Introduction
Alzheimer's disease (AD) is a progressive
neurodegenerative disorder which has become a worldwide public
health problem (1). The pathogenesis
of AD is complex, and it may be contributed by genetic and
environmental factors. The external environment can affect DNA
methylation to change phenotype and gene expression (2). DNA methylation is an important
epigenetic mechanism regulating the expression of aging genes in
brain (3). The expression and
closure of methylation regulatory genes are closely related to the
human nervous system (4) and
cognitive function (5).
The major challenge of AD is to identify new
therapeutic targets and to develop new therapies for this disease
(6). AD is closely related to tau
hyperphosphorylation, oxidative stress, amyloid-β (Aβ) production,
neuronal apoptosis, gene mutation, apolipoprotein E (APOE).
Bridging integrator 1 (BIN1) is an important gene in the modulation
of tau pathology, and BIN1 knockdown was shown to significantly
suppress tau-mediated neurotoxicity (7). Sortilin-related receptor 1 (SORL1) is a
member of the low-density lipoprotein receptor family that reduces
amyloid-β (Aβ) production by regulating the intracellular transport
and processing of APP (8). Nerve
growth factor (NGF) contributes to the survival, regeneration and
death of neurons during aging and in neurodegenerative diseases
(9). PSEN2 is a transmembrane
protein and AD-related presenilin mutations can alter intracellular
calcium signaling, which leads to Aβ aggregation to form brain
plaques and neuronal cell death (10). Genetic variation within these genes
is associated with an increased risk of AD (9,11–14). The
8-oxoguanine DNA glycosylase 1 (OGG1) is a bifunctional
enzyme with both glycosylase and AP lyase activities (15). Decreased OGG1 activity occurs
early in the progression of AD (16). OGG1 was largely hypomethylated
in LOAD and control blood DNA, and they do not support an increased
promoter methylation of OGG1 in blood DNA of AD patients
(17). Dihydrolipoamide
succinyltransferase (DLST) is a subunit enzyme of the
a-ketoglutarate dehydrogenase complex in the Krebs cycle.
Polymorphisms of DLST were associated with AD in both
Japanese and Caucasian populations (18–20).
In the present study, we aimed to validate the
association of the five AD-associated variants (OGG1
rs1052133, BIN1 rs744373, SORL1 rs1133174,
PSEN2 rs8383, and NGF rs6330) with AD in Xinjiang
population. We also tested the association of OGG1 and
DLST promoter methylation with AD.
Materials and methods
Epidemiological investigation was carried out in
Xinjiang province of China between 2014 and 2015. A total of 17 AD
patients (75.65±5.86 years) and 34 well-matched controls
(77.59±7.41 years) were selected for the present study (Table I). This study was approved by the
First Affiliated Hospital of Xinjiang Medical University Ethics
Committee. All the patients gave their written informed consent
forms for the current study. The clinical diagnosis of AD was done
according to the criteria of the Diagnostic and Statistical
Manual-IV (DSM-IV). The details were the same as previously
described (21). Whole blood was
stored in EDTA tube at −80°C. Genomic DNA was extracted and
dissolved in TE buffer, and then it was stored at −20°C. Polymerase
chain reaction (PCR) was carried out in 40 µl volume containing 2
µl of each primer, 4 µl genomic DNA, 12 µl ddH2O and 20 µl 2X
HotTaq Master Mix. PCR was performed in a Veriti 96-well thermal
cycler (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Genotyping was done using the Sanger sequencing.
The primer sequences were TTACTTCTCCACGGACAAC and
CAAGATTCTAACAGGACTCATC for the forward and the reverse primers of
PSEN2 rs8383 genotyping, GCCAGTCCATCTTCTTCT and
ACCACATCTTAGCCACAG for the forward and the reverse primers of
BIN1 rs744373 genotyping, CATCCATACTGCCTGAGTC and
CCTGTGAGTCCTGTTGAAG for the forward and the reverse primers of
NGF rs6330 genotyping, GTGGATTCTCATTGCCTTC and
AAACTGACTGCTTGATTTGG for the forward and the reverse primers of
OGG1 rs1052133 genotyping, and TGTGACTTGTGCTGTATGAT and
ACGCTAGAAGAAGGCTTATC for the forward and the reverse primers of
SORL1 rs1133174 genotyping. PCR consisted of an initial
melting step at 95°C for 10 min, 35 cycles (NGF, BIN1, and
OGG1) or 37 cycles (PSEN2) or 40 cycles
(SORL1), and a final extension step at 72°C for 2 min. The
cycling program was 95°C for 30 sec, 58°C (NGF and
BIN1) or 54°C (OGG1) or 57°C (PSEN2) or 53°C
(SORL1) for 45 sec for annealing, and 72°C for 30 sec. DNA
bisulphite conversion was done using the EZ DNA Methylation-Gold™
Kit (Zymo Research Corp., Irvine, CA, USA). The details of
bisulphite conversion were the same as previously described
(22). Promoter methylation status
of OGG1 and DLST were examined utilizing quantitative
methylation-specific PCR (qMSP). The primer sequences were
CGGTGGTTGAGTTTTATTTTC and CTCCTTACGACTTATCTTCTC for the upstream
and the downstream primers of OGG1, respectively. And the
upstream and the downstream primer sequences of DLST were
GTTGTAGTCGGGATATTGG and CGAAACGAACCACTAACA, respectively.
