Introduction
Atherosclerosis is a multi-factorial process and a
major cause of coronary heart disease (1,2).
Atherosclerosis is accompanied by lipid metabolism dysregulation
and inflammatory infiltrates in the arterial walls (3). These processes are vital in the
initiation and progression of atherosclerosis, eventually leading
to rupture of the atherosclerotic plaque (4). Atherosclerosis is therefore considered
to be a chronic cytokine-mediated inflammatory disease that
involves substantial remodeling of the arteries (3–5). During
the initiation of atherosclerosis, monocytes migrate into the area
in response to locally produced chemokines, differentiate into
macrophages and increase the expression of several pattern
recognition receptors (6). These
macrophages subsequently accumulate cholesterol and lipids and
become foam cells (7–9). Monocytes, macrophages and other immune
cells, as well as inflammatory cytokines, growth factors and the
accumulation of cholesterol and lipids serve a role in the
pathogenesis of atherosclerosis (6–9).
Flavonoids have been reported to exhibit diverse
health benefits; a number of epidemiological studies have reported
the potential effects of flavonoids in ameliorating cardiovascular
disease (CVD) (10–13). Luteolin is a natural flavone, a
subtype of flavonoid, which is abundant in edible plants, including
broccoli, green chilies, onion leaf, French beans, carrots, white
radish, clover blossom and ragweed pollen (14). A number of previous studies have
reported that luteolin possesses beneficial medicinal properties,
including anti-oxidant, anti-inflammatory and anti-aging actions
(15–17). However, the effect of luteolin on
atherosclerosis remains unclear. Recently, a number of studies have
demonstrated that luteolin protects against tumor necrosis factor-α
(TNF-α)-induced vascular inflammation and monocyte adhesion to
endothelial cells (ECs) in vitro and in vivo
(18–20). In addition, luteolin suppresses THP-1
cell differentiation and inhibits transendothelial migration of
monocytes and the formation of lipid-laden macrophages (21,22).
These observations suggest that luteolin serves a protective role
in the vasculature and inflammatory process during atherosclerosis
development.
The aim of the present study was to investigate the
effects of luteolin on atherosclerotic plaque development and lipid
accumulation in the abdominal aortas and plasma of low-density
lipoprotein receptor-deficient (LDLR−/−) mice fed with a
western diet. The protective effect of monocyte migration and
inflammation in the aortal root was also investigated. Furthermore,
the effect of luteolin on lipid deposition, inflammation and
AMP-activated protein kinase (AMPK)-Sirtuin (SIRT)1 signaling in
ox-LDL-induced THP-1-derived macrophages was assessed. The results
indicate that luteolin as a dietary supplement has potential
protective effects, preventing the development of atherosclerosis
via decreasing macrophage inflammation.
Materials and methods
Animal experiments
A total of 30 9-week-old male LDLR−/−
knockout mice with a C57BL/6 background (Body weight, 24.5±0.37 g)
were purchased from Beijing Vital River Laboratory Animal
Technology (Beijing, China) and housed in ventilated cages
maintained at 22±2°C, 55±5% relative humidity with a 12 h
light/dark cycle. Food and water were administered ad
libitum. Mice were randomly divided into three groups (n=10)
and fed with the following; chow diet, western-type diet (Research
Diets D12108C formula containing 4.5 kcal/g, 40% of energy from fat
and 1.25% cholesterol; Research Diets, Inc., New Brunswick, NJ,
USA) or western-type diet supplemented with luteolin (>98%
purity; 100 mg/kg diet; Sigma Aldrich; Merck KGaA, Darmstadt,
Germany) for 14 weeks. All animal experimental procedure protocols
were reviewed and approved by the Institutional Animal Care and Use
Committee of Capital Medical University (Beijing, China). At the
end of the treatment period, mice were sacrificed and the plasma
and tissues were collected and immediately frozen.
Assessment of aortic
atherosclerosis
Whole abdominal aortas were collected, fixed with
10% formalin for 24 h and stained with oil red O for 2.5 h at room
temperature (25°C) to detect the lipid deposition in lesions.
Images of the entire aortic intimal surface were captured using a
digital camera and digitally scanned (Nikon Corporation; Tokyo,
Japan). Oil red O-positive stained lesions were identified by
assessing staining intensity with Image-Pro Plus Version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA). Quantification of the
atherosclerotic lesion area was expressed as a positive area
percentage.
Lipoprotein measurement
After 14 weeks, mice were euthanized with
CO2 asphyxiation in a 7.07-l chamber with 1.5 l/min flow
rate and a final concentration of 100% CO2. To confirm
sacrifice was successful, mice remained in the chamber filled with
100% CO2 for an additional time of ~3 min. Subsequently,
their blood were harvested. To obtain plasma, blood was left
overnight at 4°C and centrifuged at 5,000 g (4°C) for 15 min.
Plasma total cholesterol, triglyceride, high density
lipoprotein-cholesterol, LDL-cholesterol (LDL-c) were measured
using a colorimetric enzymatic kit including triglyceride,
cholesterol reagents or HDL cholesterol precipitating reagent kits
(ALPCO, Salem, NH, USA) according to the manufacturer's
protocol.
