C and He X: Effect of
tetrandrine combined with epirubicin on the growth of human breast
carcinoma multidrug resistance cell line. Yakugaku Zasshi.
128:663–666. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Tsai YH and Tseng SH: The
potential of tetrandrine as a protective agent for ischemic stroke.
Molecules. 16:8020–8032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie W and Du L: Diabetes is an
inflammatory disease: Evidence from traditional Chinese medicines.
Diabetes Obes Metab. 13:289–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Idec-Sadkowska I, Andrzejak R,
Antonowicz-Juchniewicz J and Kaczmarek-Wdowiak B: Trials of casual
treatment of silicosis. Med Pr. 57:271–280. 2006.(In Polish).
PubMed/NCBI
|
|
17
|
Perera PK, Peng C, Xue L, Li Y and Han C:
Ex vivo and in vivo effect of Chinese herbal pill Yi Shen Juan Bi
(YJB) on experimental arthritis. J Ethnopharmacol. 134:171–175.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin B, Zhang H, Zhao XX, Rahman K, Wang Y,
Ma XQ, Zheng CJ, Zhang QY, Han T and Qin LP: Inhibitory effects of
the root extract of Litsea cubeba (lour.) pers. on adjuvant
arthritis in rats. J Ethnopharmacol. 147:327–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davies NM and Jamali F: COX-2 selective
inhibitors cardiac toxicity: Getting to the heart of the matter. J
Pharm Pharm Sci. 7:332–336. 2004.PubMed/NCBI
|
|
20
|
Steiniger B and van der Meide PH:
High-dose interferon-gamma alters the distribution of B lymphocytes
and macrophages in rat spleen and lymph nodes. Immunology.
78:461–467. 1993.PubMed/NCBI
|
|
21
|
Koshy L, Anju AL, Harikrishnan S, Kutty
VR, Jissa VT, Kurikesu I, Jayachandran P, Jayakumaran Nair A,
Gangaprasad A, Nair GM and Sudhakaran PR: Evaluating genomic DNA
extraction methods from human whole blood using endpoint and
real-time PCR assays. Mol Biol Rep. 44:97–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ge S, Cui L and Wang P: Research progress
on pharmacological effects of tetrandrine. Chin Traditional Herbal
Drugs. 31:W004–W006. 2000.
|
|
24
|
Bhagya N and Chandrashekar KR:
Tetrandrine-A molecule of wide bioactivity. Phytochemistry.
125:5–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rao MR: Effects of tetrandrine on cardiac
and vascular remodeling. Acta Pharmacol Sin. 23:1075–1085.
2002.PubMed/NCBI
|
|
26
|
Li DG, Wang ZR and Lu HM: Pharmacology of
tetrandrine and its therapeutic use in digestive diseases. World J
Gastroenterol. 7:627–629. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu T, Liu X and Li W: Tetrandrine, a
Chinese plant-derived alkaloid, is a potential candidate for cancer
chemotherapy. Oncotarget. 7:40800–40815. 2016.PubMed/NCBI
|
|
28
|
Xie QM, Tang HF, Chen JQ and Bian RL:
Pharmacological actions of tetrandrine in inflammatory pulmonary
diseases. Acta Pharmacol Sin. 23:1107–1113. 2002.PubMed/NCBI
|
|
29
|
Hsu YC, Chiu YT, Cheng CC, Wu CF, Lin YL
and Huang YT: Antifibrotic effects of tetrandrine on hepatic
stellate cells and rats with liver fibrosis. J Gastroenterol
Hepatol. 22:99–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chan CM, Chan YW, Lau CH, Lau TW, Lau KM,
Lam FC, Che CT, Leung PC, Fung KP, Lau CB and Ho YY: Influence of
an anti-diabetic foot ulcer formula and its component herbs on
tissue and systemic glucose homeostasis. J Ethnopharmacol.
109:10–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lai JH: Immunomodulatory effects and
mechanisms of plant alkaloid tetrandrine in autoimmune diseases.