 | Table I.Baseline clinical data of included
subjects. |
Table I.
Baseline clinical data of included
subjects.
|
Characteristics | Cases (n=17) | Controls
(n=34) | P-value |
|---|
| Age (years) | 75.65±5.86 | 77.59±7.41 | 0.35 |
| SBP (mmHg) | 132.94±16.40 | 136.24±20.05 | 0.56 |
| DBP (mmHg) | 75.35±9.66 | 77.21±11.13 | 0.56 |
| TG (mmol/l) | 2.03±1.53 | 1.52±1.27 | 0.31 |
| TC (mmol/l) | 3.94±1.65 | 4.37±1.60 | 0.38 |
| HDL (mmol/l) | 1.41±0.30 | 1.29±0.45 | 0.33 |
| LDL (mmol/l) | 2.71±0.68 | 2.75±1.05 | 0.88 |
| FBG (mmol/l) | 4.85±0.80 | 5.15±1.10 | 0.33 |
| Male/Female | 7/10 | 17/17 | 0.55 |
|
Diabetes/Non-diabetes | 1/16 | 7/27 | 0.34 |
|
Hypertension/Non-hypertension | 9/8 | 20/14 | 0.69 |
|
Smoking/Non-smoking | 2/15 | 4/30 | 1.00 |
| Drinking/No
drinking | 1/16 | 2/32 | 1.00 |
| APOE ε4/Not APOE
ε4 | 8/9 | 2/31 | 0.002 |
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for the statistical analysis. Comparison of demographical
parameters between cases and controls was performed using the
Student's t test for continuous variables and the χ2 test for
categorical data. Spearman rank correlation test was used to
analyze the associations between gene methylation and metabolic
characteristics. P<0.05 was considered to indicate a
statistically significant difference.
Results
The characteristics of AD and control groups were
presented in Table I. Our results
showed the two groups were well paired according to the facts that
there were no significant difference on gender, age, hypertension,
diabetes, lipid levels, smoking and drinking status between the AD
group and control group (P>0.05). As shown in Table II, there were no associations of the
five genetic polymorphisms with AD. Further APOE ε4 based
subgroup analysis indicated there were no significant interaction
of APOE ε4 with the five genetic variants (Table III, P>0.05).
 | Table II.Genotype and allele frequencies
between cases and controls. |
Table II.
Genotype and allele frequencies
between cases and controls.
| SNP | Case; control
(MM/Mm/mm) | P-value | Case; control
(M/m) | P-value |
|---|
| PSEN2 (rs8383,
C>T) | 6/6/5; 7/22/5 | 0.12 | 18/16; 36/32 | 1.00 |
| NGF (rs6330,
C>T) | 13/4/0;
23/10/1 | 0.83 | 30/4; 56/12 | 0.44 |
| SORL1 (rs1133174,
A>G) | 9/2/6; 19/7/8 | 0.57 | 20/14; 45/23 | 0.47 |
| OGG1 (rs1052133,
G>C) | 5/8/4; 6/20/8 | 0.60 | 18/16; 32/36 | 0.58 |
| BIN1 (rs744373,
T>C) | 5/7/5; 4/19/11 | 0.29 | 17/17; 27/41 | 0.32 |
 | Table III.Analysis of the interaction between
APOE ε4 and other variants. |
Table III.
Analysis of the interaction between
APOE ε4 and other variants.