Cell culture
Human THP-1 cells (ATCC, Manassas, VA, USA) were
cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) medium and maintained at 37°C in humidified
atmosphere containing 5% CO2 in air and differentiation
into adherent macrophages was induced by incubating at 37°C with
100 nM phorbol myristate acetate for 48 h. The medium was replaced
with new medium containing 0, 5, 10 and 20 µM of luteolin for 24 h.
Next, the cells were treated with 100 µg/ml ox-LDL (Alfa Aesar,
Tewksbury, MA, USA) for 5 h at 37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the mice proximal aorta
or cultured cells with TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. cDNA was synthesized by RT using reverse transcriptase
(Takara Biotechnology Co., Ltd., Dalian, China). For reverse
transcription, 2.5 µl of 20 µM primer stock was combined with RNA
sample and heated with 70°C for 3 min. A total of 4 µl 5X
First-Strand Buffer, 2 µl dNTP mix, 2 µl 100 mM DTT and 0.5 µl
SMART MMLV reverse transcriptase were mixed and incubated at 42°C
for 60 min. The reaction was terminated by heating at 70°C for 15
min. qPCR was performed on an iCycler (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) using a SYBR PCR kit (Takara Biotechnology Co.,
Ltd.) according to the manufacturer's protocol. The thermocycling
conditions used were as follows: qPCR was performed at 42°C for 5
min, 95°C 10 sec for one cycle, followed by 40 cycles at 95°C for 5
sec, 60°C for 20 sec; and melting curve analysis was performed at
65°C for 15 sec, and increased to 95°C by 0.1°C/sec. Results were
quantified using the 2−∆∆Cq method (23). β-actin served as the housekeeping
gene for the comparisons of the gene expression data. The primer
sequences for qPCR are listed in Table
I.
 | Table I.Primers for the quantitative
polymerase chain reaction. |
Table I.
Primers for the quantitative
polymerase chain reaction.
| Gene | Forward primer
(5′-3′) | Reverse primers
(5′-3′) |
|---|
| CD68 |
TGTCTGATCTTGCTAGGACCG |
GAGAGTAACGGCCTTTTTGTGA |
| CCL2 |
CCCCAAGAAGGAATGGGTCC |
GGTTGTGGAAAAGGTAGTGG |
| IL-6 |
CCTTCCTACCCCAATTTCCAA |
AGATGAATTGGATGGTCTTGGTC |
| TNF-α |
ACGGCATGGATCTCAAAGAC |
AGATAGCAAATCGGCTGACG |
| β-actin |
CATCCGTAAAGACCTCTATGCCAAC |
ATGGAGCCACCGATCCACA |
Western blotting
Following treatment, cells were lysed in
radioimmunoprecipitation assay buffer containing 10 mM
phenylmethylsulfonyl fluoride (Beyotime Institute of Biotechnology,
Haimen, China). Total cellular protein (30 µg) determined by the
BCA method was separated by 10% SDS-PAGE, transferred onto
nitrocellulose membranes and blocked with 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 2 h at room
temperature. Subsequently, the membrane was incubated with primary
antibodies directed against SIRT1 (1:1,000; cat. no. 2028; Cell
Signaling Technology, Inc., Danvers, MA, USA), AMPK (1:1,000; cat.
no. 5832; Cell Signaling Technology, Inc.) and Thr172
phosphorylated-AMPK (pAMPK; 1:1,000; cat. no. 2535; Cell Signaling
Technology, Inc.). Following incubation with the rabbit horseradish
peroxidase-conjugated secondary antibodies (1:5,000, cat. no. 7074;
Cell Signaling Technology, Inc.). Following incubation with the
secondary antibodies, the immunoreactive bands were detected with
an enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.).
Immunoblots were quantified using ImageQuant TL 7.0 software (GE
Healthcare, Chicago, IL, USA) and expressed at a ratio of pAMPK to
AMPK or SIRT1 to β-actin (1:1,000, cat. no. 4970; Cell Signaling
Technology, Inc.).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. One-way analysis of variance with Duncan's post hoc tests
were used for the mouse assays and in vitro assays P<0.05
was considered to indicate a statistically significant difference.
SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) was used as statistical
software.