Acta Pharmacol Sin. 23:1093–1101. 2002.PubMed/NCBI
|
|
32
|
Hu GX, Hu Y, Fang DC and Jiang MX:
Hemodynamic effects of tetrandrine in conscious rats. Zhongguo Yao
Li Xue Bao. 8:325–328. 1987.(In Chinese). PubMed/NCBI
|
|
33
|
Ho LJ and Lai JH: Chinese herbs as
immunomodulators and potential disease-modifying antirheumatic
drugs in autoimmune disorders. Curr Drug Metab. 5:181–192. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Caplazi P, Baca M, Barck K, Carano RA,
DeVoss J, Lee WP, Bolon B and Diehl L: Mouse models of rheumatoid
arthritis. Vet Pathol. 52:819–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chieng LO, Madhavan K and Vanni S: Pooled
data analysis on anterior versus posterior approach for rheumatoid
arthritis at the craniovertebral junction. Neurosurg Focus.
38:E182015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Olumuyiwa-Akeredolu OO and Pretorius E:
Platelet and red blood cell interactions and their in rheumatoid
arthritis. Rheumatol Int. 35:1955–1964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kerola AM, Kauppi MJ, Nieminen T,
Rantalaiho V, Kautiainen H, Kerola T, Virta LJ, Pohjolainen T and
Puolakka K: Psychiatric and cardiovascular comorbidities as causes
of long-term work disability among individuals with recent-onset
rheumatoid arthritis. Scand J Rheumatol. 44:87–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Murata K, Yoshitomi H, Furu M, Ishikawa M,
Shibuya H, Ito H and Matsuda S: MicroRNA-451 down-regulates
neutrophil chemotaxis via p38 MAPK. Arthritis Rheumatol.
66:549–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuno H, Yudoh K, Katayama R, Nakazawa
F, Uzuki M, Sawai T, Yonezawa T, Saeki Y, Panayi GS, Pitzalis C and
Kimura T: The role of TNF-alpha in the pathogenesis of inflammation
and joint destruction in rheumatoid arthritis (RA): A study using a
human RA/SCID mouse chimera. Rheumatology (Oxford). 41:329–337.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Walker JG, Ahern MJ, Coleman M, Weedon H,
Papangelis V, Beroukas D, Roberts-Thomson PJ and Smith MD:
Expression of Jak3, STAT1, STAT4, and STAT6 in inflammatory
arthritis: Unique Jak3 and STAT4 expression in dendritic cells in
seropositive rheumatoid arthritis. Ann Rheum Dis. 65:149–156. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Smolen JS, Agarwal SK, Ilivanova E, Xu XL,
Miao Y, Zhuang Y, Nnane I, Radziszewski W, Greenspan A, Beutler A
and Baker D: A randomised phase II study evaluating the efficacy
and safety of subcutaneously administered ustekinumab and
guselkumab in patients with active rheumatoid arthritis despite
treatment with methotrexate. Ann Rheum Dis. 76:831–839. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Serag E, l-Dien MM, Abdou AG, Asaad NY,
Abd El-Wahed MM and Kora MA: Intratumoral FOXP3+ regulatory T cells
in diffuse large B-cell lymphoma. Appl Immunohistochem Mol Morphol.
Feb 9–2016.(Epub ahead of print).
|
|
43
|
Liu JY, Feng CP, Li X, Chang MC, Meng JL
and Xu LJ: Immunomodulatory and antioxidative activity of Cordyceps
militaris polysaccharides in mice. Int J Biol Macromol. 86:594–598.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu L, Yang B, Wang J, Zhao L, Luo W, Jiang
Q and Yang J: Time course change of COX2-PGI2/TXA2 following global
cerebral ischemia reperfusion injury in rat hippocampus. Behav
Brain Funct. 10:422014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoon HY, Lee EG, Lee H, Cho IJ, Choi YJ,
Sung MS, Yoo HG and Yoo WH: Kaempferol inhibits IL-1β-induced
proliferation of rheumatoid arthritis synovial fibroblasts and the
production of COX-2, PGE2 and MMPs. Int J Mol Med. 32:971–977.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yorifuji M, Sawaji Y, Endo K, Kosaka T and
Yamamoto K: Limited efficacy of COX-2 inhibitors on nerve growth
factor and metalloproteinases expressions in human synovial
fibroblasts. J Orthop Sci. 21:381–388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Choy EH and Panayi GS: Cytokine pathways
and joint inflammation in rheumatoid arthritis. N Engl J Med.
344:907–916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Choi EM and Hwang JK: Effects of
methanolic extract and fractions from Litsea cubeba bark on the
production of inflammatory mediators in RAW264.7 cells.
Fitoterapia. 75:141–148. 2004. View Article : Google Scholar : PubMed/NCBI
|