|
| Genotype | APOE ε4 |
| Non-APOE ε4 |
|
|---|
|
|
|
|
|
|
|
|---|
| SNP | Allele | Case | Control | P-value | Case | Control | P-value |
|---|
| NGF | CC/CT/TT | 6/2/0 | 2/0/0 | 1.000 | 7/2/0 | 20/10/1 | 0.763 |
| rs6330 | C/T | 14/2 | 4/0 | 1.000 | 16/2 | 50/12 | 0.647 |
| PSEN2 | CC/CT/TT | 3/3/2 | 0/1/1 | 1.000 | 3/3/3 | 6/21/4 | 0.156 |
| rs8383 | C/T | 9/7 | 1/3 | 0.582 | 9/9 | 33/29 | 0.809 |
| SORL1 | AA/AG/GG | 3/4/1 | 0/1/1 | 0.667 | 6/2/1 | 20/6/5 | 1.000 |
| rs1133174 | A/G | 10/6 | 1/3 | 0.285 | 14/4 | 46/16 | 1.000 |
| OGG1 | CC/CG/GG | 2/3/3 | 0/2/0 | 0.667 | 3/5/1 | 6/18/7 | 0.677 |
| rs1052133 | C/G | 7/9 | 2/2 | 1.000 | 11/7 | 30/32 | 0.342 |
| BIN1 | CC/CT/TT | 2/5/1 | 0/2/0 | 1.000 | 3/2/2 | 3/17/11 | 0.144 |