Results
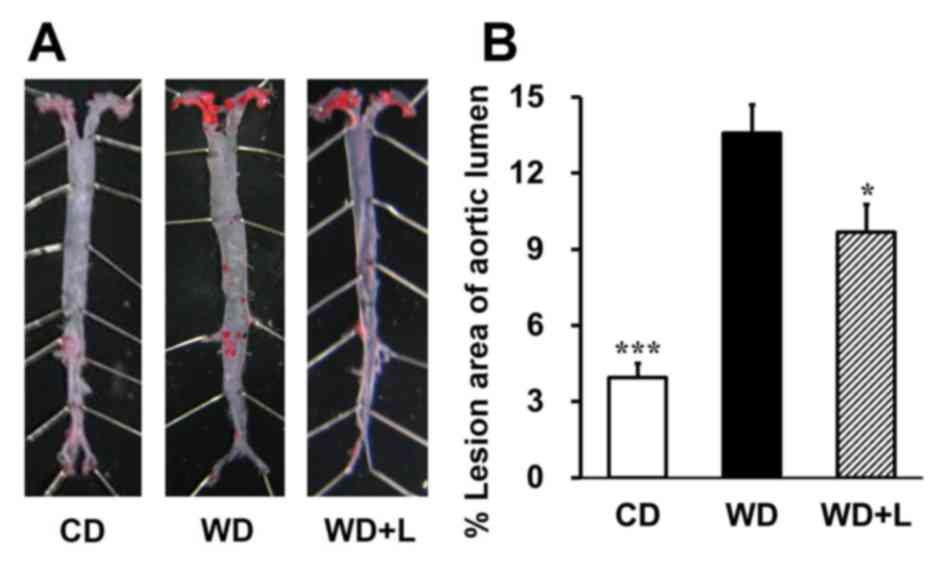
Luteolin prevents atherosclerotic plaque development
in LDLR−/− mice fed with a western diet. To
investigate the role of luteolin in atherosclerosis, the effects of
luteolin on lesion formation in LDLR−/− mice fed with a
western diet were examined. Oil red O staining revealed that
LDLR−/− mice had significant atherosclerotic lesions
compared with chow diet-fed mice (P<0.001; Fig. 1). Notably, supplementation with 100
mg/kg luteolin for 14 weeks significantly reduced the lesion area
by 28.8% in mice fed with a western diet (P<0.05; Fig. 1). The results demonstrate that
dietary luteolin prevents aortic lipid accumulation and
atherosclerotic plaque development in LDLR−/− mice fed
with a western diet.
Luteolin normalizes metabolic
parameters in LDLR−/− mice fed with a western diet
Plasma total cholesterol (P<0.001; Fig. 2A) and triglyceride (P<0.01;
Fig. 2B) levels were significantly
higher in mice fed with a western diet compared to the control
group; however, total cholesterol (P<0.05; Fig. 2A) and triglyceride ~30% following
luteolin treatment. Consistently, plasma LDL-c levels exhibited a
32.8% reduction in the luteolin treatment group compared with the
western diet group (P<0.05; Fig.
2C). However, dietary luteolin had no significant effect on
high-density lipoprotein cholesterol levels in mice fed with a
western diet (Fig. 2D). These data
indicate that luteolin ameliorates lipid accumulation in the plasma
and prevents dyslipidemia in LDLR−/− mice fed with a
western diet.
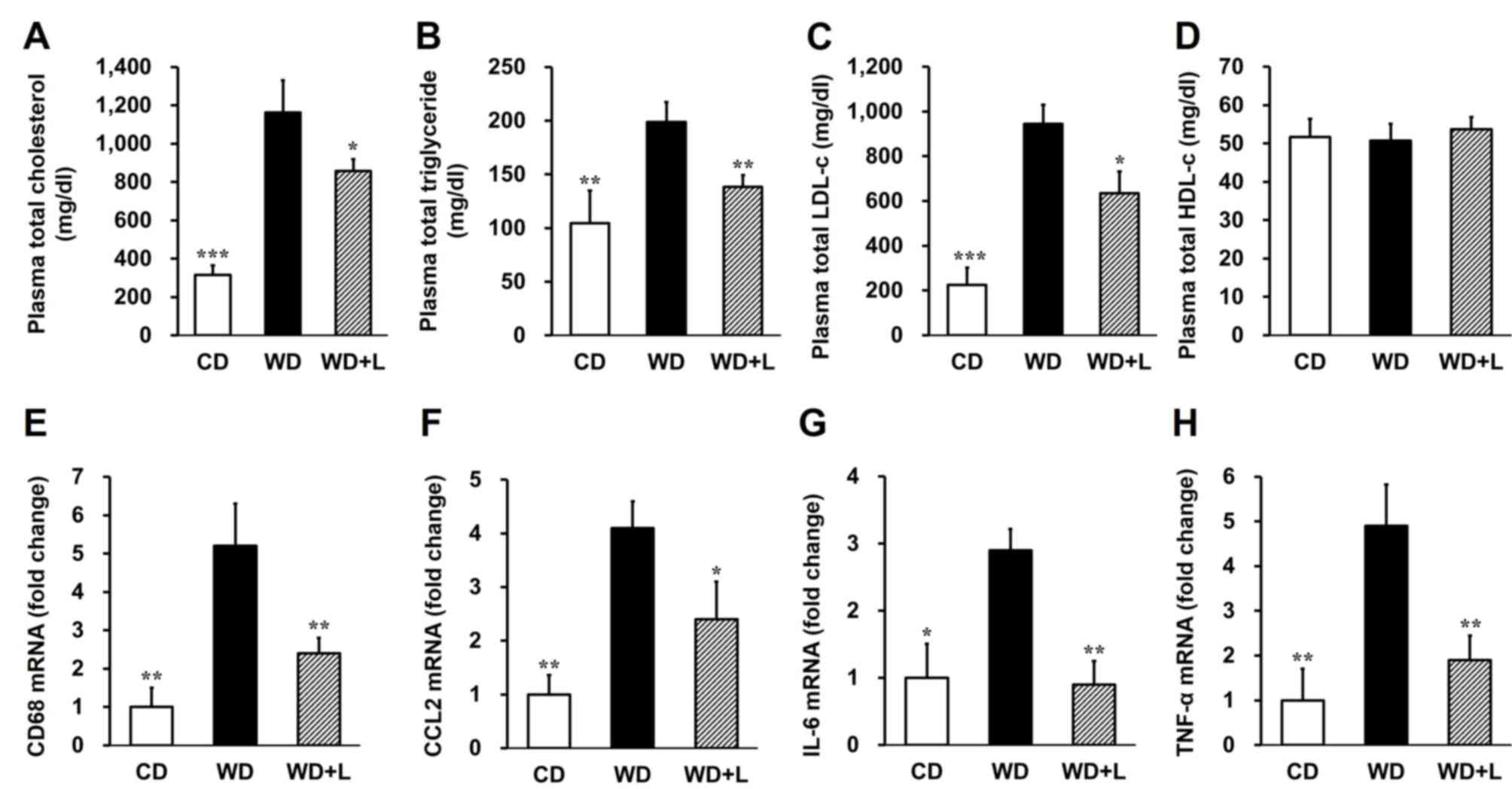 | Figure 2.Luteolin improves the metabolic
parameters of plasma in LDLR−/− mice fed with a WD. (A)
Total plasma cholesterol, (B) total plasma triglycerides, (C) total
plasma LDL-c and (D) total plasma HDL-c were quantified in
LDLR−/− mice fed with CD, WD and WD+L. mRNA expression
of (E) CD68, (F) CCL2, (G) IL-6 and (H) TNF-α in the aortic root of
LDLR−/− mice fed with CD, WD and WD+L. β-actin was used
as a reference gene. n=10 per group. *P<0.05, **P<0.01 and
***P<0.001 vs. WD mice. LDLR−/−, low-density
lipoprotein receptor-deficient; LDL-c, low-density lipoprotein