| rs744373 | C/T | 9/7 | 2/2 | 1.000 | 8/6 | 23/39 | 0.168 |
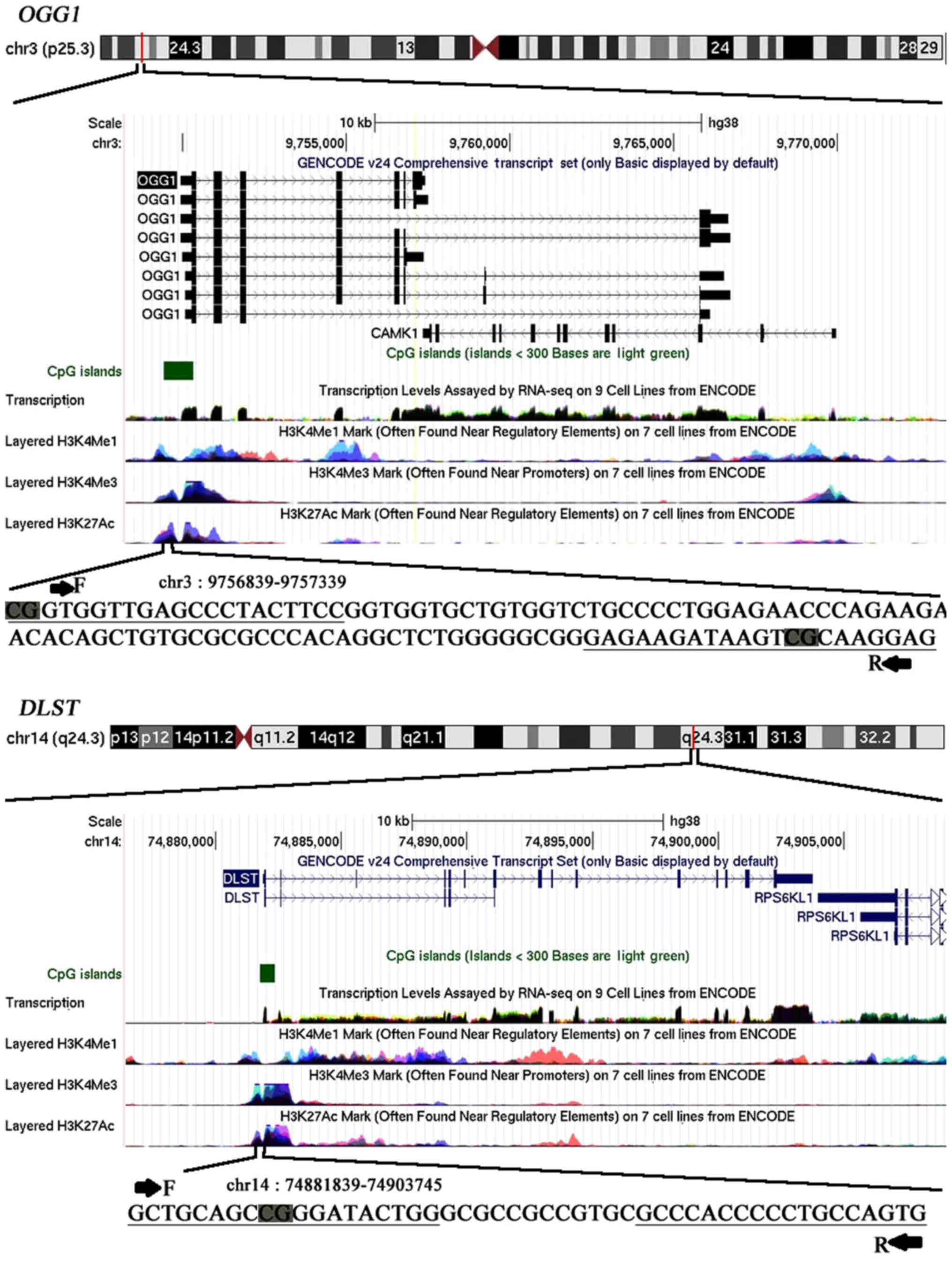
The promoter regions of OGG1 and DLST
were selected for the current methylation study (Fig. 1). In this study, we investigated the
association of the methylation levels of OGG1 and
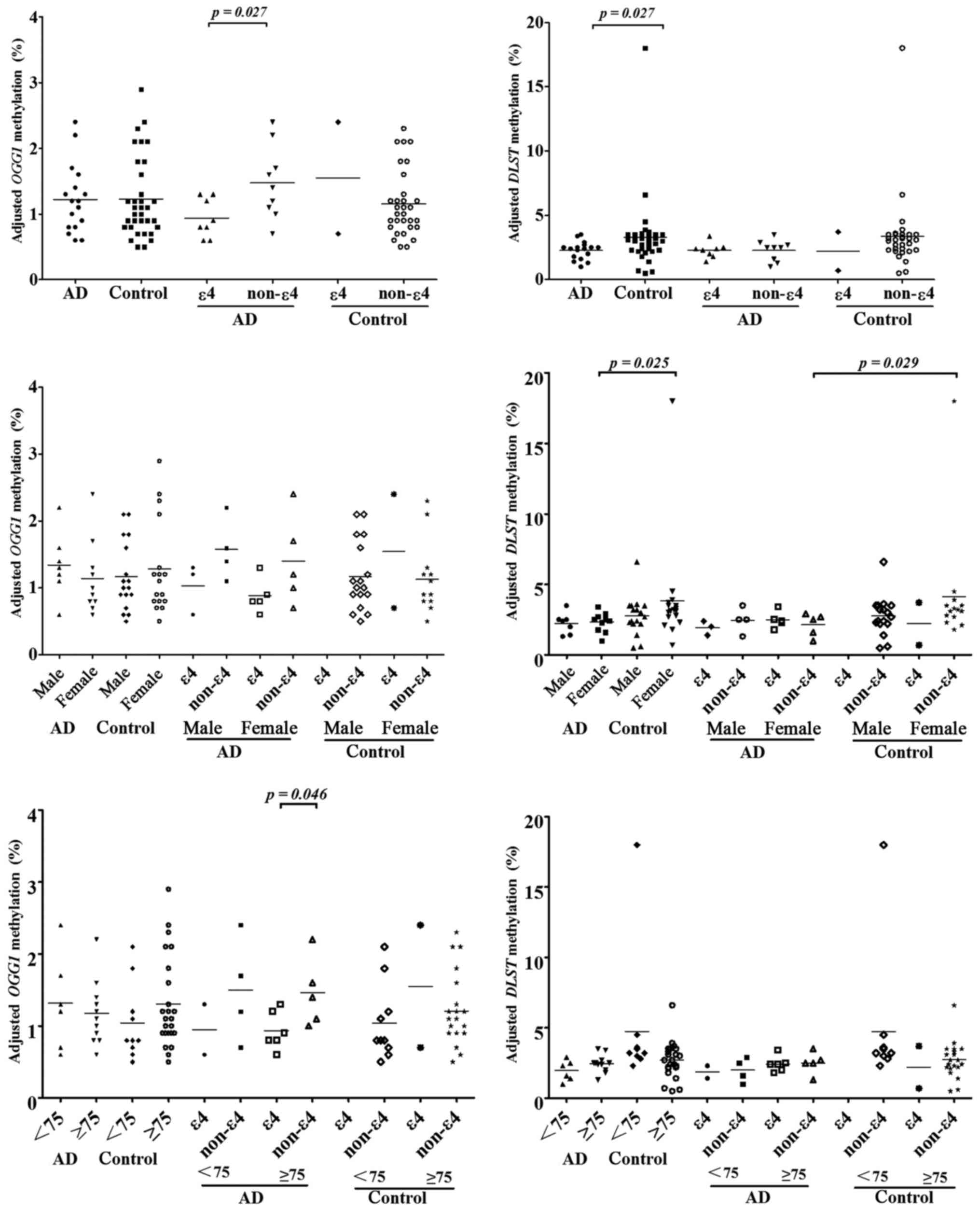
DLST genes with AD (Fig. 2).
Although OGG1 methylation was not associated with AD, our
results showed that OGG1 methylation was significantly lower
in AD patients with APOE ε4 allele than AD patients with
APOE non-ε4 allele (P=0.027). Among AD patients older than
75 years old, the levels of OGG1 methylation were
significantly lower in AD patients carrying APOE ε4 allele
than AD patients who did not carry APOE ε4 allele
(P=0.046).