cholesterol; HDL-c, high-density lipoprotein cholesterol; CD, chow
diet; WD, western diet; WD+L, WD supplemented with 100 mg/kg
luteolin; CD68, cluster of differentiation 68; CCL2, macrophage
chemoattractant protein 2; IL, interleukin; TNF-α, tumor necrosis
factor-α. |
Luteolin attenuates the macrophage
number and vascular inflammation in the aortic root
The activation of monocytes and macrophages is an
important initial step in atherosclerosis, sustaining inflammation
within atheromasia and possibly influencing plaque stability
(7–9). The macrophage recruitment and
inflammation in the aortic root was therefore examined in the
present study. RT-qPCR revealed that the macrophage marker cluster
of differentiation 68 (CD68; P<0.01; Fig. 2E) and macrophage chemoattractant
protein 2 (CCL2; P<0.05; Fig. 2F)
were significantly reduced in the aortic root of mice fed a western
diet supplemented with luteolin compared with mice fed with a
western diet alone. Additionally, the levels of inflammatory
cytokine interleukin (IL)-6 and TNF-α were significantly decreased
in the aortic root following luteolin treatment (P<0.01;
Fig. 2G and H). These results
demonstrate that luteolin ameliorates atherosclerosis by decreasing
macrophage infiltration into the plaque and inflammation in the
aorta of LDLR−/− mice.
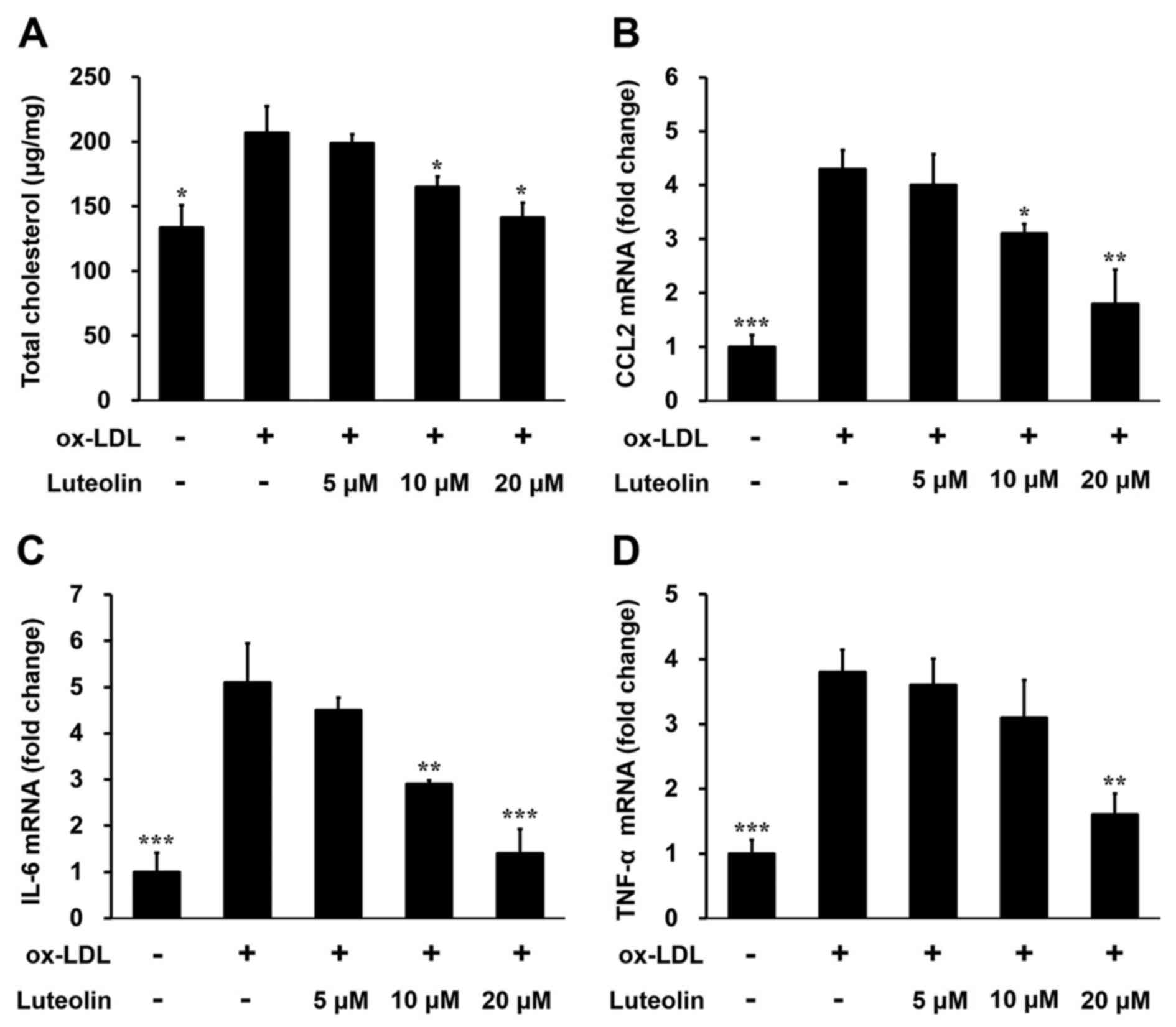
Luteolin decreases foam cell formation
and inflammatory factors in ox-LDL induced THP-1-derived
macrophages
In order to verify the effect of luteolin on
macrophage inflammation in atherosclerosis, differentiated
THP-1-derived macrophages were treated with increasing
concentrations of luteolin. Incubation of THP-1 cells with 100
µg/ml ox-LDL significantly increased the uptake of lipids, as
demonstrated by the higher total cholesterol level (P<0.05;
Fig. 3A). Treatment with 10 or 20 µM
of luteolin for 24 h significantly reduced the cholesterol content
in THP-1 cells in a dose-dependent manner (P<0.05; Fig. 3A). Consistent with these results,
luteolin also inhibited CCL2 (P<0.05; Fig. 3B), IL-6 (P<0.01; Fig. 3C) and TNF-α (P<0.01; Fig. 3D) expression in ox-LDL induced THP-1
cells in a dose-dependent manner. These results provide further
evidence that luteolin decreases foam cell formation and
inflammatory factors in macrophages, alleviating
atherosclerosis.
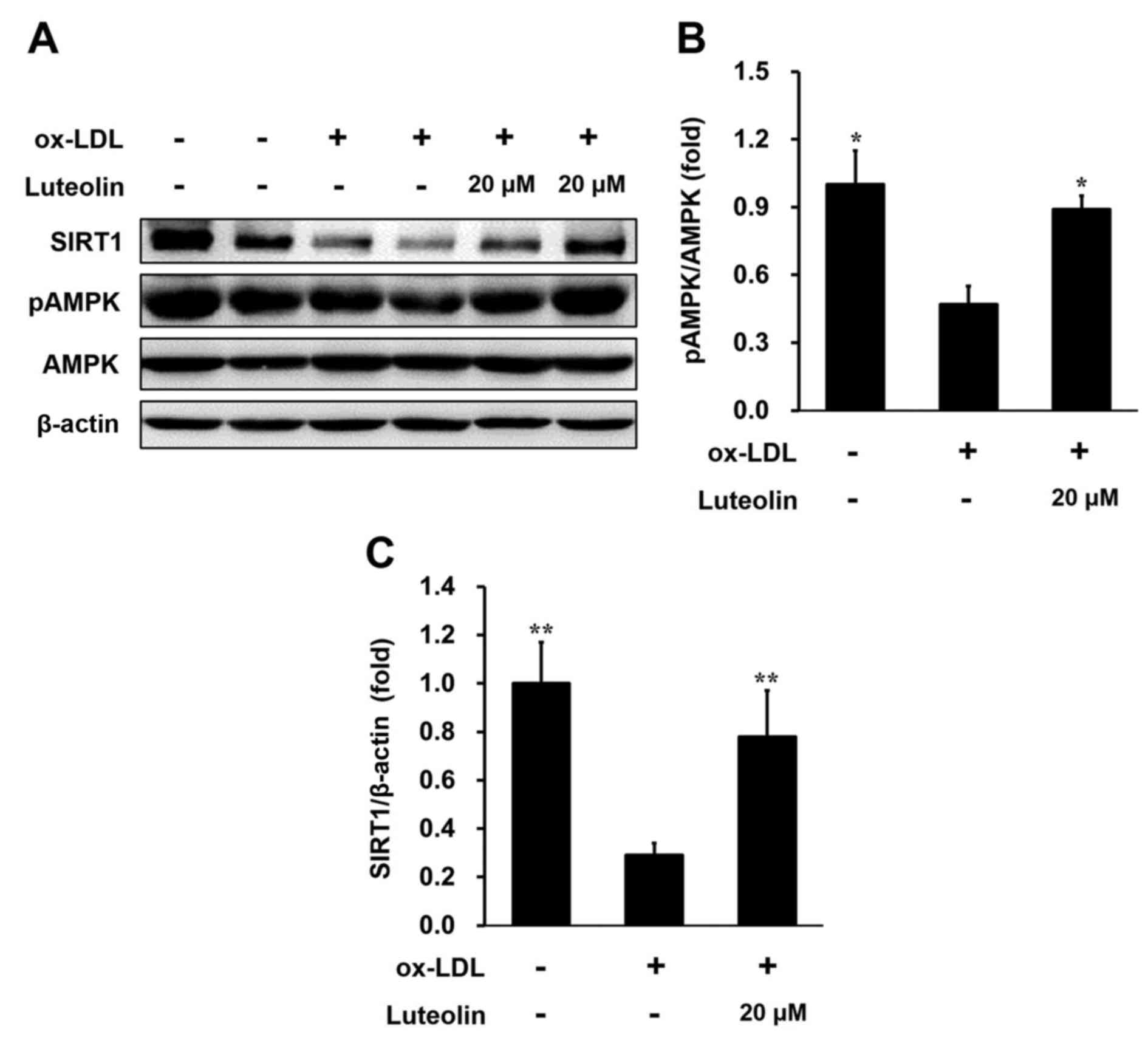
Luteolin inhibits lipid deposition and
inflammation and activates AMPK-SIRT1 signaling in ox-LDL-induced
THP-1-derived macrophages
AMPK-SIRT1 signaling has been reported to be
associated with the attenuation of atherosclerosis and vascular
dysfunction (21–25); luteolin may be able to activate this
signaling in macrophages (26). In
order to explore the effect of luteolin on lipid deposition and
inflammation in macrophages, AMPK-SIRT1 signaling in
luteolin-treated THP-1 cells was assessed. Western blot analysis
revealed that 20 µM luteolin, an effective concentration for the
inhibition of inflammation in THP-1 cells, significantly increased
the levels of phosphorylated AMPK compared with cells without
luteolin (P<0.05; Fig. 4A and B).
Furthermore, luteolin significantly ameliorated the ox-LDL-induced
decrease in SIRT1 expression in THP-1-derived macrophages
(P<0.01; Fig. 4C). In aggregates,
these results demonstrated that luteolin inhibits macrophage
inflammation via a mechanism that is associated with activation of
AMPK-SIRT1 signaling.
Discussion
CVD is multifactorial and the risk of developing CVD
is increased by predisposing factors, including obesity,
hyperlipidemia, arterial hypertension, diabetes inadequate exercise
and smoking (27,28). Imbalanced lipid metabolism and
vascular inflammation serve a significant role in the pathogenesis
of atherosclerosis. Early development of atherosclerosis begins
with the adhesion of monocytes to the vascular endothelium,
followed by accumulation of lipid and eventually maturation in the
intima (6–9,29). In
the present study, dietary luteolin supplements were demonstrated
to have beneficial effects, preventing plaque formation by
regulating macrophage recruitment and inflammation in
LDLR−/− mice fed with a western diet. Luteolin was also