As shown in Fig. 2,
DLST methylation levels were significantly lower in AD
patients (P=0.027). Further subgroup analysis by gender showed that
the association of DLST methylation with AD was specific in
females (P=0.025). Further subgroup analysis by APOE ε4
locus showed that DLST methylation was associated with AD in
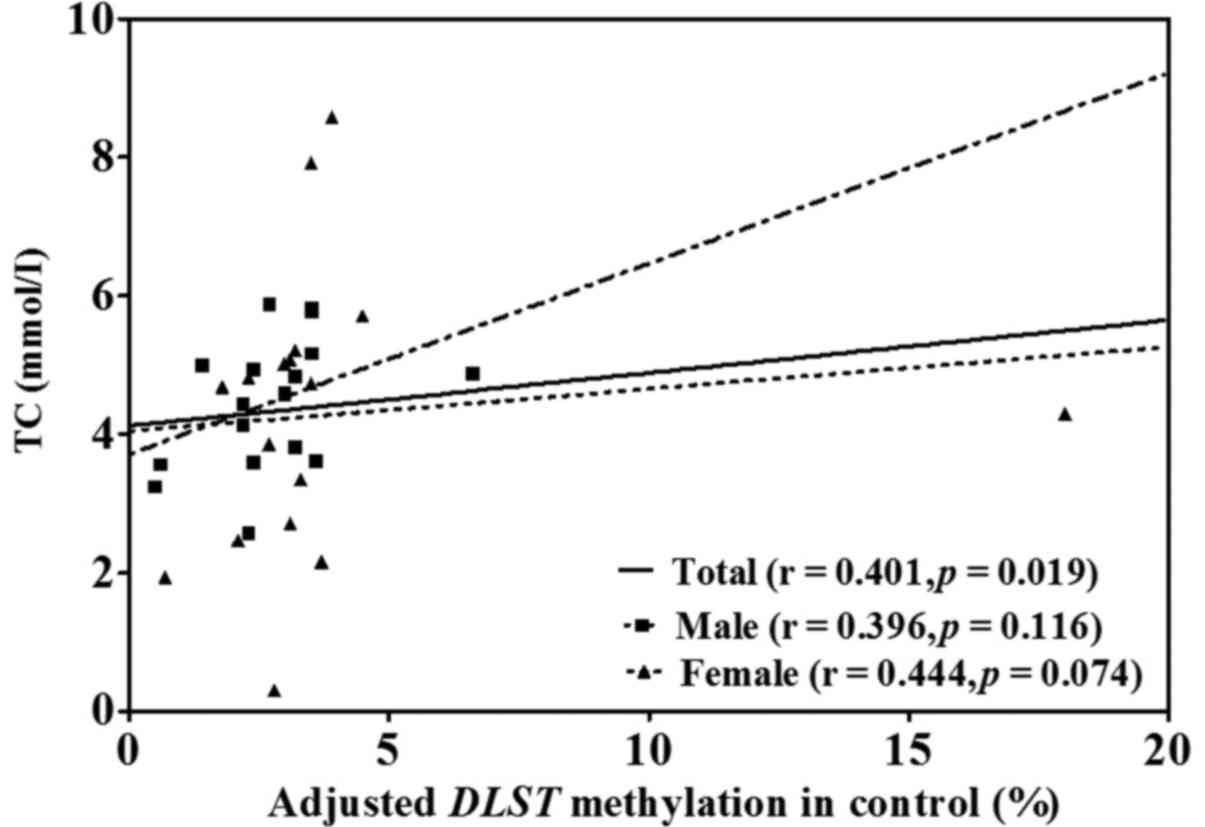
the APOE non-ε4 individuals (P=0.029). In the control group,
the level of DLST methylation was positively correlated with
TC (r=0.401, P=0.019; Table IV,
Fig. 3). Further stratification by
gender showed age and OGG1 methylation levels were
significantly correlated in AD group (male: r=0.762, P=0.046;
female: r=−0.753, P=0.012; Table V,
Fig. 4). In control group, the level of DLST methylation was
inversely correlated with age (r=−0.414, P=0.015), and further
stratified by gender showed that there was an inverse correlation
between age and DLST methylation in males (r=−0.607,
P=0.010).
 | Table IV.Correlation tests between the DNA
methylation and important parameters. |
Table IV.
Correlation tests between the DNA
methylation and important parameters.
|
| OGG1 | DLST |
|---|
|
|
|
|
|---|
|
| Case | Control | Case | Control |
|---|
|
|
|
|
|
|
|---|
| Variable | r | p | r | p | r | p | r | p |
|---|
| Total |
|
FBG | 0.309 | 0.228 | −0.285 | 0.113 | −0.336 | 0.187 | −0.081 | 0.648 |
| TG | −0.178 | 0.494 | 0.274 | 0.116 | −0.020 | 0.939 | 0.009 | 0.962 |
| TC | 0.259 | 0.315 | 0.039 | 0.825 | 0.440 | 0.077 | 0.401 | 0.019 |
|
HDL | 0.008 | 0.977 | 0.198 | 0.261 | 0.322 | 0.207 | 0.217 | 0.218 |
|
LDL | 0.283 | 0.271 | −0.019 | 0.914 | 0.322 | 0.207 | 0.217 | 0.218 |
| Female |
|
FBG | 0.347 | 0.326 | −0.458 | 0.064 | −0.623 | 0.055 | 0.156 | 0.550 |
| TG | −0.197 | 0.586 | 0.198 | 0.447 | 0.197 | 0.586 | −0.014 | 0.957 |
| TC | 0.282 | 0.430 | −0.033 | 0.899 | 0.241 | 0.552 | 0.444 | 0.074 |
|
HDL | −0.357 | 0.312 | 0.247 | 0.338 | 0.463 | 0.178 | 0.163 | 0.533 |
|
LDL | 0.464 | 0.177 | 0.051 | 0.845 | 0.111 | 0.760 | 0.145 | 0.579 |
| Male |
|
FBG | 0.635 | 0.125 | 0.006 | 0.983 | 0.208 | 0.654 | −0.294 | 0.252 |
| TG | −0.247 | 0.593 | 0.325 | 0.203 | −0.194 | 0.676 | −0.091 | 0.730 |
| TC | 0.382 | 0.398 | 0.279 | 0.278 | 0.675 | 0.096 | 0.396 | 0.116 |
|
HDL | 0.110 | 0.814 | 0.093 | 0.721 | 0.662 | 0.105 | 0.252 | 0.328 |
|
LDL | 0.255 | 0.628 | −0.120 | 0.645 | 0.511 | 0.241 | 0.242 | 0.349 |
 | Table V.Correlation tests between age and
methylation levels of OGG1 and DLST. |
Table V.
Correlation tests between age and
methylation levels of OGG1 and DLST.
|
| OGG1 | DLST |
|---|
|
|
|
|
|---|
|
| Case | Control | Case | Control |
|---|
|
|
|
|
|
|
|---|
| Age | r | p | r | p | r | p | r | p |
|---|
| Total |
|
Methylation level | −0.079 | 0.763 | −0.018 | 0.921 | 0.187 | 0.472 | −0.414 | 0.015 |
| Female |
|
Methylation level | −0.753 | 0.012 | 0.236 | 0.362 | 0.203 | 0.574 | −0.076 | 0.771 |
| Male |
|
Methylation level | 0.762 | 0.046 | −0.282 | 0.273 | 0.246 | 0.595 | −0.607 | 0.010 |
Discussion
Previous studies have revealed the association of
five variants with AD, including OGG1 rs1052133, BIN1
rs744373, SORL1 rs1133174, PSEN2 rs8383, and
NGF rs6330 (15,23–27). And
BIN1 rs744373 was found to have no interaction with
APOE ε4 genotype (28). In
the present study, we were unable to repeat the association of the
above five variants with AD. And further APOE ε4 based
subgroup analysis indicated that APOE ε4 did not have
significant effects on five genetic polymorphisms. This might be
explained by the moderate power and different ethnic background in
the present pilot study. Future validation is needed in cohort with
more samples.
DLST is a core component of KGDHC
which is essential in the citric acid cycle (29). Deficiency of DLST will increase
production of free radicals thereby inducing mitochondrial damage
(29), which leads to an increase in
the generation of reactive oxygen species (ROS). ROS damage various
molecules, including DNA, protein and lipid, and induce apoptosis
(30), eventually leading to the
occurrence of AD (31). The results
of this study suggest that DLST hypomethylation may
contribute to the pathogenesis of AD in females. Women are more
likely to have AD than men because women tend live longer than men
(32–34). This finding might also help explain
the sex differences in the risk of AD (35).
There were several limitations in the current study.
Firstly, our pilot study only involved a moderate number of
subjects (17 AD cases and 34 controls). This was due to the
incidence rate of AD being low in Xinjiang. However, we chose a
total of 51 well preserved samples, for which the transport process
was reasonable and the basic informations were completed and
matched. We were unable to validate the association of five gene
polymorphisms (OGG1 rs1052133, BIN1 rs744373,
SORL1 rs1133174, PSEN2 rs8383, and NGF rs6330)
with AD in the Xinjiang population. This might be due to the
limited number of samples in this study. Secondly, we only selected
a fragment in the promoter CpG rich region to represent the
methylation of OGG1 and DLST. The methylation of
other regions of the two genes might be explored in the future.
Thirdly, Xinjiang Uygur Autonomous Region is a multi-ethnic area.