revealed to inhibit macrophage inflammation via a mechanism
including AMPK-SIRT1 signaling in ox-LDL induced THP-1-derived
macrophages.
Luteolin is a natural flavonoid that exhibits
anti-inflammatory and anti-oxidative properties (14,15). A
series of studies have reported that luteolin protects against
vascular inflammation, monocyte adhesion to endothelial cells (ECs)
and macrophage differentiation, oxidized LDL uptake and foam cell
formation of macrophages (18–20).
However, the exact effect of luteolin in atherosclerosis and the
underlying cellular mechanisms have not been elucidated. In order
to assess the effect of luteolin on atherosclerosis,
LDLR−/− mice were fed with a western diet for 14 weeks
to develop atherosclerotic plaques. Supplementing the western diet
with 100 mg/kg luteolin significantly ameliorated atherosclerotic
plaque development and decreased lipid accumulation in abdominal
aortas. These results demonstrate that luteolin may be an effective
preventative treatment for atherosclerosis, which was further
confirmed by decreased lipid concentrations in the plasma. These
results promoted us to investigate the process luteolin acted on
during atherosclerosis, including monocytes adhesion to the
vascular endothelium, lipid accumulation or plaque development.
Luteolin has been broadly reported to inhibit
macrophage inflammation in vivo and in vitro
(30–32). As macrophages serve a key role in the
initiation and progression of atherosclerosis (33), the present study focused on
macrophages and inflammation in the atherosclerotic plaque. The
results revealed that levels of the macrophage marker CD68,
macrophage chemokine CCL2 and inflammatory cytokines IL-6 and TNF-α
expression in the aortic root were decreased with luteolin
supplementation. Luteolin was also demonstrated to inhibit
macrophage chemokine and inflammatory cytokine expression in
THP-1-derived macrophages in a dose dependent manner. These results
are in agreement with earlier observations that reveal luteolin
suppresses macrophage differentiation and inhibits lipid
accumulation in macrophages (21,22).
However, luteolin has also been reported have inhibitory effects on
monocyte adhesion to ECs in vitro (18). Further studies are required to
explore whether luteolin affects the adhesive properties of ECs of
the arteries. The activated ECs in arteries also release chemokines
and cytokines, which recruits circulating immune cells, including T
cells, B cells and macrophages in atherosclerotic plaques. In
addition, ECs express adhesion proteins, including intercellular
adhesion molecule-1 and vascular cell adhesion molecule-1, which
are associated with the recruitment of immune cells (34). Consequently, the importance of
luteolin in the regulation of vascular adhesion requires further
investigation.
AMPK is important in regulating cellular glucose and
lipid metabolism and has been reported to serve a role in the
attenuation of atherosclerosis and vascular dysfunction (24–26). The
present study demonstrated that luteolin promotes AMPK
phosphorylation in ox-LDL induced THP-1 macrophages. It has also
been reported that luteolin is able to increase AMPK
phosphorylation in macrophages (30). SIRT1 is also an important regulatory
sensor of nutrient availability and a target in catabolic
metabolism, mitochondrial activation and angiogenesis (35,36).
AMPK negatively regulates lipid-induced inflammation via SIRT1 in
macrophages (37). Consequently,
luteolin may increase SIRT1 expression in THP-1 cells. These data
suggest that luteolin regulates inflammation and lipid metabolism
in macrophages via a mechanism that includes the AMPK-SIRT1
signaling pathway.
In conclusion, the results of the present study
indicate that dietary luteolin ameliorates atherosclerotic plaque
development and lipid accumulation in the abdomen and plasma of
LDLR−/− mice fed with a western diet. Dietary luteolin
also protects the aorta from monocyte migration and inflammation.
Finally, using ox-LDL-induced THP-1-derived macrophages, the
present study demonstrated that luteolin prevents inflammation
through a mechanism that includes AMPK-SIRT1 signaling.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant nos. 81400361 and 81501486) and
the Presidential Foundation of Beijing An Zhen Hospital, Capital
Medical University, China (grant no. 2015P12).
Glossary
Abbreviations
Abbreviations:
|
AMPK
|
adenosine monophosphate-activated
protein kinase
|
|
CCL2
|
macrophage chemoattractant protein
2
|
|
CD
|
chow diet
|
|
HDL
|
high-density lipoprotein
|
|
IL-6
|
interleukin-6
|
|
LDL
|
low-density lipoprotein
|
|
LDLR−/−
|
low-density lipoprotein
receptor-deficient
|
|
SIRT1
|
silent information regulator 1
|
|
TNF-α
|
tumor necrosis factor α
|
|
WD
|
western diet
|
|
WD+L
|
western diet supplemented with
luteolin
|
References
|
1
|
Glass CK and Witztum JL: Atherosclerosis.
The road ahead. Cell. 104:503–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Celermajer DS, Chow CK, Marijon E, Anstey
NM and Woo KS: Cardiovascular disease in the developing world:
Prevalences, patterns, and the potential of early disease
detection. J Am Coll Cardiol. 60:1207–1216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross R: Atherosclerosis-an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andersson J, Libby P and Hansson GK:
Adaptive immunity and atherosclerosis. Clin Immunol. 134:33–46.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weber C, Zernecke A and Libby P: The
multifaceted contributions of leukocyte subsets to atherosclerosis:
Lessons from mouse models. Nat Rev Immunol. 8:802–815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seimon T and Tabas I: Mechanisms and
consequences of macrophage apoptosis in atherosclerosis. J Lipid
Res. 50 Suppl:S382–S387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Renier G, Skamene E, DeSanctis JB and
Radzioch D: High macrophage lipoprotein lipase expression and
secretion are associated in inbred murine strains with
susceptibility to atherosclerosis. Arterioscler Thromb. 13:190–196.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Babaev VR, Fazio S, Gleaves LA, Carter KJ,
Semenkovich CF and Linton MF: Macrophage lipoprotein lipase
promotes foam cell formation and atherosclerosis in vivo. J Clin
Invest. 103:1697–1705. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arts IC and Hollman PC: Polyphenols and
disease risk in epidemiologic studies. Am J Clin Nutr. 81 1
Suppl:S317–S325. 2005. View Article : Google Scholar
|
|
11
|
Maron DJ: Flavonoids for reduction of
atherosclerotic risk. Curr Atheroscler Rep. 6:73–78. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mennen LI, Sapinho D, de Bree A, Arnault
N, Bertrais S, Galan P and Hercberg S: Consumption of foods rich in
flavonoids is related to a decreased cardiovascular risk in
apparently healthy French women. J Nutr. 134:923–926. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao J, Zhang Y, Chen W and Zhao X: The
relationship between fasting plasma concentrations of selected
flavonoids and their ordinary dietary intake. Br J Nutr.
103:249–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
López-Lázaro M: Distribution and
biological activities of the flavonoid luteolin. Mini Rev Med Chem.