Future research with larger sample sets and more ethnic populations
are required to confirm the present findings.
In summary, we found that the levels of DLST
methylation were decreased in AD patients, especially in female AD
patients. The results showed that the level of OGG1 promoter
methylation might be interacted with APOE ε4 genotype.
Acknowledgements
The authors would like to thank Professor Xiaohui
Zhou for providing guidance on the implementation of this project,
and thank Professor Shiwei Duan of the Basic Medical College of
Ningbo University in Zhejiang Province for supporting the
experiment and thesis writing.
Funding
This research was supported by the grants from the
National Natural Science Foundation of China (grant no.
U1503223).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SD, XZ and QW conceived and designed the
experiments. WC, TZ, YD, GL and XY performed the experiments. WC
and GL analyzed the data. GL and XY contributed
reagents/materials/analysis tools. WC, TZ and YD wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the First
Affiliated Hospital of Xinjiang Medical University Ethics
Committee. All the patients gave their written informed consent
forms for the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mancuso C, Bates TE, Butterfield DA,
Calafato S, Cornelius C, De Lorenzo A, Kostova Dinkova AT and
Calabrese V: Natural antioxidants in Alzheimer's disease. Expert
Opin Investig Drugs. 16:1921–1931. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feil R and Fraga MF: Epigenetics and the
environment: Emerging patterns and implications. Nat Rev Genet.
13:97–109. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiaoping L, Zhibin Y, Wenjuan L, Zeyou W,
Gang X, Zhaohui L, Ying Z, Minghua W and Guiyuan L: CPEB1, a
histone-modified hypomethylated gene, is regulated by miR-101 and
involved in cell senescence in glioma. Cell Death Dis. 4:e6752013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fabi E, Fusco A, Valiante M and Celli R:
Genetics and epigenetics of schizophrenia. Clin Ter. 164:e319–e324.
2013.(In Italian). PubMed/NCBI
|
|
5
|
Bian JT, Zhang JW, Zhang ZX and Zhao HL:
Association analysis of brain-derived neurotrophic factor (BDNF)
gene 196 A/G polymorphism with Alzheimer's disease (AD) in mainland
Chinese. Neurosci Lett. 387:11–16. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh SK, Srivastav S, Yadav AK,
Srikrishna S and Perry G: Overview of Alzheimer's disease and some
therapeutic approaches targeting Aβ by using several synthetic and
herbal compounds. Oxid Med Cell Longev. 2016:73616132016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chapuis J, Hansmannel F, Gistelinck M,
Mounier A, Van Cauwenberghe C, Kolen KV, Geller F, Sottejeau Y,
Harold D, Dourlen P, et al: Increased expression of BIN1 mediates
Alzheimer genetic risk by modulating tau pathology. Mol Psychiatry.
18:1225–1234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang F, Liu X, Wang B, Cheng Z, Zhao X,
Zhu J, Wang D, Wang Y, Dong A, Li P and Jin C: An exploratory study
of the association between SORL1 polymorphisms and sporadic
Alzheimer's disease in the Han Chinese population. Neuropsychiatr
Dis Treat. 11:1443–1448. 2014.
|
|
9
|
Xu CJ, Wang JL and Jin WL: The emerging
therapeutic role of NGF in Alzheimer's disease. Neurochem Res.
41:1211–1218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai Y, An SS and Kim S: Mutations in
presenilin 2 and its implications in Alzheimer's disease and other
dementia-associated disorders. Clin Interv Aging. 10:1163–1172.
2015.PubMed/NCBI
|
|
11
|
Schellenberg GD and Montine TJ: The
genetics and neuropathology of Alzheimer's disease. Acta
Neuropathol. 124:305–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan L, Yu JT, Zhang W, Wu ZC, Zhang Q, Liu
QY, Wang W, Wang HF, Ma XY and Cui WZ: Association of GWAS-linked
loci with late-onset Alzheimer's disease in a northern Han Chinese
population. Alzheimers Dement. 9:546–553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai
T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, et al: The
neuronal sortilin-related receptor SORL1 is genetically associated
with Alzheimer disease. Nat Genet. 39:168–177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen C, Zhou Z, Li M, Qu M, Ma Q, Zhong M,
Zhang Y and Yu Z: Presenilin-2 polymorphisms and risk of sporadic
AD: Evidence from a meta-analysis. Gene. 503:194–199. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jacob KD, Hooten Noren N, Tadokoro T,
Lohani A, Barnes J and Evans MK: Alzheimer's disease-associated
polymorphisms in human OGG1 alter catalytic activity and sensitize
cells to DNA damage. Free Radic Biol Med. 63:115–125. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shao C, Xiong S, Li GM, Gu L, Mao G,
Markesbery WR and Lovell MA: Altered 8-oxoguanine glycosylase in
mild cognitive impairment and late-stage Alzheimer's disease brain.