9:31–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nazari QA, Kume T, Takada-Takatori Y,
Izumi Y and Akaike A: Protective effect of luteolin on an
oxidative-stress model induced by microinjection of sodium
nitroprusside in mice. J Pharmacol Sci. 122:109–117. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Si H, Wyeth RP and Liu D: The flavonoid
luteolin induces nitric oxide production and arterial relaxation.
Eur J Nutr. 53:269–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jang S, Dilger RN and Johnson RW: Luteolin
inhibits microglia and alters hippocampal-dependent spatial working
memory in aged mice. J Nutr. 140:1892–1898. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia Z, Nallasamy P, Liu D, Shah H, Li JZ,
Chitrakar R, Si H, McCormick J, Zhu H, Zhen W and Li Y: Luteolin
protects against vascular inflammation in mice and
TNF-alpha-induced monocyte adhesion to endothelial cells via
suppressing IΚBα/NF-κB signaling pathway. J Nutr Biochem.
26:293–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia F, Wang C, Jin Y, Liu Q, Meng Q, Liu K
and Sun H: Luteolin protects HUVECs from TNF-α-induced oxidative
stress and inflammation via its effects on the Nox4/ROS-NF-κB and
MAPK pathways. J Atheroscler Thromb. 21:768–783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang BC, Zhang CW, Wang C, Pan DF, Xu TD
and Li DY: Luteolin attenuates foam cell formation and apoptosis in
Ox-LDL-stimulated macrophages by enhancing autophagy. Cell Physiol
Biochem. 39:2065–2076. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Makino J, Nakanishi R, Kamiya T, Hara H,
Ninomiya M, Koketsu M and Adachi T: Luteolin suppresses the
differentiation of THP-1 cells through the Inhibition of NOX2 mRNA
expression and the membrane translocation of p47phox. J Nat Prod.
76:1285–1290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim MS, Kim DS, Kim HS, Kang SW and Kang
YH: Inhibitory effects of luteolin on transendothelial migration of
monocytes and formation of lipid-laden macrophages. Nutrition.
28:1044–1054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong J, Zhang X, Zhang L, Bian HX, Xu N,
Bao B and Liu J: Quercetin reduces obesity-associated ATM
infiltration and inflammation in mice: A mechanism including
AMPKα1/SIRT1. J Lipid Res. 55:363–374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fullerton MD, Steinberg GR and Schertzer
JD: Immunometabolism of AMPK in insulin resistance and
atherosclerosis. Mol Cell Endocrinol. 366:224–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vasamsetti SB, Karnewar S, Kanugula AK,
Thatipalli AR, Kumar JM and Kotamraju S: Metformin inhibits
monocyte-to-macrophage differentiation via AMPK-mediated inhibition
of STAT3 activation: Potential role in atherosclerosis. Diabetes.
64:2028–2041. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Motoshima H, Goldstein BJ, Igata M and
Araki E: AMPK and cell proliferation-AMPK as a therapeutic target
for atherosclerosis and cancer. J Physiol. 574:63–71. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zelicoff AP: Multifactorial risk
assessment for atherosclerotic cardiovascular disease. JAMA.
313:971–972. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mahé I and Bergmann JF: Multifactorial
cardiovascular risk. Presse Med. 29:47–54. 2000.(In French).
PubMed/NCBI
|
|
29
|
Libby P, Ridker PM and Hansson GK:
Progress and challenges in translating the biology of
atherosclerosis. Nature. 473:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Han YJ, Zhang X, Wang X, Bao B,
Qu W and Liu J: Luteolin reduces obesity-associated insulin
resistance in mice by activating AMPKα1 signalling in adipose
tissue macrophages. Diabetologia. 59:2219–2228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen CY, Peng WH, Tsai KD and Hsu SL:
Luteolin suppresses inflammation-associated gene expression by
blocking NF-kappaB and AP-1 activation pathway in mouse alveolar
macrophages. Life Sci. 81:1602–1614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen D, Bi A, Dong X, Jiang Y, Rui B, Liu
J, Yin Z and Luo L: Luteolin exhibits anti-inflammatory effects by
blocking the activity of heat shock protein 90 in macrophages.
Biochem Biophys Res Commun. 443:326–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moore KJ, Sheedy FJ and Fisher EA:
Macrophages in atherosclerosis: A dynamic balance. Nat Rev Immunol.
13:709–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galkina E and Ley K: Vascular adhesion
molecules in atherosclerosis. Arterioscler Thromb Vasc Biol.
27:2292–2301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Winnik S, Stein S and Matter CM: SIRT1 -
an anti-inflammatory pathway at the crossroads between metabolic
disease and atherosclerosis. Curr Vasc Pharmacol. 10:693–696. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang MJ, Zhou Y, Chen L, Wang X, Long CY,
Pi Y, Gao CY, Li JC and Zhang LL: SIRT1 improves VSMC functions in
atherosclerosis. Prog Biophys Mol Biol. 121:11–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Z, Kahn BB, Shi H and Xue BZ:
Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK)
antagonizes fatty acid-induced inflammation through SIRT1. J Biol
Chem. 285:19051–19059. 2010. View Article : Google Scholar : PubMed/NCBI
|