Free Radic Biol Med. 45:813–819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coppedè F, Tannorella P, Stoccoro A, Chico
L, Siciliano G, Bonuccelli U and Migliore L: Methylation analysis
of DNA repair genes in Alzheimer's disease. Mech Ageing Dev.
161:105–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakano K, Ohta S, Nishimaki K, Miki T and
Matuda S: Alzheimer's disease and DLST genotype. Lancet.
350:1367–1368. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sheu KF, Brown AM, Haroutunian V, Kristal
BS, Thaler H, Lesser M, Kalaria RN, Relkin NR, Mohs RC, Lilius L,
et al: Modulation by DLST of the genetic risk of Alzheimer's
disease in a very elderly population. Ann Neurol. 45:48–53. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sheu KF, Brown AM, Kristal BS, Kalaria RN,
Lilius L, Lannfelt L and Blass JP: A DLST genotype associated with
reduced risk for Alzheimer's disease. Neurology. 52:1505–1507.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fischer BA: A review of American
psychiatry through its diagnoses: The history and development of
the diagnostic and statistical manual of mental disorders. J Nerv
Ment Dis. 200:1022–1030. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma W, Zhou X, Ji H, Luo M, Liu G, Li J,
Wang Q and Duan S: Population difference in the association of BDNF
promoter methylation with mild cognitive impairment in the Xinjiang
Uygur and Han populations. Psychiatry Res. 229:926–932. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwiatkowski D, Czarny P, Toma M, Jurkowska
N, Sliwinska A, Drzewoski J, Bachurska A, Szemraj J, Maes M, Berk
M, et al: Associations between DNA damage, DNA base excision repair
gene variability and Alzheimer's disease risk. Dement Geriatr Cogn
Disord. 41:152–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu R, Xu L and He Z: The bridging
integrator 1 gene polymorphism rs744373 and the risk of Alzheimer's
disease in caucasian and Asian populations: An updated
meta-analysis. Mol Neurobiol. 54:1419–1428. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng X, Hou D, Deng Y, Li W, Tian M and Yu
Z: SORL1 gene polymorphism association with late-onset Alzheimer's
disease. Neurosci Lett. 584:382–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue X, Zhang M, Lin Y, Xu E and Jia J:
Association between the SORL1 rs2070045 polymorphism and late-onset
Alzheimer's disease: Interaction with the ApoE genotype in the
Chinese Han population. Neurosci Lett. 559:94–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Di Maria E, Giorgio E, Uliana V, Bonvicini
C, Faravelli F, Cammarata S, Novello MC, Galimberti D, Scarpini E,
Zanetti O, et al: Possible influence of a non-synonymous
polymorphism located in the NGF precursor on susceptibility to
late-onset Alzheimer's disease and mild cognitive impairment. J
Alzheimers Dis. 29:699–705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gharesouran J, Rezazadeh M, Khorrami A,
Ghojazadeh M and Talebi M: Genetic evidence for the involvement of
variants at APOE, BIN1, CR1, and PICALM loci in risk of late-onset
Alzheimer's disease and evaluation for interactions with APOE
genotypes. J Mol Neurosci. 54:780–786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keßler M, Berger IM, Just S and Rottbauer
W: Loss of dihydrolipoyl succinyltransferase (DLST) leads to
reduced resting heart rate in the zebrafish. Basic Res Cardiol.
110:142015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanamori T, Nishimaki K, Asoh S, Ishibashi
Y, Takata I, Kuwabara T, Taira K, Yamaguchi H, Sugihara S, Yamazaki
T, et al: Truncated product of the bifunctional DLST gene involved
in biogenesis of the respiratory chain. EMBO J. 22:2913–2923. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schulz JB, Lindenau J, Seyfried J and
Dichgans J: Glutathione, oxidative stress and neurodegeneration.
Eur J Biochem. 267:4904–4911. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alzheimer's Association: 2014 Alzheimer's
disease facts and figures. Alzheimers Dement. 10:e47–e92. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mangialasche F, Kivipelto M, Solomon A and
Fratiglioni L: Dementia prevention: Current epidemiological
evidence and future perspective. Alzheimers Res Ther. 4:62012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Solomon A, Kivipelto M and Soininen H:
Prevention of Alzheimer's disease: Moving backward through the
lifespan. J Alzheimers Dis. 33 Suppl 1:S465–S469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen R, Hu Z, Wei L, Ma Y, Liu Z and
Copeland JR: Incident dementia in a defined older Chinese
population. PLoS One. 6:e248172011. View Article : Google Scholar : PubMed/NCBI
|